Introduction
The external ear is the visible part of the hearing apparatus. It is comprised of the auricle (pinna) and external auditory canal, including the lateral surface of the tympanic membrane. Together with the tympanic membrane and the middle ear, the pinna serves to amplify sound. The pinna acts as a funnel to deliver sound to the external acoustic meatus, and the external auditory canal concentrates sound onto the tympanic membrane for further transmission.[1][2][3]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The external ear’s flexible structure is maintained by elastic cartilage, which is covered in skin and attached to the skull with ligaments and muscles. The skin of the pinna is rich in sebaceous glands, which serve to protect the ear from cracking. The external auditory canal is laden with specialized ceruminous glands, which produce cerumen (earwax) that may hinder or repel the entry of insects and debris through the external acoustic meatus.
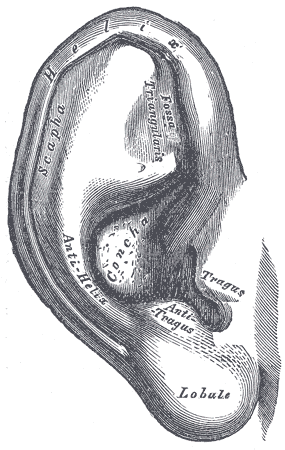
The helix is the outer posterosuperior rim of the ear and curves slightly inward towards the external acoustic meatus, giving the ear its concave geometry. The antihelix is a convex eminence located anteriorly to the helix; it curves outward from the deep concha anterior to it. Between the helix and antihelix is a shallow concavity, the scaphoid fossa. The external acoustic meatus arises from the anteriormost part of the concha and marks the beginning of the external auditory canal. The canal continues in the skull in sigmoid fashion until it meets the tympanic membrane. Given that the external acoustic meatus is not in perfect alignment with the external auditory canal, the pinna must be pulled backward and upward during an otoscopic examination to allow proper angling of the otoscope tip in the external auditory canal. Anteriorly to the acoustic meatus and concha is a cartilaginous projection called the tragus, and inferiorly to it hangs the lobule (earlobe). The lobule is devoid of cartilage and contains areolar connective tissue.
A small projection from the tragus, called Darwin’s tubercle, is present in a fraction of the population. It is homologous to the tip of the ears of pointy-eared mammals.
The ears are located symmetrically on either side of the head, which allows for binaural sound localization in the brain regarding direction and distance away. The brain centers involved in integrating information from both ears are located in the brainstem; these consist the inferior colliculus in the midbrain, and a group of pontine nuclei called the superior olivary complex, of which only the medial superior olive and superior lateral olive truly receive afferents from bilateral sources.
Arrival time and volume from each ear are registered in the brain, and the interaural time difference (ITD) and interaural level difference (ILD) are computed. ITD and ILD would increase according to the azimuth of the sound source from 0 degrees (centered in front of the subject) up to 90 degrees and decrease from 90 to 180 degrees (centered at the back of the subject). It is unclear whether interaural time or volume difference is the main aspect involved in the localization of sounds. The Duplex Theory, published by Lord Rayleigh in 1907, prioritizes one over the other based on sound frequency: ITD for lower frequency (i.e., less than 800 Hz) and ILD for higher frequency (i.e., more than 1600 Hz) sounds, with intermediate frequency, sounds presenting an overlap between ITD and ILD dominance. This is based on limitations of the human auditory system in resolving the sound spectrum of frequencies and intensities: below 800 Hz, the wavelengths are larger than the distance between the ears, delivering easily detectable phase delays calculated as ITD. Above 1600 Hz, however, the wavelengths become much smaller than the interaural distance (distance between the ears) itself, rendering phase delays impossible to resolve. Conversely, the differences in sound intensity are poorly resolved at low frequencies and much better resolved at high frequency, allowing ILD to be used at such elevated frequencies. The Duplex Theory only accounts for sounds traveling in the horizontal plane at the level of the ear and does not explain anteroposterior sound source localization.
The anterior orientation and convoluted structure of the pinna are believed to aid in further elucidating whether the sound originates from the front (0 to 90 degrees) or the back (90 to 180 degrees) and in other planes by reflecting and directing waves onto the tympanic membrane, creating a pattern of ripples in the tympanic membrane that would be interpreted by the brain to determine nuances in aurospatial orientation. This is known as the Pinna Filtering Effect theory. Further, the structure of the pinna may have evolved to enhance human sensitivity to sounds of a particular frequency, namely around 3 kHz, the frequency of human voice, which is speculated to have favored the development of speech.
