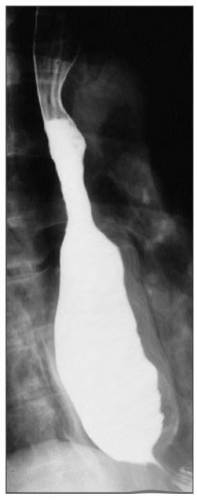
Introduction
Patients subjectively define dysphagia as difficulty swallowing and objectively defined by clinicians as an impairment in swallowing that results in an abnormal delay in the transit of a liquid or solid bolus from the oral cavity to the stomach. Dysphagia may be acute or chronic, intermittent or persistent. A globus sensation may accompany it. Dysphagia may be due to abnormalities in the oropharyngeal or esophageal phases of swallowing, or it may be mixed.
Dysphagia is a common problem, particularly in the elderly population. The underlying etiology may be a mechanical obstruction or a motility disorder. Anatomical, neuromuscular, infectious, and inflammatory diseases may all present with or contribute to dysphagia.
The medical history and physical examination are critical to determining the etiology of dysphagia and will often point to a diagnosis. However, bedside testing, barium swallow, endoscopy, and high-resolution impedance manometry are commonly used to pinpoint a diagnosis. The management of dysphagia is dependent on the cause of dysphagia and associated conditions.
Dysphagia-associated complications include an increased risk of aspiration, aspiration-induced pneumonia, malnutrition, morbidity, and mortality. Inpatient hospital stays are frequently lengthened by dysphagia and its complications. Patients with dysphagia often report a diminished quality of life.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Dysphagia, difficulty swallowing, can be acute or chronic and may occur in either the oropharyngeal or esophageal phases of swallowing. Dysphagia may coexist with odynophagia or painful swallowing and may be accompanied by a globus sensation. A globus sensation is the subjective feeling of a lump or having something stuck in the throat; it is usually painless.
Oropharyngeal Dysphagia
Oropharyngeal dysphagia is the delay in the transit of liquid or solid bolus during the oropharyngeal phase of swallowing. A variety of anatomical and neuromuscular disorders can cause oropharyngeal dysphagia.[1] Some causes of oropharyngeal dysphagia, such as that seen in the postoperative period following cervical discectomy and fusion, are multifactorial.[2][3]
Anatomical causes of oropharyngeal dysphagia are most commonly due to lesions that obstruct the lumen or cause external compression of the oropharynx. These include Zenker diverticulum, esophageal webs, oropharyngeal tumors and abscesses, goiters, and external compression by an aortic aneurysm called dysphagia aortica.[4]
Neuromuscular causes of oropharyngeal dysphagia are many. Dysphagia is common after cerebrovascular accidents, including brainstem infarctions with cranial nerve involvement. In addition, the basal ganglia lesions in Parkinson disease may cause dysphagia. Head injuries, multiple sclerosis, central nervous tumors, botulism, amyotrophic lateral sclerosis, and supranuclear palsy may all cause dysphagia. Muscular disorders such as polymyositis and myopathies may cause dysphagia. Due to its effect on the neuromuscular junction, myasthenia gravis is known to cause dysphagia.
Esophageal Dysphagia
Esophageal dysphagia may be caused by mechanical obstruction of the esophagus or a motility disorder. Mechanical obstruction is characterized primarily by dysphagia with solids, whereas motility disorders have dysphagia for both solids and liquids. Esophageal dysphagia may be intermittent or continuous.[5]
Esophageal dysphagia due to mechanical obstruction is most commonly due to a Schatzki ring, esophageal stricture or webs, esophageal carcinoma, and eosinophilic esophagitis.[6] Causes of motility disorders resulting in dysphagia include esophageal spasm, achalasia, ineffective esophageal motility, and systemic sclerosis.
Rheumatological Disorders and Dysphagia
Several rheumatological disorders are known to cause dysphagia via multiple mechanisms. Each may also exacerbate dysphagia due to other causes. For example, Sjögren syndrome, an autoimmune disease affecting exocrine glands, causes dysphagia via xerostomia and changes in the motility of the proximal esophagus. Limited cutaneous systemic sclerosis (formerly CREST syndrome) is a well-known cause of esophageal dysmotility. Other commonly encountered rheumatologic disorders that may cause or complicate dysphagia are rheumatoid arthritis, systemic lupus erythematosus, and mixed connective tissue diseases.
Medication-induced Dysphagia
Several pharmaceuticals may contribute to the severity of dysphagia. The mechanisms by which these drugs may cause dysphagia include xerostomia and changes in esophageal motility. Also, dysphagia may be secondary to the development of drug-induced esophagitis or the result of gastroesophageal reflux disease as a side effect of medication use.[7] Additionally, medications with immunosuppressant effects predispose to the development of infectious esophagitis with subsequent dysphagia. Examples of these drugs are:
- Antipsychotics (eg, olanzapine, clozapine)
- Tricyclic antidepressants
- Potassium supplements
- Non-steroidal anti-inflammatory drugs
- Bisphosphonates
- Calcium channel blockers
- Nitrates
- Theophylline
- Alcohol
- Opioids
Of note, narcotic sedatives such as opioids can lead to airway compromise and can increase the risk of aspiration in patients with dysphagia. In addition, patients with psychiatric disorders or Parkinson disease, even mild disease, can develop hypercontractile or hypertensive esophageal consequences when using opioids.
