Introduction
Ebstein anomaly is a rare congenital cardiac abnormality involving the tricuspid valve and the right ventricle (RV).[1] Ebstein anomalies comprise < 1% of congenital heart defects.[2] The anomaly was initially described by the pathologist Wilhelm Ebstein in 1866 after performing an autopsy on a 19-year-old cyanotic male with exertional dyspnea and palpitations who died of a sudden cardiac arrest.[3] Ebstein anomaly is defined by the following characteristics:
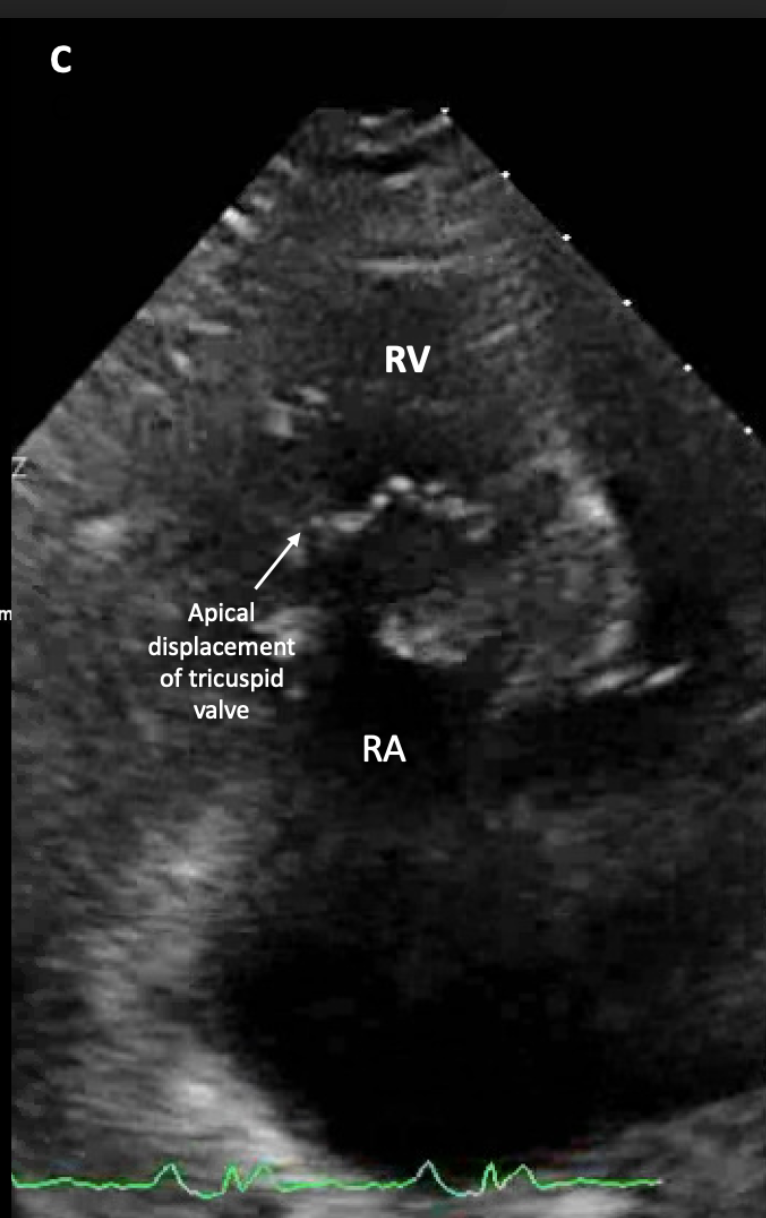
- Apical displacement of the tricuspid valve
- Adherence of the septal and posterior tricuspid leaflets to the myocardium
- Apical displacement and dilatation of the tricuspid annulus
- Dilatation of the atrialized portion of the right ventricle
- Redundancy, fenestrations, and tethering of the anterior tricuspid leaflet [2][4]
Abnormalities commonly associated with Ebstein anomaly include a secundum atrial septal defect and a variable degree of RV outflow tract obstruction. Some neonates with Ebstein anomaly may present with functional or anatomical pulmonary atresia; both are ductal-dependent lesions. Less commonly associated cardiac defects include ventricular septal defects, tetralogy of Fallot, transposition of the great arteries, and atrioventricular canal defects.[5] In general, the clinical manifestations of Ebstein anomaly can range from asymptomatic to severe, depending on the degree of tricuspid valve displacement and severity of regurgitation, the effective right ventricular volume, and the associated malformations.[6]
Atrial tachycardias, including atrial fibrillation, atrial flutter, or ectopic atrial tachycardia, can occur in 25% to 65% of patients with Ebstein anomaly. Additionally, 10% to 25% of patients have one or more accessory pathways, which increase the risk of protracted arrhythmias that can produce cardiac failure and sudden cardiac death.[4][7]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Ebstein anomaly is thought to be associated with chromosome 15q duplications during embryological cardiac development. There are also case reports suggesting a possible association of Ebstein anomaly with chromosome 11q rearrangements. Other reports indicate an association between Ebstein malformation and mutations in MYH7; MYH7 mutations are frequently associated with cardiomyopathies. Ebstein anomaly has also been associated with mutations in NKX2-5, which encodes a cardiac transcription factor responsible for the association between atrial septal defects and atrioventricular conduction defects. Mutations in NKX2-5 have also been associated with tetralogy of Fallot.[8][9]
Another known association of Ebstein anomaly is the teratogenic effect of lithium. Case-control and cohort studies of pregnant women taking lithium in the 1970s and 1980s demonstrated a risk of Ebstein anomaly in < 2% of their newborns.[10] Ebstein anomaly has also been associated with maternal exposure to benzodiazepines and varnishing materials.[7][11]
Epidemiology
Ebstein anomaly accounts for 0.3% to 0.6% of all congenital heart defects and is reported to occur in 0.2 to 0.7 per 10,000 live births.[12] Most cases of Ebstein anomaly are sporadic, with no identifiable etiology. Some studies suggest that there may be a familial inheritance, as has been observed in monozygotic twins.[13] There is a higher incidence of recurrence in the offspring of affected women (6%) than in the offspring of affected men (1%).[14]
Pathophysiology
Anatomic Abnormalities
The main pathophysiological abnormality of Ebstein anomaly is the failure of delamination of the tricuspid valve leaflets from the interventricular septum in utero.[15] The apical displacement mainly affects the posterior and septal leaflets, leading to the apical displacement of the tricuspid annulus and anteroapical displacement of the tricuspid orifice.[13][16] This apical displacement has also been described as a rotational displacement of the tricuspid leaflets towards the RV outflow tract. The displacement is usually defined as > 8mm/m2 displacement of the septal leaflet from the anatomic tricuspid annulus.[17] The anterior leaflet of the tricuspid valve often has abnormal chordal attachments and becomes hypermobile, described as "sail-like." Alternately, there may be tethering of the anterior leaflet that causes restricted motion.[15][16] There may also be fenestrations of the anterior leaflet.[4]
The RV becomes split into two portions: the "atrialized" and the "functional" RV. The atrialized RV is where the tricuspid valvular inflow should normally be, extending from the undisplaced tricuspid annulus to the functional RV. The atrialized RV receives the regurgitant flow from the tricuspid regurgitation and becomes dilated along with the right atrium (RA). The functional RV can be very small and may consist of just the RV outflow tract in cases of severe apical displacement of the septal and posterior leaflets of the tricuspid valve.[2][4]
