Introduction
Diphyllobothriasis is a parasitic infection caused by broad or fish tapeworms. Attention to diphyllobothriasis has grown recently due to increasing numbers of case reports in nonendemic areas and improved understanding of the pathogen from studies that use molecular techniques.[1] Most species causing human infection have recently been reclassified and renamed based on morphological and molecular analyses.[CDC. Diphyllosbothriasis. 2019][2]
Commonly known as broad or fish tapeworms, diphyllobothriids have the dubious distinction of being the longest intestinal zoonotic parasites to infect humans.[2] After a mammal or bird ingests fish that harbor infectious larvae of the family Diphyllobothriidae, broad tapeworms mature in the intestines of their hosts. Human infections are usually mild or asymptomatic, often noticed only when the mature worm is passed in feces.[3][1] Symptoms may include diarrhea, abdominal pain or discomfort, constipation, fatigue, the passage of proglottids, headache, and allergic reactions. The condition commonly causes B12 deficiency, but anemia is uncommon. Diphyllobothriids may rarely obstruct lumina, leading to cholangitis, intestinal obstruction, appendicitis, and cholecystitis.[4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
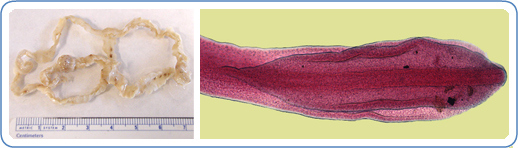
Fully grown diphyllobothriids range from 2 to 15 meters, consisting of thousands of sections called proglottids (see Images. Diphyllobothrium latus and Diphyllobothrium latus Tapeworm).[5] They can grow as many as 22 cm per day and live for 20 years or longer.[5] The anterior end or scolex of the tapeworm attaches to the intestine in the host organism via a paired groove called the bothrium on its dorsal and ventral surfaces.[5][6] New proglottids develop in the proliferative zone or neck. Proglottids, each with male and female genital organs, constitute the rest of the strobila or body. The eggs are round to ovoid, with the size and form depending on the tapeworm and the host species in which it is growing. The lack of species-specific morphological traits and intraspecies variability in egg and body characteristics across broad tapeworm species renders identification based solely on morphology unreliable.[7][2]
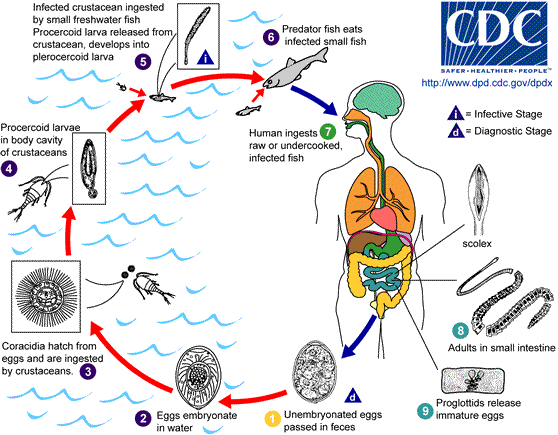
The life cycles of tapeworms in the Diphyllobothriidae family are complex, and the full life cycle of only a few species is currently known.[1][8] They are always connected to freshwater or marine environments, as the first larvae are aquatic. Larvae most commonly pass through 2 intermediate hosts before the tapeworm matures in the intestines of a definitive or accidental host (see Image. Diphyllobothriid Tapeworm Lifecycle)
The lifecycle begins when diphyllobothriid eggs are deposited or washed into fresh or marine water, where they develop into first larvae. The first intermediate hosts, usually an arthropod such as a crustacean from the subclass Copepoda, ingest the first larvae. Small freshwater or marine fish, the second intermediate hosts, eat the crustaceans and are themselves eaten by larger fish. The parasites migrate into the tissues of these larger fish and become third-stage larvae called plerocercoids. Definitive hosts become infected after eating the plerocercoids lodged in freshwater or marine fish muscles, roe, or edible organs.[CDC.Diphyllobothriasis. 2019] Mature tapeworms develop once the plerocercoids attach, usually in the ileum but sometimes in the jejunum, bile duct, or appendix. They grow into folded loops in the small intestine, producing their first eggs 2 to 6 weeks later. Adults produce up to 1 million eggs per day expelled with host feces, beginning the life cycle anew.[9]
In 2017, Waes et al proposed a new taxonomy for members of the Diphyllobothriidae family based on morphological characteristics and molecular techniques.[2] So far, 6 species from 3 genera (Dibothriocephalus, Diphyllobothrium, and Adenocephalus) are confirmed to be true human parasites.[8][1][2]
- Dibothriocephalus latus (syn Diphyllobothrium latum)
- Dibothriocephalus dendriticus (syn Diphyllobothrium dendriticum)
- Dibothriocephalus nihonkaiense (syn Diphyllobothrium nihonkaiense)
- Adenocephalus pacificus (syn Diphyllobothrium pacificum)
- Diphyllobothrium balaenopterae (syn Diphyllobothrium stemmacephalum)
- Diphyllobothrium stemmacephalum (syn Diplogonoporus grandis; syn Diplogonoporus balaenoptera)