Epidemiology
Dysphagia is a common symptom, and the incidence increases with increasing age. The prevalence of dysphagia is approximately 10% to 22% in Americans aged 50 and over.[8] In patients over age 60 years, the prevalence is approximately 40%.[9] A study reported that 63% of elderly patients who denied any history of swallowing problems demonstrated abnormalities in radiologic swallow studies.[10]
The incidence of dysphagia also varies by place of assessment. In hospitalized patients, approximately 14% to 18% of patients have symptoms of dysphagia. In senior care homes, symptomatic dysphagia is reported by 30% to 60% of patients. In patients in intensive care, a recent systematic review showed that the incidence of post-extubation dysphagia (PED) was in the range of 3% to 62%.[11] In another retrospective, observational cohort study, the prevalence of PED was as much as 84%.[12] These 2 studies indicate that the incidence of PED is relatively high in patients in the intensive care unit.[13]
The incidence of dysphagia also varies according to underlying etiology. The reported frequencies of dysphagia in patients with stroke vary between 19% and 81%.[14][15] Early detection of dysphagia in these patients is critical for reducing pulmonary complications and the length of hospital stay.[16] The incidence is more common after brainstem stroke or bilateral hemispheric stroke.[17]
The incidence of dysphagia due to an underlying pathophysiologic process may also increase with increasing age. In idiopathic achalasia, which occurs in equal frequency in males and females and is commonly diagnosed at age 50, the mean incidence is approximately 0.3 to 1.6 per 100,000 people per year in adults.[18][19][20] However, the incidence in people over 80 is approximately 17 per 100,000 annually.[21]
Over the past decade, publications on swallowing disorders have increased yearly.[22]
Pathophysiology
Swallowing is a series of events that transits food from the oral cavity to the stomach. Transit from the oral cavity to the pharynx is a voluntary process that occurs via the coordinated contraction of oropharyngeal muscles. Transit of a bolus from the oropharynx through the esophagus and into the stomach is an involuntary process. Many conditions may negatively affect this process, including neurological, muscular, mechanical, infectious, and inflammatory diseases. Each may result in dysphagia.[23]
Dysphagia can be predominately oropharyngeal or esophageal or a mixed process. As aforementioned, the incidence and prevalence of dysphagia increases with age. The pathogenesis of age-related oropharyngeal dysphagia includes dysfunction in salivary production, a loss of jaw strength, gingival and dentition problems, and age-related changes in the tongue muscles; each contributes to poor mixing of food with saliva and a delay in the oropharyngeal phase of swallowing. Age-related changes that can affect the esophageal phase of swallowing include the threshold for laryngeal elevation and a loss of upper esophageal sphincter elasticity and function.[24]
Cerebral, cerebellar, or brainstem strokes can impair swallowing function. These lesions can interrupt voluntary control of mastication and bolus transport during the oropharyngeal phase. Furthermore, cortical lesions involving the precentral gyrus cause contralateral impairment of facial muscles, lip and tongue movements, and pharyngeal peristalsis. Brainstem lesions are less common but result in significant swallowing compromise due to dysfunction of the cranial nerves emerging from the brainstem. Mouth, tongue, and cheek sensations may be compromised. Alterations may occur in the timing of pharyngeal swallow, laryngeal elevation, glottic closure, and physiological regulation of oropharyngeal swallow.[25][26]
Patients with head and neck cancer treated with platinum-based chemotherapy and radiotherapy may develop severe dysphagia related to mucositis. The esophagus is colonized with Candida species in more than 70% of these patients. The breakdown of the mucosal barrier leads to the failure of protective physiological mechanisms provided via the mucosal epithelium and T-cells, leading to opportunistic candidiasis. Mucosal candidiasis causes oropharyngeal dysfunction and dysphagia.
Achalasia frequently results in progressive dysphagia. In addition, the incidence of achalasia increases with age. Several pathogenic mechanisms may contribute to the development of achalasia.
In achalasia, chronic immune-mediated ganglionitis results in the loss of the esophageal myenteric neurons normally responsible for coordinating the relaxation of the lower esophageal sphincter. The result is aperistalsis and impaired relaxation of the lower esophageal sphincter. Antibodies against myenteric neurons have been detected in the serum of patients with achalasia, particularly in patients positive for HLA DQA1*0103 and DQB1*0603 alleles.[27] Herpes simplex virus-1, measles, and the human papillomavirus may play a role in the pathogenesis of achalasia. The evidence shows that the herpes simplex virus triggers immune activation and subsequent esophageal enteric neuron loss in genetically susceptible individuals. Lastly, polymorphisms in the HLA class II molecules, vasoactive intestinal peptide receptor-1, KIT, and interleukin-10 play a role in the pathogenesis of achalasia.[28]
A relative sparing of somatic innervation of the proximal esophagus characterizes dysphagia in patients with systemic sclerosis. The lesion usually involves impairment of innervation of the distal esophagus. The motility disorder in these patients is characterized by normal proximal esophageal motility, reduced or absent lower esophageal sphincter pressure, ineffective distal esophageal body peristalsis, and a discoordination of peristalsis and lower esophageal function.[29]
History and Physical
A thorough history and physical examination are critical in evaluating a patient with dysphagia. The history and physical examination will often point to the diagnosis.[30]
The medical history must include the nature of the dysphagia, the course of the problem, and whether to solids, liquids, or both. Difficulty swallowing solids predominately suggests obstruction or a mechanical problem such as esophageal webs or rings; this type of dysphagia may be intermittent. Alternatively, progressive dysphagia is more likely due to progressive stricture or malignancy. Dysphagia with liquids suggests a motility disorder such as achalasia or systemic sclerosis; if intermittent, then esophageal spasm.[30] Therefore, key topics to cover when gathering history include:
- Onset
- Duration and course of symptoms
- Problems with the initiation of swallowing or after a few seconds of swallowing
- Symptoms predominantly in the neck or chest
- Occurrence with solids, liquids, or both
- Self-imposed dietary changes regarding food consistency; preference for liquids or semisolids
- Eating meals slowly
- Throat clearance during or after meals
- Associated coughing, choking, or postnasal regurgitation
- History of recurrent chest infections
- Functional decline
- Neurological deficits
- Weight loss
- Feelings of embarrassment
- Social isolation, frustration, or depression
Physical Signs
- Loss of dentition or gingival disease
- Abnormal lip closure
- Local mucosal changes
- Neurological changes
Difficulty initiating swallowing is more characteristic of oropharyngeal dysphagia; esophageal dysphagia usually occurs a few seconds after swallowing. Attempts should be made to distinguish between the 2 as they are often distinct pathologic processes.
