Indications
FDA-Approved Indications
Dobutamine is approved by the US Food and Drug Administration (FDA) for short-term use in patients with decreased contractility due to heart failure or cardiac procedures resulting in cardiac decompensation.[1] Despite dobutamine's ability to improve hemodynamic balance, the drug has not demonstrated positive outcomes for heart failure patients in either the hospital or outpatient setting.
Off-Label Uses
Dobutamine is used off-labeled as temporary intravenous (IV) inotropic support until the resolution of acute inducing factors or the patient receives more definitive treatment, such as coronary revascularization, mechanical circulatory support, or heart transplant. Short-term IV inotropic support should be provided for patients in cardiogenic shock to preserve systemic blood flow and protect them from end-organ damage. Patients can reasonably receive dobutamine in continuous IV form for inotropic support to bridge patients with late-stage heart failure, stage D, refractory to guideline-directed medical therapy until patients who are candidates for and awaiting cardiac transplantation or mechanical circulatory support receive the appropriate long-term treatment.[2]
Continuous IV inotropic dobutamine support is used off-label. The medication can be given to hospitalized patients with severe systolic dysfunction who have low blood pressure and significantly reduced cardiac output to maintain systemic blood flow and protect against end-organ damage. Long-term IV inotropic dobutamine support can be given to palliative patients with late-stage heart failure, stage D, who are not candidates for mechanical circulatory support or cardiac transplantation for symptomatic control, regardless of guideline-directed medical therapy.[3] IV inotropic dobutamine can be given off-label to patients to induce pharmacological stress during stress echocardiography (ECG) if patients cannot perform an exercise stress test.[4][5]
A retrospective study of positive inotropic agents for managing acute decompensated heart failure compared the safety and efficacy of dobutamine to milrinone. In the study, the median hospital length of stay, rehospitalization within 30 days, and median length of stay in the intensive care unit (ICU) were primary endpoints, and secondary endpoints were all-cause mortality, progression to renal failure within 72 hours, rehospitalization within 90 days, and urine output within 72 hours of therapy; all endpoints were not statistically significant.
A post hoc analysis comparing primary and secondary outcomes between milrinone and dobutamine, accounting for baseline characteristics through linear and logistic regression, yielded no statistically significant findings. Overall, the study did not identify any significant differences in outcomes between the 2 treatment groups, except for a longer length of stay in the ICU for the milrinone group.[6]
Mechanism of Action
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Mechanism of Action
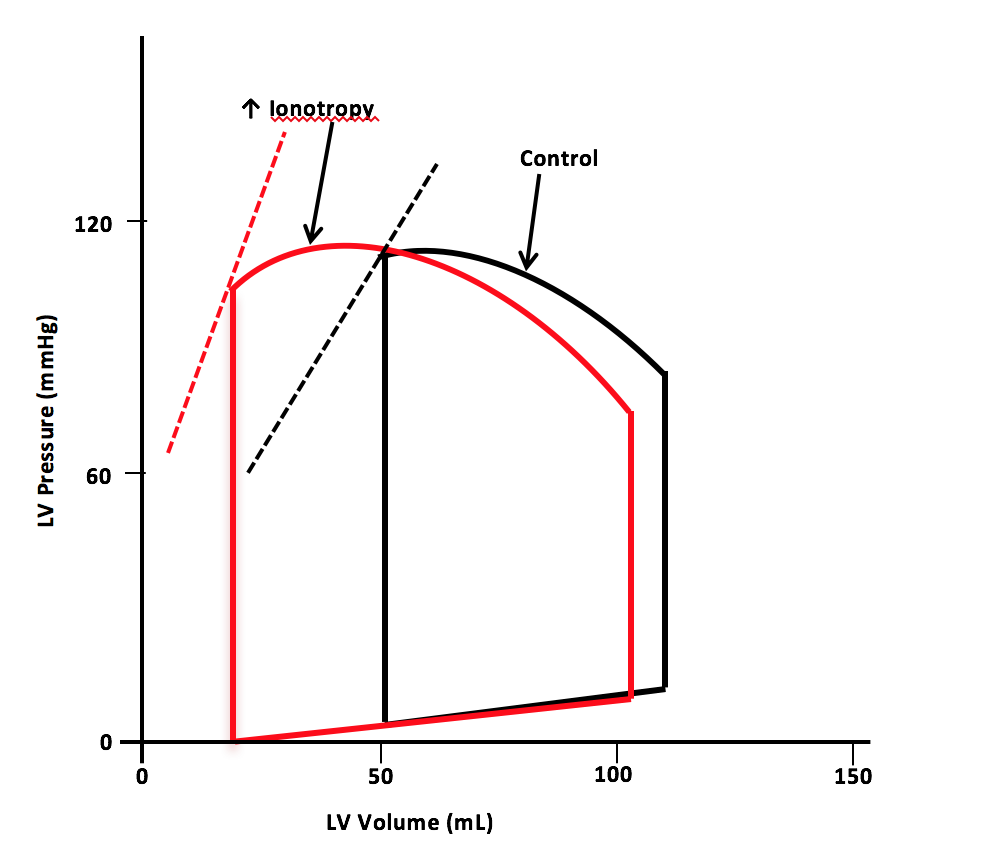
Dobutamine is a pharmacological agent with ionotropic and chronotropic effects depending on the dose. Inotropic effects on the myocardium occur by selectively binding and activating the β-1 receptors.[7] The medication is indicated for decompensated congestive heart failure because of the sympathomimetic effects. Dobutamine's ionotropic effect increases contractility, leading to decreased end-systolic volume and, therefore, increased stroke volume. The increase in stroke volume augments the heart's cardiac output (see Image. Pressure-Volume Loop for Dobutamine).[8]