D latus is assumed to be the most common species to infect humans. However, many infections are likely misidentified as D latus due to the need for molecular techniques to differentiate Diphyllobothriidae at the species level.[2] The 13 other Diphyllobothriidae species previously reported in humans were diagnosed by morphology alone and have not been confirmed by molecular techniques.[8][1]
Human infection with diphyllobothriids is known from the early Neolithic period, as evidenced by diphyllobothriid eggs found in Germany and dated between 3917 and 3905 bce.[10] Humans appear to be the principal definitive host only for D latus and possibly D nihonkaiensis.[2][1] Due to their ability to survive and reach maturity in any intestinal environment similar to their definitive host or hosts, broad tapeworms will continue to infect and adapt to other mammalian hosts, including humans.[2]
Epidemiology
Broad tapeworms are parasites of tetrapods, with marine mammals such as seals particularly affected. A complete knowledge of global distribution and host associations is hampered by erroneous species identification before molecular techniques became commonplace.[1] D latus is endemic to subarctic and temperate lakes on the Eurasian continent. D nihonkaiensis is the dominant human-associated species in northern countries bordering the Pacific Ocean. Commonly infected fish species include perch, pike, hake, salmon, and salmonids. The recent adaptation of D latus to freshwater salmonid species in South America could have significant epidemiologic and economic consequences.[7]
In 2002, the prevalence of human cases of diphyllobothriasis was estimated at 20 million individuals globally.[5][2] Comprehensive estimates of diphyllobothriid infections are difficult due to the lack of mandatory reporting in most countries. With a usually mild symptom profile and low awareness of diphyllobothriasis among the public sector and medical professionals, the underdiagnosis of diphyllobothriasis is likely.
Most cases are reported from Japan, although human infections with endemic Dipylllobothriidae species have been reported on all continents except Africa and Australia.[5][2] Infections occur primarily in countries and regions where traditional cuisine involves the consumption of raw fish, such as raw, smoked, or pickled fish in parts of Europe, sushi in Japan, smoked fish in North America, and ceviche in South America.
Improvements in sanitation have led to widespread, marked decreases in D latus infections in many regions, many of which are no longer considered endemic, eg, the UK, Austria, Czech Republic, Belgium, and the Netherlands.[11] Unfortunately, not all people in a given area may benefit from sanitation improvements; for example, the prevalence of D latus in nonindigenous adults in the Khanty-Mansi Autonomous Okrug regions of Russia decreased from 11.1% to 1.9% between 1988 to 1989 and 2018 to 2019.[12] In contrast, the decrease was minimal in indigenous populations, from 5.3% to 4.8%.[12] As the intermediate and definitive hosts for many species of tapeworms are wild animals and fishes, improved sanitation is not expected to decrease infection rates for all diphyllobothriids.[13]
Despite improvements in sanitation and the unavailability of current prevalence estimates of Diphyllobothriidae infection in humans,[5][1] multiple authors consider diphyllobothriasis a re-emerging disease. This is due to increased case reports in developed countries far from endemic zones and to global pressures predicted to increase the spread of the parasite.[2][1][7][14]
- Increasing migration, travel, fish consumption, and the popularity of foods from more diverse cuisines have widened interest and exposure to raw fish delicacies in nonendemic areas.[15][16][17][18][19]
- Rapid food transport and globalization allow fish stored on ice but not frozen to be transported long distances from countries where specific diphyllobothriids are endemic to other parts of the world.[8][16][7]
- Growing salmon smolt in lakes and possible contamination and escape of infected fish from oceanic pens have led to concerns about salmon and other aquaculture.[20] These practices could account for D latus in Brazil, Taiwan, south India, and other tropical areas.[21][22]
While the cestode has no biological preference for any age group, gender, or race,[5] clustering of infections can occur in various population subgroups with higher contact or consumption of raw fish.[23][12][5][19]
- People who consume uncooked fish regularly as part of the local cuisine, for example, in Scandinavia, Peru, and Japan (as cold smoked or salted fish, ceviche, sushi, or other raw fish products).