Symptoms Predictive of Oropharyngeal Dysphagia [31][32][33]
- A delay in oropharyngeal initiation of swallowing
- Deglutitive cough
- Nasal aspiration during swallowing
- There is a need for a repetitive swallow to clear nasal secretions
- Globus sensation
Symptoms Predictive of Esophageal Dysphagia
- Symptoms occur a few seconds after initiation of the swallow
- Associated regurgitation of undigested food
- Dysphagia felt more within the chest
Although oropharyngeal dysphasia is commonly seen in the geriatric population, a history of smoking or alcohol use, with or without unintentional weight loss, should suggest the possibility of underlying malignancy.[32]
Regurgitation of undigested food, hoarseness of voice, halitosis, the feeling of fullness in the throat, and history of aspirations may suggest Zenker diverticulum, which is believed to be the result of weakness in the cricopharyngeal muscle.[33]
Evaluation
As discussed above, a thorough history and physical examination often point to a diagnosis and are critical steps that guide further evaluation and management of dysphagia. Attempts should be made to determine if the dysphagia is oropharyngeal, esophageal, or mixed.[34]
Oropharyngeal Dysphagia
In patients with clinical evidence of oropharyngeal dysphagia, the initial test may be a clinical swallow evaluation performed by a speech pathologist or bedside nurse. However, this test has limitations and may miss over 50% of patients with significant aspiration.[35] Early detection of oropharyngeal dysphagia using screening tools may play a role in reducing complications, particularly in the older population.[36] Multiple bedside evaluation screening tools are available. However, they lack predictive value for aspiration, are of limited consistency, and are not validated.[37][38]
After the initial bedside clinical swallow evaluation, two instrumental tests are often used to assess oropharyngeal dysphagia.[32] The videofluoroscopic swallow study (VFSS) allows direct visualization of bolus passage by evaluating swallow physiology, swallow efficiency, and aspiration risk through the two-dimensional view.[39] Even though this study has significant diagnostic value, there is a need to develop scientific tools to increase reliability and decrease interreader variability, which is a limitation of this study.[40] Fiberoptic endoscopic evaluation of swallowing (FEES) is performed by passing an endoscope through the nares to observe the nasopharynx, oropharynx, and larynx to allow direct visualization and detailed analysis of swallow structures and their function during intake of food with various consistencies.[41]
Which test is chosen is dictated by patient characteristics. To perform VFSS, patients must be able to sit upright and must be able to travel to the radiology department. In contrast, FEES can be done at the bedside.[32] A recent systematic review shows that FEES is more sensitive to detecting penetration, residues, and aspiration than VFSS.[42] However, either is the gold standard for assessing Parkinson disease-related dysphagia. In addition, high-resolution manometry may be a helpful tool to complement other diagnostic strategies to evaluate upper esophageal sphincter pathology, particularly in patients with globus sensation.[43][44]
Patients with signs of malignancy, such as weight loss or bleeding, in addition to oropharyngeal dysphagia, should undergo a head and neck evaluation and a laryngoscopic examination of the pharynx and larynx. Particular attention should be paid to the oropharynx and any potential evidence of a Zenker diverticulum. An esophagogram typically diagnoses Zenker diverticulum. In addition, computed tomography of the head and neck may be considered to rule out any suspected malignancy.
Esophageal Dysphagia
Patients with no supra-esophageal symptoms, unremarkable modified barium swallow or FEES, and clinical evidence of esophageal dysphagia should be referred to a gastroenterologist for a barium video-esophagogram. This assessment should cover the anatomy and motor function of the esophagus. Barium esophagogram is relatively simple and low-cost and may be considered the first line of investigation over endoscopy in esophageal dysphagia. Endoscopy is associated with risks such as aspiration, particularly in elderly patients.[45] A timed barium video-esophagogram is also helpful in the diagnosis of achalasia. It can help objectively document treatment outcomes and detect recurrence early.[46] Barium video-esophagogram should also be considered the initial test when carcinoma, esophageal rings or strictures, or severe reflux esophagitis is suspected; it has 90% to 95% sensitivity in these conditions.[47][48]
The choice of the initial test must be based on patient characteristics and shared decision-making. The combined barium esophagogram and endoscopy approach may be the best in specific scenarios.[49] Many gastroenterologists recommend endoscopy as an initial test since most patients who underwent an esophagogram require endoscopy eventually.[50] Endoscopic evaluation offers additional benefits of therapeutic interventions such as dilation and biopsies.
High-resolution esophageal manometry, which is preferred over conventional manometry, also plays a role in evaluating esophageal dysphagia. It is helpful in the diagnosis of achalasia, diffuse esophageal spasm, jackhammer esophagus, and esophagogastric junction outflow obstruction[47][51]
Treatment / Management
Treatment of dysphagia should be tailored towards the underlying etiology and individualized based on patient characteristics. The treatment goals for dysphagia are to reduce mortality and morbidity due to aspiration, pneumonia, malnutrition, dehydration, and psychological impairments. A multidisciplinary approach must involve primary care and specialty clinicians, nurses, speech therapists, dieticians, and caregivers using compensatory and rehabilitative strategies to promote quality of life and regain physiologic swallowing, respectively.[52] Comprehensive dysphagia management is essential, particularly in the geriatric population, patients with dementia, Alzheimer disease, stroke, and Parkinson disease.[53][54](B2)
Clinicians should be aware of the psychological impacts of dysphagia on patients, including depression, anxiety, frustration, fear, vulnerability, and embarrassment. Educating patients and their caregivers about coping strategies and addressing their social needs is important.[55][56](B2)
Swallowing techniques such as head tilt, chin-tuck, head rotation, and supraglottic maneuvers have shown some benefit in minimizing or preventing aspiration; however, there is contradictory evidence of their efficacy.[52][53] Swallowing rehabilitation, including strengthening exercises and skills-based training, can be done by speech therapists.[57](B3)
Dietary modifications such as texture-modified food and thickened liquids may help patients with dysphagia. Due to the lack of data, the evidence supporting dietary modification is not strong, and the immediate and long-term effects of thickened liquids are uncertain.[58][59] Other dietary considerations that should be considered, particularly in patients with dementia or those who have had a stroke, include timing, frequency, bolus size, patient preferences, food additives, caregiver knowledge, and patient attentiveness. The lack of consistent terminology for different food textures among various countries poses a significant challenge for future studies to pool the evidence.[60](A1)
Peripheral stimulation, such as thermal, tactile stimulation at the base of the tongue and soft palate or transcranial magnetic stimulation, is a simple, safe, and noninvasive strategy that may help dysphagia due to neurological causes. However, the utility of these therapies remains uncertain in geriatric patients.[52] In patients with dysphagia due to stroke, neuromuscular electrical stimulation and traditional swallowing therapies may have potential benefits.[61] However, if the patients have mild symptoms, dilatation of the upper esophageal sphincter may be indicated.[62] Most patients with stroke-induced dysphagia will regain their swallowing function within 1 to 2 weeks after infarction.(A1)
Cricopharyngeal myotomy or dilation can be considered in patients with cricopharyngeal dysphagia or Zencker diverticulum. This can often be done via the oral route. However, if the patients have mild symptoms, dilatation of the upper esophageal sphincter may be indicated.[62]
Oropharyngeal dysphagia associated with myasthenia gravis responds to treatment with anticholinesterase inhibitors.