More than 80% of patients with Ebstein anomaly also have a secundum atrial septal defect or a patent foramen ovale through which paradoxical emboli can travel.[18] Ventricular septal defects and pulmonary atresia have been concurrently found with Ebstein malformation.[13] One study of 539 patients at the Mayo Clinic demonstrated the incidences of the following cardiac abnormalities and Ebstein anomaly: atrial septal defect or patent foramen ovale in 84%, ventricular septal defect in 4.3%, pulmonary stenosis requiring surgical intervention in 6%, and accessory conduction pathways in 14%.[7]
Ebstein anomaly associated with conduction system abnormalities is thought to be due to compression of the atrioventricular node by the septal leaflet, disrupting its function. Accessory pathways are common and often multiple.[17] Furthermore, 5% to 25% of patients with Ebstein anomaly have been reported to have Wolff-Parkinson-White (WPW) syndrome, making Ebstein anomaly the most commonly found congenital heart defect associated with WPW.[19]
Functional Abnormalities
The faulty coaptation of the tricuspid leaflets due to their anatomic displacement and tethering abnormalities leads to tricuspid regurgitation. The degree of tricuspid regurgitation is dictated by the severity of the apical displacement of the posterior and septal leaflets, the abnormality of the anterior leaflet attachments, and the integrity of the leaflets themselves.[13][15] The atrialized RV is often dyskinetic from fibrosis and myopathy, and the functional RV can become enlarged and dysfunctional from severe tricuspid regurgitation.[13][20] Right ventricular volume overload produces ventricular septal flattening with left ventricular dysfunction.[13] The frequently associated atrial septal defect or a stretched patent foramen ovale will permit right to left shunting from severe tricuspid regurgitation, resulting in hypoxemia and cyanosis.[21]
Histopathology
The degree of tricuspid leaflet delamination failure varies from mild to absent delamination. The surgical pathology of patients who underwent tricuspid valve replacement has revealed that tricuspid leaflets in Ebstein anomaly are usually large, irregularly shaped, thin, transparent, and have abnormal insertion points secondary to short chordal attachments or direct myocardial insertion. The septal leaflet often has a fibrous ridge, and leaflets can have muscularized portions.[17][22]
There is also an atypical Ebstein anomaly whereby the displacement of the septal and posterior leaflets is less than 8 mm/m2; the anterior leaflet is typically normal, and there is less severe tricuspid annular dilation, no abnormalities of the subvalvular apparatus, and no "atrialization" of the RV.[17] Rarely, studies of mitral valves in patients with Ebstein anomaly have shown primary mitral valve defects involving the mitral orifice, leaflets, papillary muscles, and cords. Approximately 50% of the mitral abnormalities were noted to have functional significance.[23][24]
History and Physical
Patients with Ebstein anomaly display a spectrum of clinical manifestations; the age at presentation varies. Most cases of Ebstein anomaly present in infancy or childhood but a significant percentage of patients present in adulthood.[4] Ebstein malformation can be detected in utero, with apical displacement of the tricuspid valve, tricuspid regurgitation, and sometimes an increased cardiothoracic ratio.[25] Fetal demise and perinatal mortality rates are high at 20% and 45%, respectively. A large multicenter study reported the fetal echocardiographic parameters associated with higher perinatal mortality to appear to be a gestational age of < 32 weeks at the time of diagnosis, a cardiothoracic area ratio of > 0.47 ± 0.12, a larger tricuspid annulus diameter by z-score, presence of pulmonary regurgitation, and pericardial effusion.[26]
The most common physical finding in neonates and infants with Ebstein anomaly is cyanosis, present in about 50% of cases.[27] Even in the presence of tricuspid regurgitation and RV dysfunction, those without cyanosis are more likely to survive into early childhood.[5] Symptoms of heart failure in early childhood may be a prominent v wave in the jugular venous pulse from severe tricuspid regurgitation; this finding may be absent in torrential tricuspid regurgitation due to rapid equalization of pressures across the tricuspid valve.[28] There may be a loud first heart sound due to anterior leaflet closure, referred to as the "sail sound," and the first heart sound may be split due to delayed tricuspid valve closure. A holosystolic murmur of tricuspid regurgitation is another common physical finding in these patients. Multiple ejection clicks can be heard in the presence of a very mobile anterior leaflet.[13][27]
