Introduction
Heart failure (HF) remains a prevailing cause of cardiovascular morbidity and mortality globally despite advancements in therapies and preventive measures. The Centers for Disease Control and Prevention (CDC) estimates that 6.7 million individuals aged 20 or older in the United States are affected by HF. It is anticipated that the prevalence of HF will increase to 8.5 million Americans by 2030.
Definition of Heart Failure
HF is a multifaceted clinical syndrome arising from functional or structural impairment in the filling or ejection of blood by the ventricles, leading to a diverse range of symptoms.
American College of Cardiology/ American Heart Association Stages of Heart Failure
The American College of Cardiology (ACC) and the American Heart Association (AHA) have outlined stages of heart failure to classify the progression and severity of the condition (Table 1. ACC/AHA Stages of Heart Failure).[1]
Patients who have resolved symptoms and signs of HF with persistent left ventricular dysfunction are categorized as stage C and should receive appropriate treatment. If all HF symptoms, signs, and structural abnormalities completely resolve, the patient is deemed to be in remission from HF.
Table 1. ACC/AHA Stages of Heart Failure
| Stages | Definition | Risk Factors | Intervention |
|
Stage A: at risk of heart failure |
Individuals at high risk of developing heart failure but without structural heart disease or symptoms. |
Hypertension, cardiovascular disease, diabetes, obesity, cardiotoxic agents, genetic variants of cardiomyopathy. |
Addressing risk factors. |
|
Stage B: pre-heart failure |
Structural heart disease is present, but there are no symptoms of heart failure. |
Structural heart disease, evidence of increased filling pressures, elevated natriuretic peptide levels, elevated cardiac troponins. | Treating the underlying structural abnormalities and risk factors. |
|
Stage C: symptomatic heart failure |
Structural heart disease is present, and there are current or past symptoms of heart failure. | Stage B risk factors with symptoms and signs of HF. | Alleviating symptoms, slowing disease progression, and improving overall quality of life. |
|
Stage D: advanced heart failure |
Advanced heart failure with persistent symptoms despite optimal medical therapy. | Stage B risk factors with marked symptoms and signs of HF and recurrent hospitalization for HF despite goal-directed medical therapy. | Advanced interventions, including heart transplantation or mechanical circulatory support. |
Classification of Heart Failure by Left Ventricular Ejection Fraction
Patients with HF are frequently classified by left ventricular ejection fraction (LVEF).[1] This classification system acknowledges the different prognoses and responses to guideline-directed medical therapy (GDMT) for patients with heart failure. The 2022 AHA/ACC/Heart Failure Society of America (HSFA) Guideline for the Management of Heart Failure identifies 4 classes of HF by LVEF (Table 2. Classification of Heart Failure by Left Ventricular Ejection Fraction).
Table 2. Classification of Heart Failure by Left Ventricular Ejection Fraction
| Type of HF According to LVEF | Definition | Serial Assessment and LVEF Trajectories |
| Heart failure with reduced ejection fraction (HFrEF) |
LVEF ≤40% |
|
| Heart failure with improved ejection fraction (HFimpEF) |
Previous LVEF ≤40% and a follow-up LVEF >40% |
|
| Heart failure with mildly reduced ejection fraction (HFmrEF) |
LVEF 41%–49% |
|
| Heart failure with preserved ejection fraction (HFpEF) |
LVEF ≥50% |
|
New York Heart Association Classification of Heart Failure
The NYHA classification of HF is a subjective evaluation by a clinician to delineate the functional capacity and symptoms of individuals diagnosed with ACC/AHA stage C or D heart failure. Serving as an independent predictor of mortality, the NYHA Classification is employed in clinical settings to assess the appropriateness of therapeutic interventions for patients in stage C or D of heart failure.[1] The 4 NYHA heart failure classes are:
- Class I (Mild HF): No restrictions in physical activity. Ordinary physical activity does not induce undue fatigue, palpitations, or dyspnea.
- Class II (Mild-to-Moderate HF): Slight limitations in physical activity. Comfortable at rest, but ordinary activities result in fatigue, palpitations, or dyspnea.
- Class III (Moderate-to-Severe HF): Significant restrictions in physical activity. Comfortable at rest, but less than ordinary activities lead to fatigue, palpitations, or dyspnea.
- Class IV (Severe HF): Unable to engage in physical activity without discomfort. Symptoms of heart failure are present at rest, and any physical activity exacerbates the discomfort.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of HF will vary among LVEF classifications. The etiology of HF may be multifactorial, and individual cases may have multiple contributing factors.
Etiologies of HFrEF: Common causes of HFrEF include myocardial infarction, ischemic heart disease, dilated cardiomyopathy, and viral infections affecting the heart muscle. Other contributing factors may include hypertension, valvular heart disease, and genetic predisposition.
Etiologies of HFmrEF: The etiologies of HFmrEF are similar to HFrEF and HFpEF. Causes of HFmrEF may include a combination of myocardial infarction, ischemic heart disease, and underlying structural heart abnormalities that fall between those seen in HFrEF and HFpEF.
Etiologies of HFpEF: Common causes of HFpEF include hypertension, atrial fibrillation, age-related cardiac changes, and underlying structural heart abnormalities such as hypertensive heart disease. Other contributing factors may include diabetes, obesity, and chronic kidney disease.
Other possible nonischemic etiologies of HF include:
- Cardiotoxic medications, including some chemotherapeutic medications
- Rheumatological or autoimmune diseases
- Endocrinopathies, including thyroid disease, acromegaly, pheochromocytoma, and diabetes
- Obesity
- Familial, genetic, or heritable cardiomyopathies and cardiac diseases
- Dysrhythmias, including tachycardias, right-ventricular pacing, or frequent premature ventricular contractions
- Hypotension
- Infiltrative cardiac diseases, including sarcoidosis, Fabry disease, hemochromatosis, and amyloidosis
- Myocarditis of any etiology
- Peripartum cardiomyopathy
- Stress cardiomyopathy such as Takotsubo and reverse Takotsubo cardiomyopathies
- Substance misuse, including alcohol, cocaine, and methamphetamines
- Congenital heart disease
- Pulmonary hypertension causing right HF
- Pulmonary embolism causing right HF.