- People who prepare fish, for example, those who taste test the Jewish dish gefilte fish before cooking.
- People who frequently consume fish parts from fresh catches, such as fishermen.
High infection rates are compounded in populations with poor access to adequate sanitation.
Pathophysiology
Once established, the tapeworm induces changes in neuromodulator concentrations in the host tissue and serum.[24] Local structural changes alter gastrointestinal tract functioning by modulating the neuroendocrine response, causing enhanced secretion and changing gut motility.[24] Damage is mediated through the induction of mast cell and eosinophilic granule cell degranulation, leading to the release of inflammatory cytokines.[24]
Prolonged or heavy Diphyllobothridae infection leads to vitamin B12 deficiency due to dissociation of B12 from the host intrinsic factor complex in the gut. The tapeworm absorbs vitamin B12 100 times faster than the human gut.[5] B12 deficiency may be found in about 40% of infections with D latus at diagnosis; less than 2% of cases develop clinical anemia.[5]
History and Physical
Human diphyllobothriid infections are often asymptomatic, noticed only incidentally on colonoscopy [25] or when proglottids are passed.[23][26] Passing of proglottids over time can have a significant emotional impact on patients.
About 25% of patients have symptoms,[4][27] which are generally mild. These include abdominal pain or distension, dyspepsia, vomiting, diarrhea, constipation, fatigue, dizziness, myalgia, and headaches.[23][26] Weight loss, symptoms of anemia, dermatologic manifestations, and luminal obstruction are rare.[4][5][27]
Unless complications are present or the patient presents with proglottids extruding from the anal orifice, physical exam is usually normal. Patients may exhibit signs of allergy. Very rarely, luminal obstruction may produce signs of an acute abdomen, or severe B12 deficiency may result in pallor, jaundice, dyspnea, or nervous system symptoms such as headaches, visual changes, or ataxia.[5][28] (Refer to the Complications section for more details on luminal obstruction and B12 deficiency).
Diagnosing broad tapeworm infection in symptomatic cases requires a thorough history with particular attention to the patient’s occupation, hobbies, eating habits, and travel and migration history.
Evaluation
The gold standard for diagnosing Diphyllobothriidae at the family level is identifying eggs or proglottids in the stool.[5] Eggs are usually plentiful, so concentrating the specimen is not needed. As few or no eggs may appear in the stool for several months if a large segment of tapeworm has been passed, repeat testing may be required.[29]
CBC and serum B12 may demonstrate megaloblastic anemia, vitamin B12 deficiency, pancytopenia, eosinophilia, and pernicious anemia.[5][30] No reliable serological tests exist to aid in the diagnosis of Diphyllobothriidae.
Due to the similarity in morphology across species and the effects of treatment or fixing agents on the morphology, identifying Diphyllobothriidae at the species level requires molecular techniques. As the treatment is the same, exact species diagnosis is clinically unnecessary. However, it can resolve diagnostic dilemmas where the microscopic characteristics of an identified tapeworm are inconsistent with the expected local epidemiology.[18]
Speciation is important to understand the epidemiology of diphyllobothriids and to determine intermediate species and appropriate infection control measures. This is done via multiplex polymerase chain reaction (PCR) amplification and detection of restriction fragment length polymorphisms, usually at the cox1 site.[31] Clinical samples for genotyping must be fresh, frozen, or fixed in a PCR-friendly solution.[2]
Radiological investigations are generally not needed. Some authors advocate the use of abdominal ultrasound for real-time diagnosis of diphyllobothriasis and treatment with praziquantel.[32][33] Ultrasound is non-invasive and widely available and may detect the hyperechoic, ribbon-like structure floating freely in the intestinal lumen in real time.[32] Tapeworms may also be detected by endoscopy, abdominal magnetic resonance imaging, and colonoscopy but are inappropriate as primary investigative tools as they are costly and invasive.[22][34]
Treatment / Management
Uncomplicated diphyllobothriasis is safely treated on an outpatient basis. As with other tapeworm infections, the treatment of choice is praziquantel.[35] Praziquantel is taken up but not metabolized by the parasite, interfering with calcium metabolism and causing flaccid paralysis.[36] The CDC recommends a single 5 to 10 mg/kg dose in children and adults.[CDC. Parasites-Diphyllobothrium-Resources for Health Professionals. 2020] Praziquantel is available in 600 mg divisible mg tablets. (B3)
Praziquantel is a category B medication, as no adequate studies have been conducted in pregnant women. However, accidental administration of praziquantel to pregnant women during mass elimination campaigns has demonstrated no difference in fetal outcomes compared to those that are not treated.[CDC. Parasites-Diphyllobothrium - Resources for Health Professionals. 2020] Safety in children younger than 4 has also not been established, although many children younger than 4 have been treated in mass elimination campaigns for schistosomiasis.[CDC. Parasites-Diphyllobothrium-Resources for Health Professionals. 2020] In individual pregnant or pediatric patients, the risk of no treatment needs to be balanced with the risk of worsening infection with no treatment.
