Introduction
Fuchs endothelial dystrophy (FED) is a bilateral, slowly progressive, and often asymmetric corneal disease characterized by endothelial cell deterioration and the development of guttata—excrescences of the Descemet membrane.[1][2] The disease advances gradually, leading to significant endothelial cell loss, impaired corneal deturgescence, and bilateral corneal edema affecting the stroma or epithelium. These changes result in ocular pain, glare, halos, and decreased visual acuity.[3]
FED is the most common corneal dystrophy affecting the endothelium and the most frequent indication for keratoplasty worldwide.[4][5] A key distinction between cornea guttata and FED is that the latter presents with corneal edema.[6] Notably, a rare nonguttate form of FED exists, in which endothelial cell degeneration causes corneal edema without excrescences of the Descemet membrane. Since nonguttate FED is rarely reported in the literature, most discussions focus on its guttate form.
FED was first described in 1910 when Viennese ophthalmologist Ernst Fuchs reported 13 older adults with bilateral central clouding. Kraupa later detailed the continuum of corneal changes in FED, and Vogt introduced the term “guttata” (gutta is Latin for "droplet") in 1921.[7] Subsequent research has expanded the understanding of guttata and its association with FED, although the precise mechanisms and progression of the disease remain under investigation. Advances in management over the past 2 decades have significantly improved the quality of life for individuals with FED.[8]
FED is a progressive corneal disease characterized by endothelial cell dysfunction and loss, resulting in corneal edema, visual impairment, and, in severe cases, painful bullous keratopathy. This ocular disorder is a common indication for corneal transplantation, particularly in older adults, and occurs more frequently in women.[9]
FED primarily affects the posterior cornea, where endothelial cells maintain corneal clarity by actively pumping excess fluid out of the stroma. Fluid accumulates as these cells deteriorate, leading to progressive visual decline, glare, and increased light sensitivity. The disease advances through distinct stages, beginning with asymptomatic guttata, followed by increasing corneal thickness due to endothelial decompensation, and ultimately progressing to bullous keratopathy, which significantly impairs vision and causes discomfort.
First described by Ernst Fuchs in 1910, FED was once considered rare. However, epidemiological studies now estimate that 4% to 5% of adults older than 40 may have some form of the disease, with women affected nearly 3 times more often than men. Advances in genetics, imaging, and surgical techniques have greatly improved the understanding and management of FED.[10]
FED typically manifests in the 5th to 6th decade of life, though early-onset variants occur, particularly in individuals with COL8A2 gene mutations. A strong genetic component is evident, as nearly 40% of patients report a family history of the condition. The pathophysiology of FED involves endothelial cell loss, oxidative stress, and genetic susceptibility. Since the corneal endothelium is a nonregenerative monolayer, any damage or cell loss results in permanent dysfunction.[11]
In FED, endothelial cells undergo apoptosis and lose their pump function, leading to excessive hydration of the corneal stroma and epithelium. The earliest sign is the formation of guttata—focal outgrowths of the Descemet membrane that appear as dark spots under specular microscopy. As the disease advances, stromal edema develops due to impaired fluid regulation, resulting in increased corneal thickness and blurred vision. In severe cases, epithelial bullae can rupture, causing pain and further visual deterioration. Corneal pachymetry often reveals significant thickening beyond 640 μm, reflecting endothelial dysfunction.
Genetic susceptibility plays a major role in FED, particularly TCF4 gene trinucleotide repeat expansions, which are identified in nearly 70% of individuals with late-onset disease, making it a strong genetic marker.[12] Other mutations, including ZEB1 and COL8A2, have been implicated in early-onset cases. Beyond genetics, oxidative stress contributes to disease progression, as FED endothelial cells demonstrate increased susceptibility to reactive oxygen species (ROS), leading to mitochondrial dysfunction and accelerated cell death. Environmental factors such as chronic UV exposure and smoking have been associated with worsening endothelial degeneration. Additionally, hormonal influences may contribute to the higher prevalence in women, with estrogen deficiency proposed as a potential risk factor.[13]
FED progresses slowly over decades, with clinical manifestations varying by disease stage. Early-stage FED is often asymptomatic and detected only through slit-lamp examination, which reveals corneal guttata. As the disease advances, morning blurry vision becomes a hallmark symptom due to worsening corneal edema, which is exacerbated upon waking by reduced evaporation during sleep. Patients also report glare, halos, and difficulty with contrast sensitivity, particularly under low-light conditions.
In moderate-stage disease, corneal thickening and endothelial dysfunction lead to persistent visual impairment throughout the day. Advanced-stage FED is marked by worsening stromal edema and the formation of painful epithelial bullae (bullous keratopathy), which can rupture and cause significant discomfort. If left untreated, chronic corneal decompensation results in irreversible fibrosis and scarring.
Diagnosis relies on clinical examination, imaging, and endothelial function assessment. Slit-lamp biomicroscopy reveals characteristic guttata in the central cornea, which initially appear as discrete excrescences and later coalesce into a more widespread "beaten metal" appearance.[14]
Specular microscopy is essential for confirming the diagnosis by assessing endothelial cell density (ECD), morphology, and pleomorphism. Normal ECD ranges from 2,500 to 3,000 cells/mm², but this value progressively declines in FED, with fewer than 1,000 cells/mm² indicating moderate disease and fewer than 500 cells/mm² suggesting severe endothelial failure. Pachymetry and anterior segment optical coherence tomography (AS-OCT) quantify corneal thickness and edema, aiding in disease staging. Fluorescein staining highlights areas of epithelial compromise in cases of bullous keratopathy.
Treatment strategies depend on disease severity. In early-stage FED, medical management focuses on reducing corneal edema and improving visual quality.[15] Hypertonic saline (5%) drops temporarily draw excess fluid from the cornea, providing symptomatic relief. Lubricating eye drops help maintain corneal hydration and prevent epithelial breakdown. Scleral contact lenses can improve vision in moderate cases by creating a smooth optical surface over the irregular cornea. However, these interventions do not halt disease progression, and worsening symptoms may necessitate surgical intervention.
Descemet membrane endothelial keratoplasty (DMEK) is the preferred surgical approach for significant corneal edema and visual impairment.[16] DMEK involves the selective transplantation of a donor endothelial layer with the Descemet membrane, offering better visual outcomes, faster recovery, and lower rejection rates than older techniques. Descemet stripping endothelial keratoplasty (DSEK), a similar but slightly thicker graft technique, serves as an alternative for patients with complex ocular histories, such as prior glaucoma surgeries. Penetrating keratoplasty (PKP) is reserved for severe cases with corneal scarring or multiple graft failures. Postoperative care includes long-term topical steroids to prevent rejection, intraocular pressure (IOP) monitoring, and routine endothelial cell assessments.
Research into nontransplant alternatives for FED is ongoing, with several promising therapies emerging.[17] ρ-Kinase (ROCK) inhibitors, such as netarsudil and ripasudil, have shown potential in enhancing endothelial cell survival and function, possibly delaying the need for keratoplasty. Cell-based therapies, such as cultured endothelial cell injections, are being investigated as a minimally invasive regenerative approach. Additionally, gene therapy targeting oxidative stress pathways is under development to protect and prolong endothelial cell function.
FED is a progressive, multifactorial disease that leads to corneal edema, visual impairment, and significant morbidity in advanced stages. While medical management provides temporary relief, keratoplasty remains the gold standard for restoring vision in severe cases. Emerging therapies, including pharmacological interventions and regenerative techniques, offer hope for treating endothelial dysfunction without corneal transplantation. Early diagnosis and timely intervention are crucial for preserving vision and improving quality of life for patients with FED.[18]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
FED inheritance follows an autosomal dominant pattern with variable expressivity and incomplete penetrance.[19][20] However, nearly half of all cases are sporadic, and many patients are unaware of a family history of the disease.[21] Genetic factors play a significant role, particularly in early-onset FED, which is often familial and linked to autosomal dominant inheritance. Pathogenic mutations in COL8A, located at chromosomal position 1p34.3–p32.3 (FCD1), are associated with early-onset FED, as these mutations disrupt the structure of the Descemet membrane.
Late-onset FED exhibits greater genetic heterogeneity, with several mutations contributing to disease development. Repeat expansion mutations in TCF4, which encodes the E2-2 protein, are strongly associated with FED across diverse populations, with the highest prevalence in Caucasians, where the mutation exhibits 80% penetrance. Additionally, mutations in DMPK have been identified as potential genetic contributors to FED.
SLC4A11 encodes an ion channel that facilitates water resorption through the endothelial layer and plays a crucial role in maintaining corneal deturgescence. Mutations in this gene are associated with corneal edema and have been correlated with FED. Similarly, mutations in ZEB1, which encodes the transcription factor zinc finger E-box binding homeodomain 1, have been linked to late-onset FED and posterior polymorphous corneal dystrophy (PPCD).
AGBL1 encodes the deglutamylase enzyme adenosine triphosphate (ATP)/guanosine triphosphate (GTP) binding protein-like 1, and mutations in this gene are associated with FED. Missense mutations in LOXHD1 have been linked to progressive hearing loss and corneal endothelial cell dysfunction in FED. Additionally, KANK4, LAMC1, and ATP1B1 have been implicated in FED due to their effects on corneal deturgescence.[22] FED can coexist with other endothelial corneal dystrophies, particularly PPCD and congenital hereditary endothelial dystrophy (CHED), which share phenotypic similarities.
FED is a progressive corneal disorder characterized by endothelial cell loss, guttata formation, and stromal edema, resulting in visual impairment. The condition is multifactorial, involving genetic predisposition, environmental influences, and oxidative stress.
FED follows an autosomal dominant inheritance pattern with variable expressivity and incomplete penetrance, often demonstrating strong familial clustering. Genetic mutations play a significant role, with TCF4 mutations identified in approximately 70% of late-onset cases. Other genes, including ZEB1 and COL8A2, have been implicated, with COL8A2 mutations primarily associated with early-onset FED.[23] Given the hereditary nature of the disease, genetic screening in high-risk families can facilitate early detection and monitoring, potentially allowing for timely interventions to preserve visual function.
Endothelial cells in FED exhibit increased susceptibility to oxidative stress, leading to progressive endothelial apoptosis. Reduced antioxidant defense mechanisms further compromise cell survival, while mitochondrial dysfunction depletes energy reserves, impairing sodium-potassium pump activity and contributing to corneal edema.[24] Given these findings, antioxidant therapy, including vitamin C and N-acetylcysteine, is under investigation as a potential protective strategy.
Hormonal factors may also influence FED progression, as the condition is more prevalent in postmenopausal women, suggesting a link to estrogen deficiency. Estrogen plays a role in maintaining endothelial cell function, and its decline may contribute to disease onset or worsening. The disproportionately higher prevalence of FED in women, estimated at approximately 70%, supports the possibility of an endocrine influence.[25] Ongoing studies are exploring the potential effects of hormone replacement therapy (HRT) on FED progression.
Environmental and lifestyle factors further modulate disease risk. UV radiation exposure induces DNA damage in endothelial cells, accelerating guttata formation. The absence of protective measures, such as sunglasses and blue-light filtering lenses, represents a modifiable risk factor.[26] Additionally, smoking exacerbates oxidative stress, promoting endothelial degeneration, while systemic conditions such as diabetes, hypertension, and metabolic syndrome may contribute to corneal decompensation. Preventive strategies include UV protection, smoking cessation, and cardiovascular risk management, which may help slow disease progression.[27]
Postoperative endothelial stress following cataract surgery can accelerate FED progression, particularly in patients with borderline endothelial function. Phacoemulsification introduces mechanical and oxidative stress, increasing the risk of rapid corneal decompensation. Intraoperative trauma and the type of viscoelastic agents used during surgery further influence endothelial cell survival. Patients with preexisting guttata or early FED may experience a faster decline in endothelial function following cataract extraction.
To mitigate surgical risks, preoperative assessment of endothelial cell count is essential for identifying patients at higher risk of postoperative decompensation. Low-energy phacoemulsification techniques and strategies to protect the corneal endothelium, such as careful fluid management and the use of dispersive viscoelastics, can help preserve endothelial viability. In patients with significant endothelial dysfunction, concurrent or staged endothelial keratoplasty may be considered to optimize visual outcomes.
FED is a complex disorder influenced by genetic, oxidative, hormonal, and environmental factors. While early detection and lifestyle modifications may slow disease progression, surgical interventions remain necessary in advanced cases to restore corneal clarity and visual function.[28]
Epidemiology
Determining the incidence and prevalence of FED is challenging, as many individuals with corneal guttata never develop the disease, and symptoms often take decades to manifest. Corneal guttata typically appear in the 4th decade of life, but most patients do not require surgical intervention until their 6th or 7th decade. FED is the leading cause of corneal endothelial transplantation worldwide.[29]
Although corneal guttata may occur in up to 4% of adults older than 40 in the U.S., they are less frequently associated with corneal edema, and many individuals with guttata do not receive a diagnosis of FED. Several studies have documented the incidence and prevalence of central corneal guttata without edema. A 2005 study in Iceland found primary corneal guttata in 11% of women and 7% of men.[30] Another study reported prevalence rates of 6.7% in Chinese Singaporeans and 3.7% in Japanese individuals.[31]
Lorenzetti et al observed central corneal guttata in 31% of eyes among individuals aged 20 to 39 and in 70% of eyes in those older than 40. Goar et al found guttata in 9.6% of patients older than 40. In 1910, Ernst Fuchs estimated that FED affected fewer than 1% of his patients. Reported incidence and prevalence vary widely, likely due to differing clinical definitions of guttata. Evidence suggests that FED is more prevalent in Europe than in other regions, with a higher proportion of corneal transplants for FED in Europe and the U.S. compared to Asia, where FED rates are lower.
Several nonheritable risk factors are associated with FED. Age and gender significantly influence disease development, with adults older than 40 and women at higher risk.[32] Reported female-to-male ratios range from 2.5:1 to 3:1. Additional risk factors include smoking, UV light exposure, and diabetes, which can affect disease severity. A rare early-onset form of FED presents in the 1st decade of life and has an equal female-to-male ratio.
Pathophysiology
The endothelial barrier and pumps are essential for maintaining corneal deturgescence and transparency, and endothelial cell dysfunction is central to the pathogenesis of FED. The corneal endothelium consists of a monolayer of hexagonal cells that regulate water flow, balancing inflow and outflow to prevent corneal swelling. These cells originate from neural crest cells and remain in the G1 phase of the cell cycle. A functional endothelium typically contains 400 to 500 cells/mm2. The aqueous humor supplies the cornea with solutes and nutrients, which diffuse through the endothelial barrier via IOP gradients.[33]
Tight junctions between endothelial cells restrict paracellular water diffusion, making sodium-potassium ATPase pumps the primary drivers of fluid movement from the cornea into the aqueous humor. While the sodium-potassium pump is the main endothelial pump, it functions alongside aquaporins and the SLC4A11-encoded sodium-borate cotransporter. The high metabolic demands of endothelial cells require ATP, chloride ions, bicarbonate ions, and carbonic anhydrase to sustain active transport processes. Corneal transparency depends on maintaining stromal hydration below 3.5 mg of water per mg of dry tissue. When endothelial function is compromised, and the number of active pumps declines, the cornea absorbs excess water, leading to swelling, hazy vision, and reduced visual acuity.
The pathophysiology of FED involves several proposed mechanisms, including channelopathies, oxidative stress, apoptosis, and epithelial-mesenchymal transition (EMT). Despite multiple hypotheses, the precise underlying mechanisms remain unclear. A key contributor to FED development is channelopathy in the corneal endothelium, characterized by ion channel dysfunction due to genetic mutations. For example, mutations in SLC4A11 disrupt normal endothelial function.
Mitochondrial dysfunction also appears to play a role. Endothelial cells in FED exhibit decreased cytochrome oxidase activity, particularly in areas of corneal edema. This deficiency suggests that impaired mitochondrial function leads to inadequate ATP production, compromising endothelial pump activity.