Epidemiology
HF is a significant public health problem with a prevalence of over 5.8 to 6.5 million in the U.S.[2][3] and around 26 million worldwide.[4] The expectation is that 8 million people in the United States will have this condition by 2030, accounting for a 46% increase in prevalence.[5] At age 45 years, the lifetime risks for HF through age 75 or 95 years were 30% to 42% in white men, 20% to 29% in black men, 32% to 39% in white women, and 24% to 46% in black and higher BP and BMI at all ages led to higher lifetime risks.[6] The increase in HF prevalence does not necessarily have links with an increase in HF incidence. The aging of the population and modern therapies for cardiac patients that led to increased survival could explain the increase in prevalence even with a reduction in the incidence.[7] Information collected during the second 25-year period of the Framingham Heart Study reveals that the lifetime risk of Heart Failure with Preserved Ejection Fraction (HFpEF) is estimated at around 19.3%. This surpasses the approximate 11.4% lifetime risk associated with Heart Failure with Reduced Ejection Fraction (HFrEF). Notably, this trend appears more pronounced in women, with an apparent lifetime risk of HFpEF at 10.7%, compared to 5.8% for HFrEF. Additionally, these risks demonstrate variability based on ethnicity. Between 13% and 24% of patients with HF have HFmrEF.[8][9]
Pathophysiology
Heart failure is a complex clinical syndrome that occurs when the heart is unable to pump or receive blood effectively, leading to inadequate perfusion of organs and tissues. The pathophysiology of heart failure involves various mechanisms, and it often develops as a result of underlying cardiovascular diseases or conditions that strain the heart.
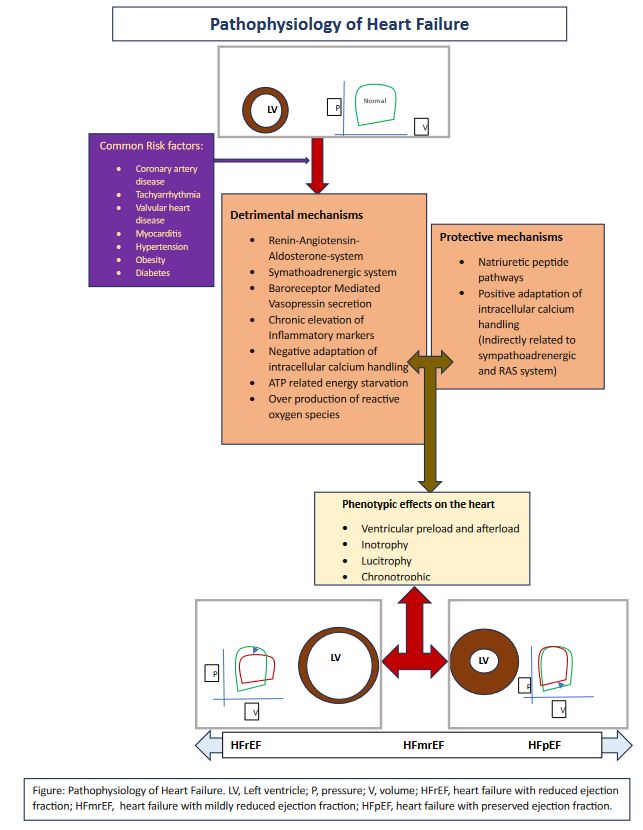
Following the initiation triggered by various risk factors such as coronary artery disease, tachyarrhythmias, valvular heart disease, myocarditis, hypertension, obesity, and diabetes, a cascade of mechanisms unfolds within the heart. Activation of the renin-angiotensin-aldosterone system, stimulation of the sympathetic adrenergic system, vasopressin secretion through beta receptor mediation leading to excessive water absorption, persistent elevation of inflammatory markers resulting in fibrosis, maladaptive intracellular calcium handling, ATP-related energy depletion, and increased production of reactive oxygen species collectively exert detrimental effects on the left ventricle. See Image. Pathophysiology of Heart Failure.
Conversely, pathways involving natriuretic peptides and positive adaptations in intracellular calcium handling exhibit protective effects on the left ventricle. These mechanisms collectively impose strain on the heart, giving rise to the phenotypic expression of the heart failure spectrum. This expression manifests as changes in ventricular preload or afterload, variations in inotropy (increased or decreased contractility), lusitropy (the ability of the ventricle to relax), and chronotropy (heart rate regulation), ultimately resulting in permanent morphological alterations in the left ventricle.
In heart failure with reduced ejection fraction, the left ventricle enlarges and weakens, and the pressure-volume relationship reveals a reduction in stroke volume, an elevation in left ventricular end-diastolic pressure, and an increase in left ventricular end-diastolic volume. Heart failure with preserved ejection fraction is associated with hypertrophy and abnormal lusitropy, and the pressure-volume relationship indicates an elevation in end-diastolic pressure along with a reduction in stroke volume and left ventricular end-diastolic volume.
Histopathology
Common histopathological findings in heart failure with preserved ejection fraction include fibrosis, hypertrophy, inflammation, and amyloidosis. Identifying amyloid traditionally involves Congo red staining, although its effectiveness varies based on staining quality and laboratory expertise. Crystal violet is an alternative stain that offers more consistency and better visibility under conventional light microscopy.
In glycogen storage disorders, enlarged myocytes with cytoplasmic vacuoles containing glycogen are observed, highlighted by periodic acid–shiff staining. Hypertrophic cardiomyopathy is characterized by myocyte disarray, requiring at least 20% of the overall section area for diagnosis. This condition presents with haphazardly arranged myocytes or myofiber bundles and increased interstitial fibrosis.
Heart failure with reduced ejection fraction is most commonly caused by coronary artery disease. Following myocardial infarction, histologic changes, including interstitial edema, can be appreciated within 4-12 hours. Early ischemia, characterized by a neutrophilic inflammatory cell infiltrate, occurs in the first 72 hours. The subsequent stage (three to five days) involves the removal of myocytes, replaced by lymphocytes, pigment-laden histiocytes, and myofibroblasts. Two to four weeks post-infarction, collagen deposition increases, and inflammation decreases. By one to two months, dense collagen scar forms, and residual inflammatory cells are minimal.
In nonischemic dilated cardiomyopathy, microscopic findings include nonspecific myocyte hypertrophy and myocardial fibrosis. Sarcoidosis exhibits patchy mononuclear inflammatory infiltrates with noncaseating granulomas, often amidst prominent myocardial fibrosis. Lyme carditis is characterized by a perivascular pattern of inflammation, resembling a "road map" and a cellular composition of lymphohistiocytic with frequent plasma cells. Giant cell myocarditis pathology involves florid myocarditis with lymphocytes, histiocytes, and multinucleated giant cells, with numerous eosinophils distinguishing it from sarcoidosis. Myocarditis histopathology involves inflammation associated with myocyte injury and various cellular compositions, including lymphocyte-predominant, lymphohistiocytic, or mixed cellular patterns with eosinophils and/or neutrophils.[10]
History and Physical
A comprehensive history and physical examination are essential for all patients suspected of having heart failure (HF), as the diagnosis relies heavily on clinical symptoms and signs. This evaluation should encompass an analysis of risk factors and potential causes of HF. Regardless of ejection fraction (EF), HF symptoms exhibit similarities.