CYP3A4 metabolizes praziquantel and thus carries the potential for drug interactions.[36] Adverse effects of praziquantel include weakness, headache, dizziness, abdominal pain, fever, or possibly urticaria.[5][37](B3)
A single dose of niclosamide is an alternative in many countries, but not in the United States. Niclosimide acts by uncoupling oxidative phosphorylation. Niclosimide is given in a single 2-gram dose for adults or 1 gram for children older than 6.[CDC. Parasites-Diphyllobothrium Infection-Resources for Health Professionals. 2020]
The patient's stool should be examined for ova and parasites 1 month after treatment to ensure the eradication of the parasite. The recommended dose of 5 to 10 mg/kg of praziquantel is lower than previous recommendations; a higher dose may be more effective with treatment failure.[5](B3)
Isolated Vitamin B12 deficiency due to diphyllobothriasis resolves with antiparasitic therapy alone. Hematologic and neurologic manifestations may require vitamin B12 supplementation.[5] (B3)
Differential Diagnosis
The differential diagnosis of diphyllobothriasis depends on the presenting complaint(s). The most common presentation for diphyllobothriasis is mild gastrointestinal symptoms such as discomfort and diarrhea, for which the differential diagnoses are protean. Order stool ova and parasites routinely whenever the history suggests travel, raw or undercooked meat consumption, or other risk factors for diphyllobothriasis or other parasitic infections. Differentiation from Taenia species by microscopy is straightforward based on the position of the gonopores in proglottids.
With a presentation of macrocytic anemia, the following should be considered:
- Pernicious anemia
- Bone marrow toxins
- Dietary restrictions
- Dietary deficiencies
- Exposure to drugs (e.g., anticonvulsants)
- Intestinal malabsorption
- Short gut syndrome
Patients very rarely present with an acute or subacute abdomen, with a differential including cholecystitis, appendicitis, or bowel obstruction.[CDC. DPDx - Diphyllobothriasis. 2019] In these cases, the diagnosis may be suspected during the workup, for example, on ultrasound or confirmed during surgical intervention such as colonoscopy.[4]
Prognosis
The prognosis of diphyllobothriasis infection is excellent as the disease is usually mild, and treatment is effective. Complications may occur due to high worm burden, comorbid conditions, or luminal obstruction. (Refer to the Complications section for more details).
Complications
Complications of diphyllobothriasis are due to the aberrant attachment of the worm scolex or heavy worm burden. These can cause intestinal obstruction, subacute appendicitis, cholecystitis, and cholangitis and require surgical intervention.[4]
Vitamin B12 deficiency commonly presents with fatigue and pallor due to megaloblastic anemia. In the setting of severe and prolonged vitamin B12 deficiency, heart failure and neurological manifestations may occur, leading to dyspnea, headaches, paresthesias, visual changes, or ataxia.
Malabsorption may be more consequential in patients with pre-existing malabsorption (eg, in HIV) or where food intake is already suboptimal.[4]
Deterrence and Patient Education
Individuals can avoid becoming infected with Diphyllobothriidae by consuming only well-cooked or previously deep-frozen fish. Undercooked, raw, pickled, smoked, or dried fish that have not been deep-frozen should be avoided. Fish mixtures such as gefilte fish should not be sampled before thoroughly cooking.[38] The CDC recommends cooking fish to an internal temperature of at least 145 °F (63 °C) or deep-freezing at -4 °F (-20 °C) or below for 7 days.[CDC. Parasites - Diphyllobothrium Infection - FAQs. 2020] Brining in 12% NaCl also kills plerocercoid larvae.[5] See the Enhancing Healthcare Team Outcomes section below for more information on commercial freezing of fish.
