Introduction
Advanced magnetic resonance (MR) neuroimaging modalities are becoming more available and useful as their value in the diagnosis and prognosis of central nervous system diseases is more fully studied and understood. Specifically, diffusion tensor imaging (DTI) has become increasingly studied and utilized in recent years. It has become incorporated by many radiologists into routine clinical practice, with most research performed on traumatic brain injury (TBI). DTI is a variant of diffusion-weighted imaging (DWI) that utilizes a tissue water diffusion rate for image production. The first application of DWI to the human brain was performed in 1986 and since has become the gold standard for detecting acute stroke.[1] DTI does not require contrast and is available on almost all modern MR scanners with relatively quick scan times for this sequence.[2]
Random thermal motion, or Brownian motion, is water molecular diffusion in three-dimensional (3D) space. Isotropy is defined as uniformity in all directions, and when applied to water molecules, isotropy occurs when the diffusion of water is entirely uninhibited (such as water movement in a glass of water). Anisotropy is when there is a directionality in the diffusion of water present, and the movement of water is no longer random (such as water movement along straws placed in a glass). The greater the anisotropy, the more directional and linear the diffusion of water molecules. Water molecules will diffuse differently through space depending on the tissue type, components, structure, architecture, and integrity; these principles allow clinically significant imaging to occur, particularly the DTI. The latter measures the movement of water along axons, analogous to the straws in a glass of water.
As early as May 2002, medical literature reported that DTI showed abnormalities in patients who suffered from mild brain trauma as compared to normal control subjects. "This study included five patients with mild traumatic brain injury (three men and two women) and ten volunteers with no known neurological disorders (five men and five women)." This study reported abnormalities in the patients with a mild brain injury that were absent in the control subjects or the uninvolved sides of the injured patients' brains: "Patients displayed a significant reduction of diffusion anisotropy in several regions compared with the homologous ones in the contralateral hemisphere. Such differences were not observed in the control subjects. Significant reduction of diffusion anisotropy was also detected when diffusion tensor results from the patients were compared with those of the controls."[3]
DWI uses volume elements (voxels) as a statistical method for data collection. When a voxel contains scalar values constituting a vector, it is known as a tensor, where DTI receives its name and explains the additional information provided through DTI.[4] DTI MR settings can measure the diffusion of water along an axon in many directions; 6, 9, 33, and 90 directions are typical parameters used, with 33 directions and above increasing confidence in the accuracy. Ninety directions typically require upwards of 20 additional minutes in the MR scanner. Therefore, it may not be suitable for routine clinical practice. In effect, DTI will provide an indirect method of assessing neuroanatomy structure on a microscopic level using water molecules' degree of anisotropy and structural orientation within a voxel. Therefore, the principal application for DTI is in the imaging of white matter, where the orientation, location, and anisotropy of the tracts can be measured and evaluated. The architecture of the axons in parallel bundles and their myelin sheaths facilitate the diffusion of the water molecules preferentially along their main direction.
There are several measures calculated using DTI that can provide quantitative power. One of the most widely used DTI measures is fractional anisotropy (FA).[5] Others include mean diffusivity or apparent diffusion coefficient (ADC), radial (perpendicular) diffusivity, and axial (parallel) diffusivity. DTI uses mean diffusivity for the rate of molecular diffusion, FA for the summative direction of the diffusion, which provides a prominent vector, axial diffusivity for the rate of diffusion parallel to the main vector, and radial diffusivity for the rate of diffusion perpendicular to the main vector.