Symptoms of Heart Failure:
- Shortness of breath, orthopnea, and paroxysmal nocturnal dyspnea
- Persistent cough or wheezing
- Edema (generalized or lower extremity)
- Fatigue and Tiredness
- Lack of appetite and nausea
- Confusion and impaired thinking
- Palpitations
Signs of Heart Failure:
- Pulses alternans
- Elevated jugular venous pressure
- Displaced LV apex beat
- Cardiac cachexia
- Sinus tachycardia
- Right ventricular heave
- Bilateral rales/ crepitations or a cardiac wheeze on lung exam
- Presence of S3
- Pitting edema in dependent areas
- Tender hepatomegaly
- Ascites
Regular assessment of HF symptoms and signs during clinic visits is crucial for monitoring therapy response and stability. Vital signs and volume status should be evaluated at each visit to ensure a comprehensive understanding of the patient's condition.
Evaluation
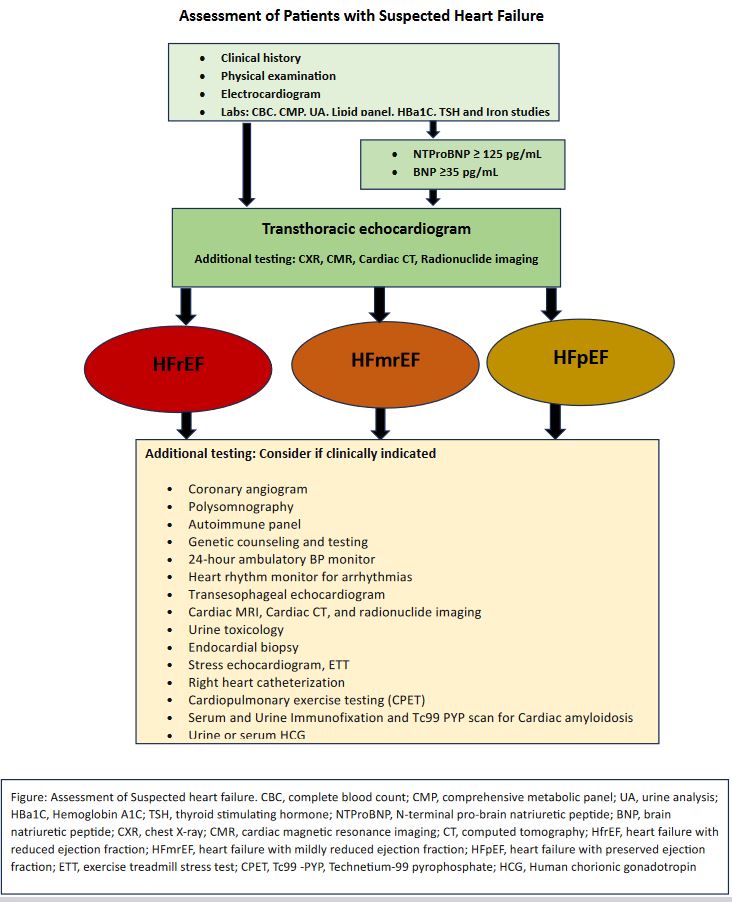
Following the initial assessment through a detailed history and physical examination, it is advisable to incorporate further diagnostic investigations. These may include an electrocardiogram, complete blood count, and a comprehensive metabolic panel encompassing liver function tests, electrolytes, and renal function tests. Additionally, considering urinalysis, lipid panel, hemoglobin A1c, thyroid-stimulating hormone, and iron studies can provide a more comprehensive understanding of the patient's health.
For a more in-depth cardiac evaluation, essential labs involve measuring NT pro-brain natriuretic peptide and brain natriuretic peptide. An NT-pro-brain natriuretic peptide exceeding 125 pg/ml and a brain natriuretic peptide equal to or greater than 35 pg/ml are reliable indicators for assessing heart failure. A transthoracic echocardiogram is crucial for assessing left ventricular ejection fraction, while a chest x-ray proves beneficial when suspecting pulmonary vascular congestion. If a transthoracic echocardiogram doesn't suffice for evaluating left ventricular function, alternative modalities like cardiac MRI, cardiac CT angiogram, or radionuclide imaging should be considered (see Image. Assessment of Patients With Suspected Heart Failure Flowsheet).[11]
Further specialized tests may be warranted when classifying heart failure based on ejection fraction. These include a stress echocardiogram, exercise treadmill stress test, cardiac CT angiogram or nuclear imaging and a coronary angiogram for suspected ischemia, a polysomnogram for suspected sleep apnea, an autoimmune panel for autoimmune diseases, genetic counseling and testing for familial cardiomyopathy (hypertrophic cardiomyopathy, idiopathic dilated cardiomyopathy, arrhythmogenic right ventricular dysplasia, isolated noncompaction cardiomyopathy), 24-hour blood pressure monitoring for hypertensive cardiomyopathy, and heart rhythm monitoring for arrhythmias.
Other diagnostic tools involve a transesophageal echocardiogram for valvular heart disease, cardiac MRI for structural heart disease, urine toxicology for drug abuse-related cardiomyopathy, and endocardial biopsy for giant cell myocarditis. Performing right heart catheterization and cardiopulmonary exercise testing frequently provides vital information for managing heart failure. Serum and urine immunofixation with a technetium 99 pyrophosphate scan for cardiac amyloidosis. Additionally, urine or serum HCG can be employed to evaluate heart failure further. (Figure 2)
Treatment / Management
Table 3.
(A1)| Medication | Recommendations | Clinical Pearls |
|
Beta-blockers (BB) |
HFrEF: Very Highly recommended HFmrEF: Very Highly recommended HFpEF: No substantial evidence |
|
|
ARNI |
HFrEF: Very Highly recommended HFmrEF: Highly recommended HFpEF: Highly recommended |
|
|
ACEi |
HFrEF: Very Highly recommended HFmrEF: Highly recommended HFpEF: No substantial evidence |
|
|
ARB |
HFrEF: Very Highly recommended HFmrEF: Highly recommended HFpEF: Highly recommended |
|
|
SGLT2i |
HFrEF: Very Highly recommended HFmrEF: Highly recommended HFpEF: Highly recommended |
|
|
MRAs |
HFrEF: Very Highly recommended HFmrEF: Highly recommended HFpEF: Highly recommended |
|
|
Hydralazine or nitrate |
HFrEF: Very Highly recommended HFmrEF: No substantial evidence HFpEF: No substantial evidence |
|
|
Ivabradine |
HFrEF: Recommended HFmrEF: No substantial evidence HFpEF: No substantial evidence |
|
|
Vericiguat |
HFrEF: Recommended HFmrEF: Recommended HFpEF: No substantial evidence |
|
|
Digoxin |
HFrEF: Recommended HFmrEF: No substantial evidence HFpEF: No substantial evidence |
|
|
PUFA
|
HFrEF: Recommended HFmrEF: No substantial evidence HFpEF: No substantial evidence |
|
|
Potassium binders |
HFrEF: Recommended HFmrEF: No substantial evidence HFpEF: No substantial evidence |
|
|
Diuretics |
HFrEF: Recommended HFmrEF: Recommended HFpEF: Recommended |
|
|
IV Iron replacement
|
HFrEF: Recommended HFmrEF: Recommended HFpEF: No substantial evidence |
|
Pharmacologic therapy for Heart Failure (HFrEF, HFmrEF, and HFpEF)
- Beta-blockers (Class I) reverse sympathetic activation in HFrEF, improving survival, reducing heart failure hospitalizations, and increasing left ventricular ejection fraction (LVEF). Randomized controlled trials support Carvedilol, metoprolol succinate, and bisoprolol.