The changes in cardiac output allow for the baroreceptor-mediated response to decrease systemic vascular resistance (SVR) and cause little to no change in arterial blood pressure. In addition to the well-known β-1 activity, dobutamine has some β-2 activity, which contributes to the reduction in SVR, and α-1 activity, to a lesser extent, whose vasoconstrictive effects are negated by the baroreceptor mediated response and β-2 activity.
Pharmacokinetics
The majority of clinical studies with dobutamine have been limited to short-term duration, typically not exceeding several hours. In the limited cases where patients were monitored for 24, 48, and 72 hours, researchers observed that some individuals exhibited a sustained elevation in cardiac output. In contrast, others experienced a return toward baseline levels. Dobutamine typically has an onset of action 1 to 2 minutes after administration. However, achieving the maximum impact of an infusion rate may take up to 10 minutes. In humans, the plasma half-life of dobutamine is approximately 2 minutes. The primary processes involved in the metabolism are catechol methylation and conjugation. The major excretion products found in human urine are the conjugates of dobutamine and 3-O-methyl dobutamine.
Administration
Available Dosage Forms and Strengths
The medication is available as dobutamine hydrochloride 1 mg/mL, 2 mg/mL, and 4 mg/mL in 250 ml and 250 mg/20 mL IV solution. Dobutamine administration is via extensive IV access and infusion pump for inotropic support in decompensated congestive heart failure, stress echocardiogram, and stress nuclear testing. Dobutamine is available in a solution as a racemic mixture of both positive and negative enantiomers for IV administration. The positive enantiomer in the solution is predominately selective for the β-sympathetic receptors, mainly β-1 and β-2. In contrast, the negative enantiomer is selective for the α-1 receptors.[9]
Adult Dosages
Dobutamine dosage for cardiac decompensation in heart failure can begin with a low dose of 0.5 to 1.0 mcg/kg/min and increase to a maximum of 40 mcg/kg/min. The lower doses of dobutamine can be prescribed at 2.5 to 5.0 mcg/kg/min, and the higher end of dobutamine doses can be 5.0 to 20.0 mcg/kg/min.[10] Before administration, parenteral drug products should be visually inspected for particulate matter and discoloration, as long as the solution and container allow for the inspection.
The infusion rate should then be adjusted at short intervals, considering the patient's response. Factors such as systemic blood pressure, frequency of ectopic activity, heart rate, urine flow, and, whenever possible, central venous pressure measurements, cardiac output, and/or pulmonary capillary wedge pressure should guide the titration process. Optimal infusion rates have varied between patients in reported trials, typically ranging from 2 to 20 mcg/kg/min, slightly below this range. In rare instances, infusion rates as high as 40 mcg/kg/min are necessary to achieve the desired effect. The stress echocardiogram and stress nuclear test dose is initiated at 5 mcg/kg/min and can be increased in intervals of 10 mcg/kg/min every 3 to 5 minutes until the target heart rate is reached.