FA quantifies the directionality of diffusivity in a summative manner and is highly sensitive to change in microstructure; however, it can be nonspecific to the cause of change. Mean diffusivity quantifies cellular and membrane density, whereas an increase in mean diffusivity indicates disease processes such as edema or necrosis. Radial diffusivity quantifies myelin neuropathology and increases with demyelination. Axial diffusivity quantifies axonal degeneration and increases with brain maturation.[6][7]
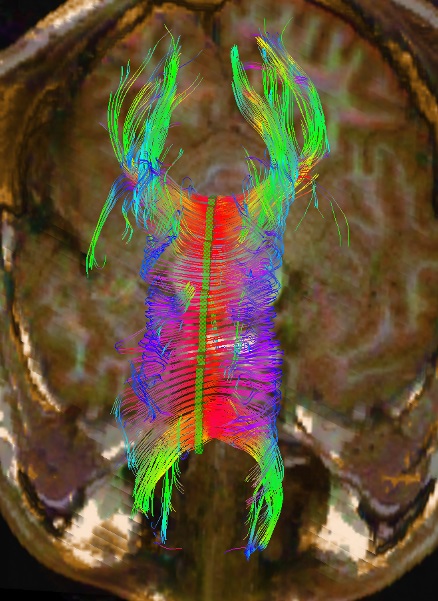
FA values are numerical values based on the anisotropy of water along the axon, which reflects the health of the axon. Abnormal FA values indicate axonal damage. FA values can be calculated utilizing the region of interest (ROI) method, whole-brain analysis (Voxel-Based analysis), or tract-based spatial statistics. The whole-brain analysis is gaining popularity due to its automation and ability to analyze more tracts. The ROI method, where the regions to be analyzed are traced by a technologist and then analyzed by a computer, remains reliable and replicable.[8][9][10][11] One of the more common and standardized ROI methods is the segmented corpus callosal values.[12][13][14] Being the largest axonal tract in the brain, damage to the corpus callosum is well described following head trauma and other pathologies (see Image. DTI of Corpus Callosum).[15]
FA values can vary depending on which of the above 3 analyzing methods is used and other factors such as MR technique and type of post-processing performed.[5] Utilizing a standardized technique, FA values are highly reproducible and not technologist-dependent. They can be subjectively interpreted by a radiologist and roughly compared to select values in the literature.
Pediatric normal values are slightly less than those of adults. However, most changes occur by age 5, and 90% of adult FA values are achieved by 11 years of age in the corpus callosum.[16] After adulthood, FA values tend to decrease with age linearly.
Additionally, FA values comparison across different scanners is now possible, even if those scanners utilize different techniques. This is achieved using 'human phantom phenomena' where a single subject is scanned on 2 different scanners, enabling a comparison between scanners by a scaling factor or even to normative databases performed on a different scanner(s).[17][18]
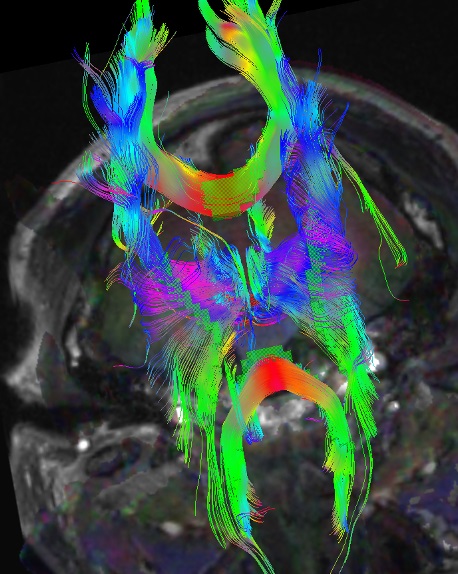
Three-dimensional reconstructions of the tensor tracts are accomplished with computer modeling and can beautifully illustrate the fiber tracts identify pathology, and aid neurosurgeons (see Image. 3D Reconstructed DTI Image of Patient with Bilateral Post-Traumatic Frontal Contusions Compared to a Normal 3D DTI).
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
DTI is generally sensitive but has a lower specificity. Specificity can be increased by combining the interpretation of DTI with the patient's clinical history and the other findings on conventional imaging.
Artifacts and Noise
Artifacts are seen in all imaging modalities, including submodalities of magnetic resonance imaging (MRI), and have many causes. Artifacts can result in misinterpretation of imaging findings if the radiologist is unaware of these artifacts. With a thorough understanding of artifacts associated with DTI, measures should be taken to decrease the artifacts and to become more aware of unavoidable artifacts.
A limitation of DTI is that it currently has a low signal-to-noise ratio (SNR), which may increase scanning times. SNR compares the level of background noise to the level of the signal obtained. When the noise is too great compared to the signal (low SNR), image quality is poor. Two ways to improve this signal-to-noise ratio are to either increase scan times or reduce image resolution.[19] Long scan times can result in patient motion artifacts, which also can cause artifacts.
Voxels continue to decrease in size, but a current limitation in DTI is the size of voxels. Low anisotropy shown in a particular voxel may seem to represent unorganized tissue contained within. However, this may just be apparent, and what actually lies in the specific voxel is multiple anisotropic structures orientated in multiple directions, resulting in isotropy. This means that diffusion anisotropy will only show on DTI when all structures coursing a voxel align on a microscopic (microstructure, myelin sheath, protein filaments) and macroscopic (axons and dendrites) scale. In the cortex, low FA occurs because of macroscopic disorganization. As image resolution improves, the cortex will likely show higher patterns of anisotropy.