Oxidative stress is another significant pathogenetic factor. The cornea is continuously exposed to UV light, generating ROS that damages both mitochondrial and nuclear DNA. This oxidative damage triggers endothelial cell apoptosis, contributing to progressive cell loss.[34] Excessive apoptosis further accelerates endothelial degeneration.[35][36] The accumulation of oxidative DNA damage around guttata disrupts mitochondrial function, leading to ongoing endothelial loss and ocular tissue degeneration.[37]
FED may also be linked to the unfolded protein response (UPR) of the endoplasmic reticulum. When misfolded or abnormal proteins accumulate in the endoplasmic reticulum, cellular mechanisms are activated to halt translation, degrade misfolded proteins, and, if unresolved, trigger apoptosis. Studies have shown that the endoplasmic reticulum enlarges, and several unfolded protein response markers are overactivated in FED.[38][39]
EMT is another proposed mechanism in FED pathogenesis. Abnormal deposition of extracellular matrix proteins, such as collagen and basement membrane components, leads to the characteristic thickening of the Descemet membrane. Histopathological analysis of FED reveals endothelial cells that have transformed into a fibroblastic or epithelial phenotype, allowing them to secrete excess extracellular matrix proteins. Genes implicated in EMT include ZEB1 and TCF4.[40]
In FED, degenerating endothelial cells produce hyaline excrescences known as guttata, which accumulate on the Descemet membrane and correlate with its thickening. These excrescences are visible both histologically and clinically. In the early stages, guttata are isolated and generally not confluent, while endothelial cells compensate for cell loss by undergoing polymegathism.[41]
Progression occurs over 2 to 3 decades, with an increase in both the number and size of guttata. As the disease advances, guttata extend to the peripheral cornea and become confluent. In stage 2, endothelial loss over guttata impairs corneal deturgescence, leading to increased corneal thickness. As FED progresses further, corneal decompensation allows excessive fluid accumulation, resulting in corneal edema. Swelling becomes more likely when ECD falls below 1,000 cells/mm².
In stage 3, worsening stromal edema may lead to epithelial bullae formation, which can rupture and cause significant discomfort. The final stage is characterized by subepithelial scarring and corneal vascularization.[42]
Histopathology
Specular microscopy relies on a smooth, transparent cornea to generate specular reflection. The utility of this diagnostic tool is limited in cases of significant corneal edema, as seen in FED. When visible, corneal guttata appear as dark, oval-to-round areas, often with a small central point of reflection. Endothelial cell counts decrease, and gaps form between cells. While specular microscopy provides rapid imaging and is a well-established technique, its effectiveness is reduced in patients with FED due to impaired visualization of the corneal stroma.
Confocal microscopy offers a superior view of the corneal endothelium compared to specular microscopy and is more effective for evaluating FED. However, this modality is not available in all clinical settings.[43] Confocal microscopy uses a gel to reduce light scattering at the corneal epithelium, making it particularly beneficial for patients with epithelial disorders or significant stromal edema. Confocal microscopy can reveal a characteristic “strawberry-like” endothelial pattern and in vivo microstructural changes. In advanced stages, findings may include fibrosis of the Descemet membrane, basal epithelial edema, and posterior stromal scarring.[44] Anterior stromal cell loss may also be detected through histology or confocal microscopy.
Light microscopy reveals intracellular epithelial edema, bullous separation, stromal thickening, endothelial loss, and Descemet membrane thickening with posterior nodules, a common finding in FED. Inflammatory cell infiltrates are not expected in patients with FED alone. Electron microscopy shows degenerating keratocytes in the corneal stroma and lipid keratopathy in most cases. Degenerating keratocytes exhibit vacuolization, cytoplasmic dissolution, loss of intracellular organelles, and nuclear chromatin clumping. The endothelium appears attenuated, particularly over posterior nodules.
Nonguttate forms of FED share many corneal changes with guttate forms, including endothelial cell pleomorphism, polymegethism, and reduced ECD, but lack guttata. Diagnosis of nonguttate FED often relies on specular or confocal microscopy.[45][46] Previous studies using phase contrast microscopy have demonstrated closely packed excrescences within thickened lamellae, even when absent on light microscopy. Additionally, oxytalan staining has revealed buried guttata on light microscopy. These findings suggest that nonguttate and guttate forms of FED may represent variations of the same disease.
Toxicokinetics
Toxicokinetics refers to the absorption, distribution, metabolism, and excretion of potentially toxic substances, including medications and surgical adjuncts used to treat FED. Understanding the toxicokinetics of various agents is crucial for optimizing the treatment of this condition while minimizing adverse effects.[47]
Hypertonic saline permeates topically with limited systemic absorption. This agent does not undergo systemic metabolism and is cleared locally via the tear film. Toxicity concerns include epithelial irritation and transient stinging. Topical corticosteroids, including prednisolone, fluorometholone, and loteprednol, rapidly penetrate the cornea and accumulate in the aqueous humor. These drugs are metabolized in the liver via CYP3A4 enzymes and excreted renally. Potential adverse effects include increased IOP, cataract formation, and corneal thinning.
Carbonic anhydrase inhibitors (CAIs) such as dorzolamide and brinzolamide are absorbed through the cornea into the aqueous humor, with minimal hepatic metabolism, and are primarily excreted through the kidneys. In individuals with endothelial dysfunction, these agents may contribute to corneal decompensation. β-Blockers, including timolol and betaxolol, are absorbed systemically via the conjunctiva, undergo hepatic metabolism, and are eliminated renally. Potential systemic side effects include bradycardia, bronchospasm, and hypotension.
ROCK inhibitors are absorbed locally in ocular tissues and metabolized in the liver, with excretion occurring through both fecal and renal pathways. Common adverse effects include conjunctival hyperemia and mild irritation. Mitomycin-C, used as an adjunct in glaucoma surgery, diffuses into ocular tissues but undergoes no metabolic transformation. This agent is slowly cleared via the aqueous humor, with toxicity concerns that include corneal toxicity and delayed wound healing.
Long-term steroid users require close IOP monitoring, and lower doses of β-blockers should be considered in patients with systemic comorbidities. Adjusting glaucoma medication regimens in patients with FED can help prevent corneal decompensation.
Toxicity Arising from Surgical Interventions
Toxicity may result from surgical interventions due to anesthesia and preservative use, endothelial keratoplasty-associated complications, and altered drug elimination in individuals with FED. Benzalkonium chloride (BAK), a common preservative in ophthalmic solutions, is known to cause endothelial toxicity in FED. Mitomycin-C, often used in glaucoma surgeries, can contribute to corneal thinning and delayed epithelial healing.[48] To mitigate these risks, preservative-free formulations should be prioritized for long-term therapy, and balanced salt solution (BSS) should be used intraoperatively to minimize exposure to toxic agents.
Endothelial keratoplasty, including DMEK and DSEK, introduces additional toxicity concerns. Donor endothelial cells are susceptible to stress from prolonged tissue manipulation, leading to apoptosis. Furthermore, preservatives in graft storage media may result in transient corneal haze postoperatively.[49] Minimizing surgical handling of donor tissue and thoroughly irrigating grafts before transplantation can help reduce these complications.
Elimination and clearance of ocular drugs may be altered in individuals with FED. Topical drugs can be systemically absorbed through nasolacrimal drainage, potentially leading to systemic side effects. Additionally, impaired endothelial function in FED may slow aqueous humor clearance, prolonging drug exposure.[50]
Long-term steroid users require close IOP monitoring, and lower doses of β-blockers should be considered in patients with systemic comorbidities. Adjusting glaucoma medication regimens in FED patients can help prevent corneal decompensation.
Understanding the toxicokinetics of commonly used drugs and surgical adjuncts in FED management is essential for optimizing therapy while preventing drug-induced complications. Minimizing exposure to cornea-toxic agents and monitoring drug clearance can help preserve long-term corneal health and improve graft survival.[51]
History and Physical
A detailed clinical history and thorough physical examination are essential for early diagnosis and appropriate FED management.[52] Assessing the progression of symptoms is essential when obtaining a clinical history, as they typically worsen over time due to endothelial dysfunction. Early symptoms in the mild stage include morning blurry vision that improves throughout the day, increased glare and halos around lights, particularly at night, and occasional fluctuating vision.[53] As endothelial dysfunction progresses to the moderate stage, patients may experience persistent blurry vision throughout the day, increased light sensitivity, contrast loss, and difficulty with night vision due to corneal edema.
In the advanced stage, characterized by corneal edema and bullous keratopathy, vision loss becomes persistent and does not improve throughout the day. Patients may also develop pain and a foreign body sensation due to epithelial bullae formation, along with progressive photophobia and discomfort.[54]
A family history of FED, which follows an autosomal dominant inheritance pattern, is a significant risk factor. Additionally, a history of cataract surgery or other intraocular procedures may exacerbate corneal decompensation. Comorbid conditions such as diabetes and glaucoma, as well as previous eye trauma, can further accelerate endothelial cell loss.[55]
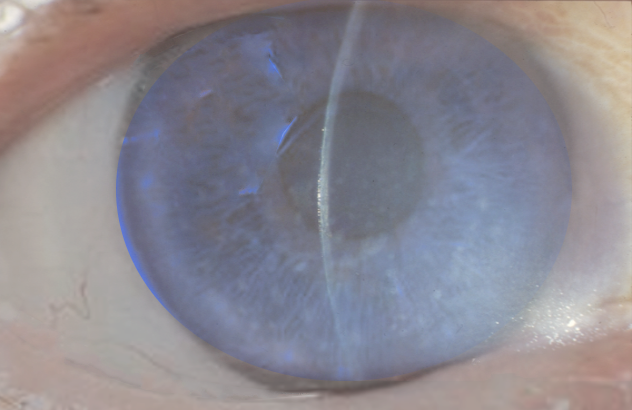
A thorough ophthalmic examination, particularly with slit-lamp biomicroscopy and specular microscopy, helps identify characteristic features of FED and assess disease severity. Slit-lamp biomicroscopy reveals corneal guttata, the hallmark sign, which appear as dark drop-like structures on the endothelium, creating a "beaten metal" appearance. As the disease progresses, the Descemet membrane thickens due to endothelial decompensation, leading to stromal edema. Corneal edema and stromal haze result in a blurry reflex on retroillumination and increased light scatter (see Image. Fuchs Endothelial Dystrophy Findings).
In advanced disease, fluid-filled microcysts or blisters, known as epithelial bullae, develop, causing pain and discomfort. Specular microscopy shows a progressive decline in ECD. Normal ECD ranges from 2,500 to 3,000 cells/mm², while early-stage FED presents with counts below 1,500 cells/mm². In advanced disease, ECD drops below 500 cells/mm², often accompanied by polymegathism and pleomorphism.[56]
Several diagnostic tests can aid in assessing corneal health and detecting complications in FED. Pachymetry measures corneal thickness and helps identify edema, with advanced cases often showing an increase beyond 640 µm. Fluorescein staining evaluates epithelial integrity and highlights features such as bullous keratopathy and microerosions. Gonioscopy and IOP measurements are used to rule out secondary glaucoma. While primary angle closure is not typically observed in FED, steroid-induced glaucoma is a possible risk.
Additional Information
FED typically manifests during the 4th decade of life and progresses through 4 stages over 20 to 30 years (see Staging). In stage 1, most individuals remain asymptomatic as endothelial cells compensate for cell loss. A routine ophthalmologic examination may reveal central corneal guttata incidentally, with biomicroscopy showing guttata, pigment on the posterior corneal surface, and a thickened Descemet membrane. Stage 1 is indistinguishable from central corneal guttata without FED and is only recognized once the disease progresses.
In stage 2, corneal decompensation leads to edema. Patients may report painless blurry vision and glare, worsening in the morning due to overnight corneal hydration from closed eyelids. Many describe using multiple pairs of glasses throughout the day as relative corneal dehydration causes a hyperopic shift. Additional findings include a hazy cornea, poor night vision, and discomfort while blinking.
In stage 3, epithelial bullae develop, further impairing vision. These bullae may rupture, leading to episodic pain. The 4th stage is marked by subepithelial scarring and significant vision loss, sometimes reducing visual acuity to the level of hand motion. Pain may be absent. In developed regions, this stage is rare due to the availability of corneal transplantation.
Slit-lamp examination may be used to assess the extent and confluence of guttata, which indicates disease progression, as well as the severity of corneal edema in advanced stages. In early disease, guttata appear centrally and expand peripherally in a predominantly horizontal pattern. Under direct illumination, guttata present as pinpoint indentations, while retroillumination reveals them as small droplets, reflecting the term's origin from the Latin word gutta. Differentiating guttata in the central cornea from Hassall-Henle bodies in the peripheral cornea is essential, as the latter represent an age-related normal finding.
As the disease advances, the Descemet membrane thickens, best visualized with broad tangential illumination, and guttata become confluent. Stromal edema initially appears as a gray haze anterior to the Descemet membrane. With increasing edema, the inelastic Descemet membrane is pushed posteriorly, forming wrinkles and folds. Full-thickness stromal edema presents with a ground-glass appearance.
Slit-lamp examination in FED may also reveal anterior corneal epithelial changes that correspond with disease progression. In stage 2, microcysts appear on the corneal surface and may be visualized using topical fluorescein and cobalt blue lighting. In stage 3, epithelial defects become evident as extracellular fluid accumulates beneath the epithelium. Stage 4 is characterized by subepithelial scarring and the formation of a connective tissue pannus. Over time, peripheral corneal vascularization develops while stromal and epithelial edema diminish. In rare cases of nonguttate FED, guttata are absent on slit-lamp examination. However, marked corneal edema and bullous keratopathy may still be present.
Pachymetry is often used to assess central corneal thickness (CCT) and guide clinical decision-making, as a CCT greater than 640 µm typically indicates corneal edema. However, some patients with inherently thinner corneas may exhibit advanced FED with a CCT of less than 600 µm. Serial CCT measurements are valuable for monitoring disease progression. The corneal central-to-peripheral thickness ratio (CPTR) has been proposed as a more objective and reproducible metric of FED severity, though it is not routinely used in clinical practice.
The differential diagnosis of FED includes several conditions with overlapping clinical features. Pseudophakic bullous keratopathy (PBK) presents as endothelial loss following cataract surgery. PPCD is typically bilateral but often asymmetric. Iridocorneal endothelial (ICE) syndrome is characterized by unilateral corneal edema with abnormal iris findings. CHED manifests as early-onset, diffuse bilateral corneal haze.
A detailed history and focused ophthalmic examination are essential for the early diagnosis and monitoring of FED. Slit-lamp findings and specular microscopy play a critical role in staging the disease and guiding treatment decisions.[57]
Evaluation
Evaluating FED requires a comprehensive ophthalmic examination with specialized imaging and functional tests. Early detection enables timely intervention to preserve vision.
History, physical examination, pachymetry, and microscopy are key evaluation tools. Additional modalities aid in staging and monitoring disease progression. Specular or confocal microscopy can assess endothelial cell loss, while CCT measurement helps monitor corneal edema and disease progression. AS-OCT has recently demonstrated utility in documenting guttata in FED, though its resolution limits quantitative analysis to a small central corneal region. Routine eye exams every 6 months are recommended to track FED progression.
Specular microscopy evaluates ECD, revealing a low ECD (<1,000 cells/mm²) along with pleomorphism and polymegathism. Pachymetry, whether ultrasound or optical, measures corneal thickness, with advanced cases often exceeding 640 μm. AS-OCT provides detailed imaging of corneal layers, showing a thickened Descemet membrane and stromal hyperreflectivity. Gonioscopy and IOP measurements help rule out secondary causes of corneal edema, confirming the absence of glaucoma-related pathology. Fluorescein staining assesses epithelial integrity, highlighting bullous keratopathy and microerosions.
Additional considerations in FED evaluation include genetic testing, which may be warranted in suspected early-onset cases associated with SLC4A11 mutations. A thorough differential diagnosis is essential to distinguish FED from PPCD, ICE syndrome, and PBK. Comprehensive assessment relies on clinical evaluation, imaging, and endothelial function testing, enabling early detection, effective monitoring, and timely surgical intervention when necessary.