Specific Patient Populations
Pregnancy considerations: Research on administering the drug to pregnant patients has not been conducted, and it should be employed only when the anticipated benefits significantly outweigh the potential risks to the developing fetus.[11]
Pediatric considerations: Dobutamine has demonstrated the ability to enhance cardiac output and systemic pressure in pediatric patients across all ages. However, dopamine is more effective in premature neonates than dobutamine in increasing systemic blood pressure without causing excessive tachycardia. Evidence does not suggest that dobutamine provides any additional advantages when administered to infants who are already receiving optimal dopamine infusions.
Older patients: The clinical trials conducted on dobutamine did not involve an adequate number of participants aged 65 and older to establish whether they exhibit different responses compared to younger individuals. However, observations from other clinical reports have not indicated any notable variations in the drug's effects between older adults and younger patients. As a general guideline, cautious dosing is recommended for patients 65 and older, typically starting at lower doses within the prescribed range. This approach considers the higher likelihood of reduced hepatic, renal, or cardiac function and the presence of concurrent illnesses or drug treatments.
Adverse Effects
Dobutamine administration can lead to possible adverse reactions, mainly due to sympathomimetic activity.[12] Most patients taking this medication have experienced a rise in systolic blood pressure from 10 to 20 mm Hg and an increase of 5 to 10 beats per minute (bpm) in their heart rate. Increased systolic blood pressure and heart rate have been reported. In about 10% of the patients, a rise of 30 bpm or more in the heart rate is expected, and in about 7.5% of patients, a 50 mm Hg or more increase in the systolic blood pressure is expected. Patients with preexisting hypertension are more susceptible to the adverse effects on systolic blood pressure when using dobutamine.[13][14]
Dobutamine increases the risk of rapid ventricular response in patients with preexisting atrial fibrillation. The recommendation for these patients is to use a regimen of digoxin before starting dobutamine to decrease the risk of developing atrial fibrillation with a rapid ventricular response. An increased risk of developing premature ventricular beats is evident during the administration of dobutamine. About 5% of patients experience premature ventricular beats.[15]
Other adverse effects caused by this medication include hypotension rarely. While increases in systolic blood pressure are common due to dobutamine, hypotension occurs less frequently due to decreased SVR. Recommendations include reducing the dose or discontinuing the drug to reverse the hypotensive effects.
Phlebitis at the site of the IV administration can occur but is an uncommon reaction.[16] Dobutamine can rarely reduce potassium concentrations to hypokalemic levels. Other rare adverse effects have occurred in 1% to 3% of the patients, including nausea, headaches, chest pain, palpitations, and shortness of breath. Dobutamine contains sulfite, which can lead to reactions in rare patients with sulfite hypersensitivity.[17]
Contraindications
According to the FDA, dobutamine use is contraindicated in patients with a noted history of allergic reactions to either previous dobutamine use or any sulfite use. The medication is contraindicated in patients with hypokalemia, idiopathic hypertrophic sub-aortic stenosis, acute myocardial infarction, unstable angina, left main stem disease, severe hypertension, arrhythmias, acute myocarditis or pericarditis, and hypokalemia.
Box Warnings
Increase in heart rate or blood pressure: Dobutamine hydrochloride can significantly elevate heart rate and/or blood pressure, particularly systolic pressure. Clinical studies have shown that approximately 10% of adult patients experience a heart rate increase of 30 bpm or more. In comparison, about 7.5% have a systolic pressure increase of 50 mm Hg or greater. However, these effects are reversible by reducing the dosage of the medication.
As dobutamine enhances atrioventricular conduction, individuals with atrial fibrillation are at risk of developing a rapid ventricular response. To mitigate this risk, the recommendation is to administer a digitalis preparation, such as digoxin, before initiating dobutamine therapy. Patients with preexisting hypertension also have a higher likelihood of experiencing an exaggerated pressure response. Therefore, careful blood pressure monitoring is essential to manage the condition effectively.
Ectopic activity: Dobutamine can induce or exacerbate ventricular ectopic activity; incidents of dobutamine directly causing ventricular tachycardia are infrequent.
Hypersensitivity: Occasional reports have indicated hypersensitivity reactions associated with the administration of dobutamine in 5% dextrose injection, USP. These reactions may include skin rash, fever, eosinophilia, and bronchospasm. Dobutamine in 5% dextrose injection contains sodium bisulfite, a sulfite compound. Sulfites have the potential to cause allergic-type reactions, ranging from anaphylactic symptoms or severe allergic reactions to less severe episodes of asthma in susceptible individuals. The prevalence of sulfite sensitivity in the general population is uncertain but believed to be low. However, sulfite sensitivity is observed more frequently in individuals with asthma than those without asthma.