Other causes resulting in artifacts can include eddy currents and motion. Changing data acquisition methods can help decrease these artifacts.[20]
Acceptance
DTI has now moved beyond investigational research and is becoming incorporated into routine clinical practice. However, some have urged caution in interpreting DTI and other advanced imaging sequences at the individual level, including a 2018 Radiological Society of North America (RSNA) guideline statement.[21][22] A minority of studies have not found a relationship between DTI and mild TBI, although the majority of the literature is supportive.[23] Given the different techniques for performing DTI and the wide variety of applications, this caution is well-taken. However, if the interpreting radiologist has experience in DTI and has developed a standardized, replicable technique concordant with the literature, particularly if a trauma database or normative database is available, these valid criticisms become moot, and DTI can be interpreted in individual patients. This approach has backing in the literature, and studies performed on individual patients demonstrate reproducibility and accuracy in diagnosing individual patients with TBI.[24][25][26] Specifically, DTI is most powerful when interpreted in concert with the clinical history and other findings on conventional imaging. The most comprehensive peer-review article on TBI and DTI concluded the following:
"Despite significant variability in sample characteristics, technical aspects of imaging, and analysis approaches, the consensus is that DTI effectively differentiates patients with TBI and controls, regardless of the severity and timeframe following injury. Furthermore, many have established a relationship between DTI measures and TBI outcomes."[5]
DTI has proven to be highly sensitive to brain injury and a variety of pathological conditions and, in many instances, is becoming a routine part of clinical practice. Further research will continue to expand the application of DTI in evaluating neurological conditions.
Clinical Significance
Although much of the research on DTI has been in TBI, other applications include aid in diagnosis, prognosis, and classifications of stroke, brain tumors, neurodegenerative diseases, developmental disorders, neuropsychiatric disorders, movement disorders, and neurogenetic developmental disorders.[27] The following topics are a few examples of described uses of DTI.
TBI/Diffuse Axonal Injury
Diffuse axonal injury (DAI) can be seen in TBI. Diffuse axonal injury is diffuse brain injury damage to axonal microstructures and can potentially appear normal on traditional computed tomography (CT) and MRI when there is an absence of macroscopic tissue disruption. Additionally, if there are macroscopic findings of DAI on CT or MRI, it indicates poorer outcomes. A predominance of published literature on DTI is about diffuse axonal injury and its close correlation with decreased FA values throughout specified regions of the brain. The clinical effectiveness of DTI has been compared to control subjects for validation in various scenarios involving MRI to help diagnose a brain injury. There are numerous peer-reviewed and case-control studies in the medical literature allowing for individual evaluations of brain-injured patients using DTI. The comparison of cases (patients with a history of TBI) and controls (no history of TBI) utilizing DTI is an accepted methodology and standard technique utilized to demonstrate the clinical utility of DTI in adding incremental diagnostic information to structural MRI, multimodal MR studies, other imaging modalities, and the clinical condition.[28][29][30][31][23] Additionally, peer-reviewed articles on DTI and TBI have demonstrated regional associations between findings on conventional imaging and FA values and 3D DTI.[32]
Carbon Monoxide Poisoning
DTI has utility for studying the brains of patients after carbon monoxide (CO) poisoning.[33] The peer-reviewed literature supports the use of DTI for identifying and longitudinally monitoring the changes in the brain that can occur secondary to CO poisoning.[34] DTI has been shown to demonstrate the evolution of white matter injury in CO encephalopathy progression over months and to correlate with neuropsychological deficits.[35]
DTI has also demonstrated evidence of demyelination with abnormal FA values in the central nervous system of patients who develop chronic neurological symptoms in the subacute phase after CO intoxication.[36] A decrease in FA values correlates with clinically observed neurological symptoms, and conversely, improvement in FA values may parallel improvement in the neurological condition.[37]
Hypoxic Brain Injury
A recent 2018 multicenter review article demonstrated that DTI might be more predictive of neurologic outcomes than conventional MRI in patients with cardiac arrest and hypoxic brain injury.[38]
Demyelination/Dysmyelination
As the brain develops, diffusivity declines, particularly in the direction myelination would be oriented. Damage to the myelin sheath affects diffusivity.[39] DTI has been used in multiple sclerosis to monitor disease progression and correlate with clinical status.[40] Suggestions are that radial diffusivity is a promising biomarker.[41]
Alzheimer disease
White matter diffusion alteration, measured by DTI in the hippocampal cingulum, correlates with tau pathology and may be a promising biomarker in preclinical Alzheimer disease.[42]
Neoplasia
The application of DTI in neoplasia is used mainly for surgical planning. With DTI and tractography, a more in-depth layout of neuroanatomy and specific eloquent white matter tracts can be seen, allowing a neurosurgeon to plan precise surgical approaches to minimize damage to the critical tracts and preserve vital functions such as motor capabilities, language, and vision. DTI has even been used intraoperatively to compensate for shifting tissue to minimize complications.