Studies suggest that slightly more than half of adult patients with Ebstein anomaly experience palpitations, with tachyarrhythmias being the first presenting clinical manifestation in almost 40% of those newly diagnosed with Ebstein anomaly.[5][13] Symptoms of exertional dyspnea are commonly observed in older patients. Dyspnea can occur as a result of right-to-left shunting with resultant hypoxemia. Chronic fatigue and lower extremity edema are other clinical symptoms in patients who develop heart failure. Rarely, patients can present with stroke, brain abscess, or myocardial infarction, all due to paradoxical emboli across an intracardiac shunt.[13] Physical examination in adults with Ebstein anomaly reveals the murmur of tricuspid regurgitation. A hyperdynamic precordium and a thrill on the left lower sternal border may be present in severe cases. The second heart sound may be widely split in the presence of an atrial septal defect or right bundle branch block.[27]
Evaluation
Electrocardiogram
The ECG of patients with Ebstein anomaly is usually abnormal. Abnormal findings include:
- Right bundle branch block (RBBB)
- Delta waves due to preexcitation from the accessory pathway
- Tall P waves that suggest right atrial enlargement
- First-degree atrioventricular (AV) block is observed in approximately 42% of patients due to intra-atrial conduction delay secondary to right atrial enlargement or structural abnormalities of the atrioventricular conduction system
- Supraventricular tachyarrhythmias, most commonly atrioventricular reentrant tachycardia, but also atrial fibrillation, atrial flutter, and atrial tachycardia.[2][17]
Cardiothoracic Imaging
Chest radiography: typically reveals cardiomegaly with right heart enlargement; the cardiac silhouette may appear globular. An increased cardiothoracic ratio from right atrial enlargement may be seen.[28]
Echocardiography: is the imaging study of choice to evaluate Ebstein anomaly. The first echocardiographic criteria to detect Ebstein anomaly is apical displacement of the septal leaflet of the tricuspid valve greater than 8 mm/m2 in the apical 4-chamber view. The mitral valve annulus is used to calculate the hinge point of the nondisplaced tricuspid septal leaflet.[29] Right atrial dilatation and interventricular septal flattening due to right ventricular volume overload may be observed. Tricuspid regurgitation can also be detected with color Doppler. Echocardiography can determine the severity of tricuspid regurgitation; this may be challenging due to the absence of systolic flow reversal in the hepatic veins due to equalization of pressures between the RA and RV from a large, low-pressure, compliant RA and the inability to calculate vena contracta and proximal isovelocity surface area due to the presence of multiple color jets.[17][20]
Echocardiographic assessment of RV size and function can be difficult using traditional methods. Typically, only a qualitative assessment based on ventricular function and septal motion is used to evaluate the RV. Right ventricular strain can be used to evaluate RV function. Transesophageal echocardiography can better visualize the tricuspid leaflet anatomy and the characteristics of the color Doppler jet while simultaneously assisting with surgical planning. Transesophageal echocardiography is usually superior to transthoracic echocardiography in detecting intracardiac shunts.[20]
Cardiac magnetic resonance imaging (cMRI): can add valuable information regarding tricuspid leaflet anatomy. cMRI is superior to echocardiography in assessing RV function because it permits quantification of the RV ejection fraction; this correlates well with echocardiographic RV strain measurements.[30] Late gadolinium enhancement is an indicator of myocardial fibrosis, and patients with Ebstein anomaly frequently demonstrate this finding in the RA and RV.[7]
Cardiac computed tomography (CT): is another modality to assess RV ejection fraction and tricuspid valve anatomy. Cardiac CT can be used for periprocedural imaging of coronary anatomy before surgical intervention on the tricuspid valve.[17]
Treatment / Management
Medical Management
In infants with Ebstein anomaly, the mainstay of treatment is supportive to reduce pulmonary vascular resistance and hypoxemia. In symptomatic infants with either heart failure or cyanosis, inhaled nitric oxide can reduce pulmonary vascular resistance.[31] In cyanotic infants, prostaglandin E1 (PGE1) infusion can maintain ductus arteriosus patency and lower pulmonary vascular resistance by increasing pulmonary vasodilation. Newborns with heart failure and cardiogenic shock may require inotropic therapy; milrinone is the drug of choice since it contributes to pulmonary vasodilation. Catecholamines such as epinephrine and norepinephrine are usually avoided in these patients, secondary to the increased risk of tachyarrhythmias.[32](A1)
Symptoms of heart failure are treated with loop diuretics and guideline-directed medical therapy. Patients with supraventricular tachyarrhythmias can receive rate-control medications such as beta blockers or calcium channel blockers; if ineffective, the class I antiarrhythmic procainamide or class III antiarrhythmic amiodarone or sotalol can be used to treat paroxysmal atrial fibrillation. Patients with intractable arrhythmias frequently require catheter ablation.