Embryology
Ear development begins in the third week from a thickening of ectoderm called the otic placode. The external ear develops from the first two pharyngeal arches, which begin forming six hillocks of His in the five weeks of development. The first three hillocks originate from the first pharyngeal arch and fuse together to form the tragus, helix, and cymba conchae. The other three hillocks originate from the second pharyngeal arch and fuse to form the concha, antihelix, and tragus. The external auditory meatus is derived from the first pharyngeal cleft, from an ectodermal diverticulum therein, which canalizes in the 18 weeks to drape the walls of the external auditory canal and external surface of the tympanic membrane in the thin stratified squamous epithelium. Thus, presumably, the fetus begins to hear in the 18 weeks. The ceruminous glands develop along with hair follicles at 5 months of gestation.
Blood Supply and Lymphatics
The auricle is irrigated by the superficial temporal artery anteriorly (toward the tragus) and the posterior auricular artery posteriorly (toward the lateral helix), both originating from the external carotid artery.
Nerves
Sensory innervation to the external ear is supplied by both cranial and spinal nerves. Branches of the trigeminal, facial, and vagus nerves (CN V, VII, X) are the cranial nerve components, while the lesser occipital (C2, C3) and greater auricular (C2, C3) nerves are the spinal nerve components involved. The lateral surface of the tympanic membrane, the external auditory canal, and the external acoustic meatus are all innervated by nervus intermedius (a branch of CN VII), the auriculotemporal nerve (CN V3), and the auricular branch of the vagus nerve. The concha receives split innervation from nervus intermedius, the auricular branch of the vagus nerve, and the greater auricular (spinal) nerve. Beyond the concha, the greater part of the pinna is innervated by the auriculotemporal and lesser occipital nerves superior to the concha, and by the greater auricular nerve inferolaterally to the lobule.
Motor innervation to muscles of the external ear is supplied by branches of the facial nerve (CN VII).
Muscles
The auricle contains both intrinsic muscles and extrinsic muscle attachments.
There are a surprising number of intrinsic ear muscles, given the human ear’s limited movement compared to other mammals’. Intrinsic ear muscles serve no role in humans and are listed helicis major and minor, tragicus, antitragicus, transverse and oblique muscles.
Extrinsic ear muscles include the superior auricular (auricularis superior), anterior auricular (auricularis anterior), and posterior auricular (auricularis posterior) muscles, located as their name indicates about the pinna. They pull the ear upward, forward, and backward, respectively. The superior and anterior auricular muscles are both fan-shaped and originate in the epicranial aponeurosis (galea aponeurotica). The superior auricular muscle is the largest of the three extrinsic ones and inserts into a tendon attached to the auricle superiorly. The anterior auricular muscle is the smallest of the group; its fibers are slight and easily overlooked. It inserts into the anterior helix. The posterior auricular muscle is comprised of 2-3 fascicles originating from the mastoid (temporal bone) and inserting into the posteroinferior part of the concha.
Clinical Significance
Ear size increases with age. Ear cartilage continues to grow throughout a person's lifetime, while the pendulous lobule elongates under the influence of gravity.
The external ear is often adorned with jewelry, particularly the cartilage-free lobule. Piercings involving the cartilaginous parts of the ear may display poor healing because of the relatively avascular nature of cartilage. Ear piercings are among the most common sites of keloid scar development in susceptible individuals. The patient may present with pain, itching, and a shiny and/or dark/pink mass at the site of the piercing, which represents the scar. African-Americans are 15 times more likely than whites to develop keloids. Susceptible individuals should avoid any further piercings and skin trauma elsewhere on their body, which may result in further keloid scar formation.