Another application of DTI in neoplasia is using FA values. Correlations have been found between FA values and cell density. In neoplasia with high cell proliferation and density, such as glioblastomas, higher FA values have been reported.
Some neoplastic disease requires radiation, and DTI has found that FA decreases in areas targeted by radiation. Observing the changes of FA in the anatomy can potentially be used to monitor the dose distribution.
A deep convolutional neural network (deep learning) is being used in classifying the grades of gliomas based on their brain white matter networks derived from DTI.[43]
Epilepsy
The addition of the DTI sequence to the routine MRI protocol is advantageous in specific pediatric patient groups with temporal lobe epilepsy or for the planning of epilepsy surgery.[44]
Fetus/Neonate Imaging
A relatively new limb of DTI research shows correlations between DTI findings and developmental neuropathology in a fetus or neonate. Discovering the utility of DTI in fetuses and neonates has been hopeful for patients with limited diagnostic imaging options.[45]
Extracranial DTI Applications
Additional DTI applications include imaging of peripheral nerves, including the brachial plexus.[46] Spinal cord DTI imaging has also demonstrated promising results.[47]
White Matter Tractography and Future Research
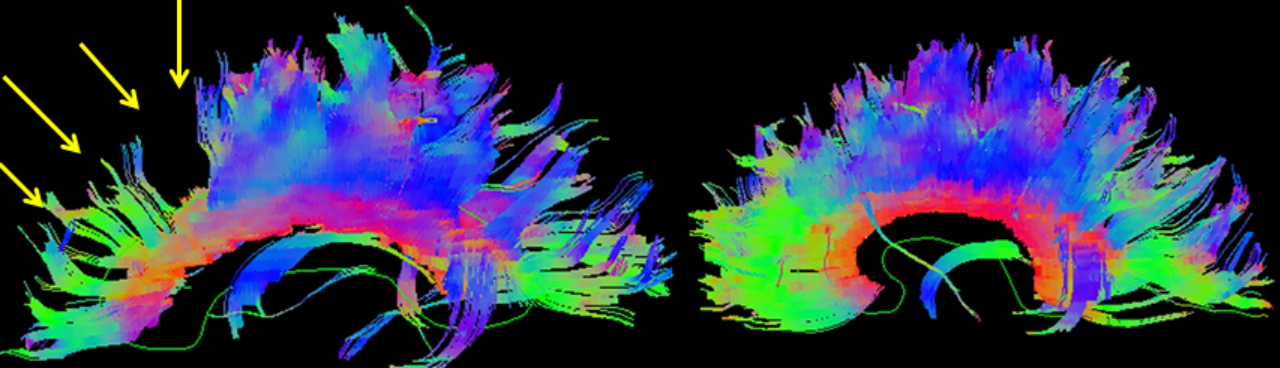
Using the assumption that diffusion anisotropy represents white matter bundles, these bundles of aligned voxels can be parcellated, providing a 3D map of white matter bundle tracts. Fiber tracking or reconstruction by starting with a voxel and propagating forward following the anisotropic path until encountering isotropy is also known as tractography and can give insight into brain connectivity (see Image. Brain Tractography). Further study and mapping of these tracts, in conjunction with functional MRI, resting state, and other MRI submodalities, remain topics of exciting cutting-edge research.[48] DTI remains in the research stage regarding neuropsychological conditions such as autism and ADHD.[49][50]
Enhancing Healthcare Team Outcomes
DTI is a specialized diagnostic imaging tool utilizing diffusion-weighted imaging of MRI. Using diffusion tensor imaging (DTI) necessitates a multifaceted approach involving various healthcare professionals, each contributing their unique skills and strategies to enhance patient-centered care, improve outcomes, ensure patient safety, and optimize interprofessional team performance. Physicians, advanced care practitioners, nurses, pharmacists, and other healthcare professionals involved in DTI must possess solid knowledge and skills in neuroimaging techniques, ensuring accurate data acquisition, analysis, and interpretation. Strategic planning is crucial in DTI, involving selecting appropriate imaging protocols and considering the clinical context of each patient, optimizing the diagnostic process and treatment decisions.
Furthermore, responsibilities extend to quality control, minimizing the risk of misdiagnosis and patient harm, and ensuring that findings are accurately reported. Interprofessional communication is essential for sharing critical information, discussing complex cases, and collectively deciding the best course of action. Effective care coordination in DTI involves seamless integration of findings into the patient's overall healthcare plan, ensuring that treatment aligns with the diagnostic results. By fostering collaboration among healthcare professionals, DTI can yield patient-centered care that not only maximizes the potential for positive outcomes but also prioritizes patient safety and team performance, making it a powerful tool in modern medicine.