- Angiotensin receptor-neprilysin inhibitor (ARNI) sacubitril/valsartan demonstrated reduced cardiovascular and all-cause mortality in HFrEF compared to enalapril in the Prospective Comparison of ARNI with ACE-I to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial. In a predefined subgroup analysis of participants in the Prospective Comparison of ARNI with ARB Global Outcomes in HFpEF (PARAGON-HF), it was found that sacubitril-valsartan, in comparison to valsartan alone, not only decreased the overall risk of heart failure-related hospitalizations but exhibited a more pronounced reduction in women with HFpEF compared to men. The average left ventricular ejection fraction (LVEF) in the study participants was 57%. It is indicated for symptomatic HFrEF, HFmrEF, and HFpEF.
- ACE inhibitors (ACEi) or angiotensin receptor blockers (ARBs) (Class IA) are the primary therapies for chronic heart failure. ACE inhibitors inhibit angiotensin-converting enzyme (ACE), preventing the formation of angiotensin II, leading to natriuresis, diuresis, and a reduction in arterial blood pressure, subsequently reducing afterload. Numerous clinical trials demonstrated survival benefits and reduced hospitalization in chronic symptomatic heart failure with reduced ejection fraction (HFrEF). While ARBs may be considered in patients intolerant to ACE inhibitors. ARB is beneficial in HFmrEF and HFpEF. However, there is no substantial evidence to support ACEI benefit in HFpEF. The routine combination of ACEi and ARB is not recommended.
- Sodium-glucose cotransporter 2 inhibitors (SGLT2i) (Class 1A recommendation for HFrEF, Class 2A recommendation for HFpEF and HFmrEF) have shown positive results in reducing HF hospitalization and mortality. Contraindications include symptomatic hypotension, SBP <95 mmHg, and eGFR <30 ml/min/1.73 m².
- Mineralocorticoid receptor antagonists (MRAs) are indicated for NYHA class II-IV patients with EF ≤35%, stable serum creatinine, eGFR >30 mL/min/1.73 m², and stable serum potassium <5.0 mEq/L. It is also beneficial in HFmrEF and HFpEF.
- Nitrates plus hydralazine (Class IA) decrease afterload and preload, which is beneficial for African American patients with NYHA class III-IV HFrEF receiving optimal medical therapy (OMT).
- Ivabradine reduces the risk of HF hospitalization in symptomatic HFrEF patients with EF <35%, sinus rhythm, and resting heart rate >70 bpm on the maximum tolerated beta-blocker dose.
- Vericiguat diminishes heart failure hospitalizations in individuals with heart failure with reduced ejection fraction (HFrEF) and heart failure with mid-range ejection fraction (HFmrEF) if they meet the criteria of NYHA II-IV, LVEF ≤45, recent heart failure hospitalization or intravenous diuretics usage, or elevated B-type natriuretic peptide (BNP) levels.
- Digoxin (Class IIA) reduces HFrEF hospitalization but does not improve survival.
- Polyunsaturated fatty acids (PUFA) are recommended in HfrEF patients with NYHA class II-IV.
- Potassium binders are recommended in HFrEF patients with hyperkalemia while on ARNi/ACEI/ARB.
- Diuretics recommended in any heart failure with symptoms and signs suggestive of volume overload. It relieves congestion, improve symptoms, and prevents worsening of heart failure. Loop diuretics are preferred over thiazide diuretics, with no discernible scientific evidence favoring one loop diuretic over another in heart failure patients.
Medications to be avoided in heart failure include non-steroidal anti-inflammatory drugs (NSAIDs), COX-2 inhibitors, calcium channel blockers (CCB) (except amlodipine and felodipine), thiazolidinediones (TZDs), the addition of an angiotensin receptor blocker (ARB) to an angiotensin-converting enzyme inhibitor (ACEi), dronedarone, metformin, and cilostazol, etc.,
Non-pharmacologic therapy for Heart Failure (HFrEF, HFmrEF, and HFpEF)
- Management of chronic heart failure requires a multidisciplinary approach, which requires primary care, general cardiology, interventional Cardiology, pulmonology-sleep medicine, cardiac rehabilitation, nurse, dietitian, and heart failure specialists.
- Nonpharmacological approaches are essential, such as lifestyle modifications, including a heart-healthy lifestyle, a low-sodium diet (less than 2 to 2.5 grams/day), regular exercise, and weight management.
- Exercise training, exercise-based cardiac rehabilitation, and weight management improved functional status, exercise performance, heart failure-specific hospitalization, and quality of life in patients with heart failure.
- Heart failure long-term management primarily depends on managing comorbidities like hypertension, diabetes, arrhythmia (eg: atrial fibrillation), obesity, coronary artery disease, valvular heart disease and sleep apnea.
- Genetic screening and counseling are recommended in first-degree relatives of patients with genetic or inherited cardiomyopathies.[40] (B3)
Non-surgical interventions
- These include implantable cardioverter-defibrillator (ICD) and cardiac resynchronization therapy (CRT). Before proceeding with device therapy, patients should be treated with Guideline Directed Medical Therapy (GDMT)for at least three months and reassess the LVEF. If EF remains less than or equal to 35% with a reasonable expectation of meaningful survival for more than 1 year, the recommendation is a referral for device therapy.
Implantable Cardioverter Defibrillator (ICD)
- ACC/AHA 2013 guidelines [41] recommend ICD for primary prevention of sudden cardiac death for non-ischemic dilated cardiomyopathy (NIDCM) or ischemic CMP (ICM) at least 40 days post-MI on chronic goal-directed medical therapy (GDMT) with either LVEF ≤ 35% and NYHA class II or III symptoms or LVEF ≤ 30% and NYHA class I symptom.