Surgical Management
Newborns with Ebstein anomaly are initially observed as the pulmonary vascular resistance decreases. If surgery is required, a systemic-to-pulmonary artery shunt is typically employed to relieve cyanosis and secure adequate pulmonary and systemic circulations. Due to high periprocedural mortality, more definitive surgery is usually delayed unless the neonate meets specific indications. Data show that delaying surgery until heart failure or RV systolic dysfunction occurs is associated with worse outcomes. This is thought to be because Ebstein anomaly is a myopathic disorder in addition to abnormal valves, and the myopathic RV may be unable to tolerate increased volumes.[33](A1)
Indications for surgery in neonates with Ebstein anomaly include:
- Right heart failure due to severe tricuspid regurgitation
- A cardiothoracic ratio > 80%
- Severe cyanosis with dependency on PGE1
- Mechanical ventilation dependency.[32][34] (A1)
In children and adults with Ebstein anomaly, indications for surgery include:
- Heart failure symptoms (New York Heart Association functional class III or IV)
- Progressive exercise intolerance
- Evidence of RV dysfunction or progressive RV dilatation
- Evidence of paradoxical emboli
- Arrhythmias refractory to medical and catheter ablation therapies
- Cyanosis with oxygen saturations < 90%
- Severe tricuspid regurgitation
- Significant concomitant lesions such as pulmonic stenosis or atrial or ventricular septal defects
- Progressive cardiomegaly
- Cardiothoracic ratio > 65%
- Reduced left ventricular function.[32][35][36] (A1)
Surgical intervention comprises tricuspid valve repair and patch closure of the atrial septal defect. For atrial arrhythmias in these patients, catheter ablation carries risks of paradoxical emboli, given the high incidence of interatrial shunts. A surgical biatrial Maze procedure during valvular repair is recommended.[37]
Multiple surgical approaches have been described for the treatment of Ebstein anomaly. Cone reconstruction is the currently preferred surgical approach in young children and adults. This approach involves mobilizing the anterior and posterior leaflets from their anomalous attachments, rotating the detached edges of these leaflets clockwise, and suturing them to the septal edge of the anterior leaflet at the level of the tricuspid valve annulus.[38] The Danielson repair is the plication of the atrialized portion of the RV to narrow the size of the tricuspid valve and create a monoleaflet tricuspid valve that is competent.[39] The Carpentier monocusp repair plicates the atrialized portion of the RV, implants a ring within the tricuspid annulus to provide support, mobilizes the anterior tricuspid valve leaflet, and attaches it to the annulus anteriorly.[40]
When the native tricuspid valve cannot be repaired, especially in older patients, it can be replaced with a bioprosthetic or mechanical valve. A bioprosthetic valve might require temporary anticoagulation; a mechanical valve will require lifelong anticoagulation. Mechanical valves are avoided when RV dysfunction is severe to mitigate disc motion abnormalities and the increased risk of valve thrombosis.[4]
Catheter Interventions
Some patients with Ebstein anomaly have a functional tricuspid valve and are not candidates for surgical valve repair. They may have a secundum atrial septal defect amenable to transcatheter device closure or tachyarrhythmias that can be treated in the cardiac catheterization laboratory.[34] (B2)
Many patients with intractable arrhythmias need definitive ablative therapy for supraventricular arrhythmias.[41]Definitive ablative therapy is often challenging due to the frequent presence of multiple accessory pathways in the setting of a markedly enlarged right heart with a displaced tricuspid annulus and distortion of the anatomic landmarks; these all complicate catheter stability.[42] Therefore, catheter ablation has a higher arrhythmia recurrence rate in patients with Ebstein anomaly than in structurally normal hearts. However, surgical cryoablation for accessory pathway-mediated tachycardia at the time of tricuspid valve repair and ASD closure can yield excellent results.[34] Patients with atrial arrhythmias with ventricular preexcitation and those with multiple accessory pathways are at significantly increased risk of complications. Holter monitoring studies should be performed for arrhythmia symptoms, such as palpitations or syncope, and referral for further electrophysiologic studies should be made if indicated. There is a low threshold for electrophysiologic intervention prior to cardiac surgery, given the susceptibility of patients with Ebstein abnormality to arrhythmias.[33](A1)
Prophylactic Management
Infective endocarditis antibiotic prophylaxis for dental procedures is recommended for patients with uncorrected Ebstein anomaly and cyanosis, prosthetic valves, any other prosthetic cardiac material in the last 6 months, or a history of endocarditis.[43] Experts suggest using oral anticoagulation for patients with Ebstein anomaly and atrial fibrillation or evidence of paradoxical embolus.[44][45]
Differential Diagnosis
Cyanotic congenital heart conditions whose presentation can mimic Ebstein anomaly include transposition of the great arteries, tetralogy of Fallot, pulmonary atresia, hypoplastic left heart syndrome, and total anomalous pulmonary venous return. Echocardiography allows for correct diagnosis.[46] Other differential diagnoses include:
Congenital tricuspid atresia: can be differentiated from Ebstein anomaly by the absence of apical displacement of the septal and posterior leaflets, no annular dilation, the presence of thickened leaflets with rolled edges, foreshortened and fused chordae, and no atrialization of the RV.[17]
Uhl anomaly: is characterized by the congenital absence of RV myocardium that leads to RV failure. Uhl anomaly is frequently associated with pulmonary atresia, RV aneurysm, and thrombi. There is no apical displacement of the septal leaflet of the tricuspid valve. The major similarity between Uhl anomaly and Ebstein malformation is the RV and right atrial dilation; distinguishing between these processes can be challenging.[47]
Aberrant tendinous tricuspid valve chords with tethering of the leaflets: is a very rare congenital heart defect characterized by aberrant tendinous tricuspid chords that impair leaflet mobility with resultant incomplete coaptation of the tricuspid valve leaflets and severe regurgitation. This is a very rare congenital heart defect.[48]
Prognosis
The main predictors of mortality in patients with Ebstein anomaly include the degree of tricuspid displacement, amount of tricuspid regurgitation, and duration and degree of RV dysfunction.[49] Cyanosis and pericardial effusion pose a high risk for fetal and perinatal mortality.[26] Concomitant pulmonary valve regurgitation in newborns with Ebstein anomaly carries a poor prognosis since it leads to a circular shunt, where flow returns from the pulmonary artery to the RV, then from the RV to the RA through the tricuspid regurgitation, then from the RA to the left atrium via a dilated patent foramen ovale or a secundum atrial septal defect. This shunt creates a low cardiac output state.[32]
Decreased survival is seen in patients with Ebstein anomaly who do not undergo surgical repair; survival rates for these patients are 90% at 1 year, 75% at 10 years, 50% at 15 years, and 40% at 20 years.[50] Sudden cardiac death due to ventricular arrhythmias has been reported as an 8.6% in a 50-year cumulative incidence from birth.[51]
In patients who undergo surgical repair, significant RV dysfunction is an independent predictor of early postsurgical mortality.[18] Late postoperative outcomes in patients with Ebstein anomaly have shown an overall survival rate of 98% at 1 year, 94% at 4 years, 90% at 10 years, 86% at 15 years, and 76% at 20 years.[18] More specifically, after cone reconstruction, only 2% of patients needed reoperation at 6 years.[52]
The Celermajer index is an established score that uses echocardiography to predict mortality. It is measured as the ratio between the area of the RA and the atrialized RV to the combined area of the functional RV, the left atrium, and the left ventricle at end-diastole. Mortality increases with increasing grade.