Treatment / Management
FED management depends on disease stage, corneal edema severity, and visual impact, with treatment ranging from conservative measures in early cases to surgical intervention in advanced stages.[58] Early FED can be managed medically, but vision is often worse in the morning than in the afternoon. Several treatments address FED symptoms, including blurry vision and ocular pain. Medical options such as hyperosmotic saline drops or ointment help dehydrate the cornea. Patients experiencing blurry vision in the morning may benefit from gently warming the cornea with dry air from a hairdryer.
Supportive treatments and surgical interventions, including phototherapeutic keratectomy, amniotic membrane transplant, anterior stromal puncture, and conjunctival flaps, can relieve pain, particularly in cases involving ruptured bullae in later stages. Surgical intervention may be considered for advanced disease affecting function and quality of life. The decision to perform cataract surgery alone or a combined DSEK and phacoemulsification procedure depends on CCT and ECD. Older adults with FED often have comorbid conditions such as cataracts, age-related macular degeneration, and cardiovascular disease, which can complicate surgical planning.
Nonsurgical Interventions
Hypertonic saline serves as a first-line therapy for early-to-moderate FED by dehydrating the cornea and reducing morning swelling. Hypertonic saline is typically instilled 4 to 6 times daily, especially upon waking. However, the effects of this fluid are temporary and do not halt disease progression. Lubricating eye drops, particularly preservative-free artificial tears, help minimize ocular surface irritation and discomfort caused by corneal edema.
Topical steroids such as fluorometholone and loteprednol have a mild anti-inflammatory role and may reduce endothelial oxidative stress, leading to transient improvements in corneal clarity. However, long-term use requires monitoring due to the risk of increased IOP.[59] Scleral contact lenses create a fluid-filled reservoir over the cornea, enhancing visual clarity and are often used in moderate FED cases with significant visual distortion.[60] (A1)
Surgical Treatments
FED is the leading indication for corneal transplantation in the U.S., accounting for 36% of nearly 47,000 corneal transplants performed in 2016. Several surgical procedures have demonstrated utility in managing the disease. PKP, Descemet stripping automated endothelial keratoplasty (DSAEK), and DMEK are definitive treatments for restoring vision.[61](B3)
PKP has been used since the 1950s to replace endothelial cells but carries risks such as high postoperative astigmatism, prolonged visual recovery, suture trauma, persistent epithelial defects, and refractive surgery complications. Due to these limitations, newer techniques such as DSAEK, DMEK, posterior lamellar keratoplasty (PLK), and deep lamellar endothelial keratoplasty (DLEK) have gained favor. DSAEK is currently the most common treatment for endothelial dysfunction, offering better visual outcomes than PKP with minimal astigmatic changes. DMEK, which transplants only the endothelial layer and the Descemet membrane, provides the fastest visual rehabilitation among keratoplasty techniques. PLK and DLEK are used less frequently due to their thick graft-host stromal interface.[62]
DMEK is the preferred surgical procedure for advanced FED, offering the best visual outcomes. This procedure involves transplanting an ultrathin donor Descemet membrane with a healthy endothelium. DMEK has a graft rejection rate of less than 2% and allows for rapid visual recovery within approximately 3 months. However, graft detachment requiring rebubbling occurs in 10% to 20% of cases.[63] DSEK is an alternative for patients with prior ocular surgery or glaucoma. The graft is slightly thicker than in DMEK, making handling easier. Visual recovery is slower, taking approximately 6 months, with a rejection rate of 5% to 10%.[64] PKP is reserved for severe corneal scarring or multiple graft failures. This procedure has a higher rejection risk of approximately 20% and requires a longer healing period of up to a year.[65](A1)
Combined phacoemulsification and DMEK or DSEK may be considered for patients with visually significant cataracts. Preoperative specular microscopy is useful in assessing endothelial function before cataract surgery.
Postoperative management includes steroid tapering, with low-dose topical steroids (eg, prednisolone 1%) maintained for at least 6 to 12 months to prevent rejection. Regular IOP monitoring is necessary to detect steroid-induced glaucoma. Lifelong UV protection and adequate corneal hydration can help reduce oxidative stress on the corneal endothelium.
Emerging Therapies
Novel modalities targeting corneal endothelial cell dysfunction include ROCK inhibitors. Y-27632, primarily used in research, has shown potential in promoting endothelial cell adhesion and proliferation. Clinically available ROCK inhibitors, such as netarsudil and ripasudil, may increase endothelial cell survival and migration and are being investigated for their ability to delay surgical intervention.[66][67][68](B3)
Other experimental approaches include corneal collagen cross-linking (CXL), which has shown mixed results in reducing corneal edema, improving visual acuity, and alleviating ocular discomfort. Preliminary research suggests that lithium, N-acetylcysteine, and sulforaphane may have therapeutic benefits in FED. Gene therapies, including adenovirus vector therapy and CRISPR gene editing, are also being explored, though their efficacy remains uncertain.
Differential Diagnosis
FED can present with corneal edema, endothelial dysfunction, and progressive visual impairment, overlapping with other ocular conditions. A thorough clinical examination, imaging, and endothelial cell analysis are crucial in distinguishing FED from other causes of corneal edema and endothelial pathology.
PBK typically occurs following cataract surgery due to endothelial cell loss from intraocular lens implantation. A history of recent intraocular surgery and specular microscopy findings of a low endothelial cell count help differentiate this condition from FED. PPCD presents with bilateral corneal edema and vesicular endothelial changes, often asymmetrical. Slit-lamp examination reveals characteristic "railroad track" endothelial bands, while specular microscopy shows polymorphic endothelial cells.
CHED manifests early in life, with diffuse bilateral corneal haze appearing in infancy or childhood. A neonatal history and genetic testing for SLC4A11 mutations confirm the diagnosis. ICE syndrome is characterized by unilateral corneal edema, abnormal iris changes, and peripheral anterior synechiae. A slit-lamp examination may show a beaten-metal appearance of the endothelium, and gonioscopy reveals peripheral angle abnormalities.
Corneal graft failure should be considered in individuals with a history of corneal transplantation who develop recurrent corneal edema. Graft clarity assessment and specular microscopy help assess endothelial function. Herpetic endotheliitis, caused by herpes simplex virus or varicella-zoster virus, typically presents with unilateral corneal edema, keratic precipitates, and a history of uveitis. Diagnosis is supported by serology, anterior chamber inflammation, and response to antiviral therapy.
Chronic angle-closure glaucoma can mimic FED, presenting with corneal edema and elevated IOP. Gonioscopy findings of closed angles, IOP exceeding 30 mm Hg, and glaucomatous optic neuropathy confirm the diagnosis. Acute endothelial toxicity results in rapid-onset corneal edema following intraocular surgery or chemical exposure. A history of recent intraocular procedures or exposure to toxic agents such as mitomycin-C or BAK is key to identifying this cause.
Other diagnoses that must be considered include the following:
- Primary central corneal guttata
- Aphakic bullous keratopathy, especially with a history of cataract surgery
- Recurrent corneal erosions
- Chandler syndrome
- Posterior polymorphous dystrophy
- Congenital hereditary stromal dystrophy
- Toxic anterior segment syndrome
- Hassall-Henle bodies
- Herpetic disciform keratitis
- Pigment dispersion syndrome
- Anterior uveitis
- Interstitial keratitis
- Herpetic stromal keratitis
Differentiating FED from other corneal endothelial disorders is essential for appropriate treatment planning. A detailed history, slit-lamp examination, and specular microscopy provide critical diagnostic insights, while additional testing helps exclude alternative causes of corneal edema.
Pertinent Studies and Ongoing Trials
Recent research and clinical trials are investigating innovative treatments for FED to enhance patient outcomes and potentially reduce reliance on traditional corneal transplantation.[69] An emerging approach is Descemetorhexis Without Endothelial Keratoplasty (DWEK) or Descemet Stripping Only (DSO), which involves removing a small section of diseased endothelium without implanting donor tissue. This technique encourages endogenous endothelial cell migration and repopulation. A study conducted by the Royal Victorian Eye and Ear Hospital demonstrated improved vision in FED patients using this method without requiring a corneal transplant.[70]
ROCK inhibitors are being investigated for their potential to promote corneal endothelial healing. The Descemet Endothelial Thickness Comparison Trial II (DETECT II) is a multicenter, placebo-controlled clinical trial comparing DMEK to DSO with adjunctive ripasudil in patients with FED. STN1010904 ophthalmic suspension is currently in a Phase IIa trial that assesses its efficacy and safety in FED management without surgical intervention. Similarly, a Phase III clinical trial is investigating the role of K-321 eye drops in promoting corneal healing and improving visual outcomes following descemetorhexis.
Further research also examines the broader impact of FED on visual function. The Mayo Clinic is conducting studies to evaluate how the disease affects vision and quality of life, aiming to clarify the progression of visual impairment and corneal transparency loss over time.[71] These ongoing investigations represent significant advancements in FED research, offering promising prospects for less invasive treatments and improved patient outcomes.
Treatment Planning
The primary goals of FED treatment are to manage corneal edema, slow disease progression, and restore vision through surgical intervention when necessary. Treatment strategies are typically stage-based, with interventions tailored to disease severity.
In the early stage, when symptoms are mild and vision remains unaffected, management focuses on symptom relief and disease monitoring. Hypertonic saline can help reduce morning corneal edema while lubricating artificial tears support corneal hydration. UV protection using sunglasses with a UV400 filter may minimize oxidative damage. ECD should be monitored every 6 to 12 months, with annual eye exams unless symptoms worsen.
As FED progresses to the moderate stage, characterized by persistent corneal edema and visual disturbances, treatment aims to reduce swelling and improve functional vision. Hypertonic saline and topical steroids can aid in edema management. Scleral contact lenses may be beneficial for patients with significant irregular astigmatism. Cataract status should be evaluated, as endothelial function assessment is crucial before surgery. ROCK inhibitors are under investigation for their potential to delay disease progression.[72] Follow-up is recommended every 3 to 6 months to monitor corneal thickness and visual function.
In the advanced stage, severe edema, vision loss, and corneal opacification necessitate surgical intervention for visual rehabilitation. DMEK is the preferred procedure for optimal visual outcomes, while DSEK is an alternative for patients with prior ocular surgery or glaucoma. PKP is reserved for cases with significant corneal scarring. When cataracts are present, combined phacoemulsification with DMEK or DSEK may be performed.[73] Postoperative follow-up should occur every 1 to 3 months, transitioning to annual evaluations once recovery stabilizes.
Surgical decision-making in FED is guided by patient-specific factors, including the severity of corneal edema, presence of cataracts, history of prior ocular surgeries, and overall corneal health. The choice of intervention aims to optimize visual outcomes while minimizing surgical risks.
For patients with mild corneal edema and no significant vision loss, medical therapy remains the preferred approach, as surgery is unnecessary. When FED is accompanied by a visually significant cataract, phacoemulsification with DMEK is the preferred option, though DSEK may also be considered. Performing a combined procedure helps prevent the need for multiple surgeries. In patients who have a history of glaucoma surgery or a filtering bleb, DSEK is favored over DMEK due to its lower risk of graft detachment. For individuals with severe corneal scarring or multiple graft failures, PKP is required, as a full-thickness transplant is necessary for vision restoration.
Long-term management of FED focuses on monitoring graft survival, optimizing postoperative care, and supporting corneal health to prevent disease progression. Annual specular microscopy and corneal thickness measurements help assess endothelial function and detect early signs of graft failure. Steroid therapy should be carefully tapered to prevent rejection while minimizing IOP elevation. Recognizing early signs of graft rejection—redness, sensitivity to light, vision loss, and pain (the RSVP mnemonic)—is essential for prompt intervention. Lifestyle and nutritional support, including adequate hydration, antioxidants, and UV protection, further contribute to corneal health.
While early-stage FED can be managed conservatively, advanced cases require timely keratoplasty, with surgical decisions tailored to patient-specific factors. Postoperative monitoring remains critical for long-term graft survival.[74]
Toxicity and Adverse Effect Management
Adverse effects associated with topical medications used in FED management vary based on the drug class, requiring careful monitoring and individualized treatment adjustments to minimize complications. Hypertonic saline often causes ocular irritation and a transient burning sensation upon application. Using preservative-free formulations can help mitigate discomfort, and applying lubricating artificial tears 10 minutes after instillation may further reduce irritation.
Corticosteroids present risks such as steroid-induced ocular hypertension and glaucoma, which can lead to optic nerve damage. Long-term use may also accelerate posterior subcapsular cataract formation and impair wound healing, increasing susceptibility to infections.[75] Regular IOP monitoring every 4 to 6 weeks is essential, particularly in patients at risk for glaucoma. Steroid-sparing agents like cyclosporine 0.05% may be considered for prolonged therapy, while weaker corticosteroids such as fluorometholone or loteprednol may be recommended for patients with a history of steroid-induced IOP elevation. Gradual tapering is necessary to prevent rebound inflammation.
IOP-lowering agents are often required for steroid-induced glaucoma but must be selected cautiously. β-Blockers can cause systemic side effects such as bradycardia, hypotension, and bronchospasm, making them unsuitable for patients with asthma or chronic obstructive pulmonary disease (COPD). Additionally, these agents may contribute to dry eye and delay corneal epithelial healing.[76] Preservative-free formulations can help reduce ocular surface irritation. CAIs are another option, but these medications may worsen corneal decompensation in patients with preexisting endothelial dysfunction, making them less suitable for individuals with advanced FED.[77]
For ROCK inhibitors, conjunctival hyperemia is a common adverse effect. These agents may be beneficial for patients experiencing steroid-induced IOP elevation who also require endothelial protection.[78]
Anesthetic and surgical agents used in FED management can pose risks to the corneal endothelium, necessitating careful selection and monitoring to prevent toxicity-related complications. Preservatives in anesthetic drops, such as BAK, may contribute to endothelial toxicity, particularly in patients undergoing keratoplasty. When possible, preservative-free anesthetics should be used to minimize damage. Additionally, mitomycin-C, sometimes utilized in conjunction with glaucoma surgery, has been associated with corneal thinning and delayed epithelial healing. Limiting mitomycin-C exposure is essential to prevent corneal toxicity.[79]
Graft-related toxicities also present challenges in posttransplant management. Some patients may develop inflammatory reactions to donor corneal endothelium, even in the absence of overt rejection. Low-grade inflammation should be monitored closely and managed with short courses of corticosteroids as needed.[80] Furthermore, despite successful outcomes following DMEK or DSEK, gradual endothelial cell loss occurs over time. To ensure long-term graft viability, annual specular microscopy assessments are necessary to track ECD and detect early signs of dysfunction.[81]
Managing toxicity and adverse effects in FED requires a comprehensive approach that includes regular monitoring, medication adjustments, and patient-specific modifications. Reducing drug-related toxicity, maintaining IOP control, and optimizing posttransplant care are essential for improving long-term outcomes and ensuring graft survival.[82]
Staging
Staging of FED is traditionally based on the extent of guttata and corneal edema. Early stages are characterized by endothelial cell loss and guttata formation on the Descemet membrane, while later stages involve all corneal layers. In 1978, Dr. Jay Krachmer and his team introduced a clinical grading scale to assess disease progression subjectively. This scale is outlined as follows:
- Grade 0 (G0): No apparent disease
- Grade 1 (G1): 0 to 12 central, nonconfluent guttata in at least 1 eye; typically asymptomatic
- Grade 2 (G2): More than 12 central, nonconfluent guttata in at least 1 eye
- Grade 3 (G3): 1- to 2-mm zone of central confluent guttata in the horizontal plane
- Grade 4 (G4): 2- to 5-mm zone of central confluent guttata in the horizontal plane
- Grade 5 (G5): Zone of central confluent guttata larger than 5 mm, with or without corneal stromal or epithelial edema [83]
Another staging system categorizes FED progression into 4 distinct stages spanning 2 to 3 decades, as follows:
- Stage 1: Asymptomatic slit-lamp examination revealing central nonconfluent guttata and thickening of the Descemet membrane
- Stage 2: Coalescence of guttata with endothelial cell polymegathism, pleomorphism, and loss; symptoms include blurry vision and glare, especially upon awakening
- Stage 3: Endothelial pump dysfunction leading to bullae formation in the epithelial and subepithelial layers, with corneal edema
- Stage 4: Corneal edema resulting in haziness, scarring, and significant vision loss
A 3rd classification further stratifies FED based on clinical features and diagnostic indicators, as follows:
- Mild: Mild guttata without corneal edema; normal pachymetry and ECD (1,500 cells/mm²)
- Moderate: Corneal edema with morning blur; increased pachymetry (>600 µm) and reduced ECD (<1,000 cells/mm²)
- Advanced: Persistent edema, bullous keratopathy, and vision loss; severe corneal thickening and critically low ECD (<500 cells/mm²)
However, with advancements in nonsurgical treatments and earlier surgical interventions, these traditional staging classifications may be losing clinical relevance.