Future research will focus on expanding DTI's clinical utility by exploring its potential in emerging fields such as personalized medicine, neurorehabilitation, and early disease detection. Collaborations between multidisciplinary teams, including neuroscientists, radiologists, computer scientists, and clinicians, will be instrumental in pushing the boundaries of DTI and translating these advancements into real-world healthcare solutions, ultimately improving our understanding of the brain's intricacies and its implications for optimal health.
Media
(Click Image to Enlarge)
3D reconstructed DTI image on the left demonstrates marked bilateral frontal axonal thinning and fiber tract gaps in this patient with bilateral post-traumatic frontal contusions on conventional imaging. The image on right is a normal 3D DTI for comparison. Travis Snyder DO, SimonMed Centers Las Vegas NV
References
Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986 Nov:161(2):401-7 [PubMed PMID: 3763909]
Soares JM, Marques P, Alves V, Sousa N. A hitchhiker's guide to diffusion tensor imaging. Frontiers in neuroscience. 2013:7():31. doi: 10.3389/fnins.2013.00031. Epub 2013 Mar 12 [PubMed PMID: 23486659]
Arfanakis K, Cordes D, Haughton VM, Carew JD, Meyerand ME. Independent component analysis applied to diffusion tensor MRI. Magnetic resonance in medicine. 2002 Feb:47(2):354-63 [PubMed PMID: 11810680]
Thaler HT, Ferber PW, Rottenberg DA. A statistical method for determining the proportions of gray matter, white matter, and CSF using computed tomography. Neuroradiology. 1978:16():133-5 [PubMed PMID: 740152]
Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR. American journal of neuroradiology. 2013 Nov-Dec:34(11):2064-74. doi: 10.3174/ajnr.A3395. Epub 2013 Jan 10 [PubMed PMID: 23306011]
Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. Journal of magnetic resonance imaging : JMRI. 2001 Apr:13(4):534-46 [PubMed PMID: 11276097]
Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2007 Jul:4(3):316-29 [PubMed PMID: 17599699]
Oh JS, Suk Park K, Chan Song I, Ju Kim S, Hwang J, Chung A, Kyoon Lyoo I. Fractional anisotropy-based divisions of midsagittal corpus callosum. Neuroreport. 2005 Mar 15:16(4):317-20 [PubMed PMID: 15729129]
Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006 Sep:32(3):989-94 [PubMed PMID: 16854598]
Kim EY, Park HJ, Kim DH, Lee SK, Kim J. Measuring fractional anisotropy of the corpus callosum using diffusion tensor imaging: mid-sagittal versus axial imaging planes. Korean journal of radiology. 2008 Sep-Oct:9(5):391-5. doi: 10.3348/kjr.2008.9.5.391. Epub [PubMed PMID: 18838846]
Rutgers DR, Fillard P, Paradot G, Tadié M, Lasjaunias P, Ducreux D. Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. AJNR. American journal of neuroradiology. 2008 Oct:29(9):1730-5. doi: 10.3174/ajnr.A1213. Epub 2008 Jul 10 [PubMed PMID: 18617586]
Li Z, Li C, Fan L, Jiang G, Wu J, Jiang T, Yin X, Wang J. Altered microstructure rather than morphology in the corpus callosum after lower limb amputation. Scientific reports. 2017 Mar 17:7():44780. doi: 10.1038/srep44780. Epub 2017 Mar 17 [PubMed PMID: 28303959]
Rimkus Cde M, Junqueira Tde F, Callegaro D, Otaduy MC, Leite Cda C. Segmented corpus callosum diffusivity correlates with the Expanded Disability Status Scale score in the early stages of relapsing-remitting multiple sclerosis. Clinics (Sao Paulo, Brazil). 2013:68(8):1115-20. doi: 10.6061/clinics/2013(08)09. Epub [PubMed PMID: 24037007]
Level 2 (mid-level) evidenceLövdén M, Bodammer NC, Kühn S, Kaufmann J, Schütze H, Tempelmann C, Heinze HJ, Düzel E, Schmiedek F, Lindenberger U. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia. 2010 Nov:48(13):3878-83. doi: 10.1016/j.neuropsychologia.2010.08.026. Epub 2010 Sep 15 [PubMed PMID: 20816877]