- Grade 1: ratio less than 0.5; mortality of 0%.
- Grade 2: ratio of 0.5 to 0.99; mortality of 10%.
- Grade 3: ratio of 1.0 to 1.49; mortality of 4%.
- Grade 4: ratio greater than 1.5; 100% mortality.[53]
The Simpson Andrews Sharland (SAS) score is another prognostic tool employing a weighted model to predict mortality. The cardiothoracic ratio, the Celermajer index, pulmonary valve flow, duct flow, and left-right ventricular ratio are graded 0, 1, or 2 points each to generate a score. In studies, a score of 5 predicted 100% mortality, and a score of 3 or less predicted 91% survival.[54]
Complications
Complications in Patients With Unrepaired Ebstein Anomaly
The most common complications in this subset of patients include:
- Heart failure resulting from right or biventricular dysfunction or tachyarrhythmias
- Intractable atrial or atrioventricular arrhythmias
- Paradoxical emboli resulting in myocardial infarction or cerebrovascular accident
- Sudden cardiac death due to tachyarrhythmias or heart failure.[4]
Long-term predictors of death in patients with unrepaired Ebstein anomaly include:
- Severe tricuspid regurgitation
- Cardiothoracic ratio ≥ 65%
- Impaired pulmonary growth and function from cardiomegaly
- Impaired left ventricular function from RV dilatation with consequent compression and displacement of the interventricular septum
- NYHA functional class III or IV
- Cyanosis
- Early age at diagnosis.[50]
Complications After Surgical Intervention in Patients With Ebstein Anomaly
Short-term complications include:
- Symptomatic arrhythmias
- Reoperation due to significant residual tricuspid valve dysfunction, either insufficiency or stenosis.
Long-term complications with the need for reoperation include:
- Ongoing atrial arrhythmias from right atrial dilatation or surgical scarring
- Progressive tricuspid valve dysfunction.[36]
Deterrence and Patient Education
Ebstein anomaly is a rare congenital heart defect with variable presentation. Disease severity varies across a broad spectrum from newborns who do not survive beyond infancy to asymptomatic adults diagnosed incidentally in the sixth and seventh decades of life. Patients should be educated about symptoms of worsening function, such as cyanosis, symptoms of heart failure, and those of arrhythmia, such as palpitations, presyncope, and syncope.[33]
Pearls and Other Issues
- Ebstein anomaly is a malformation with upward displacement of the tricuspid valve resulting in tricuspid regurgitation and variable right ventricular structural abnormalities.
- The clinical presentation of patients with Ebstein anomaly is heterogeneous.
- Most patients with Ebstein anomaly will require surgical intervention, especially when diagnosed as neonates or young children.
- Atrial tachyarrhythmias and accessory pathways are frequent and predispose to lethal ventricular tachyarrhythmias.
- Given the frequency of arrhythmia, there is a low threshold for further electrophysiologic studies and intervention, especially before defect repair.
- Cone reconstruction of the tricuspid valve demonstrates promising short- and long-term results.
- Delaying surgery until RV systolic dysfunction or heart failure develops is associated with worse outcomes. Some studies suggest improved outcomes with early intervention.[7]
Enhancing Healthcare Team Outcomes
Infants with moderate to severe Ebstein anomaly come to attention soon after birth. These infants need NICU monitoring, and hence, the ICU nurse must be familiar with the management of congenital heart disorders. While some infants have an isolated heart anomaly, others may also have involvement in other organ systems. Hence, appropriate consultations are required. Once the diagnosis is made, surgery is usually required. Postsurgery, these children require close monitoring for an extended period until pacing wires and chest tubes have been removed and the child is breathing without the aid of a mechanical ventilator.[41] As a result, the management of Ebstein anomaly requires robust interdisciplinary coordination between pediatricians or primary care physicians, congenital heart disease specialists, cardiothoracic and pediatric surgeons, and intensivists to improve patient outcomes.
As the Ebstein malformation varies in severity, some patients reach adulthood with few or minimal symptoms, which can appear late as worsening dyspnea, with or without cyanosis, during exercise, and arrhythmias from atrial flutter/fibrillation or atrioventricular reentry tachycardia via accessory pathways, which are common in these patients. Patient management for CHF, cyanosis, and arrhythmias requires a cardiologist or a pediatric and congenital electrophysiologist. Worsening tricuspid regurgitation might necessitate surgery. Other forms of imaging, such as cardiac MRI or cardiac CT, might be required, especially when transthoracic echocardiography is challenging. Transesophageal echocardiography might be needed for further tricuspid valve assessment, especially before surgery. It has been acknowledged that pediatric patients with congenital heart disease should undergo neurodevelopmental or neuropsychological evaluation and treatment and that older pediatric and adult patients with congenital heart disease should be evaluated and treated for depression and anxiety to enhance their academic, behavioral, and psychosocial functioning.[33]