Warnings and Precautions
General: When administering dobutamine, continuously monitoring the patient's ECG and blood pressure is essential. This monitoring allows for close observation of any changes in heart rhythm and blood pressure response. Additionally, monitoring pulmonary wedge pressure and cardiac output can provide valuable information for ensuring the safe and effective dobutamine infusion. Hypovolemia should be assessed before initiating treatment with dobutamine.
Administration following acute myocardial infarction: Clinical knowledge regarding the use of dobutamine after myocardial infarction is limited, making it challenging to determine the drug's safety in this specific scenario. Caution is necessary because any medication that enhances contractile force and heart rate might exacerbate ischemia, potentially leading to a more significant infarction. However, it is uncertain if dobutamine has such an effect.
Drug interactions: Dobutamine is contraindicated for concomitant use with dihydroergotamine (synergistic effects) or phenelzine (additive effects). Both combinations can increase the risk of severe hypertension, including hypertensive crisis.
Research suggests that when dobutamine and nitroprusside are coadministered, it generally leads to increased cardiac output and lower pulmonary wedge pressure than when either drug is used individually. Combining dobutamine with catechol-O-methyltransferase (COMT) inhibitors such as entacapone may have potential effects such as an elevated heart rate, arrhythmias, and alterations in blood pressure.
Monitoring
With the administration of dobutamine, continuous monitoring using a cardiac monitor and blood pressure checks are recommended because dobutamine is typically given to unstable patients and can lead to serious effects quickly that require monitoring and corrective action. The clinician can reduce the dose of dobutamine or stop the medication if the patient experiences adverse effects.
Toxicity
Dobutamine toxicity is rare, and the half-life is short at 2 minutes. Symptoms are generally due to sympathetic overstimulation and can include chest pain, palpitations, headaches, tremors, shortness of breath, nausea, and vomiting. IV metoprolol can be given to reverse the tachycardia caused by dobutamine.
Enhancing Healthcare Team Outcomes
Dobutamine is utilized in the ICU for managing low blood pressure, and although it is considered safe, dobutamine necessitates close monitoring due to its potential to elevate blood pressure and induce arrhythmias. Optimal therapeutic efficacy and avoidance of adverse effects are ensured through the engagement of an interprofessional healthcare team comprising ordering clinicians, specialists, nurses, and pharmacists. Nurses commonly oversee IV dobutamine administration and should, therefore, possess familiarity with dosing and monitoring parameters, alerting the ordering clinician to any observed patient concerns. Pharmacists, particularly those specializing in cardiology, verify dosing, check for drug interactions, and collaborate with other team members as necessary. The effects of dobutamine are transient, and the hemodynamic parameters reverse as soon as the infusion ceases. Effective treatment management with dobutamine for patients in scenarios involving decreased contractility due to heart failure or surgical procedures leading to decompensation relies on collaboration and open communication among all interprofessional team members to achieve optimal patient outcomes.