Pearls and Other Issues
Key facts to keep in mind about diphyllobothriasis are as follows:
- Diphyllobothriasis is an intestinal zoonotic infection caused by fish tapeworms of the Diphyllobothriidae family.
- A high index of suspicion and a thorough patient history lead to the diagnosis, asking about occupational history, hobbies, travel history, and eating habits.
- Most people infected with diphyllobothriids are asymptomatic, but others present with a mild illness characterized by abdominal pain or discomfort, diarrhea, and fatigue or by the expulsion of proglottids.
- Laboratory testing is most commonly normal, although vitamin B12 deficiency and, less commonly, eosinophilia or megaloblastic anemia may be found.
- Treatment is with a single dose of praziquantel for children and adults, including during pregnancy.
- Individuals can prevent infection by avoiding the consumption of raw, pickled, smoked, or dried fish that has not been previously deep-frozen.
- Control of diphyllobothriasis requires a cross-disciplinary approach across professionals from multiple disciplines, such as healthcare, engineering, parasitology, and food harvesting, processing, and transportation.
Enhancing Healthcare Team Outcomes
A team-based approach is required to provide comprehensive patient-centered care and prevent the spread and acquisition of Diphyllobothriidae infections at a population level. Control of diphyllobothriids in wild fish is impossible, so prevention must be implemented during fish storage, processing, and preparation.[39]
Clinicians, nurse practitioners, nurses with specialty training in infectious diseases, and pharmacists should be aware of the re-emergence of diphyllobothriasis to ensure prompt patient diagnosis, treatment, and understanding of food safety measures to prevent repeat infection.
Public education by public health and food safety agencies regarding consuming raw, pickled, smoked, or dried fish that have not been deep-frozen is essential to reduce the risk of infection. This should be directed mainly towards travelers and locals in endemic areas and consumers more broadly due to the increased popularity of raw or undercooked fish dishes and the globalization of supply chains.
Public Health authorities and sanitation engineers must address water contamination worldwide, as diphyllobothriids are easily spread in regions with poor sanitary infrastructure. Ineffective sewage treatment can play a major role in maintaining broad tapeworm populations, of which humans are the definitive host.[38][40] Biologists, epidemiologists, and public health researchers can assist in anticipating the effect of climate change on parasites.
Adequate freezing and parasitologic screening of fish before exportation could help prevent the global spread of Diphyllobothriidae species.[7] Fish needs to be frozen until solid and kept at a very low temperature for a minimum time to kill the plerocercoids - lower temperatures allow for shorter freezing times.[CDC. Parasites - Diphyllobothrium Infection. 2020]
- At -4 °F (-20 °C) or below for 7 days (total time), or
- At -31°F (-35 °C) or below until solid, and storing at -31 °F (-35 °C) or below for 15 hours, or
- At -31°F (-35 °C) or below until solid and storing at -4 °F (-20 °C) or below for 24 hours.