Media
(Click Image to Enlarge)
References
Mulla S, Asuka E, Bora V, Siddiqui WJ. Tricuspid Regurgitation. StatPearls. 2024 Jan:(): [PubMed PMID: 30252377]
Attenhofer Jost CH, Connolly HM, Dearani JA, Edwards WD, Danielson GK. Ebstein's anomaly. Circulation. 2007 Jan 16:115(2):277-85 [PubMed PMID: 17228014]
Mazurak M, Kusa J. The Two Anomalies of Wilhelm Ebstein. Texas Heart Institute journal. 2017 Jun:44(3):198-201. doi: 10.14503/THIJ-16-6063. Epub 2017 Jun 1 [PubMed PMID: 28761400]
Holst KA, Connolly HM, Dearani JA. Ebstein's Anomaly. Methodist DeBakey cardiovascular journal. 2019 Apr-Jun:15(2):138-144. doi: 10.14797/mdcj-15-2-138. Epub [PubMed PMID: 31384377]
Kumar TKS. Ebstein's anomaly in the neonate. Indian journal of thoracic and cardiovascular surgery. 2021 Jan:37(Suppl 1):17-25. doi: 10.1007/s12055-020-00942-z. Epub 2020 Mar 21 [PubMed PMID: 33603283]
Possner M, Gensini FJ, Mauchley DC, Krieger EV, Steinberg ZL. Ebstein's Anomaly of the Tricuspid Valve: an Overview of Pathology and Management. Current cardiology reports. 2020 Oct 9:22(12):157. doi: 10.1007/s11886-020-01412-z. Epub 2020 Oct 9 [PubMed PMID: 33037480]
Level 3 (low-level) evidenceNeumann S, Rüffer A, Sachweh J, Biermann D, Herrmann J, Jerosch-Herold M, Hazekamp M, Sinning C, Zengin E, Blankenberg S, Girdauskas E, Reichenspurner H, Kehl T, Müller G, Kozlik-Feldmann R, Rickers C. Narrative review of Ebstein's anomaly beyond childhood: Imaging, surgery, and future perspectives. Cardiovascular diagnosis and therapy. 2021 Dec:11(6):1310-1323. doi: 10.21037/cdt-20-771. Epub [PubMed PMID: 35070800]
Level 3 (low-level) evidenceYuan SM. Ebstein's Anomaly: Genetics, Clinical Manifestations, and Management. Pediatrics and neonatology. 2017 Jun:58(3):211-215. doi: 10.1016/j.pedneo.2016.08.004. Epub 2016 Nov 19 [PubMed PMID: 28017577]
Miranda-Fernández MC, Ramírez-Oyaga S, Restrepo CM, Huertas-Quiñones VM, Barrera-Castañeda M, Quero R, Hernández-Toro CJ, Tamar Silva C, Laissue P, Cabrera R. Identification of a New Candidate Locus for Ebstein Anomaly in 1p36.2. Molecular syndromology. 2018 May:9(3):164-169. doi: 10.1159/000488820. Epub 2018 Apr 28 [PubMed PMID: 29928183]
Cohen LS, Friedman JM, Jefferson JW, Johnson EM, Weiner ML. A reevaluation of risk of in utero exposure to lithium. JAMA. 1994 Jan 12:271(2):146-50 [PubMed PMID: 8031346]
Level 3 (low-level) evidenceCorrea-Villaseñor A, Ferencz C, Neill CA, Wilson PD, Boughman JA. Ebstein's malformation of the tricuspid valve: genetic and environmental factors. The Baltimore-Washington Infant Study Group. Teratology. 1994 Aug:50(2):137-47 [PubMed PMID: 7801301]
Level 2 (mid-level) evidenceSharma N, Lalnunnem TJ, Nandwani M, Santa SA, Synrang BW. Ebstein Anomaly with Pregnancy: A Rare Case. Journal of reproduction & infertility. 2018 Apr-Jun:19(2):119-122 [PubMed PMID: 30009147]
Level 3 (low-level) evidenceFuchs MM, Connolly HM. Ebstein Anomaly in the Adult Patient. Cardiology clinics. 2020 Aug:38(3):353-363. doi: 10.1016/j.ccl.2020.04.004. Epub 2020 Jun 6 [PubMed PMID: 32622490]
Connolly HM, Warnes CA. Ebstein's anomaly: outcome of pregnancy. Journal of the American College of Cardiology. 1994 Apr:23(5):1194-8 [PubMed PMID: 8144788]
Dearani JA, Mora BN, Nelson TJ, Haile DT, O'Leary PW. Ebstein anomaly review: what's now, what's next? Expert review of cardiovascular therapy. 2015 Oct:13(10):1101-9. doi: 10.1586/14779072.2015.1087849. Epub 2015 Sep 10 [PubMed PMID: 26357983]
Anderson KR, Zuberbuhler JR, Anderson RH, Becker AE, Lie JT. Morphologic spectrum of Ebstein's anomaly of the heart: a review. Mayo Clinic proceedings. 1979 Mar:54(3):174-80 [PubMed PMID: 431123]
Stephens EH, Dearani JA, Qureshi MY, Ammash N, Maleszewski JJ. The Congenital Tricuspid Valve Spectrum: From Ebstein to Dysplasia. World journal for pediatric & congenital heart surgery. 2020 Nov:11(6):783-791. doi: 10.1177/2150135120949235. Epub [PubMed PMID: 33164686]
Brown ML, Dearani JA, Danielson GK, Cetta F, Connolly HM, Warnes CA, Li Z, Hodge DO, Driscoll DJ, Mayo Clinic Congenital Heart Center. The outcomes of operations for 539 patients with Ebstein anomaly. The Journal of thoracic and cardiovascular surgery. 2008 May:135(5):1120-36, 1136.e1-7. doi: 10.1016/j.jtcvs.2008.02.034. Epub [PubMed PMID: 18455593]