Prognosis
FED is a progressive condition, with significant visual impairment often emerging in the 6th or 7th decade of life, necessitating surgical intervention. Prognosis depends on the stage at diagnosis, rate of endothelial cell loss, and timeliness of treatment. Early-stage cases may be managed conservatively, while advanced disease typically requires corneal transplantation (eg, DMEK or DSEK) for visual rehabilitation.[84]
Without treatment, FED follows a predictable progression, with endothelial cell loss gradually leading to worsening corneal edema and vision impairment. In the early stage, patients remain asymptomatic or experience mild morning blurring due to transient corneal edema. Vision remains functional for years with minimal interventions, such as hypertonic saline drops. However, if endothelial decompensation occurs, persistent corneal edema may develop.[85]
As the disease advances to the moderate stage, patients experience increased glare, halos, and fluctuating vision, which can interfere with activities like night driving and reading. Without intervention, corneal thickening progresses, and bullous keratopathy becomes inevitable. Corneal scarring further increases the likelihood of requiring surgical treatment.
In the advanced stage, severe endothelial failure results in chronic stromal edema, epithelial bullae, and persistent visual deterioration. Without surgery, functional vision is lost, significantly impacting daily life. Left untreated, bullous keratopathy can lead to recurrent erosions, pain, and permanent corneal scarring, making corneal transplantation the only viable option for visual rehabilitation.
DMEK offers the best visual outcomes among all surgical options, with a 90% to 95% graft survival rate at 5 years. Compared to other procedures, DMEK has the lowest rejection rates (<2%), but graft detachment requiring rebubbling occurs in 10% to 20% of cases (see Image. Long-Term Graft Survival Rates of Corneal Transplants in Fuchs Endothelial Dystrophy).[86]
DSEK has an 85% to 90% graft survival rate at 5 years and is more suitable for patients with prior ocular surgery or glaucoma. However, this approach has a higher rejection rate (5% to 10%) than DMEK, and visual recovery is slightly longer due to interface haze.[87]
PKP is reserved for cases with severe corneal scarring when DMEK or DSEK is not viable. Recovery is prolonged, taking approximately 1 year, and rejection rates are higher (~20%) compared to lamellar keratoplasty (see Image. Prognosis of Fuchs Endothelial Dystrophy After Surgery).[88]
If endothelial cells remain functional, DMEK and DSEK can provide stable vision for over a decade. Long-term graft survival depends on appropriate steroid tapering and rejection monitoring. Late graft failure occurring more than 10 years postoperatively may necessitate repeat keratoplasty in some cases. Patients with FED can maintain functional vision for decades with early diagnosis, proper medical management, and timely surgical intervention. Advances in DMEK and DSEK have significantly improved long-term visual rehabilitation with lower complication rates. Regular follow-up and adherence to postoperative care remain essential for sustained success.
Most patients who undergo keratoplasty for FED experience significant visual improvement.[89][90] A Cochrane review by Nanavaty et al in 2014 found no difference in final visual outcomes between patients who received endothelial keratoplasty (DSEK, DSAEK, DMEK, or femtosecond laser-assisted endothelial keratoplasty) and those who underwent PKP.[91] However, endothelial keratoplasty provides faster and more reliable visual recovery than PKP while preserving the structural integrity of the eye.[92] Additionally, PKP is associated with a higher risk of complications. By 2013, DMEK had surpassed PKP as the preferred treatment for endothelial disorders, and endothelial keratoplasty techniques, eg, DSAEK and DMEK, have since become the standard of care for FED.[93] Patients generally achieve good visual outcomes.
Long-term visual outcomes following keratoplasty depend on endothelial cell function, postoperative management, and patient adherence to care. When endothelial cells remain functional, DMEK and DSEK can sustain vision for more than a decade. Steroid tapering and regular monitoring for graft rejection are essential for long-term graft survival. However, late graft failure, occurring more than 10 years postoperatively, may necessitate repeat keratoplasty in some patients.
With early diagnosis, appropriate medical management, and timely surgical intervention, individuals with FED can maintain functional vision for decades. Advances in DMEK and DSEK have significantly improved prognosis, providing durable visual rehabilitation with low complication rates. Consistent follow-up and adherence to postoperative care remain critical for long-term success.[94]
Complications
FED can lead to complications such as corneal scarring and progressive vision loss. Without treatment, endothelial cell loss may result in corneal decompensation and the need for transplantation. However, while surgical interventions can restore vision, they also carry risks (see Image. Fuchs Endothelial Dystrophy Postsurgical Complications). Early recognition, interprofessional care, close follow-up, and patient education regarding these complications are essential for optimizing outcomes.[95]
Complications of untreated FED primarily stem from progressive endothelial cell loss, leading to corneal edema, vision impairment, and increased surgical risks. Loss of endothelial cells allows fluid to accumulate in the stroma, increasing corneal thickness and reducing transparency. In the early stages, transient morning blurring occurs due to overnight swelling, improving as the day progresses. As the disease advances, persistent vision haze, glare, and difficulty with night vision develop. Severe cases progress to bullous keratopathy, characterized by fluid-filled epithelial blisters that cause significant pain and discomfort.
Chronic endothelial failure results in permanent stromal scarring, leading to corneal opacification and progressive visual deterioration. Patients experience increasing corneal haze, loss of contrast sensitivity, and functional vision loss. In advanced cases, corneal transplantation with DMEK, DSEK, or PKP is the only effective treatment.
FED by itself also increases the risk of complications from intraocular surgery. Cataract surgery accelerates endothelial cell loss, worsening corneal edema and increasing the likelihood of postoperative decompensation, particularly when preoperative ECD is low. Proper assessment of corneal thickness and endothelial function is essential before cataract surgery in these patients.[96]
Surgical management of FED with DMEK, DSEK, or PKP carries risks that can impact long-term graft survival and visual outcomes. Graft dislocation, the most common complication of DMEK, occurs in 10% to 20% of cases due to incomplete attachment of the donor Descemet membrane. Patients may experience reduced vision postoperatively, with persistent corneal edema detected on slit-lamp and AS-OCT examination.[97] Management involves a rebubbling procedure with air or gas injection to reattach the graft, along with strict supine positioning to promote adherence.[98]
Graft rejection, occurring in 1% to 10% of cases, is more frequent in DSEK and PKP than in DMEK. Immune-mediated rejection of the donor endothelium presents with redness, photophobia, vision loss, and pain due to inflammation and corneal decompensation.[99] Treatment includes intensive application of topical corticosteroids such as prednisolone 1% administered hourly, with oral steroids reserved for severe cases. Close monitoring is essential to detect recurrent rejection episodes.
Steroid-induced glaucoma results from prolonged corticosteroid use after DMEK or DSEK, leading to trabecular meshwork dysfunction and elevated IOP. Patients with preexisting glaucoma or extended steroid use exceeding 6 months are at higher risk. Management includes regular IOP monitoring and the use of β-blockers, CAIs, or ROCK inhibitors while avoiding prostaglandin analogs that may exacerbate inflammation.
Primary graft failure, occurring in 3% to 5% of cases, is characterized by persistent corneal edema beyond 3 months postoperatively due to poor-quality donor tissue, surgical trauma, or preexisting endothelial dysfunction. When endothelial cells fail to function, repeat endothelial keratoplasty may be necessary.[100]
Infectious keratitis is more common in PKP than in DMEK or DSEK due to the presence of sutures, which create entry points for microbial infection. Long-term steroid use further increases susceptibility. Clinical signs include corneal infiltrates with ulceration, increased discharge, and pain. Management involves intensive topical antibiotic therapy, corneal cultures to guide treatment, and suture removal if they contribute to infection.[101]
Late graft failure, which can occur more than 5 to 10 years postoperatively, results from gradual endothelial cell loss leading to corneal decompensation. Chronic IOP elevation and repeated graft rejection episodes increase the risk. Regular endothelial cell counts and pachymetry measurements help monitor progression. Repeat endothelial keratoplasty may be considered for visual rehabilitation.[102]
Graft detachment rates after DSAEK range from 0.9% to 36.4%, while a large multicenter study reported a 36.4% detachment rate after DMEK.[103][104] Detachments are typically managed with an additional procedure to reattach the graft using air or sulfur hexafluoride. Marques et al demonstrated that patients who underwent DMEK had better-corrected distance visual acuity and a 60% lower rejection rate than those who received DSAEK, though DMEK required more rebubblings due to graft detachment.[105]
Individuals who undergo PKP face risks such as postoperative astigmatism and wound rupture. Given these potential complications, endothelial keratoplasty techniques are the standard of care for FED. Cataract progression is also a concern after keratoplasty.
Other potential perioperative complications of surgical treatments for FED include aqueous leakage and vitreoretinal injury, which require prompt evaluation and appropriate surgical intervention. Meanwhile, long-term sequelae necessitate ongoing management and rehabilitation. Lifelong immunosuppression may be required to prevent chronic graft rejection, often in the form of low-dose topical steroids. However, prolonged steroid use increases the risk of glaucoma and cataract formation, making regular monitoring essential.
Postoperative astigmatism or irregular corneal curvature can result in residual refractive error and visual distortion, sometimes requiring spectacle correction or scleral lenses. Additionally, some patients, particularly those who undergo DSEK, experience persistent glare and halos at night due to graft-host interface haze. Wavefront-guided corneal topography can help evaluate and address residual aberrations.[106]
Even in successfully transplanted corneas, ECD continues to decline over time. Long-term graft survival depends on strategies to preserve endothelial function and early intervention if signs of decompensation emerge.[107]
Effective preventive strategies can minimize complications associated with keratoplasty and improve long-term outcomes. Preoperative risk assessment involves using specular microscopy and pachymetry to evaluate endothelial reserve before cataract surgery. Careful IOP control is also essential to prevent secondary endothelial damage.[108]
Postoperative care optimization includes a strict steroid tapering protocol to balance the risk of rejection with the potential for steroid-induced IOP elevation. In DMEK, timely rebubbling is necessary to prevent prolonged corneal edema and ensure graft adherence.[109]
Long-term monitoring involves annual ECD assessments to detect late-stage graft dysfunction. Additionally, patient education on recognizing early signs of graft rejection using the RSVP mnemonic can facilitate prompt intervention.
Postoperative and Rehabilitation Care
Postoperative care following DMEK, DSEK, or PKP is critical for graft survival, optimal visual recovery, and minimizing complications. Proper rehabilitation ensures long-term success and prevents endothelial rejection.
In the first 4 weeks after surgery, positioning plays a crucial role in graft adherence. Patients who underwent DMEK should remain supine for 24 to 48 hours to facilitate graft attachment, while individuals who received DSEK may have fewer restrictions. Topical corticosteroids such as prednisolone 1% or fluorometholone are administered every 1 to 2 hours initially, followed by a gradual taper over several months. Antibiotic drops, including moxifloxacin or tobramycin, are prescribed for 1 to 2 weeks to prevent infection. IOP monitoring is essential when prolonged steroid use is required, given the risk of steroid-induced glaucoma. Patients must avoid eye rubbing, heavy lifting, or straining, as these activities may cause graft dislocation. Exposure to swimming pools and dusty environments should also be minimized to reduce infection risk.
Over the next several weeks, regular follow-up is necessary to monitor graft adherence, endothelial function, and corneal edema resolution. Slit-lamp and specular microscopy examinations help assess these factors. IOP monitoring remains a priority, as steroid-induced IOP elevation is common and may necessitate steroid dose adjustments or the introduction of IOP-lowering drops. Detecting early signs of graft rejection is crucial, as symptoms such as redness, sensitivity to light, vision loss, and pain require urgent steroid escalation to prevent graft failure.
Between 3 to 12 months postoperatively, long-term rehabilitation focuses on visual recovery and ongoing graft surveillance. Steroid tapering must be carefully managed over 6 to 12 months to balance the prevention of graft rejection with the risk of steroid-related complications. Visual outcomes differ by surgical technique. Patients post-DMEK often achieve their best vision within 3 months. Patients who undergo DSEK may require 6 months or longer. Some individuals may need spectacles or scleral lenses to address residual astigmatism. Chronic monitoring includes annual endothelial cell counts and corneal optical coherence tomography to assess long-term graft function, along with routine glaucoma screening due to prolonged steroid exposure.
Lifestyle modifications, such as wearing UV-blocking sunglasses to reduce oxidative stress and minimize rejection risks, can further support long-term graft survival. Maintaining hydration and a low-sodium diet helps regulate corneal hydration levels. Regular follow-up examinations every 3 to 6 months ensure continued monitoring of graft health.
A structured postoperative and rehabilitation plan is essential for successful corneal graft survival and vision restoration in FED patients. Close monitoring, proper steroid tapering, and patient education significantly improve long-term outcomes.
Consultations
An interprofessional approach ensures comprehensive FED management by coordinating care among ophthalmic subspecialists, primary care physicians, and specialists in systemic disease management. This collaboration optimizes vision preservation, surgical outcomes, and overall patient care.[110]
A cornea specialist or anterior segment ophthalmologist serves as the primary consultant, assessing endothelial function and corneal thickness through slit-lamp examination, specular microscopy, pachymetry, and AS-OCT. This specialist determines disease severity and whether medical or surgical intervention, such as DMEK, DSEK, or PKP, is required while also managing postoperative care, rejection surveillance, and endothelial graft function.[111] Comprehensive ophthalmologists and optometrists play a role in early detection during routine eye exams, prescribing hypertonic saline drops for mild corneal edema and fitting scleral contact lenses for irregular corneal astigmatism in individuals not yet requiring surgery. These specialists monitor vision changes and refer patients to a corneal specialist when necessary.[112]
If concurrent cataracts are present, a cataract surgeon can evaluate lens status and determine whether combined cataract surgery with DMEK or DSEK is needed. Specular microscopy helps assess endothelial reserve before phacoemulsification, and endothelium-safe techniques, such as low-flow phacoemulsification and dispersive viscoelastic use, are selected to minimize endothelial trauma.[113] In cases where steroid-induced IOP spikes or FED-related glaucoma develop, a glaucoma specialist monitors postoperative IOP and recommends IOP-lowering agents, such as β-blockers, CAIs, or ROCK inhibitors, when needed.[114] If FED is associated with posterior segment pathology, a retina specialist evaluates macular edema, diabetic retinopathy, or age-related macular degeneration to ensure retinal function remains stable before corneal transplantation.[115][116]
Systemic conditions that contribute to corneal endothelial dysfunction necessitate monitoring by a primary care physician or internist who manages hypertension, diabetes, and autoimmune diseases while assessing overall surgical fitness for keratoplasty.[117] In cases of familial FED, genetic consultation is beneficial, as the condition follows an autosomal dominant inheritance pattern. Screening family members allows for early disease detection, and genetic counseling is particularly recommended for individuals with early-onset or rapidly progressing disease.
Deterrence and Patient Education
FED is a slowly progressive corneal disorder, often with an unknown cause, that can lead to blurred vision, corneal swelling, and eventual vision loss. Although FED is not preventable, its progression can be slowed. Patients should have regular follow-up appointments to monitor disease progression through routine eye examinations. Patients need to understand that the condition will eventually require surgical intervention.[118] Below is a guide for patient education and strategies for slowing progression.