- ESC 2016 guidelines [42] recommend ICD for primary prevention in symptomatic HF (NYHA II-III) with LVEF ≤ 35% despite ≥ three months of GDMT in ICM (IA) or NIDCM. (A1)
Cardiac Resynchronization Therapy (CRT)
- ACC/AHA 2013 guidelines recommend CRT for patients with HF with sinus rhythm, LVEF ≤ 35%, left bundle branch block (LBBB) with QRS duration ≥ 150 ms, and NYHA class II to ambulatory IV on GDMT.
- ESC 2016 guidelines recommend CRT for symptomatic HF with sinus rhythm and LVEF ≤ 35% despite GDMT with LBBB with QRSd ≥ 150 ms or 130 to 149 ms.
Invasive therapies
- Coronary revascularization is indicated for patients with HF (HFpEF, HFrEF, or HFmrEF) on GDMT with angina and suitable coronary anatomy.[43]
- The utilization of the CardioMEMS pulmonary artery pressure hemodynamic monitoring system enhances the quality of life. It reduces hospitalizations in individuals with heart failure and recurrent hospitalizations. Furthermore, the use of CardioMEMS has demonstrated improvements in underlying metabolic comorbidities concurrent with enhancements in pulmonary artery pressures.
- Transcatheter edge-to-edge mitral valve repair proves advantageous for individuals classified under NYHA class II-IV, presenting severe secondary mitral regurgitation with suitable anatomy and left ventricular ejection fraction ranging from 20-50%, a left ventricular end-systolic diameter less than 7 cm, and a pulmonary artery systolic pressure below 70 mmHg.
- Catheter ablation- pulmonary venous isolation is indicated in patients with heart failure with reduced ejection fraction associated with symptoms attributable to atrial fibrillation.
Surgical treatment of HF
- Surgical interventions for heart failure are typically reserved for more advanced cases when medical treatments alone prove inadequate. Among the surgical options for managing heart failure is Coronary Artery Bypass Grafting (CABG), employed in cases of coronary artery disease (CAD) with heart failure. Another surgical approach involves the repair or replacement of damaged heart valves, aiming to enhance the heart's functionality. This procedure focuses on addressing issues with heart valves, ultimately contributing to improved cardiac function.
- Despite the advances in the medical management of HF, there are some circumstances in which surgery is the best treatment option. Surgical approaches to HF treatment include heart transplantation and procedures that reshape the heart, repair the heart, or replace all or part of the heart function. The basis for any decision to surgically treat HF depends on functional status, prognosis, and severity of the underlying HF and comorbidities. It should take place in centers with multidisciplinary medical and surgical teams.
Heart Transplantation
- According to Heart Failure Society of America (HFSA) 2010 heart failure guidelines, it is recommended to evaluate patients for heart transplantation in severe HF, debilitating refractory symptoms, ventricular arrhythmia, or congenital heart disease that remains uncontrolled despite drug, device, or alternative surgical therapy (strength of evidence B).[44] Data from the registry of the International Society of Heart and Lung Transplantation indicates a current 1-year survival of 84.5% and 5-year survival of 72.5%; subsequently, survival decreases linearly by approximately 3.4% per year.[45] (A1)
Ventricular Assist Devices (VADs)
- Blood is extracted from the failing ventricle and directed into a pump that channels the blood to either the aorta (in the case of left ventricular failure and LVAD) or the pulmonary artery (in the case of right ventricle failure and RVAD). Devices designed for this process include the left ventricular assist device (LVAD), right ventricular assist device (RVAD), or biventricular assist device (BiVAD). The BiVAD is inserted into the left ventricle, drawing blood from it and expelling it into the ascending aorta. These ventricular assist devices (VADs) serve as bridge therapy to heart transplantation in refractory stage D heart failure, as recommended by the ACCF/AHA, ESC, and HFSA guidelines. Alternatively, they may be used as destination therapy when the patient is not a candidate for heart transplantation. In such cases, LVADs as destination therapy have shown superiority over medical therapy, as demonstrated by the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial.[41][46][44][45][47] (A1)
Acute Decompensated Heart Failure (ADHF)
If clinical and hemodynamic indicators suggest cardiogenic shock, a multidisciplinary shock team assessment, and intervention have demonstrated improvements in 30-day all-cause mortality and reduced in-hospital mortality. The Society for Cardiovascular Angiography and Interventions (SCAI) has proposed a classification system for cardiogenic shock, which categorizes patients into different stages based on the severity of their condition.
-
Stage A: At Risk
- Patients at risk for cardiogenic shock due to a medical condition but without evidence of hypoperfusion.
- Cardiac power output (CPO), pulmonary artery pulsatility index (PAPi), Lactate, and blood pressure within normal limits.
-
Stage B: Beginning
- Patients with evidence of hypoperfusion (such as elevated lactate) but without hypotension.
- Early stages of cardiogenic shock with compensatory mechanisms still maintain blood pressure.
- Cardiac power output (CPO), pulmonary artery pulsatility index (PAPi), and blood pressure within normal limits. Lactate ↑.
-
Stage C: Classic
- Patients with hypoperfusion and hypotension despite adequate fluid resuscitation.
- This represents classic cardiogenic shock with overt signs of circulatory failure.
- Cardiac power output (CPO) ↓ or pulmonary artery pulsatility index (PAPi) ↓. Blood pressure ↓. Lactate↑.
-
Stage D: Deteriorating
- Patients with refractory shock despite initial treatment measures.
- Includes those who require advanced hemodynamic support, such as mechanical circulatory support devices.
- Cardiac power output (CPO) ↓ or pulmonary artery pulsatility index (PAPi) ↓. Blood pressure ↓. Lactate↑.
-
Stage E: Extremis
- Patients in profound refractory shock with multiorgan failure.
- This represents the most severe stage of cardiogenic shock.
- Cardiac power output (CPO) ↓ or pulmonary artery pulsatility index (PAPi) ↓. Blood pressure ↓. Lactate↑.
The SCAI Stages of Cardiogenic Shock provide a framework for clinicians to categorize and manage patients based on the severity of their condition. The goal is to guide appropriate interventions and support strategies tailored to each stage.
The Forrester Classification is a system used to categorize patients with acute myocardial infarction based on their hemodynamic profile. These hemodynamic profiles help guide therapeutic decisions, such as using inotropic agents, vasopressors, and other interventions to improve cardiac output and address volume status. The classification system helps clinicians tailor their approach to the specific hemodynamic characteristics of the patient. See Image. Forrester Classification and Management of Heart Failure.