Khositseth A, Danielson GK, Dearani JA, Munger TM, Porter CJ. Supraventricular tachyarrhythmias in Ebstein anomaly: management and outcome. The Journal of thoracic and cardiovascular surgery. 2004 Dec:128(6):826-33 [PubMed PMID: 15573066]
Qureshi MY, O'Leary PW, Connolly HM. Cardiac imaging in Ebstein anomaly. Trends in cardiovascular medicine. 2018 Aug:28(6):403-409. doi: 10.1016/j.tcm.2018.01.002. Epub 2018 Jan 12 [PubMed PMID: 29409687]
Voges I, Al-Mallah MH, Scognamiglio G, Di Salvo G. Right Heart-Pulmonary Circulation Unit in Congenital Heart Diseases. Heart failure clinics. 2018 Jul:14(3):283-295. doi: 10.1016/j.hfc.2018.02.005. Epub [PubMed PMID: 29966627]
Barbara DW, Edwards WD, Connolly HM, Dearani JA. Surgical pathology of 104 tricuspid valves (2000-2005) with classic right-sided Ebstein's malformation. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2008 May-Jun:17(3):166-71. doi: 10.1016/j.carpath.2007.07.005. Epub 2007 Oct 24 [PubMed PMID: 18402795]
Gerlis LM, Ho SY, Sweeney AE. Mitral valve anomalies associated with Ebstein's malformation of the tricuspid valve. The American journal of cardiovascular pathology. 1993:4(4):294-301 [PubMed PMID: 8305192]
Álvarez Macedo MR, Vázquez Antona CA. Uncommon mitral valve anomalies associated with Ebstein anomaly. Revista espanola de cardiologia (English ed.). 2021 Aug:74(8):717-719. doi: 10.1016/j.rec.2021.01.013. Epub 2021 Mar 27 [PubMed PMID: 33785267]
Hernandez-Andrade E, Patwardhan M, Cruz-Lemini M, Luewan S. Early Evaluation of the Fetal Heart. Fetal diagnosis and therapy. 2017:42(3):161-173. doi: 10.1159/000477564. Epub 2017 Jul 5 [PubMed PMID: 28675906]
Freud LR, Escobar-Diaz MC, Kalish BT, Komarlu R, Puchalski MD, Jaeggi ET, Szwast AL, Freire G, Levasseur SM, Kavanaugh-McHugh A, Michelfelder EC, Moon-Grady AJ, Donofrio MT, Howley LW, Tierney ES, Cuneo BF, Morris SA, Pruetz JD, van der Velde ME, Kovalchin JP, Ikemba CM, Vernon MM, Samai C, Satou GM, Gotteiner NL, Phoon CK, Silverman NH, McElhinney DB, Tworetzky W. Outcomes and Predictors of Perinatal Mortality in Fetuses With Ebstein Anomaly or Tricuspid Valve Dysplasia in the Current Era: A Multicenter Study. Circulation. 2015 Aug 11:132(6):481-9. doi: 10.1161/CIRCULATIONAHA.115.015839. Epub 2015 Jun 9 [PubMed PMID: 26059011]
Level 2 (mid-level) evidenceCiepłucha A, Trojnarska O, Kociemba A, Łanocha M, Barczynski M, Rozmiarek S, Kramer L, Pyda M. Clinical aspects of myocardial fibrosis in adults with Ebstein's anomaly. Heart and vessels. 2018 Sep:33(9):1076-1085. doi: 10.1007/s00380-018-1141-5. Epub 2018 Feb 21 [PubMed PMID: 29468473]
Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation. 2008 Dec 2:118(23):e714-833. doi: 10.1161/CIRCULATIONAHA.108.190690. Epub 2008 Nov 7 [PubMed PMID: 18997169]
Level 1 (high-level) evidenceShiina A, Seward JB, Edwards WD, Hagler DJ, Tajik AJ. Two-dimensional echocardiographic spectrum of Ebstein's anomaly: detailed anatomic assessment. Journal of the American College of Cardiology. 1984 Feb:3(2 Pt 1):356-70 [PubMed PMID: 6693624]
Kühn A, Meierhofer C, Rutz T, Rondak IC, Röhlig C, Schreiber C, Fratz S, Ewert P, Vogt M. Non-volumetric echocardiographic indices and qualitative assessment of right ventricular systolic function in Ebstein's anomaly: comparison with CMR-derived ejection fraction in 49 patients. European heart journal. Cardiovascular Imaging. 2016 Aug:17(8):930-5. doi: 10.1093/ehjci/jev243. Epub 2015 Oct 8 [PubMed PMID: 26453545]
Level 2 (mid-level) evidenceAtz AM, Munoz RA, Adatia I, Wessel DL. Diagnostic and therapeutic uses of inhaled nitric oxide in neonatal Ebstein's anomaly. The American journal of cardiology. 2003 Apr 1:91(7):906-8 [PubMed PMID: 12667588]
Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019 Apr 2:139(14):e698-e800. doi: 10.1161/CIR.0000000000000603. Epub [PubMed PMID: 30586767]
Level 1 (high-level) evidenceStout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2019 Apr 2:73(12):e81-e192. doi: 10.1016/j.jacc.2018.08.1029. Epub 2018 Aug 16 [PubMed PMID: 30121239]
Level 1 (high-level) evidenceOxenius A, Attenhofer Jost CH, Prêtre R, Dave H, Bauersfeld U, Kretschmar O, Seifert B, Balmer C, Valsangiacomo Buechel ER. Management and outcome of Ebstein's anomaly in children. Cardiology in the young. 2013 Feb:23(1):27-34. doi: 10.1017/S1047951112000224. Epub 2012 Mar 15 [PubMed PMID: 22417890]
Level 2 (mid-level) evidenceHolst KA, Dearani JA, Said SM, Davies RR, Pizarro C, Knott-Craig C, Kumar TKS, Starnes VA, Kumar SR, Pasquali SK, Thibault DP, Meza JM, Hill KD, Chiswell K, Jacobs JP, Jacobs ML. Surgical Management and Outcomes of Ebstein Anomaly in Neonates and Infants: A Society of Thoracic Surgeons Congenital Heart Surgery Database Analysis. The Annals of thoracic surgery. 2018 Sep:106(3):785-791. doi: 10.1016/j.athoracsur.2018.04.049. Epub 2018 May 16 [PubMed PMID: 29777671]