Parizel PM, Ozsarlak, Van Goethem JW, van den Hauwe L, Dillen C, Verlooy J, Cosyns P, De Schepper AM. Imaging findings in diffuse axonal injury after closed head trauma. European radiology. 1998:8(6):960-5 [PubMed PMID: 9683701]
Feldman HM, Yeatman JD, Lee ES, Barde LH, Gaman-Bean S. Diffusion tensor imaging: a review for pediatric researchers and clinicians. Journal of developmental and behavioral pediatrics : JDBP. 2010 May:31(4):346-56. doi: 10.1097/DBP.0b013e3181dcaa8b. Epub [PubMed PMID: 20453582]
Palacios EM, Martin AJ, Boss MA, Ezekiel F, Chang YS, Yuh EL, Vassar MJ, Schnyer DM, MacDonald CL, Crawford KL, Irimia A, Toga AW, Mukherjee P, TRACK-TBI Investigators. Toward Precision and Reproducibility of Diffusion Tensor Imaging: A Multicenter Diffusion Phantom and Traveling Volunteer Study. AJNR. American journal of neuroradiology. 2017 Mar:38(3):537-545. doi: 10.3174/ajnr.A5025. Epub 2016 Dec 22 [PubMed PMID: 28007768]
Venkatraman VK, Gonzalez CE, Landman B, Goh J, Reiter DA, An Y, Resnick SM. Region of interest correction factors improve reliability of diffusion imaging measures within and across scanners and field strengths. NeuroImage. 2015 Oct 1:119():406-16. doi: 10.1016/j.neuroimage.2015.06.078. Epub 2015 Jul 2 [PubMed PMID: 26146196]
Farrell JA, Landman BA, Jones CK, Smith SA, Prince JL, van Zijl PC, Mori S. Effects of signal-to-noise ratio on the accuracy and reproducibility of diffusion tensor imaging-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5 T. Journal of magnetic resonance imaging : JMRI. 2007 Sep:26(3):756-67 [PubMed PMID: 17729339]
Rohde GK, Barnett AS, Basser PJ, Marenco S, Pierpaoli C. Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magnetic resonance in medicine. 2004 Jan:51(1):103-14 [PubMed PMID: 14705050]
Meltzer CC, Sze G, Rommelfanger KS, Kinlaw K, Banja JD, Wolpe PR. Guidelines for the ethical use of neuroimages in medical testimony: report of a multidisciplinary consensus conference. AJNR. American journal of neuroradiology. 2014 Apr:35(4):632-7. doi: 10.3174/ajnr.A3711. Epub 2013 Aug 29 [PubMed PMID: 23988754]
Level 3 (low-level) evidenceWintermark M, Sanelli PC, Anzai Y, Tsiouris AJ, Whitlow CT, American College of Radiology Head Injury Institute. Imaging evidence and recommendations for traumatic brain injury: advanced neuro- and neurovascular imaging techniques. AJNR. American journal of neuroradiology. 2015 Feb:36(2):E1-E11. doi: 10.3174/ajnr.A4181. Epub 2014 Nov 25 [PubMed PMID: 25424870]
Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, Snyder AZ, Raichle ME, Witherow JR, Fang R, Flaherty SF, Brody DL. Detection of blast-related traumatic brain injury in U.S. military personnel. The New England journal of medicine. 2011 Jun 2:364(22):2091-100. doi: 10.1056/NEJMoa1008069. Epub [PubMed PMID: 21631321]
Level 2 (mid-level) evidenceBouix S, Pasternak O, Rathi Y, Pelavin PE, Zafonte R, Shenton ME. Increased gray matter diffusion anisotropy in patients with persistent post-concussive symptoms following mild traumatic brain injury. PloS one. 2013:8(6):e66205. doi: 10.1371/journal.pone.0066205. Epub 2013 Jun 11 [PubMed PMID: 23776631]
Kim N, Branch CA, Kim M, Lipton ML. Whole brain approaches for identification of microstructural abnormalities in individual patients: comparison of techniques applied to mild traumatic brain injury. PloS one. 2013:8(3):e59382. doi: 10.1371/journal.pone.0059382. Epub 2013 Mar 26 [PubMed PMID: 23555665]