Lifestyle modifications can help slow the progression of FED and support overall eye health. Wearing UV-blocking sunglasses protects endothelial cells from oxidative stress, and avoiding prolonged sun exposure helps prevent endothelial dysfunction.[119] Chronic eye rubbing can worsen endothelial damage, so preservative-free artificial tears should be used for dry eye symptoms instead.[120] A healthy diet rich in antioxidants and low in sodium supports corneal health while staying hydrated helps maintain ocular hydration.[121] Managing systemic conditions such as diabetes and hypertension, along with avoiding ROS from sources such as cigarettes and alcohol, can prevent accelerated endothelial cell loss.[122]
Preventative strategies are essential for individuals at high risk of FED. The condition's genetic component necessitates routine specular microscopy screening for 1st-degree relatives. Cataract surgery can stress the endothelial layer, so in high-risk cases, combined cataract surgery with DMEK and endothelium-protective techniques like low-flow phacoemulsification should be considered.[123] Regular eye examinations with annual corneal endothelial cell counts are crucial, and if pachymetry shows corneal thickening above 640 μm, a referral to a corneal specialist is recommended.[124]
Medical management can delay the progression of FED. Hypertonic saline drops can reduce morning fogging by pulling fluid out of the cornea, with usage recommended every 6 to 8 hours as needed.[125] IOP control is essential to prevent accelerated endothelial loss, with β-blockers, CAIs, and ROCK inhibitors as potential treatment options.[126]
When discussing surgical treatment options for FED, clinicians should consider the patient's specific condition and risk factors. DMEK is preferred in most cases due to its superior vision outcomes and lower rejection rates, though it carries a higher risk of graft dislocation. DSEK is suitable for patients with prior intraocular surgery, but it has a higher rejection risk (5%-10%) than DMEK. PKP is reserved for severe cases involving corneal scarring, with a longer recovery time and increased rejection risk.[127]
Addressing common patient concerns and misconceptions is essential for effective communication. Many patients worry, "I will go blind from Fuchs dystrophy," but modern surgical techniques like DMEK and DSEK restore vision in most cases. Others believe, "Cataract surgery will fix my vision completely," but cataract surgery alone may worsen corneal edema in FED, and combined cataract surgery with DMEK may be necessary. Patients may also say, "There is no treatment for Fuchs dystrophy," but medical management can delay progression, and surgery can restore vision when needed.[128]
Patient support and rehabilitation are vital for managing FED. Psychological support is important as vision changes can lead to anxiety and depression. Patients should be reassured about available treatment options and encouraged to join support groups.[129] Postoperative care education is essential, emphasizing long-term follow-up for graft rejection detection and the need for long-term steroid drops to prevent rejection. Financial and insurance counseling ensures patients are informed about coverage for keratoplasty procedures and follow-up care.
Educating patients about early symptoms, preventative measures, and treatment options empowers them to make informed decisions. A proactive, interprofessional approach optimizes visual outcomes, reduces anxiety, and enhances quality of life.[130]
Pearls and Other Issues
Key clinical insights about FED include the following:
- Early morning vision fluctuation: Symptoms of FED typically worsen upon waking due to overnight corneal swelling, improving as the day progresses.[131]
- Gender predilection: FED is more common in women, often presenting in the 5th to 7th decade of life.[132]
- Role of specular microscopy and pachymetry: Endothelial cell loss, which precedes corneal edema, makes specular microscopy and pachymetry essential diagnostic tools. Corneal thickness greater than 640 μm often signals clinically significant edema, which may require surgical intervention.
- Surgical timing considerations: DMEK is preferred over DSEK for better visual outcomes and reduced rejection risk. Cataract surgery combined with DMEK may be considered for patients with concurrent lens opacities.
- Medical therapy as a temporary measure: Hypertonic saline drops or ointment can temporarily reduce morning vision haze, while topical ROCK inhibitors are being investigated for endothelial cell regeneration.
Disposition in long-term management of FED involves a comprehensive plan for ongoing monitoring, potential surgical intervention, and continued care to address disease progression and optimize visual outcomes.
- Progressive course without treatment: The disease worsens gradually over years, with eventual corneal decompensation leading to blurred vision and bullous keratopathy.[133]
- Success rates of surgical interventions: DMEK graft survival exceeds 90% at 5 years, making it the preferred treatment in advanced cases. The risk of rejection is lower in DMEK than in DSEK due to thinner grafts and better integration.
- Need for lifelong monitoring: Even after transplantation, steroid therapy must be tapered gradually to avoid graft rejection or steroid-induced glaucoma. Patients should have regular ECD assessments posttransplant.[134]
Emerging imaging modalities are advancing the diagnosis and management of FED.
- Specular microscopy, combined with artificial intelligence-assisted endothelial analysis, allows automated monitoring of ECD.
- AS-OCT can detect early corneal edema before clinical symptoms manifest.
- Confocal microscopy is useful for evaluating endothelial cell morphology and detecting stress changes in FED.[135]
Novel therapies are also showing promise in FED management. Besides ROCK inhibitors, tissue engineering and cultured endothelial cells offer innovative approaches to restore endothelial function without requiring full keratoplasty.[136]
Several pitfalls can complicate accurate assessment and intervention in managing FED, including the following:
- Early signs of FED may be overlooked as normal aging changes. Specular microscopy is essential for high-risk patients, such as those who have a significant family history or a predisposition to develop cataracts.
- Cataract extraction alone may worsen corneal edema in moderate-to-severe FED. Combined phacoemulsification and DMEK should be considered when the endothelial function is borderline.
- Postoperative complications, such as graft dislocation requiring rebubbling and IOP spikes due to prolonged steroid use, should be monitored and managed proactively.
Prevention and risk reduction in FED involve key strategies that can significantly impact disease progression.
- Avoidance of excessive UV exposure: UV exposure can accelerate oxidative stress in endothelial cells, so sunglasses with UV protection are recommended.
- Caution with intraocular surgery: In patients with mild FED, phacoemulsification should be performed carefully with minimal fluid turbulence to reduce endothelial stress.[137]
- Early referral for surgical planning: Patients with persistent morning fogging, corneal edema, or pachymetry exceeding 640 μm should be referred to a corneal specialist before bullous keratopathy develops.
- Genetic counseling in family members: Family members may benefit from early screening since FED follows an autosomal dominant inheritance pattern.
Understanding FED's natural course, early diagnostic clues, and treatment nuances is essential for timely intervention and optimal surgical outcomes. An interprofessional team approach can help prevent vision loss and improve patients' quality of life.
Posttransplant Management: Additional Insights
Postoperative medication protocols include the use of topical corticosteroids such as prednisolone acetate 1% or fluorometholone to prevent graft rejection, with gradual tapering over 6 to 12 months to reduce the risk of secondary glaucoma. IOP monitoring is crucial, as prolonged steroid use can cause ocular hypertension. If necessary, antiglaucoma therapy, including β-blockers, CAIs, and ROCK inhibitors, may be employed. Hypertonic saline drops remain useful in managing residual corneal edema during early postoperative recovery.[138]
Early signs of graft rejection, often remembered by the mnemonic "RSVP," require urgent intervention with high-dose corticosteroids if symptoms appear. Graft survival rates for DMEK are 90% to 95% at 5 years, with better visual outcomes due to thinner grafts and minimal interface haze.[139] DSEK, while preferred for patients with prior intraocular surgery or glaucoma, has slightly higher rejection rates (5%-10%) compared to DMEK.
When comparing DMEK and DSEK, several aspects of graft survival, visual recovery, rejection rate, and suitability for different patient profiles must be considered.
- DMEK offers superior outcomes in terms of graft survival and visual recovery, with faster and better quality vision due to its thinner graft.
- DMEK also has a lower rejection rate (1%-2%) and is preferred for younger patients with minimal ocular history.
- DSEK has a slightly lower graft survival rate (85%-90%) and slower visual recovery due to the thicker graft.
- DSEK's rejection rate is higher compared to DMEK, and it is typically preferred for individuals who are older or have had prior ocular surgery.
- While DMEK has minimal interface haze, DSEK results in mild haze.
To prevent postoperative complications, UV400 protection from sunglasses is advised to reduce oxidative stress on corneal endothelial cells. Patients should undergo specular microscopy every 6 to 12 months after surgery for endothelial monitoring.
Enhancing Healthcare Team Outcomes
Managing FED requires interprofessional collaboration. Given the progressive nature of the disease, the patient’s quality of vision and life must be central to management decisions. An interprofessional team approach ensures optimal patient outcomes and long-term corneal health through effective communication, care coordination, and shared responsibilities.[140]
Ophthalmologists and corneal specialists use specular microscopy, pachymetry, and AS-OCT to monitor disease progression in FED, focusing on endothelial cell count and corneal edema. These specialists manage the condition with hypertonic saline drops, IOP-lowering drugs, and topical anti-inflammatory agents when needed. Surgical interventions these professionals may provide include DMEK for early-to-moderate cases, DSEK for more advanced damage, and PKP for severe corneal scarring or graft failure.
Optometrists play a key role in early detection and referral during routine eye exams. These specialists can fit scleral lenses to correct corneal irregularities and assist with postoperative rehabilitation through vision therapy and optical correction following endothelial keratoplasty.
Nurses and allied healthcare professionals play a key role in promoting compliance and providing support. These professionals provide preoperative and postoperative counseling, educating patients about surgical expectations and signs of graft rejection. These providers also assist with diagnostic tests such as pachymetry and slit-lamp imaging to monitor disease progression and help reinforce adherence to postoperative steroid drop schedules to prevent graft rejection.[141] Coordination between the ophthalmic surgeon, social workers, and low-vision therapists after surgery is vital, especially in older individuals.[142]
Early medical management should involve pharmacists, who can advise patients on the importance of medication compliance. These professionals can also educate patients on the use of hypertonic saline drops, corticosteroids, and IOP-lowering drugs. Pharmacists may also monitor for drug interactions, particularly in individuals with glaucoma who may develop steroid-induced ocular hypertension.[143]
Primary care physicians manage systemic risk factors that affect corneal healing, such as diabetes, hypertension, and autoimmune diseases. These providers also facilitate timely referrals to ophthalmologists for early intervention.[144]
Care coordination and a patient-centered approach are essential for successful FED management. Presurgical team meetings should focus on determining patient suitability for DMEK versus DSEK, while postoperative monitoring schedules help ensure early graft rejection detection through close follow-ups. Communication across disciplines is critical, with primary care physicians and pharmacists informed about steroid tapering regimens.[145]
Ethical considerations in FED management include informed consent and decision-making. Patients should fully understand the risks, benefits, and alternatives to keratoplasty before surgery and retain autonomy in deciding the timing of the procedure. Equitable access to corneal transplants must be ensured, with transparent donor cornea allocation based on medical necessity and improved access for underserved populations.[146]
Improving the patient experience involves setting clear preoperative expectations regarding visual recovery timelines and potential risks. Addressing psychosocial concerns and providing emotional support for anxiety related to vision loss are also key. Financial counseling ensures patients are aware of insurance coverage for endothelial keratoplasty.[147]
Key indicators for evaluating team performance in FED management should center on clinical outcomes and patient quality of life. The recommended metrics include achieving 95% graft survival at 5 years for DMEK, optimizing steroid regimens to reduce postoperative IOP spikes and prevent secondary glaucoma, assessing patient satisfaction through improvements in visual function and quality of life, and minimizing delays in corneal transplant surgery from diagnosis.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)

Long-Term Graft Survival Rates of Corneal Transplants in Fuchs Endothelial Dystrophy. This chart compares the long-term graft survival rates of different corneal transplant procedures—Descemet membrane endothelial keratoplasty (DMEK), Descemet stripping endothelial keratoplasty (DSEK), and penetrating keratoplasty (PKP)—in patients with Fuchs endothelial dystrophy. DMEK produces better graft survival rates than DSEK and PKP.
Bharat Gurnani, MBBS, DNB, FCRS, FICO, FAICO Cornea, FAICO Refractive Surgery, MRCS Ed, MNAMS, FACS, FIACLE
(Click Image to Enlarge)

Prognosis of Fuchs Endothelial Dystrophy After Surgery. This table summarizes the outcomes after Descemet membrane endothelial keratoplasty (DMEK), Descemet stripping endothelial keratoplasty (DSEK), and penetrating keratoplasty (PKP). DMEK has the best outcomes and may be offered to patients with minimal ocular history.
Bharat Gurnani, MBBS, DNB, FCRS, FICO, FAICO Cornea, FAICO Refractive, MRCS Ed, MNAMS, FACS, FIACLE
(Click Image to Enlarge)

Fuchs Endothelial Dystrophy Postsurgical Complications. This table summarizes the risks associated with surgery for Fuchs endothelial dystrophy, as well as the management strategies for each condition.