Simply putting fish on ice is inadequate.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Scholz T, Kuchta R, Brabec J. Broad tapeworms (Diphyllobothriidae), parasites of wildlife and humans: Recent progress and future challenges. International journal for parasitology. Parasites and wildlife. 2019 Aug:9():359-369. doi: 10.1016/j.ijppaw.2019.02.001. Epub 2019 Apr 1 [PubMed PMID: 31341771]
Waeschenbach A, Brabec J, Scholz T, Littlewood DTJ, Kuchta R. The catholic taste of broad tapeworms - multiple routes to human infection. International journal for parasitology. 2017 Nov:47(13):831-843. doi: 10.1016/j.ijpara.2017.06.004. Epub 2017 Aug 3 [PubMed PMID: 28780153]
Ito A, Budke CM. Culinary delights and travel? A review of zoonotic cestodiases and metacestodiases. Travel medicine and infectious disease. 2014 Nov-Dec:12(6 Pt A):582-91. doi: 10.1016/j.tmaid.2014.06.009. Epub 2014 Jul 15 [PubMed PMID: 25069407]
Level 3 (low-level) evidenceSharma K, Wijarnpreecha K, Merrell N. Diphyllobothrium latum Mimicking Subacute Appendicitis. Gastroenterology research. 2018 Jun:11(3):235-237. doi: 10.14740/gr989w. Epub 2018 May 31 [PubMed PMID: 29915635]
Scholz T, Garcia HH, Kuchta R, Wicht B. Update on the human broad tapeworm (genus diphyllobothrium), including clinical relevance. Clinical microbiology reviews. 2009 Jan:22(1):146-60, Table of Contents. doi: 10.1128/CMR.00033-08. Epub [PubMed PMID: 19136438]
Level 3 (low-level) evidenceSTUNKARD HW. Diphyllobothrium stemmacephalum Cobbold, 1858 and D. latum (Linn., 1758). The Journal of parasitology. 1949 Dec:35(6):613-24 [PubMed PMID: 15394410]
Level 3 (low-level) evidenceKuchta R, Radačovská A, Bazsalovicsová E, Viozzi G, Semenas L, Arbetman M, Scholz T. Host Switching of Zoonotic Broad Fish Tapeworm (Dibothriocephalus latus) to Salmonids, Patagonia. Emerging infectious diseases. 2019 Nov:25(11):2156-2158. doi: 10.3201/eid2511.190792. Epub [PubMed PMID: 31625847]
Kuchta R, Serrano-Martínez ME, Scholz T. Pacific Broad Tapeworm Adenocephalus pacificus as a Causative Agent of Globally Reemerging Diphyllobothriosis. Emerging infectious diseases. 2015 Oct:21(10):1697-703 [PubMed PMID: 26402440]
Yoneva A, Kuchta R, Scholz T. First study of vitellogenesis of the broad fish tapeworm Diphyllobothrium latum (Cestoda, Diphyllobothriidea), a human parasite with extreme fecundity. Parasitology international. 2014 Dec:63(6):747-53. doi: 10.1016/j.parint.2014.07.002. Epub 2014 Jul 12 [PubMed PMID: 25025756]
Level 3 (low-level) evidenceLe Bailly M, Bouchet F. Diphyllobothrium in the past: Review and new records. International journal of paleopathology. 2013 Sep:3(3):182-187. doi: 10.1016/j.ijpp.2013.05.004. Epub 2013 Jun 17 [PubMed PMID: 29539453]
Ryan H, Flammer PG, Nicholson R, Loe L, Reeves B, Allison E, Guy C, Doriga IL, Waldron T, Walker D, Kirchhelle C, Larson G, Smith AL. Reconstructing the history of helminth prevalence in the UK. PLoS neglected tropical diseases. 2022 Apr:16(4):e0010312. doi: 10.1371/journal.pntd.0010312. Epub 2022 Apr 21 [PubMed PMID: 35446843]
Kozlov A, Vershubskaya G. The prevalence of helminthiases in North-Western Siberia rural indigenous and long-term resident people in 1988-89 and 2018-19. International journal of circumpolar health. 2021 Dec:80(1):1917270. doi: 10.1080/22423982.2021.1917270. Epub [PubMed PMID: 33899703]
Kuchta R, Brabec J, Kubáčková P, Scholz T. Tapeworm Diphyllobothrium dendriticum (Cestoda)--neglected or emerging human parasite? PLoS neglected tropical diseases. 2013:7(12):e2535. doi: 10.1371/journal.pntd.0002535. Epub 2013 Dec 26 [PubMed PMID: 24386497]