Level 2 (mid-level) evidenceLipton ML, Kim N, Park YK, Hulkower MB, Gardin TM, Shifteh K, Kim M, Zimmerman ME, Lipton RB, Branch CA. Robust detection of traumatic axonal injury in individual mild traumatic brain injury patients: intersubject variation, change over time and bidirectional changes in anisotropy. Brain imaging and behavior. 2012 Jun:6(2):329-42. doi: 10.1007/s11682-012-9175-2. Epub [PubMed PMID: 22684769]
Tae WS, Ham BJ, Pyun SB, Kang SH, Kim BJ. Current Clinical Applications of Diffusion-Tensor Imaging in Neurological Disorders. Journal of clinical neurology (Seoul, Korea). 2018 Apr:14(2):129-140. doi: 10.3988/jcn.2018.14.2.129. Epub 2018 Feb 28 [PubMed PMID: 29504292]
Yuh EL, Cooper SR, Mukherjee P, Yue JK, Lingsma HF, Gordon WA, Valadka AB, Okonkwo DO, Schnyer DM, Vassar MJ, Maas AI, Manley GT, TRACK-TBI INVESTIGATORS. Diffusion tensor imaging for outcome prediction in mild traumatic brain injury: a TRACK-TBI study. Journal of neurotrauma. 2014 Sep 1:31(17):1457-77. doi: 10.1089/neu.2013.3171. Epub 2014 Jul 9 [PubMed PMID: 24742275]
Aoki Y, Inokuchi R. A voxel-based meta-analysis of diffusion tensor imaging in mild traumatic brain injury. Neuroscience and biobehavioral reviews. 2016 Jul:66():119-26. doi: 10.1016/j.neubiorev.2016.04.021. Epub 2016 Apr 28 [PubMed PMID: 27133211]
Level 1 (high-level) evidenceEwing-Cobbs L, Johnson CP, Juranek J, DeMaster D, Prasad M, Duque G, Kramer L, Cox CS, Swank PR. Longitudinal diffusion tensor imaging after pediatric traumatic brain injury: Impact of age at injury and time since injury on pathway integrity. Human brain mapping. 2016 Nov:37(11):3929-3945. doi: 10.1002/hbm.23286. Epub [PubMed PMID: 27329317]
Ware JB, Biester RC, Whipple E, Robinson KM, Ross RJ, Nucifora PG. Combat-related Mild Traumatic Brain Injury: Association between Baseline Diffusion-Tensor Imaging Findings and Long-term Outcomes. Radiology. 2016 Jul:280(1):212-9. doi: 10.1148/radiol.2016151013. Epub 2016 Mar 29 [PubMed PMID: 27022770]
Kou Z, Wu Z, Tong KA, Holshouser B, Benson RR, Hu J, Haacke EM. The role of advanced MR imaging findings as biomarkers of traumatic brain injury. The Journal of head trauma rehabilitation. 2010 Jul-Aug:25(4):267-82. doi: 10.1097/HTR.0b013e3181e54793. Epub [PubMed PMID: 20611045]
Xu J, Rasmussen IA, Lagopoulos J, Håberg A. Diffuse axonal injury in severe traumatic brain injury visualized using high-resolution diffusion tensor imaging. Journal of neurotrauma. 2007 May:24(5):753-65 [PubMed PMID: 17518531]
Terajima K, Igarashi H, Hirose M, Matsuzawa H, Nishizawa M, Nakada T. Serial assessments of delayed encephalopathy after carbon monoxide poisoning using magnetic resonance spectroscopy and diffusion tensor imaging on 3.0T system. European neurology. 2008:59(1-2):55-61 [PubMed PMID: 17917459]
Level 3 (low-level) evidenceChang CC, Chang WN, Lui CC, Wang JJ, Chen CF, Lee YC, Chen SS, Lin YT, Huang CW, Chen C. Longitudinal study of carbon monoxide intoxication by diffusion tensor imaging with neuropsychiatric correlation. Journal of psychiatry & neuroscience : JPN. 2010 Mar:35(2):115-25 [PubMed PMID: 20184809]
Beppu T, Nishimoto H, Ishigaki D, Fujiwara S, Yoshida T, Oikawa H, Kamada K, Sasaki M, Ogasawara K. Assessment of damage to cerebral white matter fiber in the subacute phase after carbon monoxide poisoning using fractional anisotropy in diffusion tensor imaging. Neuroradiology. 2010 Aug:52(8):735-43. doi: 10.1007/s00234-009-0649-x. Epub 2010 Jan 12 [PubMed PMID: 20066405]
Beppu T. The role of MR imaging in assessment of brain damage from carbon monoxide poisoning: a review of the literature. AJNR. American journal of neuroradiology. 2014 Apr:35(4):625-31. doi: 10.3174/ajnr.A3489. Epub 2013 Apr 18 [PubMed PMID: 23598831]