Media
(Click Image to Enlarge)
References
McNally EM. Can we do better than dobutamine? Circulation research. 2013 Aug 2:113(4):355-7. doi: 10.1161/CIRCRESAHA.113.302000. Epub [PubMed PMID: 23908327]
Stevenson LW. Clinical use of inotropic therapy for heart failure: looking backward or forward? Part I: inotropic infusions during hospitalization. Circulation. 2003 Jul 22:108(3):367-72 [PubMed PMID: 12876135]
Martens P, Vercammen J, Ceyssens W, Jacobs L, Luwel E, Van Aerde H, Potargent P, Renaers M, Dupont M, Mullens W. Effects of intravenous home dobutamine in palliative end-stage heart failure on quality of life, heart failure hospitalization, and cost expenditure. ESC heart failure. 2018 Aug:5(4):562-569. doi: 10.1002/ehf2.12248. Epub 2018 Jan 17 [PubMed PMID: 29341466]
Level 2 (mid-level) evidenceCurrie GM. Pharmacology, Part 4: Nuclear Cardiology. Journal of nuclear medicine technology. 2019 Jun:47(2):97-110. doi: 10.2967/jnmt.118.219675. Epub 2019 Feb 15 [PubMed PMID: 30770476]
Lattanzi F, Picano E, Adamo E, Varga A. Dobutamine stress echocardiography: safety in diagnosing coronary artery disease. Drug safety. 2000 Apr:22(4):251-62 [PubMed PMID: 10789822]
Kelly J, Cheng J, Malloy R, Lupi K. Comparison of Positive Inotropic Agents in the Management of Acute Decompensated Heart Failure. Journal of cardiovascular pharmacology. 2020 May:75(5):455-459. doi: 10.1097/FJC.0000000000000811. Epub [PubMed PMID: 32091426]
Alhayek S, Preuss CV. Beta 1 Receptors. StatPearls. 2024 Jan:(): [PubMed PMID: 30422499]
Kislitsina ON, Rich JD, Wilcox JE, Pham DT, Churyla A, Vorovich EB, Ghafourian K, Yancy CW. Shock - Classification and Pathophysiological Principles of Therapeutics. Current cardiology reviews. 2019:15(2):102-113. doi: 10.2174/1573403X15666181212125024. Epub [PubMed PMID: 30543176]
Ruffolo RR Jr. The pharmacology of dobutamine. The American journal of the medical sciences. 1987 Oct:294(4):244-8 [PubMed PMID: 3310640]
Erlemeier HH, Kupper W, Bleifeld W. Intermittent infusion of dobutamine in the therapy of severe congestive heart failure--long-term effects and lack of tolerance. Cardiovascular drugs and therapy. 1992 Aug:6(4):391-8 [PubMed PMID: 1520649]
Level 1 (high-level) evidenceJeejeebhoy FM, Zelop CM, Lipman S, Carvalho B, Joglar J, Mhyre JM, Katz VL, Lapinsky SE, Einav S, Warnes CA, Page RL, Griffin RE, Jain A, Dainty KN, Arafeh J, Windrim R, Koren G, Callaway CW, American Heart Association Emergency Cardiovascular Care Committee, Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Cardiovascular Diseases in the Young, and Council on Clinical Cardiology. Cardiac Arrest in Pregnancy: A Scientific Statement From the American Heart Association. Circulation. 2015 Nov 3:132(18):1747-73. doi: 10.1161/CIR.0000000000000300. Epub 2015 Oct 6 [PubMed PMID: 26443610]
Russell JA. Vasopressor therapy in critically ill patients with shock. Intensive care medicine. 2019 Nov:45(11):1503-1517. doi: 10.1007/s00134-019-05801-z. Epub 2019 Oct 23 [PubMed PMID: 31646370]
Mertes H, Sawada SG, Ryan T, Segar DS, Kovacs R, Foltz J, Feigenbaum H. Symptoms, adverse effects, and complications associated with dobutamine stress echocardiography. Experience in 1118 patients. Circulation. 1993 Jul:88(1):15-9 [PubMed PMID: 8319327]
Lewis TC, Aberle C, Altshuler D, Piper GL, Papadopoulos J. Comparative Effectiveness and Safety Between Milrinone or Dobutamine as Initial Inotrope Therapy in Cardiogenic Shock. Journal of cardiovascular pharmacology and therapeutics. 2019 Mar:24(2):130-138. doi: 10.1177/1074248418797357. Epub 2018 Sep 2 [PubMed PMID: 30175599]
Level 2 (mid-level) evidenceDavid S, Zaks JM. Arrhythmias associated with intermittent outpatient dobutamine infusion. Angiology. 1986 Feb:37(2):86-91 [PubMed PMID: 3954157]
Silva EVC, Ochiai ME, Vieira KRN, Pereira Barretto AC. The use of peripherally inserted central catheter reduced the incidence of phlebitis in heart failure patients: A randomized trial. The journal of vascular access. 2023 Sep:24(5):942-947. doi: 10.1177/11297298211059650. Epub 2021 Nov 23 [PubMed PMID: 34812074]
Level 1 (high-level) evidenceKang SY, Lee JW, Park DE, Bae BR, Lee HS, Kim BK, Kim JY, Lim KH, Park HW, Koo BK, Cho SH, Song WJ. Hypereosinophilia with rash to dobutamine infusion; sulfite hypersensitivity diagnosed by in vitro stimulation assays. Allergology international : official journal of the Japanese Society of Allergology. 2016 Oct:65(4):477-480. doi: 10.1016/j.alit.2016.01.008. Epub 2016 Apr 30 [PubMed PMID: 27137991]