Bharat Gurnani, MBBS, DNB, FCRS, FICO, FAICO Refractive, FAICO Cornea, MRCS Ed, MNAMS, FACS, FIACLE
References
Eghrari AO, Gottsch JD. Fuchs' corneal dystrophy. Expert review of ophthalmology. 2010 Apr:5(2):147-159 [PubMed PMID: 20625449]
Adamis AP, Filatov V, Tripathi BJ, Tripathi RC. Fuchs' endothelial dystrophy of the cornea. Survey of ophthalmology. 1993 Sep-Oct:38(2):149-68 [PubMed PMID: 8235998]
Level 3 (low-level) evidenceFeizi S. Corneal endothelial cell dysfunction: etiologies and management. Therapeutic advances in ophthalmology. 2018 Jan-Dec:10():2515841418815802. doi: 10.1177/2515841418815802. Epub 2018 Dec 7 [PubMed PMID: 30560230]
Level 3 (low-level) evidenceHamill CE, Schmedt T, Jurkunas U. Fuchs endothelial cornea dystrophy: a review of the genetics behind disease development. Seminars in ophthalmology. 2013 Sep-Nov:28(5-6):281-6. doi: 10.3109/08820538.2013.825283. Epub [PubMed PMID: 24138036]
Nanda GG, Alone DP. REVIEW: Current understanding of the pathogenesis of Fuchs' endothelial corneal dystrophy. Molecular vision. 2019:25():295-310 [PubMed PMID: 31263352]
Level 3 (low-level) evidenceRepp DJ, Hodge DO, Baratz KH, McLaren JW, Patel SV. Fuchs' endothelial corneal dystrophy: subjective grading versus objective grading based on the central-to-peripheral thickness ratio. Ophthalmology. 2013 Apr:120(4):687-94. doi: 10.1016/j.ophtha.2012.09.022. Epub 2013 Jan 28 [PubMed PMID: 23369486]
Level 2 (mid-level) evidenceSon HS, Villarreal G Jr, Meng H, Eberhart CG, Jun AS. On the origin of 'guttae'. The British journal of ophthalmology. 2014 Sep:98(9):1308-10. doi: 10.1136/bjophthalmol-2014-305069. Epub 2014 Jul 10 [PubMed PMID: 25012533]
Level 3 (low-level) evidenceMoshirfar M, Ding Y, Shah TJ. A Historical Perspective on Treatment of Fuchs' Endothelial Dystrophy: We have Come a Long Way. Journal of ophthalmic & vision research. 2018 Jul-Sep:13(3):339-343. doi: 10.4103/jovr.jovr_94_18. Epub [PubMed PMID: 30090191]
Level 3 (low-level) evidenceZhang Y, Zhang S, Zhang K, Lu Y, Zhu X. Characteristics of the Corneal Endothelium in Elderly Adults with High Myopia. Phenomics (Cham, Switzerland). 2024 Dec:4(6):562-569. doi: 10.1007/s43657-024-00186-6. Epub 2025 Jan 27 [PubMed PMID: 40061825]
Nakagawa T, Honda T, Yuasa T, Nishiuchi G, Sato M, Tokunaga A, Nakahara M, Tourtas T, Schlötzer-Schrehardt U, Kruse F, Padmanabhan P, Chatterjee A, Sathe G, Ghose V, Janakiraman N, Blake DJ, Koizumi N, Elchuri S, Okumura N. The TCF4 Gene Regulates Apoptosis of Corneal Endothelial Cells in Fuchs Endothelial Corneal Dystrophy. Investigative ophthalmology & visual science. 2025 Mar 3:66(3):16. doi: 10.1167/iovs.66.3.16. Epub [PubMed PMID: 40048186]
Gurnani B, Kaur K, Tripathy K. Is there a genetic link between Keratoconus and Fuch's endothelial corneal dystrophy? Medical hypotheses. 2021 Dec:157():110699. doi: 10.1016/j.mehy.2021.110699. Epub 2021 Oct 9 [PubMed PMID: 34666260]
Ong Tone S, Jurkunas U. Imaging the Corneal Endothelium in Fuchs Corneal Endothelial Dystrophy. Seminars in ophthalmology. 2019:34(4):340-346. doi: 10.1080/08820538.2019.1632355. Epub 2019 Jun 19 [PubMed PMID: 31215821]
Gupta N, Price FW Jr, Price MO. Long-Term Efficacy and Safety of Fluorometholone 0.1% Use After Descemet Membrane Endothelial Keratoplasty. Cornea. 2025 Mar 5:():. doi: 10.1097/ICO.0000000000003846. Epub 2025 Mar 5 [PubMed PMID: 40043309]
Friedrich M, Son HS, Lind J, Hammer M, Chychko L, Yildirim TM, Auffarth GU, Augustin VA. Preoperative edema severity affects outcomes after Descemet membrane endothelial keratoplasty for Fuchs endothelial corneal dystrophy: a cohort study. Eye and vision (London, England). 2025 Mar 1:12(1):9. doi: 10.1186/s40662-025-00425-5. Epub 2025 Mar 1 [PubMed PMID: 40022251]
Kaur K, Gurnani B. Specular Microscopy. StatPearls. 2024 Jan:(): [PubMed PMID: 36256774]
Tzamalis A, Dermenoudi M, Diafas A, Oustoglou E, Matsou A, Ziakas N, Tsinopoulos I. Safety and efficacy of hypertonic saline solution (5%) versus placebo in the treatment of postoperative corneal edema after uneventful phacoemulsification: a randomized double-blind study. International ophthalmology. 2020 Sep:40(9):2139-2150. doi: 10.1007/s10792-020-01395-4. Epub 2020 May 5 [PubMed PMID: 32372161]
Level 1 (high-level) evidenceGurnani B, Kaur K, Lalgudi VG, Tripathy K. Risk Factors for Descemet Membrane Endothelial Keratoplasty Rejection: Current Perspectives- Systematic Review. Clinical ophthalmology (Auckland, N.Z.). 2023:17():421-440. doi: 10.2147/OPTH.S398418. Epub 2023 Feb 1 [PubMed PMID: 36755886]
Level 1 (high-level) evidenceFutterknecht S, Chatzimichail E, Gugleta K, Panos GD, Gatzioufas Z. The Role of Rho Kinase Inhibitors in Corneal Diseases. Drug design, development and therapy. 2024:18():97-108. doi: 10.2147/DDDT.S435522. Epub 2024 Jan 19 [PubMed PMID: 38264539]
Zhang J, McGhee CNJ, Patel DV. The Molecular Basis of Fuchs' Endothelial Corneal Dystrophy. Molecular diagnosis & therapy. 2019 Feb:23(1):97-112. doi: 10.1007/s40291-018-0379-z. Epub [PubMed PMID: 30607871]
Zhang J, Patel DV. The pathophysiology of Fuchs' endothelial dystrophy--a review of molecular and cellular insights. Experimental eye research. 2015 Jan:130():97-105. doi: 10.1016/j.exer.2014.10.023. Epub 2014 Nov 1 [PubMed PMID: 25446318]
Sarnicola C, Farooq AV, Colby K. Fuchs Endothelial Corneal Dystrophy: Update on Pathogenesis and Future Directions. Eye & contact lens. 2019 Jan:45(1):1-10. doi: 10.1097/ICL.0000000000000469. Epub [PubMed PMID: 30005051]
Level 3 (low-level) evidenceAfshari NA, Igo RP Jr, Morris NJ, Stambolian D, Sharma S, Pulagam VL, Dunn S, Stamler JF, Truitt BJ, Rimmler J, Kuot A, Croasdale CR, Qin X, Burdon KP, Riazuddin SA, Mills R, Klebe S, Minear MA, Zhao J, Balajonda E, Rosenwasser GO, Baratz KH, Mootha VV, Patel SV, Gregory SG, Bailey-Wilson JE, Price MO, Price FW Jr, Craig JE, Fingert JH, Gottsch JD, Aldave AJ, Klintworth GK, Lass JH, Li YJ, Iyengar SK. Genome-wide association study identifies three novel loci in Fuchs endothelial corneal dystrophy. Nature communications. 2017 Mar 30:8():14898. doi: 10.1038/ncomms14898. Epub 2017 Mar 30 [PubMed PMID: 28358029]
Level 2 (mid-level) evidenceOng Tone S, Kocaba V, Böhm M, Wylegala A, White TL, Jurkunas UV. Fuchs endothelial corneal dystrophy: The vicious cycle of Fuchs pathogenesis. Progress in retinal and eye research. 2021 Jan:80():100863. doi: 10.1016/j.preteyeres.2020.100863. Epub 2020 May 8 [PubMed PMID: 32438095]
Scioli MG, Storti G, D'Amico F, Rodríguez Guzmán R, Centofanti F, Doldo E, Céspedes Miranda EM, Orlandi A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. Journal of clinical medicine. 2020 Jun 25:9(6):. doi: 10.3390/jcm9061995. Epub 2020 Jun 25 [PubMed PMID: 32630452]
Sanders KM, Kotowicz MA, Nicholson GC. Potential role of the antioxidant N-acetylcysteine in slowing bone resorption in early post-menopausal women: a pilot study. Translational research : the journal of laboratory and clinical medicine. 2007 Oct:150(4):215 [PubMed PMID: 17900508]
Level 3 (low-level) evidenceMcCrohon JA, Adams MR, McCredie RJ, Robinson J, Pike A, Abbey M, Keech AC, Celermajer DS. Hormone replacement therapy is associated with improved arterial physiology in healthy post-menopausal women. Clinical endocrinology. 1996 Oct:45(4):435-41 [PubMed PMID: 8959082]
Niemann B, Rohrbach S, Miller MR, Newby DE, Fuster V, Kovacic JC. Oxidative Stress and Cardiovascular Risk: Obesity, Diabetes, Smoking, and Pollution: Part 3 of a 3-Part Series. Journal of the American College of Cardiology. 2017 Jul 11:70(2):230-251. doi: 10.1016/j.jacc.2017.05.043. Epub [PubMed PMID: 28683970]
Chen HC, Huang CW, Yeh LK, Hsiao FC, Hsueh YJ, Meir YJ, Chen KJ, Cheng CM, Wu WC. Accelerated Corneal Endothelial Cell Loss after Phacoemulsification in Patients with Mildly Low Endothelial Cell Density. Journal of clinical medicine. 2021 May 24:10(11):. doi: 10.3390/jcm10112270. Epub 2021 May 24 [PubMed PMID: 34073857]
Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, Thuret G. Global Survey of Corneal Transplantation and Eye Banking. JAMA ophthalmology. 2016 Feb:134(2):167-73. doi: 10.1001/jamaophthalmol.2015.4776. Epub [PubMed PMID: 26633035]
Level 3 (low-level) evidenceZoega GM, Fujisawa A, Sasaki H, Kubota A, Sasaki K, Kitagawa K, Jonasson F. Prevalence and risk factors for cornea guttata in the Reykjavik Eye Study. Ophthalmology. 2006 Apr:113(4):565-9 [PubMed PMID: 16581419]
Level 2 (mid-level) evidenceKitagawa K, Kojima M, Sasaki H, Shui YB, Chew SJ, Cheng HM, Ono M, Morikawa Y, Sasaki K. Prevalence of primary cornea guttata and morphology of corneal endothelium in aging Japanese and Singaporean subjects. Ophthalmic research. 2002 May-Jun:34(3):135-8 [PubMed PMID: 12097795]
Zhang X, Igo RP Jr, Fondran J, Mootha VV, Oliva M, Hammersmith K, Sugar A, Lass JH, Iyengar SK, Fuchs' Genetics Multi-Center Study Group. Association of smoking and other risk factors with Fuchs' endothelial corneal dystrophy severity and corneal thickness. Investigative ophthalmology & visual science. 2013 Aug 27:54(8):5829-35. doi: 10.1167/iovs.13-11918. Epub 2013 Aug 27 [PubMed PMID: 23882692]
Level 2 (mid-level) evidenceBonanno JA. Molecular mechanisms underlying the corneal endothelial pump. Experimental eye research. 2012 Feb:95(1):2-7. doi: 10.1016/j.exer.2011.06.004. Epub 2011 Jun 15 [PubMed PMID: 21693119]
Level 3 (low-level) evidenceYuen HK, Rassier CE, Jardeleza MS, Green WR, de la Cruz Z, Stark WJ, Gottsch JD. A morphologic study of Fuchs dystrophy and bullous keratopathy. Cornea. 2005 Apr:24(3):319-27 [PubMed PMID: 15778606]
Borderie VM, Baudrimont M, Vallée A, Ereau TL, Gray F, Laroche L. Corneal endothelial cell apoptosis in patients with Fuchs' dystrophy. Investigative ophthalmology & visual science. 2000 Aug:41(9):2501-5 [PubMed PMID: 10937560]
Li QJ, Ashraf MF, Shen DF, Green WR, Stark WJ, Chan CC, O'Brien TP. The role of apoptosis in the pathogenesis of Fuchs endothelial dystrophy of the cornea. Archives of ophthalmology (Chicago, Ill. : 1960). 2001 Nov:119(11):1597-604 [PubMed PMID: 11709009]
Halilovic A, Schmedt T, Benischke AS, Hamill C, Chen Y, Santos JH, Jurkunas UV. Menadione-Induced DNA Damage Leads to Mitochondrial Dysfunction and Fragmentation During Rosette Formation in Fuchs Endothelial Corneal Dystrophy. Antioxidants & redox signaling. 2016 Jun 20:24(18):1072-83. doi: 10.1089/ars.2015.6532. Epub 2016 Apr 11 [PubMed PMID: 26935406]
Jun AS, Meng H, Ramanan N, Matthaei M, Chakravarti S, Bonshek R, Black GC, Grebe R, Kimos M. An alpha 2 collagen VIII transgenic knock-in mouse model of Fuchs endothelial corneal dystrophy shows early endothelial cell unfolded protein response and apoptosis. Human molecular genetics. 2012 Jan 15:21(2):384-93. doi: 10.1093/hmg/ddr473. Epub 2011 Oct 14 [PubMed PMID: 22002996]
Level 3 (low-level) evidenceKim EC, Toyono T, Berlinicke CA, Zack DJ, Jurkunas U, Usui T, Jun AS. Screening and Characterization of Drugs That Protect Corneal Endothelial Cells Against Unfolded Protein Response and Oxidative Stress. Investigative ophthalmology & visual science. 2017 Feb 1:58(2):892-900. doi: 10.1167/iovs.16-20147. Epub [PubMed PMID: 28159976]
Okumura N, Minamiyama R, Ho LT, Kay EP, Kawasaki S, Tourtas T, Schlötzer-Schrehardt U, Kruse FE, Young RD, Quantock AJ, Kinoshita S, Koizumi N. Involvement of ZEB1 and Snail1 in excessive production of extracellular matrix in Fuchs endothelial corneal dystrophy. Laboratory investigation; a journal of technical methods and pathology. 2015 Nov:95(11):1291-304. doi: 10.1038/labinvest.2015.111. Epub 2015 Aug 24 [PubMed PMID: 26302187]
WOLTER JR, LARSON BF. Pathology of cornea guttata. American journal of ophthalmology. 1959 Jul:48(1, Part 2):161-9 [PubMed PMID: 13670277]
Amin SR, Baratz KH, McLaren JW, Patel SV. Corneal abnormalities early in the course of Fuchs' endothelial dystrophy. Ophthalmology. 2014 Dec:121(12):2325-33. doi: 10.1016/j.ophtha.2014.07.001. Epub 2014 Aug 22 [PubMed PMID: 25156138]
Level 2 (mid-level) evidenceHara M, Morishige N, Chikama T, Nishida T. Comparison of confocal biomicroscopy and noncontact specular microscopy for evaluation of the corneal endothelium. Cornea. 2003 Aug:22(6):512-5 [PubMed PMID: 12883342]
Level 2 (mid-level) evidenceGrupcheva CN, Craig JP, Sherwin T, McGhee CN. Differential diagnosis of corneal oedema assisted by in vivo confocal microscopy. Clinical & experimental ophthalmology. 2001 Jun:29(3):133-7 [PubMed PMID: 11446452]
Level 3 (low-level) evidenceAbbott RL, Fine BS, Webster RG Jr, Paglen PG, Spencer WH. Specular microscopic and histologic observations in nonguttate corneal endothelial degeneration. Ophthalmology. 1981 Aug:88(8):788-800 [PubMed PMID: 6976545]
Level 3 (low-level) evidenceBrooks AM, Grant G, Gillies WE. A comparison of corneal endothelial morphology in cornea guttata, Fuchs' dystrophy and bullous keratopathy. Australian and New Zealand journal of ophthalmology. 1988 May:16(2):93-100 [PubMed PMID: 3263136]
Du J, Aleff RA, Soragni E, Kalari K, Nie J, Tang X, Davila J, Kocher JP, Patel SV, Gottesfeld JM, Baratz KH, Wieben ED. RNA toxicity and missplicing in the common eye disease fuchs endothelial corneal dystrophy. The Journal of biological chemistry. 2015 Mar 6:290(10):5979-90. doi: 10.1074/jbc.M114.621607. Epub 2015 Jan 15 [PubMed PMID: 25593321]
Goldstein MH, Silva FQ, Blender N, Tran T, Vantipalli S. Ocular benzalkonium chloride exposure: problems and solutions. Eye (London, England). 2022 Feb:36(2):361-368. doi: 10.1038/s41433-021-01668-x. Epub 2021 Jul 14 [PubMed PMID: 34262161]