Structural causes such as benign esophageal strictures can be treated in various ways. If the stricture is mild, control of underlying gastroesophageal reflux may be adequate, with or without endoscopic dilation. Refractory cases can be treated with steroid injection, incision, or stent placement. Patients with dysphagia related to head and neck tumors will require long-term swallow rehabilitation. Usually, their dysphagia continues despite the surgical excision of the tumor mass, radiation therapy, or chemotherapy.[63] Dysphagia caused by an esophageal malignancy is usually managed by a team of surgeons, gastroenterologists, medical and radiation oncologists, and the rehabilitation team.[64](A1)
A gastrostomy tube may be indicated in patients who fail to respond to the above-stated measures.[1]
Because the underlying pathophysiology of dysphagia due to diffuse esophageal spasm is unknown, treatment is directed at controlling patient symptoms.[65] Drugs such as concentrated peppermint oil, nitrates, phosphodiesterase-5 inhibitors, calcium channel blockers, and tricyclic anti-depressants may be used. Other treatments, such as peroral endoscopic myotomy, botulinum toxin, and esophageal dilatation, may be considered.
Scleroderma can cause aperistalsis or low-amplitude esophageal contractions of the distal esophagus and a hypotensive lower esophageal sphincter. Usually, these patients have dysphagia with both solids and liquids. The treatment is focused on symptomatic management. No pharmacological intervention has been shown to be promising. Prokinetic agents that stimulate gut peristalsis, such as metoclopramide, erythromycin, bethanechol, buspirone, and domperidone, may help.[66] Non-pharmacological approaches, such as acupuncture stimulation or transcutaneous electrical nerve stimulation (TENS), may be indicated in the management of advanced cases.[67](B3)
Achalasia
Based on high-resolution esophageal manometry results, 3 subtypes of achalasia can be identified; the subtype forms the basis of achalasia treatment.[68][69] (A1)
Medical management can be attempted using nitrates or calcium channel blockers to decrease the resting pressure of the esophageal sphincter; side effects occur in one-third of patients and include hypotension, headache, and dizziness. Endoscopic injection of botulinum toxin A into the lower esophageal sphincter may be offered. While more than 80% of patients undergoing this intervention have a clinical response, recurrence of symptoms is common.[70]
Procedural or surgical intervention for achalasia is the mainstay of therapy. Types I and II achalasia are best managed with pneumatic dilatation, laparoscopic Heller myotomy, or gastric peroral endoscopic myotomy (POEM). POEM is the preferred treatment for Type III achalasia. As with any procedure, there are risks and benefits with each approach.
Pneumatic dilatation uses air-filled balloons to dilate the lower esophageal sphincter under fluoroscopic guidance. However, nearly one-third of patients have a recurrence of their symptoms in 4 to 6 years. Pneumatic dilatation is contraindicated in patients with poor cardiopulmonary status and those with comorbidities that prevent surgical intervention should an esophageal rupture occur.
Laparoscopic Heller myotomy of the distal esophageal muscle and lower esophageal sphincter significantly reduces reflux when combined with fundoplication.[71] The surgery is safe, with a reported mortality of 0.1%. The main complication is perforation of the esophagus or gastric mucosa.
POEM requires a highly skilled operator using a high-definition endoscope. The procedure takes approximately 2 to 3 hours, and patients must stay in the hospital for 2 to 3 days for monitoring and intravenous antibiotics.[68][72](A1)
Differential Diagnosis
Dysphagia may be acute or chronic and can be oropharyngeal, esophageal, or mixed. There are many underlying etiologies of dysphagia.[73][74] These include but are not limited to:
- Benign esophageal stricture
- Cerebrovascular accident (stroke)
- Diffuse esophageal spasm
- Eosinophilic esophagitis
- Esophageal malignancies
- Esophageal webs and rings
- Gastroesophageal reflux disease (GERD)
- Hiatal hernia
- Multiple sclerosis
- Parkinson disease
- Paterson-Kelly syndrome
- Zenker diverticulum
A form of dysphagia that is unique to childhood is dysphagia lusoria. This form of dysphagia is due to compression of the esophagus by a vascular abnormality. The most common abnormalities are an aberrant right subclavian artery arising from the left side of the aortic or double aortic arch.
Prognosis
The prognosis of dysphagia depends upon the underlying etiology. For example, dysphagia in stroke patients normalizes gradually, from 3 weeks to approximately 6 months or longer. Dysphagia due to achalasia or mechanical obstruction may resolve with surgical intervention.
Complications
Dysphagia can significantly impair the quality of life of those affected. Patients with dysphagia may experience social isolation because of cough, embarrassment, and difficulties in swallowing. Dysphagia may increase the risk of chronic aspiration and pneumonia. Dehydration and malnutrition may occur. Rehabilitation after injury or illness may be reduced.[75]
Deterrence and Patient Education
Patients should be educated about the underlying etiology of their dysphagia, including any etiology-specific needs. However, patients or their caregivers are advised to feed in an upright posture with the neck flexed to help swallowing and reduce the risk of aspiration. Patients with xerostomia may benefit from artificial saliva or lubricants. Patients should be advised to refrain from talking while eating. High-caloric food should be taken. Speech therapists may teach a range of exercises to assist swallowing.
Pearls and Other Issues
Oropharyngeal dysphagia should be differentiated from esophageal dysphagia, and each should be considered a distinct category. While the medical history and physical examination are essential for making such a differentiation, investigations may help identify the underlying cause of the dysphagia.
Enhancing Healthcare Team Outcomes
Most patients with dysphagia may present to the primary care provider or the emergency department. The public, nurses, pharmacists, and patients should be educated about dysphagia. At schools, students with severe disabilities or orthopedic impairments may require help because of dysphagia. In essence, such schools' support teams may need a speech-language pathologist with adequate training in swallowing evaluation and management. No one should provide such services without proper official up-to-date training in this area—a team of speech therapists, neurologists, otolaryngologists, oncologists, dietitians, and nurses usually manage patients with oropharyngeal dysphagia. Surgeons, gastroenterologists, radiation oncologists, and rehabilitation teams typically care for patients with esophageal malignancy.