Level 3 (low-level) evidenceCai YC, Chen SH, Yamasaki H, Chen JX, Lu Y, Zhang YN, Li H, Ai L, Chen HN. Four Human Cases of Diphyllobothrium nihonkaiense (Eucestoda: Diphyllobothriidae) in China with a Brief Review of Chinese Cases. The Korean journal of parasitology. 2017 Jun:55(3):319-325. doi: 10.3347/kjp.2017.55.3.319. Epub 2017 Jun 30 [PubMed PMID: 28719957]
Level 3 (low-level) evidenceAndo Y, Ono Y, Ono S. Diphyllobothriasis from Eating Sushi. The American journal of tropical medicine and hygiene. 2021 Apr 12:104(6):1953-1954. doi: 10.4269/ajtmh.21-0105. Epub 2021 Apr 12 [PubMed PMID: 33844646]
Autier B, Belaz S, Degeilh B, Gangneux JP, Robert-Gangneux F. Dibothriocephalus nihonkaiensis: an emerging foodborne parasite in Brittany (France)? Parasites & vectors. 2019 May 28:12(1):267. doi: 10.1186/s13071-019-3516-6. Epub 2019 May 28 [PubMed PMID: 31138323]
Ikuno H, Akao S, Yamasaki H. Epidemiology of Diphyllobothrium nihonkaiense Diphyllobothriasis, Japan, 2001-2016. Emerging infectious diseases. 2018 Aug:24(8):1428-34. doi: 10.3201/eid2408.171454. Epub [PubMed PMID: 30016246]
Greigert V, Brunet J, Pfaff AW, Lemoine JP, Candolfi E, Abou-Bacar A. Locally acquired infection with Dibothriocephalus nihonkaiense (=Diphyllobothrium nihonkaiense) in France: the importance of molecular diagnosis. Parasitology research. 2020 Feb:119(2):513-518. doi: 10.1007/s00436-019-06566-6. Epub 2019 Dec 17 [PubMed PMID: 31848744]
Broglia A, Kapel C. Changing dietary habits in a changing world: emerging drivers for the transmission of foodborne parasitic zoonoses. Veterinary parasitology. 2011 Nov 24:182(1):2-13. doi: 10.1016/j.vetpar.2011.07.011. Epub 2011 Jul 14 [PubMed PMID: 21835548]
Level 3 (low-level) evidenceCabello FC. Salmon aquaculture and transmission of the fish tapeworm. Emerging infectious diseases. 2007 Jan:13(1):169-71 [PubMed PMID: 17370539]
Level 3 (low-level) evidenceSampaio JL, de Andrade VP, Lucas Mda C, Fung L, Gagliardi SM, Santos SR, Mendes CM, Eduardo MB, Dick T. Diphyllobothriasis, Brazil. Emerging infectious diseases. 2005 Oct:11(10):1598-600 [PubMed PMID: 16318703]
Level 3 (low-level) evidenceAn YC, Sung CC, Wang CC, Lin HC, Chen KY, Ku FM, Chen RM, Chen ML, Huang KY. Molecular Identification of Diphyllobothrium latum from a Pediatric Case in Taiwan. The Korean journal of parasitology. 2017 Aug:55(4):425-428. doi: 10.3347/kjp.2017.55.4.425. Epub 2017 Aug 31 [PubMed PMID: 28877575]
Level 3 (low-level) evidenceLee EB, Song JH, Park NS, Kang BK, Lee HS, Han YJ, Kim HJ, Shin EH, Chai JY. A case of Diphyllobothrium latum infection with a brief review of diphyllobothriasis in the Republic of Korea. The Korean journal of parasitology. 2007 Sep:45(3):219-23 [PubMed PMID: 17876168]
Level 3 (low-level) evidenceDezfuli BS, Pironi F, Simoni E, Shinn AP, Giari L. Selected pathological, immunohistochemical and ultrastructural changes associated with an infection by Diphyllobothrium dendriticum (Nitzsch, 1824) (Cestoda) plerocercoids in Coregonus lavaretus (L.) (Coregonidae). Journal of fish diseases. 2007 Aug:30(8):471-82 [PubMed PMID: 17640250]
Level 3 (low-level) evidenceLal S, Steinhart AH. Diphyllobothrium latum: a case of an incidental finding. World journal of gastroenterology. 2007 Mar 28:13(12):1875-6 [PubMed PMID: 17465485]
Level 3 (low-level) evidenceChoi HJ, Lee J, Yang HJ. Four human cases of Diphyllobothrium latum infection. The Korean journal of parasitology. 2012 Jun:50(2):143-6. doi: 10.3347/kjp.2012.50.2.143. Epub 2012 May 24 [PubMed PMID: 22711926]
Level 3 (low-level) evidenceTsuboi M, Hayakawa K, Yamasaki H, Katanami Y, Yamamoto K, Kutsuna S, Takeshita N, Kanagawa S, Ohmagari N, Kato Y. Clinical characteristics and epidemiology of intestinal tapeworm infections over the last decade in Tokyo, Japan: A retrospective review. PLoS neglected tropical diseases. 2018 Feb:12(2):e0006297. doi: 10.1371/journal.pntd.0006297. Epub 2018 Feb 20 [PubMed PMID: 29462133]