Romano D, Aiello F, Parekh M, Levis HJ, Gadhvi KA, Moramarco A, Viola P, Fontana L, Semeraro F, Romano V. Incidence and management of early postoperative complications in lamellar corneal transplantation. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2023 Nov:261(11):3097-3111. doi: 10.1007/s00417-023-06073-6. Epub 2023 Apr 27 [PubMed PMID: 37103622]
Patel A, Cholkar K, Agrahari V, Mitra AK. Ocular drug delivery systems: An overview. World journal of pharmacology. 2013:2(2):47-64 [PubMed PMID: 25590022]
Level 3 (low-level) evidenceKhawaja AP, Chan MP, Broadway DC, Garway-Heath DF, Luben R, Yip JL, Hayat S, Wareham NJ, Khaw KT, Foster PJ. Systemic medication and intraocular pressure in a British population: the EPIC-Norfolk Eye Study. Ophthalmology. 2014 Aug:121(8):1501-7. doi: 10.1016/j.ophtha.2014.02.009. Epub 2014 Apr 2 [PubMed PMID: 24702754]
Camburu G, Zemba M, Tătaru CP, Purcărea VL. The measurement of Central Corneal Thickness. Romanian journal of ophthalmology. 2023 Apr-Jun:67(2):168-174. doi: 10.22336/rjo.2023.29. Epub [PubMed PMID: 37522018]
Subudhi P, Agarwal P. Myopia. StatPearls. 2025 Jan:(): [PubMed PMID: 35593836]
Gurnani B, Kaur K. Pseudophakic Bullous Keratopathy. StatPearls. 2024 Jan:(): [PubMed PMID: 34662019]
Chen HC, Lee CY, Chang YL, Huang JY, Yang SF, Chang CK. Risk Factors for Corneal Endothelial Decompensation after Penetrating Keratoplasty: A Population-Based Cohort Study. Journal of clinical medicine. 2024 Jan 26:13(3):. doi: 10.3390/jcm13030718. Epub 2024 Jan 26 [PubMed PMID: 38337412]
Park KS, Lieu AC, Ang MJ, Afshari NA. Reticular Bullous Epithelial Corneal Edema after Netarsudil Use for Elevated Intraocular Pressure with Concurrent Fuchs Endothelial Corneal Dystrophy: A Case Report. Case reports in ophthalmology. 2024 Jan-Dec:15(1):369-373. doi: 10.1159/000538119. Epub 2024 Apr 17 [PubMed PMID: 38633448]
Level 3 (low-level) evidenceWongchaisuwat N, Metheetrairat A, Chonpimai P, Nujoi W, Prabhasawat P. Comparison of central corneal thickness measurements in corneal edema using ultrasound pachymetry, Visante anterior-segment optical coherence tomography, Cirrus optical coherence tomography, and Pentacam Scheimpflug camera tomography. Clinical ophthalmology (Auckland, N.Z.). 2018:12():1865-1873. doi: 10.2147/OPTH.S172159. Epub 2018 Sep 25 [PubMed PMID: 30310265]
Fung AT, Tran T, Lim LL, Samarawickrama C, Arnold J, Gillies M, Catt C, Mitchell L, Symons A, Buttery R, Cottee L, Tumuluri K, Beaumont P. Local delivery of corticosteroids in clinical ophthalmology: A review. Clinical & experimental ophthalmology. 2020 Apr:48(3):366-401. doi: 10.1111/ceo.13702. Epub 2020 Jan 22 [PubMed PMID: 31860766]
Semp DA, Beeson D, Sheppard AL, Dutta D, Wolffsohn JS. Artificial Tears: A Systematic Review. Clinical optometry. 2023:15():9-27. doi: 10.2147/OPTO.S350185. Epub 2023 Jan 10 [PubMed PMID: 36647552]
Level 1 (high-level) evidenceHarthan JS, Shorter E. Therapeutic uses of scleral contact lenses for ocular surface disease: patient selection and special considerations. Clinical optometry. 2018:10():65-74. doi: 10.2147/OPTO.S144357. Epub 2018 Jul 11 [PubMed PMID: 30319297]
Vedana G, Villarreal G Jr, Jun AS. Fuchs endothelial corneal dystrophy: current perspectives. Clinical ophthalmology (Auckland, N.Z.). 2016:10():321-30. doi: 10.2147/OPTH.S83467. Epub 2016 Feb 18 [PubMed PMID: 26937169]
Level 3 (low-level) evidenceFernandez MM, Afshari NA. Endothelial Keratoplasty: From DLEK to DMEK. Middle East African journal of ophthalmology. 2010 Jan:17(1):5-8. doi: 10.4103/0974-9233.61210. Epub [PubMed PMID: 20543930]
Stuart AJ, Romano V, Virgili G, Shortt AJ. Descemet's membrane endothelial keratoplasty (DMEK) versus Descemet's stripping automated endothelial keratoplasty (DSAEK) for corneal endothelial failure. The Cochrane database of systematic reviews. 2018 Jun 25:6(6):CD012097. doi: 10.1002/14651858.CD012097.pub2. Epub 2018 Jun 25 [PubMed PMID: 29940078]
Level 1 (high-level) evidenceMaghsoudlou P, Sood G, Gurnani B, Akhondi H. Cornea Transplantation. StatPearls. 2024 Jan:(): [PubMed PMID: 30969512]
Gurnani B, Kaur K. Penetrating Keratoplasty. StatPearls. 2025 Jan:(): [PubMed PMID: 37276324]
Macsai MS, Shiloach M. Use of Topical Rho Kinase Inhibitors in the Treatment of Fuchs Dystrophy After Descemet Stripping Only. Cornea. 2019 May:38(5):529-534. doi: 10.1097/ICO.0000000000001883. Epub [PubMed PMID: 30720541]
Koizumi N, Okumura N, Ueno M, Kinoshita S. New therapeutic modality for corneal endothelial disease using Rho-associated kinase inhibitor eye drops. Cornea. 2014 Nov:33 Suppl 11():S25-31. doi: 10.1097/ICO.0000000000000240. Epub [PubMed PMID: 25289721]
Level 3 (low-level) evidencePagano L, Lee JW, Posarelli M, Giannaccare G, Kaye S, Borgia A. ROCK Inhibitors in Corneal Diseases and Glaucoma-A Comprehensive Review of These Emerging Drugs. Journal of clinical medicine. 2023 Oct 25:12(21):. doi: 10.3390/jcm12216736. Epub 2023 Oct 25 [PubMed PMID: 37959203]
Liu JX, Chiang TL, Hung KF, Sun YC. Therapeutic future of Fuchs endothelial corneal dystrophy: An ongoing way to explore. Taiwan journal of ophthalmology. 2024 Jan-Mar:14(1):15-26. doi: 10.4103/tjo.TJO-D-23-00115. Epub 2024 Jan 5 [PubMed PMID: 38654984]
Nakagawa H, Alemi H, Wang S, Kahale F, Blanco T, Liu C, Yin J, Dohlman TH, Dana R. Descemet Stripping Only Technique for Corneal Endothelial Damage in Mice. Cornea. 2023 Apr 1:42(4):470-475. doi: 10.1097/ICO.0000000000003223. Epub 2022 Dec 22 [PubMed PMID: 36728991]
Wacker K, Baratz KH, Bourne WM, Patel SV. Patient-Reported Visual Disability in Fuchs' Endothelial Corneal Dystrophy Measured by the Visual Function and Corneal Health Status Instrument. Ophthalmology. 2018 Dec:125(12):1854-1861. doi: 10.1016/j.ophtha.2018.06.018. Epub 2018 Aug 10 [PubMed PMID: 30104038]
Chow SC, Chan JC. Review on the Use of Topical Ocular Hypertonic Saline in Corneal Edema. Cornea. 2021 Apr:40(4):533-539. doi: 10.1097/ICO.0000000000002652. Epub [PubMed PMID: 33470681]
Bayyoud T, Gelisken F, Rohrbach JM, Blumenstock G, Bartz-Schmidt KU, Thaler S. Outcomes after Descemet membrane endothelial keratoplasty over a period of 7 years at a tertiary referral center: endothelial cell density, central corneal thickness, and visual acuity. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2021 Jul:259(7):1907-1914. doi: 10.1007/s00417-021-05152-w. Epub 2021 Mar 15 [PubMed PMID: 33723638]
Nguyen NX,Seitz B,Martus P,Langenbucher A,Cursiefen C, Long-term topical steroid treatment improves graft survival following normal-risk penetrating keratoplasty. American journal of ophthalmology. 2007 Aug [PubMed PMID: 17659972]
Level 1 (high-level) evidencePrice MO, Price DA, Price FW Jr. Long-Term Risk of Steroid-Induced Ocular Hypertension/Glaucoma With Topical Prednisolone Acetate 1% After Descemet Stripping Endothelial Keratoplasty. Cornea. 2024 Mar 1:43(3):323-326. doi: 10.1097/ICO.0000000000003312. Epub 2023 Jun 7 [PubMed PMID: 37155339]
Noecker RJ. The management of glaucoma and intraocular hypertension: current approaches and recent advances. Therapeutics and clinical risk management. 2006 Jun:2(2):193-206 [PubMed PMID: 18360593]
Level 3 (low-level) evidenceZhao JC, Chen T. Brinzolamide induced reversible corneal decompensation. The British journal of ophthalmology. 2005 Mar:89(3):389-90 [PubMed PMID: 15722328]
Wang J, Wang H, Dang Y. Rho-Kinase Inhibitors as Emerging Targets for Glaucoma Therapy. Ophthalmology and therapy. 2023 Dec:12(6):2943-2957. doi: 10.1007/s40123-023-00820-y. Epub 2023 Oct 14 [PubMed PMID: 37837578]
Rybkin I, Gerometta R, Fridman G, Candia O, Danias J. Model systems for the study of steroid-induced IOP elevation. Experimental eye research. 2017 May:158():51-58. doi: 10.1016/j.exer.2016.07.013. Epub 2016 Jul 20 [PubMed PMID: 27450911]
Di Zazzo A, Lee SM, Sung J, Niutta M, Coassin M, Mashaghi A, Inomata T. Variable Responses to Corneal Grafts: Insights from Immunology and Systems Biology. Journal of clinical medicine. 2020 Feb 21:9(2):. doi: 10.3390/jcm9020586. Epub 2020 Feb 21 [PubMed PMID: 32098130]
Price MO, Kanapka L, Kollman C, Lass JH, Price FW Jr. Descemet Membrane Endothelial Keratoplasty: 10-Year Cell Loss and Failure Rate Compared With Descemet Stripping Endothelial Keratoplasty and Penetrating Keratoplasty. Cornea. 2024 Nov 1:43(11):1403-1409. doi: 10.1097/ICO.0000000000003446. Epub 2023 Dec 20 [PubMed PMID: 38128100]
Blitzer AL, Colby KA. Update on the Surgical Management of Fuchs Endothelial Corneal Dystrophy. Ophthalmology and therapy. 2020 Dec:9(4):757-765. doi: 10.1007/s40123-020-00293-3. Epub 2020 Aug 25 [PubMed PMID: 32840804]
Krachmer JH, Purcell JJ Jr, Young CW, Bucher KD. Corneal endothelial dystrophy. A study of 64 families. Archives of ophthalmology (Chicago, Ill. : 1960). 1978 Nov:96(11):2036-9 [PubMed PMID: 309758]
Soh YQ, Kocaba V, Pinto M, Mehta JS. Fuchs endothelial corneal dystrophy and corneal endothelial diseases: East meets West. Eye (London, England). 2020 Mar:34(3):427-441. doi: 10.1038/s41433-019-0497-9. Epub 2019 Jul 2 [PubMed PMID: 31267087]
Vallabh NA, Kennedy S, Vinciguerra R, McLean K, Levis H, Borroni D, Romano V, Willoughby CE. Corneal Endothelial Cell Loss in Glaucoma and Glaucoma Surgery and the Utility of Management with Descemet Membrane Endothelial Keratoplasty (DMEK). Journal of ophthalmology. 2022:2022():1315299. doi: 10.1155/2022/1315299. Epub 2022 Jan 30 [PubMed PMID: 35637682]
Price DA, Kelley M, Price FW Jr, Price MO. Five-Year Graft Survival of Descemet Membrane Endothelial Keratoplasty (EK) versus Descemet Stripping EK and the Effect of Donor Sex Matching. Ophthalmology. 2018 Oct:125(10):1508-1514. doi: 10.1016/j.ophtha.2018.03.050. Epub 2018 May 3 [PubMed PMID: 29731147]
Wakimasu K, Kitazawa K, Kayukawa K, Yokota I, Inatomi T, Hieda O, Sotozono C, Kinoshita S. Five-year follow-up outcomes after Descemet's stripping automated endothelial keratoplasty: a retrospective study. BMJ open ophthalmology. 2020:5(1):e000354. doi: 10.1136/bmjophth-2019-000354. Epub 2020 Jan 29 [PubMed PMID: 32154369]
Level 2 (mid-level) evidenceBorderie VM, Georgeon C, Sandali O, Bouheraoua N. Long-term outcomes of deep anterior lamellar versus penetrating keratoplasty for keratoconus. The British journal of ophthalmology. 2023 Dec 18:108(1):10-16. doi: 10.1136/bjo-2023-324230. Epub 2023 Dec 18 [PubMed PMID: 37890880]
Trousdale ER, Hodge DO, Baratz KH, Maguire LJ, Bourne WM, Patel SV. Vision-related quality of life before and after keratoplasty for Fuchs' endothelial dystrophy. Ophthalmology. 2014 Nov:121(11):2147-52. doi: 10.1016/j.ophtha.2014.04.046. Epub 2014 Jul 9 [PubMed PMID: 25015214]
Level 2 (mid-level) evidencevan der Meulen IJ, Patel SV, Lapid-Gortzak R, Nieuwendaal CP, McLaren JW, van den Berg TJ. Quality of vision in patients with fuchs endothelial dystrophy and after descemet stripping endothelial keratoplasty. Archives of ophthalmology (Chicago, Ill. : 1960). 2011 Dec:129(12):1537-42. doi: 10.1001/archophthalmol.2011.247. Epub 2011 Aug 8 [PubMed PMID: 21825178]
Level 2 (mid-level) evidenceNanavaty MA, Wang X, Shortt AJ. Endothelial keratoplasty versus penetrating keratoplasty for Fuchs endothelial dystrophy. The Cochrane database of systematic reviews. 2014 Feb 14:2014(2):CD008420. doi: 10.1002/14651858.CD008420.pub3. Epub 2014 Feb 14 [PubMed PMID: 24526345]
Level 1 (high-level) evidencePrice MO, Gupta P, Lass J, Price FW Jr. EK (DLEK, DSEK, DMEK): New Frontier in Cornea Surgery. Annual review of vision science. 2017 Sep 15:3():69-90. doi: 10.1146/annurev-vision-102016-061400. Epub 2017 Jul 11 [PubMed PMID: 28697678]
Röck T, Landenberger J, Bramkamp M, Bartz-Schmidt KU, Röck D. The Evolution of Corneal Transplantation. Annals of transplantation. 2017 Dec 15:22():749-754 [PubMed PMID: 29242495]
Wilhelm TI, Gauché L, Böhringer D, Maier P, Heinzelmann S, Glegola M, Kammrath Betancor P, Reinhard T. Ten-year outcomes after DMEK, DSAEK, and PK: insights on graft survival, endothelial cell density loss, rejection and visual acuity. Scientific reports. 2025 Jan 8:15(1):1249. doi: 10.1038/s41598-025-85138-4. Epub 2025 Jan 8 [PubMed PMID: 39774993]
Kim YW, Kim MK, Wee WR. Long-term evaluation of endothelial cell changes in Fuchs corneal dystrophy: the influence of phacoemulsification and penetrating keratoplasty. Korean journal of ophthalmology : KJO. 2013 Dec:27(6):409-15. doi: 10.3341/kjo.2013.27.6.409. Epub 2013 Nov 15 [PubMed PMID: 24311925]
Lee NS, Ong K. Risk factors for corneal endothelial cell loss after phacoemulsification. Taiwan journal of ophthalmology. 2024 Jan-Mar:14(1):83-87. doi: 10.4103/tjo.TJO-D-23-00146. Epub 2024 Jan 30 [PubMed PMID: 38654985]
Basak SK, Basak S. Complications and management in Descemet's stripping endothelial keratoplasty: analysis of consecutive 430 cases. Indian journal of ophthalmology. 2014 Feb:62(2):209-18. doi: 10.4103/0301-4738.116484. Epub [PubMed PMID: 24008797]
Level 3 (low-level) evidenceKramer N, Unterlauft JD, Girbardt C. The need of rebubbling in case of small graft detachments after Descemet Membrane Endothelial Keratoplasty (DMEK). European journal of ophthalmology. 2023 May:33(3):1347-1353. doi: 10.1177/11206721221146579. Epub 2022 Dec 19 [PubMed PMID: 36536596]
Level 3 (low-level) evidenceGurnani B, Czyz CN, Mahabadi N, Havens SJ. Corneal Graft Rejection. StatPearls. 2024 Jan:(): [PubMed PMID: 30085585]