Media
References
Rommel N, Hamdy S. Oropharyngeal dysphagia: manifestations and diagnosis. Nature reviews. Gastroenterology & hepatology. 2016 Jan:13(1):49-59. doi: 10.1038/nrgastro.2015.199. Epub 2015 Dec 2 [PubMed PMID: 26627547]
Ebot J, Domingo R, Nottmeier E. Post-operative dysphagia in patients undergoing a four level anterior cervical discectomy and fusion (ACDF). Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2020 Feb:72():211-213. doi: 10.1016/j.jocn.2019.12.002. Epub 2019 Dec 12 [PubMed PMID: 31839384]
Ohba T, Hatsushika K, Ebata S, Koyama K, Akaike H, Yokomichi H, Masuyama K, Haro H. Risk Factors and Assessment Using an Endoscopic Scoring System for Early and Persistent Dysphagia After Anterior Cervical Decompression and Fusion Surgery. Clinical spine surgery. 2020 May:33(4):E168-E173. doi: 10.1097/BSD.0000000000000945. Epub [PubMed PMID: 32011353]
Choi SHJ, Yang GK, Gagnon J. Dysphagia aortica secondary to thoracoabdominal aortic aneurysm resolved after endograft placement. Journal of vascular surgery cases and innovative techniques. 2019 Dec:5(4):501-505. doi: 10.1016/j.jvscit.2019.08.008. Epub 2019 Nov 13 [PubMed PMID: 31763508]
Level 3 (low-level) evidencePhilpott H, Garg M, Tomic D, Balasubramanian S, Sweis R. Dysphagia: Thinking outside the box. World journal of gastroenterology. 2017 Oct 14:23(38):6942-6951. doi: 10.3748/wjg.v23.i38.6942. Epub [PubMed PMID: 29097867]
Mari A, Tsoukali E, Yaccob A. Eosinophilic Esophagitis in Adults: A Concise Overview of an Evolving Disease. Korean journal of family medicine. 2020 Mar:41(2):75-83. doi: 10.4082/kjfm.18.0162. Epub 2020 Feb 17 [PubMed PMID: 32062959]
Level 3 (low-level) evidenceSnyder DL, Crowell MD, Horsley-Silva J, Ravi K, Lacy BE, Vela MF. Opioid-Induced Esophageal Dysfunction: Differential Effects of Type and Dose. The American journal of gastroenterology. 2019 Sep:114(9):1464-1469. doi: 10.14309/ajg.0000000000000369. Epub [PubMed PMID: 31403963]
Howden CW. Management of acid-related disorders in patients with dysphagia. The American journal of medicine. 2004 Sep 6:117 Suppl 5A():44S-48S [PubMed PMID: 15478852]
Ney DM, Weiss JM, Kind AJ, Robbins J. Senescent swallowing: impact, strategies, and interventions. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2009 Jun-Jul:24(3):395-413. doi: 10.1177/0884533609332005. Epub [PubMed PMID: 19483069]
Ekberg O, Feinberg MJ. Altered swallowing function in elderly patients without dysphagia: radiologic findings in 56 cases. AJR. American journal of roentgenology. 1991 Jun:156(6):1181-4 [PubMed PMID: 2028863]
Level 3 (low-level) evidenceSkoretz SA, Flowers HL, Martino R. The incidence of dysphagia following endotracheal intubation: a systematic review. Chest. 2010 Mar:137(3):665-73. doi: 10.1378/chest.09-1823. Epub [PubMed PMID: 20202948]
Level 1 (high-level) evidenceMacht M, Wimbish T, Clark BJ, Benson AB, Burnham EL, Williams A, Moss M. Postextubation dysphagia is persistent and associated with poor outcomes in survivors of critical illness. Critical care (London, England). 2011:15(5):R231. doi: 10.1186/cc10472. Epub 2011 Sep 29 [PubMed PMID: 21958475]
Level 2 (mid-level) evidenceZuercher P, Moret CS, Dziewas R, Schefold JC. Dysphagia in the intensive care unit: epidemiology, mechanisms, and clinical management. Critical care (London, England). 2019 Mar 28:23(1):103. doi: 10.1186/s13054-019-2400-2. Epub 2019 Mar 28 [PubMed PMID: 30922363]
Barer DH. The natural history and functional consequences of dysphagia after hemispheric stroke. Journal of neurology, neurosurgery, and psychiatry. 1989 Feb:52(2):236-41 [PubMed PMID: 2564884]
Meng NH, Wang TG, Lien IN. Dysphagia in patients with brainstem stroke: incidence and outcome. American journal of physical medicine & rehabilitation. 2000 Mar-Apr:79(2):170-5 [PubMed PMID: 10744192]
Level 2 (mid-level) evidenceMartino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005 Dec:36(12):2756-63 [PubMed PMID: 16269630]
Level 1 (high-level) evidenceSloan MA, Haley EC Jr. The syndrome of bilateral hemispheric border zone ischemia. Stroke. 1990 Dec:21(12):1668-73 [PubMed PMID: 2264072]
Level 2 (mid-level) evidenceBecker J, Niebisch S, Ricchiuto A, Schaich EJ, Lehmann G, Waltgenbach T, Schafft A, Hess T, Lenze F, Venerito M, Hüneburg R, Lingohr P, Matthaei H, Seewald S, Scheuermann U, Kreuser N, Veits L, Wouters MM, Gockel HR, Lang H, Vieth M, Müller M, Eckardt AJ, von Rahden BH, Knapp M, Boeckxstaens GE, Fimmers R, Nöthen MM, Schulz HG, Gockel I, Schumacher J. Comprehensive epidemiological and genotype-phenotype analyses in a large European sample with idiopathic achalasia. European journal of gastroenterology & hepatology. 2016 Jun:28(6):689-95. doi: 10.1097/MEG.0000000000000602. Epub [PubMed PMID: 26882171]
Level 2 (mid-level) evidenceSadowski DC, Ackah F, Jiang B, Svenson LW. Achalasia: incidence, prevalence and survival. A population-based study. Neurogastroenterology and motility. 2010 Sep:22(9):e256-61. doi: 10.1111/j.1365-2982.2010.01511.x. Epub 2010 May 11 [PubMed PMID: 20465592]