In the management of cardiogenic shock, right heart catheterization plays a crucial role. If there is a reduction in cardiac power output (CPO) or pulmonary artery pulsatility index (PAPi), the consideration of inotropic agents such as Milrinone or Dobutamine with or without mechanical ventricular assisted device is warranted. In cases where CPO or PAPi and blood pressure are decreased, a combination of vasopressors (such as Norepinephrine or Epinephrine) and inotropic agents with or without mechanical ventricular-assisted device is recommended. This approach addresses compromised cardiac function and optimizes hemodynamics for effective shock management and correction of precipitating factors like arrhythmias and ischemia.
Key components of therapy for patients experiencing acute decompensated Heart Failure without shock include appropriate ventilatory support and diuresis. Generally, loop diuretics are favored over thiazide diuretics, with no discernible scientific evidence favoring one loop diuretic over another in acutely decompensated heart failure patients. The Diuretic Optimization Strategies Evaluation (DOSE) trial found no clinically significant differences in symptomatic improvement between bolus and continuous intravenous loop diuretics. Some cases may necessitate a combination of diuretic classes, such as thiazide, thiazide-like, or acetazolamide. It is important to note that adding metolazone increases the risk of adverse outcomes compared to loop diuretics alone. Conversely, the Acetazolamide in Decompensated Heart Failure with Volume OveRload (ADVOR) trial demonstrated that adding acetazolamide to loop diuretics increases the incidence of successful decongestion in patients with acute heart failure within 3 days compared to placebo.
After the initial stabilization of hemodynamics and ventilation, the paramount consideration is the assessment and management of ischemia, arrhythmias, and other precipitating factors and comorbidities. Urgent revascularization is recommended in cases of acute coronary syndrome. For patients with hypertensive urgency or emergency and pulmonary edema, intravenous or topical nitroglycerin is the preferred choice, as it aids in decreasing preload in patients with pulmonary edema.
Short-term renal replacement therapy or ultrafiltration should be contemplated for symptomatic hypervolemic patients with poor renal function and minimal urine output, irrespective of supportive therapy and stable hemodynamics. Patients with poor baseline renal function or end-stage renal disease are at a significantly heightened risk of requiring renal replacement therapy.
Initiating angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), angiotensin receptor-Naprosyn inhibitors (ARNIs), mineralocorticoid receptor antagonists (MRAs), sodium-glucose cotransporter-2 inhibitors (SGLT2i) therapy, or beta-blockers early in stabilized acute decompensated heart failure with reduced ejection fraction (HFrEF) and heart failure with mid-range ejection fraction (HFmrEF) patients before hospital discharge offers some benefits. However, these medications are not recommended for use in unstable patients experiencing acutely decompensated heart failure with preserved ejection fraction (HFpEF). Thiazolidinediones and dipeptidyl peptidase-4 (DPP-4) inhibitors like sitagliptin or saxagliptin are contraindicated in cases of acutely decompensated heart failure.
Differential Diagnosis
The diagnosis of heart failure is mostly clinical, as mentioned above, but many other medical conditions can cause the same signs and symptoms. There is a broad differential diagnosis of heart failure, which includes but is not limited to bacterial pneumonia, chronic obstructive pulmonary disease (COPD), liver cirrhosis, acute kidney injury, idiopathic pulmonary fibrosis, nephrotic syndrome, pulmonary embolism respiratory failure, primary pulmonary hypertension, anemia, and venous insufficiency.
- Acute kidney injury
- Bacterial pneumonia
- Acute respiratory distress syndrome
- Cardiogenic pulmonary edema
- Interstitial (non-idiopathic) pulmonary fibrosis
- Chronic obstructive pulmonary disease
- Cirrhosis
- Goodpasture syndrome
- Community-acquired pneumonia
- Idiopathic pulmonary fibrosis
- Myocardial infarction
- Pulmonary embolism
- Nephrotic syndrome
- Neurogenic pulmonary edema
- Pneumothorax
- Respiratory failure
- Viral pneumonia
- Venous insufficiency
Treatment Planning
Table 4.
| Medication | Initial Daily Dose | Target Dose |
| Metoprolol succinate |
12.5–25 mg once daily |
200 mg once daily |
|
Carvedilol |
3.125 mg twice daily |
25–50 mg twice daily |
|
Bisoprolol |
1.25 mg once daily |
10 mg once daily |
|
Captopril |
6.25 mg 3 times daily |
50 mg 3 times daily |
|
Enalapril |
2.5 mg twice daily |
10–20 mg twice daily |
|
Fosinopril |
5–10 mg once daily |
40 mg once daily |
|
Lisinopril |
2.5–5 mg once daily |
20–40 mg once daily |
|
Perindopril |
2 mg once daily |
8–16 mg once daily |
|
Quinapril |
5 mg twice daily |
20 mg twice daily |
|
Ramipril |
1.25–2.5 mg once daily |
10 mg once daily |
|
Candesartan |
4–8 mg once daily |
32 mg once daily |
|
Losartan |
25–50 mg once daily |
50–150 mg once daily |
|
Valsartan |
20–40 mg once daily |
160 mg twice daily |
|
Sacubitril-valsartan |
49 mg sacubitril and 51 mg valsartan twice daily |
97 mg sacubitril and 103 mg valsartan twice daily |
|
Spironolactone |
12.5–25 mg once daily |
25–50 mg once daily |
|
Eplerenone |
25 mg once daily |
50 mg once daily |
|
Dapagliflozin |
10 mg once daily |
10 mg once daily |
|
Empagliflozin |
10 mg once daily |
10 mg once daily |
|
Isosorbide dinitrate and hydralazine |
20–30 mg isosorbide dinitrate and 25–50 mg hydralazine 3–4 times daily |
120 mg isosorbide dinitrate total daily in divided doses and 300 mg hydralazine total daily in divided doses |
|
Ivabradine |
5 mg twice daily |
7.5 mg twice daily |
|
Vericiguat |
2.5 mg once daily |
10 mg once daily |
|
Digoxin |
0.125–0.25 mg daily | Achieve serum digoxin concentration 0.5–<0.9 ng/mL |
Toxicity and Adverse Effect Management
Heart failure medications can be associated with various complications or side effects. It's important to note that the benefits of these medications often outweigh the potential risks, and individual responses can vary. Here are some potential complications associated with commonly prescribed heart failure medications:
-
ACE Inhibitors (Angiotensin-Converting Enzyme Inhibitors):
- Cough: ACE inhibitors may cause a persistent dry cough in some individuals.
- Hyperkalemia: These medications can lead to elevated potassium levels in the blood.
- Hypotension: ACE inhibitors can cause low blood pressure, especially when initiating treatment or increasing the dosage.
-
ARBs (Angiotensin II Receptor Blockers):
- Hyperkalemia: Similar to ACE inhibitors, ARBs can increase potassium levels.