Sun MJ, Duong AT, Tran KD, Straiko MMW, Stoeger CG, Sales CS. Primary Graft Failure, Infection, and Endothelial Cell Density in Corneal Transplants With Increased Death-to-Preservation Time. Cornea. 2021 Nov 1:40(11):1462-1465. doi: 10.1097/ICO.0000000000002697. Epub [PubMed PMID: 33734162]
Gurnani B, Kaur K. Anti-infective therapies for Pythium insidiosum keratitis. Expert review of anti-infective therapy. 2024 Oct:22(10):805-817. doi: 10.1080/14787210.2024.2403146. Epub 2024 Sep 13 [PubMed PMID: 39268901]
Gurnani B, Kaur K, Chaudhary S, Kaur RP, Nayak S, Mishra D, Balakrishnan H, Parkash RO, Morya AK, Porwal A. Pediatric corneal transplantation: techniques, challenges, and outcomes. Therapeutic advances in ophthalmology. 2024 Jan-Dec:16():25158414241237906. doi: 10.1177/25158414241237906. Epub 2024 Mar 25 [PubMed PMID: 38533487]
Level 3 (low-level) evidenceNahum Y, Leon P, Mimouni M, Busin M. Factors Associated With Graft Detachment After Primary Descemet Stripping Automated Endothelial Keratoplasty. Cornea. 2017 Mar:36(3):265-268. doi: 10.1097/ICO.0000000000001123. Epub [PubMed PMID: 28079683]
Ang M, Wilkins MR, Mehta JS, Tan D. Descemet membrane endothelial keratoplasty. The British journal of ophthalmology. 2016 Jan:100(1):15-21. doi: 10.1136/bjophthalmol-2015-306837. Epub 2015 May 19 [PubMed PMID: 25990654]
Marques RE, Guerra PS, Sousa DC, Gonçalves AI, Quintas AM, Rodrigues W. DMEK versus DSAEK for Fuchs' endothelial dystrophy: A meta-analysis. European journal of ophthalmology. 2019 Jan:29(1):15-22. doi: 10.1177/1120672118757431. Epub 2018 Apr 16 [PubMed PMID: 29661044]
Level 1 (high-level) evidenceAnshu A, Planchard B, Price MO, da R Pereira C, Price FW Jr. A cause of reticular interface haze and its management after descemet stripping endothelial keratoplasty. Cornea. 2012 Dec:31(12):1365-8. doi: 10.1097/ICO.0b013e31823d027d. Epub [PubMed PMID: 22751144]
Ghareeb AE, Figueiredo MS, Pradhan SP, Curnow E, Armitage WJ, Figueiredo FC. Long-Term Graft Survival and Decline in Endothelial Cell Density Following Penetrating Keratoplasty with Organ-Cultured Corneas. Ophthalmology and therapy. 2022 Jun:11(3):1131-1146. doi: 10.1007/s40123-022-00481-3. Epub 2022 Mar 18 [PubMed PMID: 35303284]
Chang VS, Gibbons A, Osigian C. Phacoemulsification in the Setting of Corneal Endotheliopathies: A Review. International ophthalmology clinics. 2020 Summer:60(3):71-89. doi: 10.1097/IIO.0000000000000315. Epub [PubMed PMID: 32576725]
Schaub F, Mestanoglu M, Cursiefen C, Hos D. Impact of early intensified postoperative corticosteroids on immune reaction rates after Descemet membrane endothelial keratoplasty (DMEK). Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2022 Feb:260(2):693-695. doi: 10.1007/s00417-021-05393-9. Epub 2021 Sep 1 [PubMed PMID: 34468833]
Bernhardt BA, Zayac C, Pyeritz RE. Why is genetic screening for autosomal dominant disorders underused in families? The case of hereditary hemorrhagic telangiectasia. Genetics in medicine : official journal of the American College of Medical Genetics. 2011 Sep:13(9):812-20. doi: 10.1097/GIM.0b013e31821d2e6d. Epub [PubMed PMID: 21637104]
Level 3 (low-level) evidenceParkash RO, Gurnani B, Kaur K, Parkash TO, Vajpayee RB. Novel trocar assisted intraocular lens and capsular bag complex fixation. European journal of ophthalmology. 2024 Mar:34(2):583-588. doi: 10.1177/11206721231208662. Epub 2023 Oct 26 [PubMed PMID: 37882171]
Kaur K, Srividya KS, Kabra N, Saranath R, Gurnani B, Venkatesh R. Patterns of ophthalmic emergencies presenting to a tertiary eye care hospital in India. Indian journal of ophthalmology. 2024 Feb 1:72(2):296-297. doi: 10.4103/IJO.IJO_1578_23. Epub 2024 Jan 25 [PubMed PMID: 38273691]
Parmar GS, Agrawal A, Meena A, Mutha P, Gurnani B. Corset suture: A novel technique of overlay appositional continuous sutures with air tamponade for management of large acute corneal hydrops. Indian journal of ophthalmology. 2024 Apr 1:72(4):592-595. doi: 10.4103/IJO.IJO_1006_23. Epub 2024 Mar 8 [PubMed PMID: 38546470]
Gurnani B, Kaur K. Rare coexistence of Mittendorf dot with persistent hyaloid artery in a young Asian child. Journal francais d'ophtalmologie. 2024 Jan:47(1):103933. doi: 10.1016/j.jfo.2023.07.006. Epub 2023 Aug 30 [PubMed PMID: 37658031]
Kaur K, Gurnani B. Early postoperative complications of manual small-incision cataract surgery. Indian journal of ophthalmology. 2023 Jun:71(6):2611-2612. doi: 10.4103/IJO.IJO_2910_22. Epub [PubMed PMID: 37322701]
Morya AK, Ramesh PV, Nishant P, Kaur K, Gurnani B, Heda A, Salodia S. Diabetic retinopathy: A review on its pathophysiology and novel treatment modalities. World journal of methodology. 2024 Dec 20:14(4):95881. doi: 10.5662/wjm.v14.i4.95881. Epub 2024 Dec 20 [PubMed PMID: 39712561]
Gurnani B, Natarajan R, Mohan M, Kaur K. Breaking-Down Barriers: Proposal of Using Cellulose Biosynthesis Inhibitors and Cellulase Enzyme as a Novel Treatment Modality for Vision Threatening Pythium Insidiosum Keratitis. Clinical ophthalmology (Auckland, N.Z.). 2024:18():765-776. doi: 10.2147/OPTH.S450665. Epub 2024 Mar 11 [PubMed PMID: 38495678]
Hutchinson AK, Morse CL, Hercinovic A, Cruz OA, Sprunger DT, Repka MX, Lambert SR, Wallace DK, American Academy of Ophthalmology Preferred Practice Pattern Pediatric Ophthalmology/Strabismus Panel. Pediatric Eye Evaluations Preferred Practice Pattern. Ophthalmology. 2023 Mar:130(3):P222-P270. doi: 10.1016/j.ophtha.2022.10.030. Epub 2022 Dec 19 [PubMed PMID: 36543602]
Backes C, Religi A, Moccozet L, Behar-Cohen F, Vuilleumier L, Bulliard JL, Vernez D. Sun exposure to the eyes: predicted UV protection effectiveness of various sunglasses. Journal of exposure science & environmental epidemiology. 2019 Oct:29(6):753-764. doi: 10.1038/s41370-018-0087-0. Epub 2018 Oct 31 [PubMed PMID: 30382242]
Najmi H, Mobarki Y, Mania K, Altowairqi B, Basehi M, Mahfouz MS, Elmahdy M. The correlation between keratoconus and eye rubbing: a review. International journal of ophthalmology. 2019:12(11):1775-1781. doi: 10.18240/ijo.2019.11.17. Epub 2019 Nov 18 [PubMed PMID: 31741868]
Level 2 (mid-level) evidenceCong Y, Zhang Y, Han Y, Wu Y, Wang D, Zhang B. Recommendations for nutritional supplements for dry eye disease: current advances. Frontiers in pharmacology. 2024:15():1388787. doi: 10.3389/fphar.2024.1388787. Epub 2024 May 30 [PubMed PMID: 38873421]
Level 3 (low-level) evidenceMorya AK, Ramesh PV, Kaur K, Gurnani B, Heda A, Bhatia K, Sinha A. Diabetes more than retinopathy, it's effect on the anterior segment of eye. World journal of clinical cases. 2023 Jun 6:11(16):3736-3749. doi: 10.12998/wjcc.v11.i16.3736. Epub [PubMed PMID: 37383113]
Level 3 (low-level) evidenceOkoye GS, Bonabe D, Obasi CU, Munikrishna D, Osho F, Mutali M, Ogwumu K, Oke-Ifidon EO, Nathan IG, Enaholo ES, Suleman AI, Chukwuyem C, Enang AE, Oji RC, Ogechukwu VN, Chidera SP, Ogechukwu HC, Kaur K, Gurnani B. Visual outcomes and complications after phacoemulsification and small incision manual cataract surgery in two eye hospitals. Journal francais d'ophtalmologie. 2025 Jan:48(1):104353. doi: 10.1016/j.jfo.2024.104353. Epub 2024 Nov 18 [PubMed PMID: 39561679]
Murugan SRB, Sanjay S, Somanath A, Mahendradas P, Patil A, Kaur K, Gurnani B. Artificial Intelligence in Uveitis: Innovations in Diagnosis and Therapeutic Strategies. Clinical ophthalmology (Auckland, N.Z.). 2024:18():3753-3766. doi: 10.2147/OPTH.S495307. Epub 2024 Dec 14 [PubMed PMID: 39703602]
Meena A, Agrawal A, Parmar G, Gurnani B. Subconjunctival dexamethasone-assisted conjunctival autograft harvesting versus normal saline during pterygium surgery - A randomized clinical trial. Indian journal of ophthalmology. 2024 Feb 1:72(2):217-222. doi: 10.4103/IJO.IJO_969_23. Epub 2023 Dec 15 [PubMed PMID: 38099381]
Level 1 (high-level) evidenceMorya AK, Shrivastava AK, Janti SS, Tejaswini A, Gupta R, Gurnani B, Venkatesh D, Prasad R. Effect of Asanas in Yoga on Intraocular Pressure of Practicing Healthy Individuals: a Prospective Observational Study. Maedica. 2023 Jun:18(2):238-245. doi: 10.26574/maedica.2023.18.2.238. Epub [PubMed PMID: 37588839]
Level 2 (mid-level) evidenceTrindade BLC, Eliazar GC. Descemet membrane endothelial keratoplasty (DMEK): an update on safety, efficacy and patient selection. Clinical ophthalmology (Auckland, N.Z.). 2019:13():1549-1557. doi: 10.2147/OPTH.S178473. Epub 2019 Aug 16 [PubMed PMID: 31496646]
Kraft SA, Porter KM, Korngiebel DM, James C, Constantine M, Kelley M, Capron AM, Diekema D, Lee SS, Cho MK, Magnus D, Wilfond BS. Research on Medical Practices: Why Patients Consider Participating and the Investigational Misconception. IRB. 2017 Jul-Aug:39(4):10-16 [PubMed PMID: 30387977]
Delavar A, Bu JJ, Radha Saseendrakumar B, Weinreb RN, Baxter SL. Mental health and social support among glaucoma patients enrolled in the NIH All of Us COVID-19 Participant Experience (COPE) survey. BMC ophthalmology. 2023 Feb 13:23(1):63. doi: 10.1186/s12886-023-02771-1. Epub 2023 Feb 13 [PubMed PMID: 36782129]
Level 3 (low-level) evidenceGurnani B, Kaur K. Therapeutic Keratoplasty. StatPearls. 2025 Jan:(): [PubMed PMID: 37276297]
Wacker K, McLaren JW, Kane KM, Patel SV. Corneal Optical Changes Associated with Induced Edema in Fuchs Endothelial Corneal Dystrophy. Cornea. 2018 Mar:37(3):313-317. doi: 10.1097/ICO.0000000000001465. Epub [PubMed PMID: 29408827]
Young A, Okuyemi OT. Malignant Salivary Gland Tumors. StatPearls. 2025 Jan:(): [PubMed PMID: 33079504]
Okumura N, Okazaki Y, Inoue R, Kakutani K, Nakano S, Kinoshita S, Koizumi N. Effect of the Rho-Associated Kinase Inhibitor Eye Drop (Ripasudil) on Corneal Endothelial Wound Healing. Investigative ophthalmology & visual science. 2016 Mar:57(3):1284-92. doi: 10.1167/iovs.15-18586. Epub [PubMed PMID: 26998714]
Beşek NK, Yalçınkaya G, Kırgız A, Çakmak S, Genç S, Nacaroğlu ŞA, Yıldız BK, Yıldırım Y, Ağca A. Graft survival and clinical outcomes of Descemet membrane endothelial keratoplasty: long-term results. International ophthalmology. 2022 Jan:42(1):269-279. doi: 10.1007/s10792-021-02078-4. Epub 2021 Oct 12 [PubMed PMID: 34637061]
Level 2 (mid-level) evidenceShao Y, Jie Y, Liu ZG, Expert Workgroup of Guidelines for the application of artificial intelligence in the diagnosis of anterior segment diseases (2023); Ophthalmic Imaging and Intelligent Medicine Branch of Chinese Medicine Education Association; Ophthalmology Committee of International Association of Translational Medicine; Chinese Ophthalmic Imaging Study Groups. Guidelines for the application of artificial intelligence in the diagnosis of anterior segment diseases (2023). International journal of ophthalmology. 2023:16(9):1373-1385. doi: 10.18240/ijo.2023.09.03. Epub 2023 Sep 18 [PubMed PMID: 37724278]
Parekh M, Miall A, Chou A, Buhl L, Deshpande N, Price MO, Price FW, Jurkunas UV. Enhanced Migration of Fuchs Corneal Endothelial Cells by Rho Kinase Inhibition: A Novel Ex Vivo Descemet's Stripping Only Model. Cells. 2024 Jul 19:13(14):. doi: 10.3390/cells13141218. Epub 2024 Jul 19 [PubMed PMID: 39056800]
Ivanov IV, Mappes T, Schaupp P, Lappe C, Wahl S. Ultraviolet radiation oxidative stress affects eye health. Journal of biophotonics. 2018 Jul:11(7):e201700377. doi: 10.1002/jbio.201700377. Epub 2018 Apr 24 [PubMed PMID: 29603665]
Taberna M, Gil Moncayo F, Jané-Salas E, Antonio M, Arribas L, Vilajosana E, Peralvez Torres E, Mesía R. The Multidisciplinary Team (MDT) Approach and Quality of Care. Frontiers in oncology. 2020:10():85. doi: 10.3389/fonc.2020.00085. Epub 2020 Mar 20 [PubMed PMID: 32266126]
Level 2 (mid-level) evidenceHill JC, Ivey A. Corticosteroids in corneal graft rejection: double versus single pulse therapy. Cornea. 1994 Sep:13(5):383-8 [PubMed PMID: 7995059]
Level 1 (high-level) evidenceBosch B, Mansell H. Interprofessional collaboration in health care: Lessons to be learned from competitive sports. Canadian pharmacists journal : CPJ = Revue des pharmaciens du Canada : RPC. 2015 Jul:148(4):176-9. doi: 10.1177/1715163515588106. Epub [PubMed PMID: 26448769]
Kaur K, Zeppieri M, Gurnani B. Primary Congenital Glaucoma. StatPearls. 2025 Jan:(): [PubMed PMID: 34662067]
Cicinelli MV, Marmamula S, Khanna RC. Comprehensive eye care - Issues, challenges, and way forward. Indian journal of ophthalmology. 2020 Feb:68(2):316-323. doi: 10.4103/ijo.IJO_17_19. Epub [PubMed PMID: 31957719]
Fini ME, Schwartz SG, Gao X, Jeong S, Patel N, Itakura T, Price MO, Price FW Jr, Varma R, Stamer WD. Steroid-induced ocular hypertension/glaucoma: Focus on pharmacogenomics and implications for precision medicine. Progress in retinal and eye research. 2017 Jan:56():58-83. doi: 10.1016/j.preteyeres.2016.09.003. Epub 2016 Sep 22 [PubMed PMID: 27666015]
Wiggins MN, Landes RD, Bhaleeya SD, Uwaydat SH. Primary care physicians' knowledge of the ophthalmic effects of diabetes. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2013 Aug:48(4):265-8. doi: 10.1016/j.jcjo.2013.03.011. Epub [PubMed PMID: 23931464]
Moshirfar M, Thomson AC, Ronquillo Y. Corneal Endothelial Transplantation. StatPearls. 2025 Jan:(): [PubMed PMID: 32965936]
Wancata LM, Hinshaw DB. Rethinking autonomy: decision making between patient and surgeon in advanced illnesses. Annals of translational medicine. 2016 Feb:4(4):77. doi: 10.3978/j.issn.2305-5839.2016.01.36. Epub [PubMed PMID: 27004224]
Demmin DL, Silverstein SM. Visual Impairment and Mental Health: Unmet Needs and Treatment Options. Clinical ophthalmology (Auckland, N.Z.). 2020:14():4229-4251. doi: 10.2147/OPTH.S258783. Epub 2020 Dec 3 [PubMed PMID: 33299297]