Gennaro N, Portale G, Gallo C, Rocchietto S, Caruso V, Costantini M, Salvador R, Ruol A, Zaninotto G. Esophageal achalasia in the Veneto region: epidemiology and treatment. Epidemiology and treatment of achalasia. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2011 Mar:15(3):423-8. doi: 10.1007/s11605-010-1392-7. Epub 2010 Nov 30 [PubMed PMID: 21116729]
Level 2 (mid-level) evidenceFarrukh A, DeCaestecker J, Mayberry JF. An epidemiological study of achalasia among the South Asian population of Leicester, 1986-2005. Dysphagia. 2008 Jun:23(2):161-4 [PubMed PMID: 18027026]
Level 2 (mid-level) evidenceSun W, Kang X, Zhao N, Dong X, Li S, Zhang G, Liu G, Yang Y, Zheng C, Yu G, Shuai L, Feng Z. Study on dysphagia from 2012 to 2021: A bibliometric analysis via CiteSpace. Frontiers in neurology. 2022:13():1015546. doi: 10.3389/fneur.2022.1015546. Epub 2022 Dec 15 [PubMed PMID: 36588913]
Trate DM, Parkman HP, Fisher RS. Dysphagia. Evaluation, diagnosis, and treatment. Primary care. 1996 Sep:23(3):417-32 [PubMed PMID: 8888335]
Sura L, Madhavan A, Carnaby G, Crary MA. Dysphagia in the elderly: management and nutritional considerations. Clinical interventions in aging. 2012:7():287-98. doi: 10.2147/CIA.S23404. Epub 2012 Jul 30 [PubMed PMID: 22956864]
Martino R, Terrault N, Ezerzer F, Mikulis D, Diamant NE. Dysphagia in a patient with lateral medullary syndrome: insight into the central control of swallowing. Gastroenterology. 2001 Aug:121(2):420-6 [PubMed PMID: 11487551]
Level 3 (low-level) evidenceErtekin C, Aydogdu I, Tarlaci S, Turman AB, Kiylioglu N. Mechanisms of dysphagia in suprabulbar palsy with lacunar infarct. Stroke. 2000 Jun:31(6):1370-6 [PubMed PMID: 10835459]
Verne GN, Sallustio JE, Eaker EY. Anti-myenteric neuronal antibodies in patients with achalasia. A prospective study. Digestive diseases and sciences. 1997 Feb:42(2):307-13 [PubMed PMID: 9052511]
Level 3 (low-level) evidenceVackova Z, Niebisch S, Triantafyllou T, Becker J, Hess T, Kreuser N, Kanoni S, Deloukas P, Schüller V, Heinrichs SK, Thieme R, Nöthen MM, Knapp M, Spicak J, Gockel I, Schumacher J, Theodorou D, Martinek J. First genotype-phenotype study reveals HLA-DQβ1 insertion heterogeneity in high-resolution manometry achalasia subtypes. United European gastroenterology journal. 2019 Feb:7(1):45-51. doi: 10.1177/2050640618804717. Epub 2018 Oct 3 [PubMed PMID: 30788115]
Emmanuel A. Current management of the gastrointestinal complications of systemic sclerosis. Nature reviews. Gastroenterology & hepatology. 2016 Aug:13(8):461-72. doi: 10.1038/nrgastro.2016.99. Epub 2016 Jul 6 [PubMed PMID: 27381075]
Nigam GB, Vasant DH, Dhar A. Curriculum review : investigation and management of dysphagia. Frontline gastroenterology. 2022:13(3):254-261. doi: 10.1136/flgastro-2021-101917. Epub 2021 Aug 3 [PubMed PMID: 35493628]
Johnston BT. Oesophageal dysphagia: a stepwise approach to diagnosis and management. The lancet. Gastroenterology & hepatology. 2017 Aug:2(8):604-609. doi: 10.1016/S2468-1253(17)30001-8. Epub [PubMed PMID: 28691686]
Thiyagalingam S, Kulinski AE, Thorsteinsdottir B, Shindelar KL, Takahashi PY. Dysphagia in Older Adults. Mayo Clinic proceedings. 2021 Feb:96(2):488-497. doi: 10.1016/j.mayocp.2020.08.001. Epub [PubMed PMID: 33549267]
Law R, Katzka DA, Baron TH. Zenker's Diverticulum. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014 Nov:12(11):1773-82; quiz e111-2. doi: 10.1016/j.cgh.2013.09.016. Epub 2013 Sep 18 [PubMed PMID: 24055983]
Gretarsdottir HM, Jonasson JG, Björnsson ES. Etiology and management of esophageal food impaction: a population based study. Scandinavian journal of gastroenterology. 2015 May:50(5):513-8. doi: 10.3109/00365521.2014.983159. Epub 2015 Feb 22 [PubMed PMID: 25704642]
Level 2 (mid-level) evidenceSplaingard ML, Hutchins B, Sulton LD, Chaudhuri G. Aspiration in rehabilitation patients: videofluoroscopy vs bedside clinical assessment. Archives of physical medicine and rehabilitation. 1988 Aug:69(8):637-40 [PubMed PMID: 3408337]
Sheikhany AR, Shohdi SS, Aziz AA, Abdelkader OA, Abdel Hady AF. Screening of dysphagia in geriatrics. BMC geriatrics. 2022 Dec 19:22(1):981. doi: 10.1186/s12877-022-03685-1. Epub 2022 Dec 19 [PubMed PMID: 36536306]
O'Horo JC, Rogus-Pulia N, Garcia-Arguello L, Robbins J, Safdar N. Bedside diagnosis of dysphagia: a systematic review. Journal of hospital medicine. 2015 Apr:10(4):256-65. doi: 10.1002/jhm.2313. Epub 2015 Jan 12 [PubMed PMID: 25581840]
Level 1 (high-level) evidenceRogus-Pulia N, Wirth R, Sloane PD. Dysphagia in Frail Older Persons: Making the Most of Current Knowledge. Journal of the American Medical Directors Association. 2018 Sep:19(9):736-740. doi: 10.1016/j.jamda.2018.07.018. Epub [PubMed PMID: 30149842]
Martin-Harris B, Canon CL, Bonilha HS, Murray J, Davidson K, Lefton-Greif MA. Best Practices in Modified Barium Swallow Studies. American journal of speech-language pathology. 2020 Jul 10:29(2S):1078-1093. doi: 10.1044/2020_AJSLP-19-00189. Epub 2020 Jul 10 [PubMed PMID: 32650657]