- Hypotension: ARBs may lead to low blood pressure, particularly at the beginning of treatment.
-
Beta-Blockers:
- Bradycardia: Beta-blockers can slow the heart rate, leading to bradycardia.
- Hypotension: These medications may cause low blood pressure.
- Fatigue: Some individuals may experience fatigue or weakness.
-
Diuretics:
- Electrolyte Imbalance: Diuretics can lead to electrolyte imbalances, such as low potassium levels (hypokalemia).
- Dehydration: Excessive diuretic use may result in dehydration.
- Renal Impairment: Long-term use of diuretics can affect kidney function.
-
Mineralocorticoid receptor antagonist (Spironolactone, Eplerenone):
- Hyperkalemia: Aldosterone antagonists can cause an increase in potassium levels.
- Renal Impairment: These medications may impact kidney function.
-
ARNIs (Angiotensin Receptor-Neprilysin Inhibitors):
- Hypotension: ARNIs may lead to low blood pressure.
- Hyperkalemia: They can cause an elevation in potassium levels.
-
Hydralazine and Isosorbide Dinitrate:
- Headache: Combination therapy may cause headaches.
- Fluid Retention: Fluid retention may occur.
-
Digoxin:
- Arrhythmias: Digoxin can increase the risk of arrhythmias, especially at higher levels.
- Nausea and Vomiting: Some individuals may experience nausea and vomiting.
-
SGLT2 Inhibitors (Sodium-Glucose Cotransporter-2 Inhibitors):
- Genital Infections: SGLT2 inhibitors are associated with an increased risk of genital infections.
- Hypotension: These medications may lead to low blood pressure.
-
Ivabradine:
- Bradycardia: Ivabradine can cause a reduction in heart rate.
- Phosphenes (Visual Phenomena): Some individuals may experience visual disturbances.
Individuals taking heart failure medications should report any new or worsening symptoms to their healthcare provider promptly. Adjustments in medication dosages or alternative therapies may be considered to minimize complications.
Prognosis
- The Seattle Heart Failure Model, CHARM Risk Score, CORONA Risk Score, and MAGGIC Risk Score are predictive tools applicable to all cases of chronic heart failure. For heart failure with reduced ejection fraction (HFrEF), risk assessment tools like PARADIGM-HF, HF-ACTION, GUIDE-IT, and TOPCAT risk scores are commonly used. In the context of chronic heart failure with preserved ejection fraction (HFpEF), the I-PRESERVE Score and TOPCAT Risk Score are relevant. For acute decompensated heart failure, outcome predictions are provided by tools such as the ADHERE Classification and Regression Tree (CART) Model, AHA Get With The Guidelines Score, and EFFECT Risk Score.[11]
- Changes in EF over time are more prognostic of HF than baseline EF. Patients who progress from HFmrEF to HFrEF have a worse prognosis than those who progress to HFpEF or remain stable in HFmrEF. The mortality rate is higher in HFrEF than HFmrEF and HFpEF, according to the OPTIMIZE-HF trail [48], showed a mortality rate of 3.9% for HFrEF, 3% for HFmrEF, and 2.9% for HFpEF. The mortality rate is also higher in symptomatic patients.
Complications
HF may cause multiple complications, including but not limited to:
- Arrhythmias: Atrial fibrillation (Afib) can be a cause or a consequence of HF and may present in 10% to 50% of chronic HF patients, and those patients with HF and Afib have a poor prognosis. Malignant ventricular arrhythmias (like sustained monomorphic ventricular tachycardia, sustained polymorphic ventricular tachycardia, and torsades de pointes) are common in end-stage heart failure, especially if precipitating or factors are present like electrolyte disturbance, prolonged QT interval, and digoxin toxicity. Bradyarrhythmias may also happen.[49]
- Thromboembolism: HF is a cause of stroke in 9% of patients.[50] Between 10 and 24% of patients with stroke have HF.[51][52] There is a high relative risk for deep venous thrombosis (DVT) and pulmonary embolism (PE) in patients with HF, especially those who are under 60 years of age.[53]
- Gastrointestinal: liver shock (ischemic hepatitis), liver cirrhosis, and cardiac cachexia due to decreased intestinal blood flow in patients with HF.[54]
- Renal: renal function may get worse in both acute and chronic HF, and that predicts poor prognosis and even a small transient rise in creatinine will be clinically relevant.[55]
- Respiratory: pulmonary congestion, respiratory muscle weakness, and rarely pulmonary hypertension
Deterrence and Patient Education
Emphasizing diet and medical compliance to patients with heart failure is essential. One of the most common causes of readmission due to heart failure is the failure to comply with diet or medications. A single-session intervention could be beneficial. A randomized control trial of 605 patients with heart failure found that the incidence of all-cause hospitalization or mortality was not significantly reduced in patients receiving multisession self-care training compared to those receiving a single-session intervention.[56]
Printable patient education handout materials are available here:
What is Heart Failure? This printable patient education handout includes an explanation of systolic heart failure, which is also known as heart failure with reduced ejection fraction (HFrEF), heart failure with mildly reduced ejection fraction (HFmrEF), or heart failure with improved ejection fraction (HFimpEF). This patient education includes an explanation of diastolic heart failure, which is also called heart failure with preserved ejection fraction, or HFpEF. Heart failure symptoms and ejection fraction are discussed.
Coping With Heart Failure. This discusses ways to feel better and how to ask for help.
Heart Failure: How to be Active. This discusses how to safely stay active.
Heart Failure: Assessing Your Heart. This discusses which tests may be used to diagnose heart failure and what is typically included in a treatment plan.
Heart Failure: Making Changes to Your Diet. The buildup of excess fluid and a daily sodium goal are discussed. How to read food labels, how to limit salt and fluids, and when to call a healthcare provider are also discussed.
Heart Failure: Procedures That May Help. This patient education handout explains procedures, such as angioplasty and valve repair, used to treat artery and valve problems. Devices used to treat heart rhythm problems are explained. These include a pacemaker, biventricular pacing/cardiac resynchronization therapy (a special type of pacemaker), implantable cardioverter defibrillator (ICD), and wearable cardioverter defibrillator (WCD). A discussion on ventricular assist devices (VADs), heart transplant, and ultrafiltration for more serious cases of heart failure is provided.
Heart Failure: Tracking Your Weight. How to use a weight chart and when to call your doctor regarding weight gain are discussed.
Heart Failure: Warning Signs of a Flare-Up. Warning signs, including swelling, weight gain, and shortness of breath, are discussed.
Taking Medicine to Control Heart Failure. Diuretics, SGLT2 inhibitors, aldosterone blockers, beta-blockers, angiotensin receptor neprilysin inhibitor, angiotensin receptor blockers (ARBs), ACE inhibitors, hydralazine and nitrates, digoxin, sinus node I-f channel blocker, and statins are explained. This handout also includes tips for taking heart failure medications.