Velly L, Perlbarg V, Boulier T, Adam N, Delphine S, Luyt CE, Battisti V, Torkomian G, Arbelot C, Chabanne R, Jean B, Di Perri C, Laureys S, Citerio G, Vargiolu A, Rohaut B, Bruder N, Girard N, Silva S, Cottenceau V, Tourdias T, Coulon O, Riou B, Naccache L, Gupta R, Benali H, Galanaud D, Puybasset L, MRI-COMA Investigators. Use of brain diffusion tensor imaging for the prediction of long-term neurological outcomes in patients after cardiac arrest: a multicentre, international, prospective, observational, cohort study. The Lancet. Neurology. 2018 Apr:17(4):317-326. doi: 10.1016/S1474-4422(18)30027-9. Epub 2018 Feb 27 [PubMed PMID: 29500154]
Aung WY, Mar S, Benzinger TL. Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging in medicine. 2013 Oct 1:5(5):427-440 [PubMed PMID: 24795779]
Sbardella E, Tona F, Petsas N, Pantano P. DTI Measurements in Multiple Sclerosis: Evaluation of Brain Damage and Clinical Implications. Multiple sclerosis international. 2013:2013():671730. doi: 10.1155/2013/671730. Epub 2013 Mar 31 [PubMed PMID: 23606965]
Klistorner A, Wang C, Fofanova V, Barnett MH, Yiannikas C, Parratt J, You Y, Graham SL. Diffusivity in multiple sclerosis lesions: At the cutting edge? NeuroImage. Clinical. 2016:12():219-26. doi: 10.1016/j.nicl.2016.07.003. Epub 2016 Jul 5 [PubMed PMID: 27489769]
Chen Q, Abrigo J, Deng M, Shi L, Wang YX, Chu WCW, Alzheimer’s Disease Neuroimaging Initiative. Diffusion Changes in Hippocampal Cingulum in Early Biologically Defined Alzheimer's Disease. Journal of Alzheimer's disease : JAD. 2023:91(3):1007-1017. doi: 10.3233/JAD-220671. Epub [PubMed PMID: 36530082]
Vidyadharan S, Prabhakar Rao BVVSN, Perumal Y, Chandrasekharan K, Rajagopalan V. Deep Learning Classifies Low- and High-Grade Glioma Patients with High Accuracy, Sensitivity, and Specificity Based on Their Brain White Matter Networks Derived from Diffusion Tensor Imaging. Diagnostics (Basel, Switzerland). 2022 Dec 19:12(12):. doi: 10.3390/diagnostics12123216. Epub 2022 Dec 19 [PubMed PMID: 36553224]
Szmuda M, Szmuda T, Springer J, Rogowska M, Sabisz A, Dubaniewicz M, Mazurkiewicz-Bełdzińska M. Diffusion tensor tractography imaging in pediatric epilepsy - A systematic review. Neurologia i neurochirurgia polska. 2016:50(1):1-6. doi: 10.1016/j.pjnns.2015.10.003. Epub 2015 Oct 29 [PubMed PMID: 26851683]
Level 1 (high-level) evidenceZanin E, Ranjeva JP, Confort-Gouny S, Guye M, Denis D, Cozzone PJ, Girard N. White matter maturation of normal human fetal brain. An in vivo diffusion tensor tractography study. Brain and behavior. 2011 Nov:1(2):95-108. doi: 10.1002/brb3.17. Epub [PubMed PMID: 22399089]
Cauley KA, Filippi CG. Diffusion-tensor imaging of small nerve bundles: cranial nerves, peripheral nerves, distal spinal cord, and lumbar nerve roots--clinical applications. AJR. American journal of roentgenology. 2013 Aug:201(2):W326-35. doi: 10.2214/AJR.12.9230. Epub [PubMed PMID: 23883249]
Rutman AM, Peterson DJ, Cohen WA, Mossa-Basha M. Diffusion Tensor Imaging of the Spinal Cord: Clinical Value, Investigational Applications, and Technical Limitations. Current problems in diagnostic radiology. 2018 Jul-Aug:47(4):257-269. doi: 10.1067/j.cpradiol.2017.07.005. Epub 2017 Jul 29 [PubMed PMID: 28869104]
Chung MK, Hanson JL, Adluru N, Alexander AL, Davidson RJ, Pollak SD. Integrative Structural Brain Network Analysis in Diffusion Tensor Imaging. Brain connectivity. 2017 Aug:7(6):331-346. doi: 10.1089/brain.2016.0481. Epub 2017 Jun 28 [PubMed PMID: 28657774]
van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neuroscience and biobehavioral reviews. 2012 Apr:36(4):1093-106. doi: 10.1016/j.neubiorev.2012.01.003. Epub 2012 Jan 24 [PubMed PMID: 22305957]
Level 1 (high-level) evidenceChen R, Jiao Y, Herskovits EH. Structural MRI in autism spectrum disorder. Pediatric research. 2011 May:69(5 Pt 2):63R-8R. doi: 10.1203/PDR.0b013e318212c2b3. Epub [PubMed PMID: 21289538]