Stoeckli SJ, Huisman TA, Seifert B, Martin-Harris BJ. Interrater reliability of videofluoroscopic swallow evaluation. Dysphagia. 2003 Winter:18(1):53-7 [PubMed PMID: 12497197]
Miller CK, Schroeder JW Jr, Langmore S. Fiberoptic Endoscopic Evaluation of Swallowing Across the Age Spectrum. American journal of speech-language pathology. 2020 Jul 10:29(2S):967-978. doi: 10.1044/2019_AJSLP-19-00072. Epub 2020 Jul 10 [PubMed PMID: 32650653]
Giraldo-Cadavid LF, Leal-Leaño LR, Leon-Basantes GA, Bastidas AR, Garcia R, Ovalle S, Abondano-Garavito JE. Accuracy of endoscopic and videofluoroscopic evaluations of swallowing for oropharyngeal dysphagia. The Laryngoscope. 2017 Sep:127(9):2002-2010. doi: 10.1002/lary.26419. Epub 2016 Nov 15 [PubMed PMID: 27859291]
Suttrup I, Suttrup J, Suntrup-Krueger S, Siemer ML, Bauer J, Hamacher C, Oelenberg S, Domagk D, Dziewas R, Warnecke T. Esophageal dysfunction in different stages of Parkinson's disease. Neurogastroenterology and motility. 2017 Jan:29(1):. doi: 10.1111/nmo.12915. Epub 2016 Jul 31 [PubMed PMID: 27477636]
Peng L, Patel A, Kushnir V, Gyawali CP. Assessment of upper esophageal sphincter function on high-resolution manometry: identification of predictors of globus symptoms. Journal of clinical gastroenterology. 2015 Feb:49(2):95-100. doi: 10.1097/MCG.0000000000000078. Epub [PubMed PMID: 24492407]
Level 2 (mid-level) evidenceSifrim D, Blondeau K, Mantillla L. Utility of non-endoscopic investigations in the practical management of oesophageal disorders. Best practice & research. Clinical gastroenterology. 2009:23(3):369-86. doi: 10.1016/j.bpg.2009.03.005. Epub [PubMed PMID: 19505665]
Ponds FA, Oors JM, Smout AJPM, Bredenoord AJ. Rapid drinking challenge during high-resolution manometry is complementary to timed barium esophagogram for diagnosis and follow-up of achalasia. Neurogastroenterology and motility. 2018 Nov:30(11):e13404. doi: 10.1111/nmo.13404. Epub 2018 Jul 10 [PubMed PMID: 29989262]
Chan MQ, Balasubramanian G. Esophageal Dysphagia in the Elderly. Current treatment options in gastroenterology. 2019 Dec:17(4):534-553. doi: 10.1007/s11938-019-00264-z. Epub [PubMed PMID: 31741211]
Podboy A, Katzka DA, Enders F, Larson JJ, Geno D, Kryzer L, Alexander J. Oesophageal narrowing on barium oesophagram is more common in adult patients with eosinophilic oesophagitis than PPI-responsive oesophageal eosinophilia. Alimentary pharmacology & therapeutics. 2016 Jun:43(11):1168-77. doi: 10.1111/apt.13601. Epub 2016 Mar 30 [PubMed PMID: 27028344]
Allen BC, Baker ME, Falk GW. Role of barium esophagography in evaluating dysphagia. Cleveland Clinic journal of medicine. 2009 Feb:76(2):105-11. doi: 10.3949/ccjm.76a.08032. Epub [PubMed PMID: 19188476]
Level 3 (low-level) evidenceVaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. The American journal of gastroenterology. 2013 Aug:108(8):1238-49; quiz 1250. doi: 10.1038/ajg.2013.196. Epub 2013 Jul 23 [PubMed PMID: 23877351]
Hernandez JC, Ratuapli SK, Burdick GE, Dibaise JK, Crowell MD. Interrater and intrarater agreement of the chicago classification of achalasia subtypes using high-resolution esophageal manometry. The American journal of gastroenterology. 2012 Feb:107(2):207-14. doi: 10.1038/ajg.2011.353. Epub 2011 Oct 18 [PubMed PMID: 22008895]
Wirth R, Dziewas R, Beck AM, Clavé P, Hamdy S, Heppner HJ, Langmore S, Leischker AH, Martino R, Pluschinski P, Rösler A, Shaker R, Warnecke T, Sieber CC, Volkert D. Oropharyngeal dysphagia in older persons - from pathophysiology to adequate intervention: a review and summary of an international expert meeting. Clinical interventions in aging. 2016:11():189-208. doi: 10.2147/CIA.S97481. Epub 2016 Feb 23 [PubMed PMID: 26966356]
Perry L, Hamilton S, Williams J. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2006 Mar:37(3):765 [PubMed PMID: 16505343]
Level 3 (low-level) evidenceGandolfi M, Smania N, Bisoffi G, Squaquara T, Zuccher P, Mazzucco S. Improving post-stroke dysphagia outcomes through a standardized and multidisciplinary protocol: an exploratory cohort study. Dysphagia. 2014 Dec:29(6):704-12. doi: 10.1007/s00455-014-9565-2. Epub 2014 Aug 13 [PubMed PMID: 25115857]
Level 2 (mid-level) evidenceMartino R, Beaton D, Diamant NE. Perceptions of psychological issues related to dysphagia differ in acute and chronic patients. Dysphagia. 2010 Mar:25(1):26-34. doi: 10.1007/s00455-009-9225-0. Epub 2009 Aug 6 [PubMed PMID: 19657695]
Ninfa A, Crispiatico V, Pizzorni N, Bassi M, Casazza G, Schindler A, Delle Fave A. The care needs of persons with oropharyngeal dysphagia and their informal caregivers: A scoping review. PloS one. 2021:16(9):e0257683. doi: 10.1371/journal.pone.0257683. Epub 2021 Sep 23 [PubMed PMID: 34555044]
Level 2 (mid-level) evidenceChristmas C, Rogus-Pulia N. Swallowing Disorders in the Older Population. Journal of the American Geriatrics Society. 2019 Dec:67(12):2643-2649. doi: 10.1111/jgs.16137. Epub 2019 Aug 20 [PubMed PMID: 31430395]