Brown ML, Dearani JA, Danielson GK, Cetta F, Connolly HM, Warnes CA, Li Z, Hodge DO, Driscoll DJ. Functional status after operation for Ebstein anomaly: the Mayo Clinic experience. Journal of the American College of Cardiology. 2008 Aug 5:52(6):460-6. doi: 10.1016/j.jacc.2008.03.064. Epub [PubMed PMID: 18672167]
Stulak JM, Sharma V, Cannon BC, Ammash N, Schaff HV, Dearani JA. Optimal surgical ablation of atrial tachyarrhythmias during correction of Ebstein anomaly. The Annals of thoracic surgery. 2015 May:99(5):1700-5; discussion 1705. doi: 10.1016/j.athoracsur.2015.01.037. Epub 2015 Mar 29 [PubMed PMID: 25825196]
Sainathan S, da Fonseca da Silva L, da Silva JP. Ebstein's anomaly: contemporary management strategies. Journal of thoracic disease. 2020 Mar:12(3):1161-1173. doi: 10.21037/jtd.2020.01.18. Epub [PubMed PMID: 32274197]
Danielson GK, Driscoll DJ, Mair DD, Warnes CA, Oliver WC Jr. Operative treatment of Ebstein's anomaly. The Journal of thoracic and cardiovascular surgery. 1992 Nov:104(5):1195-202 [PubMed PMID: 1434695]
Carpentier A, Chauvaud S, Macé L, Relland J, Mihaileanu S, Marino JP, Abry B, Guibourt P. A new reconstructive operation for Ebstein's anomaly of the tricuspid valve. The Journal of thoracic and cardiovascular surgery. 1988 Jul:96(1):92-101 [PubMed PMID: 3386297]
Wackel P, Cannon B, Dearani J, Sessions K, Holst K, Johnson J, Cetta F. Arrhythmia after cone repair for Ebstein anomaly: The Mayo Clinic experience in 143 young patients. Congenital heart disease. 2018 Jan:13(1):26-30. doi: 10.1111/chd.12566. Epub 2018 Jan 8 [PubMed PMID: 29316261]
Wei W, Zhan X, Xue Y, Fang X, Liao H, Deng H, Liang Y, Wu S. Features of accessory pathways in adult Ebstein's anomaly. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2014 Nov:16(11):1619-25. doi: 10.1093/europace/euu028. Epub 2014 Mar 9 [PubMed PMID: 24614573]
Level 2 (mid-level) evidenceRajani R, Klein JL. Infective endocarditis: A contemporary update. Clinical medicine (London, England). 2020 Jan:20(1):31-35. doi: 10.7861/clinmed.cme.20.1.1. Epub [PubMed PMID: 31941729]
Moe TG, Abrich VA, Rhee EK. Atrial Fibrillation in Patients with Congenital Heart Disease. Journal of atrial fibrillation. 2017 Jun-Jul:10(1):1612. doi: 10.4022/jafib.1612. Epub 2017 Jun 30 [PubMed PMID: 29250225]
Martín de Miguel I, Ávila P. Atrial Fibrillation in Congenital Heart Disease. European cardiology. 2021 Feb:16():e06. doi: 10.15420/ecr.2020.41. Epub 2021 Mar 9 [PubMed PMID: 33737960]
Deeg KH. Echocardiographic differential diagnosis of the cyanotic newborn. Ultraschall in der Medizin (Stuttgart, Germany : 1980). 2015 Apr:36(2):104-18; quiz 119-20. doi: 10.1055/s-0034-1385493. Epub 2014 Dec 4 [PubMed PMID: 25474186]
Mihos CG, Larrauri-Reyes M, Yucel E, Santana O. Clinical presentation and echocardiographic characteristics of Uhl's anomaly. Echocardiography (Mount Kisco, N.Y.). 2017 Feb:34(2):299-302. doi: 10.1111/echo.13430. Epub 2016 Dec 29 [PubMed PMID: 28032368]
Kobza R, Kurz DJ, Oechslin EN, Prêtre R, Zuber M, Vogt P, Jenni R. Aberrant tendinous chords with tethering of the tricuspid leaflets: a congenital anomaly causing severe tricuspid regurgitation. Heart (British Cardiac Society). 2004 Mar:90(3):319-23 [PubMed PMID: 14966058]
Level 2 (mid-level) evidenceCelermajer DS, Bull C, Till JA, Cullen S, Vassillikos VP, Sullivan ID, Allan L, Nihoyannopoulos P, Somerville J, Deanfield JE. Ebstein's anomaly: presentation and outcome from fetus to adult. Journal of the American College of Cardiology. 1994 Jan:23(1):170-6 [PubMed PMID: 8277076]
Level 2 (mid-level) evidenceAttie F, Rosas M, Rijlaarsdam M, Buendia A, Zabal C, Kuri J, Granados N. The adult patient with Ebstein anomaly. Outcome in 72 unoperated patients. Medicine. 2000 Jan:79(1):27-36 [PubMed PMID: 10670407]
Level 2 (mid-level) evidenceAttenhofer Jost CH, Tan NY, Hassan A, Vargas ER, Hodge DO, Dearani JA, Connolly H, Asirvatham SJ, McLeod CJ. Sudden death in patients with Ebstein anomaly. European heart journal. 2018 Jun 1:39(21):1970-1977a. doi: 10.1093/eurheartj/ehx794. Epub [PubMed PMID: 29315367]
Holst KA, Dearani JA, Said S, Pike RB, Connolly HM, Cannon BC, Sessions KL, O'Byrne MM, O'Leary PW. Improving Results of Surgery for Ebstein Anomaly: Where Are We After 235 Cone Repairs? The Annals of thoracic surgery. 2018 Jan:105(1):160-168. doi: 10.1016/j.athoracsur.2017.09.058. Epub 2017 Nov 24 [PubMed PMID: 29174783]
Celermajer DS, Cullen S, Sullivan ID, Spiegelhalter DJ, Wyse RK, Deanfield JE. Outcome in neonates with Ebstein's anomaly. Journal of the American College of Cardiology. 1992 Apr:19(5):1041-6 [PubMed PMID: 1552092]
Level 2 (mid-level) evidenceAndrews RE, Tibby SM, Sharland GK, Simpson JM. Prediction of outcome of tricuspid valve malformations diagnosed during fetal life. The American journal of cardiology. 2008 Apr 1:101(7):1046-50. doi: 10.1016/j.amjcard.2007.11.049. Epub 2008 Feb 6 [PubMed PMID: 18359329]