Level 2 (mid-level) evidenceBjörkenheim B. Optic neuropathy caused by vitamin-B12 deficiency in carriers of the fish tapeworm, Diphyllobothrium latum. Lancet (London, England). 1966 Mar 26:1(7439):688-90 [PubMed PMID: 4159602]
Level 3 (low-level) evidenceKitamoto H, Inoue S, Yamamoto S, Okamoto K, Inokuma T. Human diphyllobothriasis - Authors' reply. Lancet (London, England). 2020 Sep 12:396(10253):755-756. doi: 10.1016/S0140-6736(20)31178-8. Epub [PubMed PMID: 32919508]
Jimenez JA, Rodriguez S, Gamboa R, Rodriguez L, Garcia HH, Cysticercosis Working Group in Peru. Diphyllobothrium pacificum infection is seldom associated with megaloblastic anemia. The American journal of tropical medicine and hygiene. 2012 Nov:87(5):897-901. doi: 10.4269/ajtmh.2012.12-0067. Epub 2012 Sep 17 [PubMed PMID: 22987655]
Fang FC, Billman ZP, Wallis CK, Abbott AN, Olson JC, Dhanireddy S, Murphy SC. Human Diphyllobothrium nihonkaiense infection in Washington State. Journal of clinical microbiology. 2015 Apr:53(4):1355-7. doi: 10.1128/JCM.00065-15. Epub 2015 Jan 21 [PubMed PMID: 25609724]
Level 3 (low-level) evidenceKitamoto H, Inoue S, Okamoto K, Inokuma T. Scanning early catches the worm: abdominal ultrasound as a possible screening method for intestinal cestodes. Lancet (London, England). 2019 Oct 5:394(10205):1264. doi: 10.1016/S0140-6736(19)32132-4. Epub [PubMed PMID: 31591983]
Hase R, Mito H, Yano Y, Morishima Y, Hasegawa Y. Tapeworm (Diphyllobothrium nihonkaiense) detected by abdominal ultrasonography. The Brazilian journal of infectious diseases : an official publication of the Brazilian Society of Infectious Diseases. 2023 May-Jun:27(3):102773. doi: 10.1016/j.bjid.2023.102773. Epub 2023 May 5 [PubMed PMID: 37156469]
Maithel S, Duong AK, Zhang J, Nguyen DL. An unusual cause of vitamin B12 and iron deficiency. Cleveland Clinic journal of medicine. 2015 Jul:82(7):406-8. doi: 10.3949/ccjm.82a.14151. Epub [PubMed PMID: 26185938]
Craig P, Ito A. Intestinal cestodes. Current opinion in infectious diseases. 2007 Oct:20(5):524-32 [PubMed PMID: 17762788]
Level 3 (low-level) evidenceLloyd AE, Honey BL, John BM, Condren M. Treatment Options and Considerations for Intestinal Helminthic Infections. The Journal of pharmacy technology : jPT : official publication of the Association of Pharmacy Technicians. 2014 Aug:30(4):130-139. doi: 10.1177/8755122514533667. Epub 2014 Apr 28 [PubMed PMID: 34860931]
Groll E. Praziquantel for cestode infections in man. Acta tropica. 1980 Sep:37(3):293-6 [PubMed PMID: 6106371]
Menconi V, Zoppi S, Pastorino P, Di Blasio A, Tedeschi R, Pizzul E, Mugetti D, Tomasoni M, Dondo A, Prearo M. Relationship between the prevalence of Dibothriocephalus latus (Cestoda: Diphyllobothriidea) and the load of Escherichia coli: New findings in a neglected fish-borne parasitic zoonosis. Zoonoses and public health. 2021 Dec:68(8):965-972. doi: 10.1111/zph.12891. Epub 2021 Sep 6 [PubMed PMID: 34486231]
Cantatore DMP, Lanfranchi AL, Canel D, Levy E, Timi JT. Plerocercoids of Adenocephalus pacificus in Argentine hakes: Broad distribution, low zoonotic risk. International journal of food microbiology. 2023 Apr 16:391-393():110142. doi: 10.1016/j.ijfoodmicro.2023.110142. Epub 2023 Feb 22 [PubMed PMID: 36841077]
Menconi V, Pastorino P, Momo I, Mugetti D, Bona MC, Levetti S, Tomasoni M, Pizzul E, Ru G, Dondo A, Prearo M. Occurrence and Spatial Distribution of Dibothriocephalus Latus (Cestoda: Diphyllobothriidea) in Lake Iseo (Northern Italy): An Update. International journal of environmental research and public health. 2020 Jul 14:17(14):. doi: 10.3390/ijerph17145070. Epub 2020 Jul 14 [PubMed PMID: 32674519]