Beck AM, Kjaersgaard A, Hansen T, Poulsen I. Systematic review and evidence based recommendations on texture modified foods and thickened liquids for adults (above 17 years) with oropharyngeal dysphagia - An updated clinical guideline. Clinical nutrition (Edinburgh, Scotland). 2018 Dec:37(6 Pt A):1980-1991. doi: 10.1016/j.clnu.2017.09.002. Epub 2017 Sep 9 [PubMed PMID: 28939270]
Level 1 (high-level) evidenceFlynn E, Smith CH, Walsh CD, Walshe M. Modifying the consistency of food and fluids for swallowing difficulties in dementia. The Cochrane database of systematic reviews. 2018 Sep 24:9(9):CD011077. doi: 10.1002/14651858.CD011077.pub2. Epub 2018 Sep 24 [PubMed PMID: 30251253]
Level 1 (high-level) evidenceCichero JA, Lam P, Steele CM, Hanson B, Chen J, Dantas RO, Duivestein J, Kayashita J, Lecko C, Murray J, Pillay M, Riquelme L, Stanschus S. Development of International Terminology and Definitions for Texture-Modified Foods and Thickened Fluids Used in Dysphagia Management: The IDDSI Framework. Dysphagia. 2017 Apr:32(2):293-314. doi: 10.1007/s00455-016-9758-y. Epub 2016 Dec 2 [PubMed PMID: 27913916]
Alamer A, Melese H, Nigussie F. Effectiveness of Neuromuscular Electrical Stimulation on Post-Stroke Dysphagia: A Systematic Review of Randomized Controlled Trials. Clinical interventions in aging. 2020:15():1521-1531. doi: 10.2147/CIA.S262596. Epub 2020 Sep 3 [PubMed PMID: 32943855]
Level 1 (high-level) evidenceFan HS, Stavert B, Chan DL, Talbot ML. Management of Zenker's diverticulum using flexible endoscopy. VideoGIE : an official video journal of the American Society for Gastrointestinal Endoscopy. 2019 Feb:4(2):87-90. doi: 10.1016/j.vgie.2018.12.007. Epub 2019 Jan 30 [PubMed PMID: 30766952]
Deschuymer S, Nevens D, Duprez F, Daisne JF, Dok R, Laenen A, Voordeckers M, De Neve W, Nuyts S. Randomized clinical trial on reduction of radiotherapy dose to the elective neck in head and neck squamous cell carcinoma; update of the long-term tumor outcome. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2020 Feb:143():24-29. doi: 10.1016/j.radonc.2020.01.005. Epub 2020 Feb 7 [PubMed PMID: 32044165]
Level 1 (high-level) evidenceJoseph R, Laks S, Meyers M, McRee AJ. Multidisciplinary Approach to the Management of Esophageal Malignancies. World journal of surgery. 2017 Jul:41(7):1726-1733. doi: 10.1007/s00268-017-4009-4. Epub [PubMed PMID: 28361298]
Goel S, Nookala V. Diffuse Esophageal Spasm. StatPearls. 2023 Jan:(): [PubMed PMID: 31082150]
McMahan ZH, Hummers LK. Gastrointestinal involvement in systemic sclerosis: diagnosis and management. Current opinion in rheumatology. 2018 Nov:30(6):533-540. doi: 10.1097/BOR.0000000000000545. Epub [PubMed PMID: 30234725]
Level 3 (low-level) evidenceSallam HS, McNearney TA, Chen JD. Acupuncture-based modalities: novel alternative approaches in the treatment of gastrointestinal dysmotility in patients with systemic sclerosis. Explore (New York, N.Y.). 2014 Jan-Feb:10(1):44-52. doi: 10.1016/j.explore.2013.10.001. Epub 2013 Oct 17 [PubMed PMID: 24439095]
Cappell MS, Stavropoulos SN, Friedel D. Updated Systematic Review of Achalasia, with a Focus on POEM Therapy. Digestive diseases and sciences. 2020 Jan:65(1):38-65. doi: 10.1007/s10620-019-05784-3. Epub 2019 Aug 27 [PubMed PMID: 31451984]
Level 1 (high-level) evidenceBoeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet (London, England). 2014 Jan 4:383(9911):83-93. doi: 10.1016/S0140-6736(13)60651-0. Epub 2013 Jul 17 [PubMed PMID: 23871090]
Vaezi MF, Felix VN, Penagini R, Mauro A, de Moura EG, Pu LZ, Martínek J, Rieder E. Achalasia: from diagnosis to management. Annals of the New York Academy of Sciences. 2016 Oct:1381(1):34-44. doi: 10.1111/nyas.13176. Epub 2016 Aug 29 [PubMed PMID: 27571581]
Wilfong C, Ross S, Musumeci M, Spence J, Gravetz A, Sucandy I, Rosemurgy A. Doing more with less: our decade of experience with laparo-endoscopic single site Heller myotomy supports its application. Surgical endoscopy. 2020 Oct:34(10):4481-4485. doi: 10.1007/s00464-019-07232-9. Epub 2020 Mar 16 [PubMed PMID: 32180003]
Khashab MA, Vela MF, Thosani N, Agrawal D, Buxbaum JL, Abbas Fehmi SM, Fishman DS, Gurudu SR, Jamil LH, Jue TL, Kannadath BS, Law JK, Lee JK, Naveed M, Qumseya BJ, Sawhney MS, Yang J, Wani S. ASGE guideline on the management of achalasia. Gastrointestinal endoscopy. 2020 Feb:91(2):213-227.e6. doi: 10.1016/j.gie.2019.04.231. Epub 2019 Dec 13 [PubMed PMID: 31839408]
Vinit C, Dieme A, Courbage S, Dehaine C, Dufeu CM, Jacquemot S, Lajus M, Montigny L, Payen E, Yang DD, Dupont C. Eosinophilic esophagitis: Pathophysiology, diagnosis, and management. Archives de pediatrie : organe officiel de la Societe francaise de pediatrie. 2019 Apr:26(3):182-190. doi: 10.1016/j.arcped.2019.02.005. Epub 2019 Mar 1 [PubMed PMID: 30827775]
Azer SA, Reddivari AKR. Reflux Esophagitis. StatPearls. 2023 Jan:(): [PubMed PMID: 32119349]
Eslick GD, Talley NJ. Dysphagia: epidemiology, risk factors and impact on quality of life--a population-based study. Alimentary pharmacology & therapeutics. 2008 May:27(10):971-9. doi: 10.1111/j.1365-2036.2008.03664.x. Epub 2008 Feb 28 [PubMed PMID: 18315591]
Level 2 (mid-level) evidence