Cauliflower ear is seen in boxers and individuals who experience frequent physical trauma to the pinna. The deformity develops over time as hematomas form between the cartilage and its perichondrium, resulting in the death of the cartilage as it is cut off from its supply of nutrients. Fibrosis sets in, deforming the ear. [4]
Otitis externa is the inflammation of the external auditory canal. A notorious cause of otitis externa is swimmer’s ear, which is an infection acquired in contaminated swimming pools or lakes. The pathogens typically include Pseudomonas aeruginosa and Staphylococcus aureus. Otitis externa is diagnosed via otoscopic examination, which reveals an erythematous and swollen auditory canal. In some cases, the first otoscopic finding in a patient with ear canal pain from otitis externa may be a lack of cerumen, as the infection impedes its production. Treatment is topical and rarely requires oral antibiotics. Swimmer’s ear must be differentiated from surfer’s ear, which is abnormal growth of bone to form an exostosis in the external auditory canal. It occurs as a result of frequent exposure to cold and wet conditions and is seen in surfers, divers, and other water sports enthusiasts. Ear myiasis is maggot infestation of the ear. Children may insert small objects into their auditory canals, which may lead to conductive hearing loss and otitis externa secondary to foreign body insertion.[5]
As cerumen continuously is secreted in the healthy ear, it may collect in the external auditory canal even to the point of complete or partial obstruction. The misuse of cotton swabs is associated with wax impaction, as cotton swabs that are inserted beyond the external acoustic meatus may push the cerumen further into the ear canal, rather than clearing it out. The patient may present with conductive hearing loss and possibly otalgia if there is infection beyond the obstruction. Intra-otic drops may help to soften the impacted wax overnight, and the patient should return on the following day for irrigation to dislodge the solid wax. Instrumentation of the ear canal should be done with caution as its vagal innervation may cause bradycardia or coughing.
The ear is relatively exposed and thus merits special attention with regards to sun damage. The pinna is a frequent site of actinic keratosis, cutaneous horns, and skin cancer. [6]Conversely, the external ear is particularly susceptible to frostbite.
Abnormalities in the shape of the external ear, such as microtia, anotia, auricular dysplasia, external auditory canal atresia, etc. may be associated with genetic syndromes. Examples include the autosomal-dominant Treacher Collins syndrome and Crouzon syndrome.[7][8]
The area immediately anterior to the tragus should be observed for preauricular pits or tags, which may be associated with genetic syndromes like branchio-oto-renal syndrome, Beckwith-Wiedemann syndrome. Neonates with preauricular pits or tags should undergo a renal ultrasound to verify for renal abnormalities. Pits are believed to originate from hillock fusion abnormalities, while tags may represent supernumerary hillocks.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
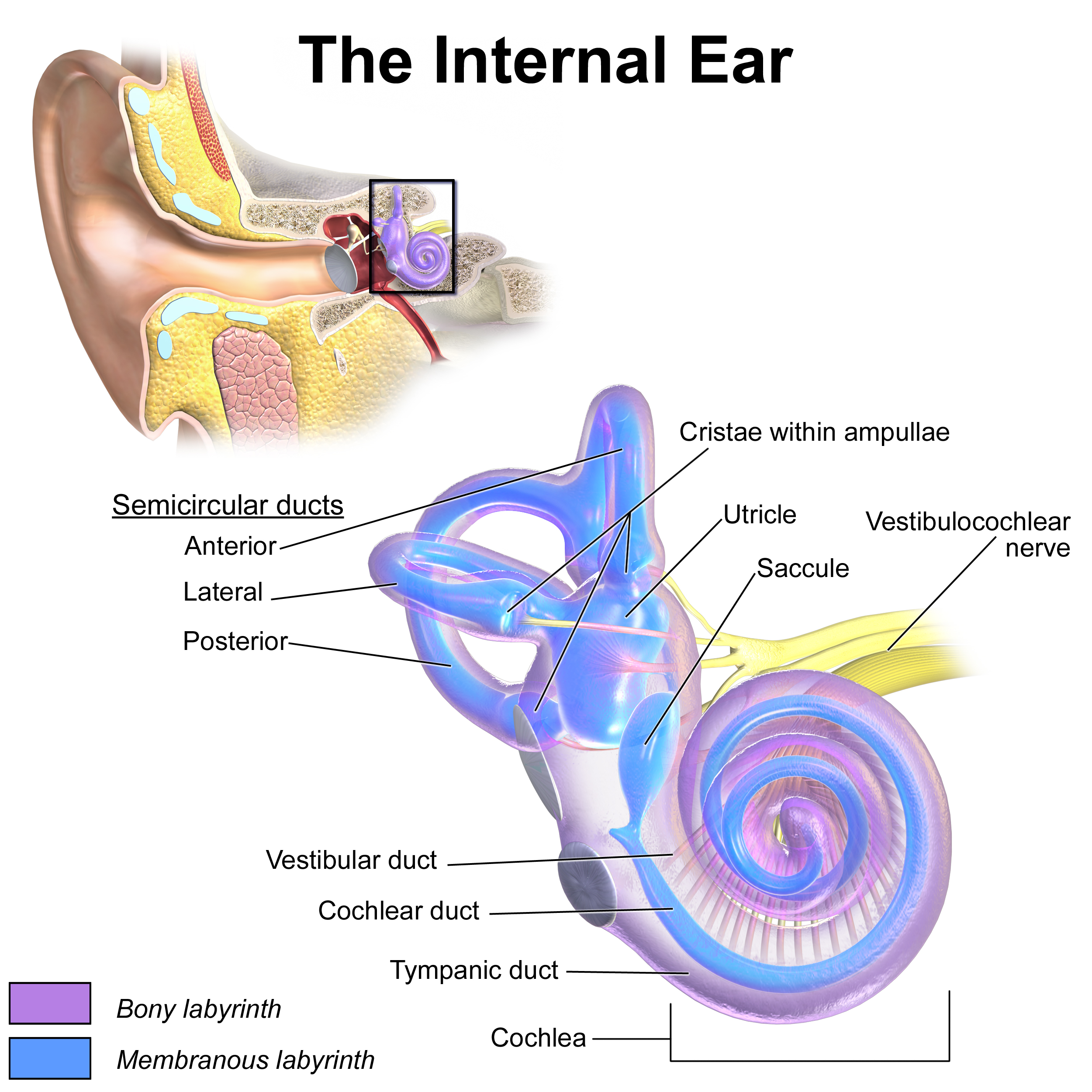
Inner Ear Anatomy. This illustration shows the semicircular ducts and parts of the cochlea.
Blausen.com staff. Medical Gallery of Blausen Medical 2014. WikiJournal of Medicine. doi: 10.15347/wjm/2014.010.
ISSN 2002-4436. [CC BY 3.0 (https://creativecommons.org/licenses/by/3.0)] via Wikimedia Commons.
References
White HJ, Helwany M, Biknevicius AR, Peterson DC. Anatomy, Head and Neck, Ear Organ of Corti. StatPearls. 2023 Jan:(): [PubMed PMID: 30855919]
Altafulla J, Iwanaga J, Lachkar S, Prickett J, Dupont G, Yilmaz E, Ishak B, Litvack Z, Tubbs RS. The Great Auricular Nerve: Anatomical Study with Application to Nerve Grafting Procedures. World neurosurgery. 2019 May:125():e403-e407. doi: 10.1016/j.wneu.2019.01.087. Epub 2019 Jan 28 [PubMed PMID: 30703599]
Young AS, Rosengren SM, Welgampola MS. Disorders of the inner-ear balance organs and their pathways. Handbook of clinical neurology. 2018:159():385-401. doi: 10.1016/B978-0-444-63916-5.00025-2. Epub [PubMed PMID: 30482329]
Krogmann RJ, Jamal Z, King KC. Auricular Hematoma. StatPearls. 2023 Jan:(): [PubMed PMID: 30285394]
Szmuilowicz J, Young R. Infections of the Ear. Emergency medicine clinics of North America. 2019 Feb:37(1):1-9. doi: 10.1016/j.emc.2018.09.001. Epub [PubMed PMID: 30454772]
Osborn HA, Goldsmith TA, Varvares MA. Assessing functional outcomes in head and neck surgical oncology. Head & neck. 2019 Jul:41(7):2051-2057. doi: 10.1002/hed.25656. Epub 2019 Jan 30 [PubMed PMID: 30698897]
Markkanen S, Niemi P, Rautiainen M, Saarenpää-Heikkilä O, Himanen SL, Satomaa AL, Peltomäki T. Craniofacial and occlusal development in 2.5-year-old children with obstructive sleep apnoea syndrome. European journal of orthodontics. 2019 May 24:41(3):316-321. doi: 10.1093/ejo/cjz009. Epub [PubMed PMID: 30925192]
Birgfeld C, Heike C. Craniofacial Microsomia. Clinics in plastic surgery. 2019 Apr:46(2):207-221. doi: 10.1016/j.cps.2018.12.001. Epub [PubMed PMID: 30851752]