All 9 heart failure patient education handouts are free and printable.
Living Well With Heart Failure is an interactive guide for patients, families, and caregivers. This 78-page guide is a toolkit with quizzes, monitoring tools, videos, animations, and audio instruction. Included in the tool kit are a symptom chart, symptom action plan, sodium and meal log, medication list, activity log, healthcare team tracker, and resource list. The information is also printable. The interactive guide helps individuals learn how to maintain their health after experiencing heart failure. Chapters include the following:
- You Can Live Well with Heart Failure!
- Monitoring Symptoms
- Following Low-Sodium Diet
- Taking Medication
- Living with a Chronic Condition
- Steps for a Healthier Heart
Enhancing Healthcare Team Outcomes
Heart failure is a leading cause of hospitalization and represents a significant clinical and economic burden. The long-term goal of treatment is to avoid exacerbation of HF and decrease hospital readmission rates. It needs an interprofessional approach involving patients, physicians, nurses, pharmacists, families, and caretakers. Those strategies include early identification of high-risk patients, patient education, improving medication and dietary compliance, assuring close follow-up, introducing end-of-care issues, and tele-home monitoring if available. Primary care and emergency department providers often are the first to make this diagnosis. Referral to cardiologists is often appropriate. Cardiology, medical/surgical, and critical care nurses administer treatment, provide education, monitor patients, and communicate with the rest of the team so that everyone on the healthcare team operates from the same data set. The managing clinician would do well to consult with a board-certified cardiology pharmacist when initiating pharmaceutical care in HF cases. Pharmacists also review medicines, check the dosages, detect drug-drug interactions, and stress to patients and their families the importance of compliance. In end-stage cases, hospice care and nurses can work with the patient and their family to provide comfort care. These interprofessional collaborations will optimize patient outcomes in HF cases.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
. Correction to: 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2023 Apr 4:147(14):e674. doi: 10.1161/CIR.0000000000001142. Epub 2023 Apr 3 [PubMed PMID: 37011077]
Level 1 (high-level) evidenceWriting Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation. 2016 Jan 26:133(4):447-54. doi: 10.1161/CIR.0000000000000366. Epub [PubMed PMID: 26811276]
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017 Mar 7:135(10):e146-e603. doi: 10.1161/CIR.0000000000000485. Epub 2017 Jan 25 [PubMed PMID: 28122885]
Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC heart failure. 2014 Sep:1(1):4-25. doi: 10.1002/ehf2.12005. Epub [PubMed PMID: 28834669]
Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016 Jan 26:133(4):e38-360. doi: 10.1161/CIR.0000000000000350. Epub 2015 Dec 16 [PubMed PMID: 26673558]
Huffman MD, Berry JD, Ning H, Dyer AR, Garside DB, Cai X, Daviglus ML, Lloyd-Jones DM. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. Journal of the American College of Cardiology. 2013 Apr 9:61(14):1510-7. doi: 10.1016/j.jacc.2013.01.022. Epub [PubMed PMID: 23500287]
Level 2 (mid-level) evidenceNajafi F, Jamrozik K, Dobson AJ. Understanding the 'epidemic of heart failure': a systematic review of trends in determinants of heart failure. European journal of heart failure. 2009 May:11(5):472-9. doi: 10.1093/eurjhf/hfp029. Epub 2009 Feb 27 [PubMed PMID: 19251729]
Level 1 (high-level) evidenceTsuji K, Sakata Y, Nochioka K, Miura M, Yamauchi T, Onose T, Abe R, Oikawa T, Kasahara S, Sato M, Shiroto T, Takahashi J, Miyata S, Shimokawa H, CHART-2 Investigators. Characterization of heart failure patients with mid-range left ventricular ejection fraction-a report from the CHART-2 Study. European journal of heart failure. 2017 Oct:19(10):1258-1269. doi: 10.1002/ejhf.807. Epub 2017 Mar 31 [PubMed PMID: 28370829]
Golla MSG, Shams P. Heart Failure With Preserved Ejection Fraction (HFpEF). StatPearls. 2024 Jan:(): [PubMed PMID: 38320083]
Duncanson ER, Mackey-Bojack SM. Histologic Examination of the Heart in the Forensic Autopsy. Academic forensic pathology. 2018 Sep:8(3):565-615. doi: 10.1177/1925362118797736. Epub 2018 Aug 31 [PubMed PMID: 31240060]
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3:145(18):e895-e1032. doi: 10.1161/CIR.0000000000001063. Epub 2022 Apr 1 [PubMed PMID: 35363499]
Level 1 (high-level) evidence. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet (London, England). 1999 Jan 2:353(9146):9-13 [PubMed PMID: 10023943]
Level 1 (high-level) evidence. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet (London, England). 1999 Jun 12:353(9169):2001-7 [PubMed PMID: 10376614]
Level 1 (high-level) evidencePacker M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann-Zalan I, DeMets DL, Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002 Oct 22:106(17):2194-9 [PubMed PMID: 12390947]
Level 1 (high-level) evidenceDocherty KF, Vaduganathan M, Solomon SD, McMurray JJV. Sacubitril/Valsartan: Neprilysin Inhibition 5 Years After PARADIGM-HF. JACC. Heart failure. 2020 Oct:8(10):800-810. doi: 10.1016/j.jchf.2020.06.020. Epub [PubMed PMID: 33004114]
Berg DD, Samsky MD, Velazquez EJ, Duffy CI, Gurmu Y, Braunwald E, Morrow DA, DeVore AD. Efficacy and Safety of Sacubitril/Valsartan in High-Risk Patients in the PIONEER-HF Trial. Circulation. Heart failure. 2021 Feb:14(2):e007034. doi: 10.1161/CIRCHEARTFAILURE.120.007034. Epub 2021 Feb 3 [PubMed PMID: 33530704]
CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The New England journal of medicine. 1987 Jun 4:316(23):1429-35 [PubMed PMID: 2883575]
Level 1 (high-level) evidenceSOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The New England journal of medicine. 1991 Aug 1:325(5):293-302 [PubMed PMID: 2057034]
Level 1 (high-level) evidencePacker M, Poole-Wilson PA, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Rydén L, Thygesen K, Uretsky BF. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation. 1999 Dec 7:100(23):2312-8 [PubMed PMID: 10587334]
Level 1 (high-level) evidencePfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. The New England journal of medicine. 1992 Sep 3:327(10):669-77 [PubMed PMID: 1386652]
Level 1 (high-level) evidenceCohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. The New England journal of medicine. 2001 Dec 6:345(23):1667-75 [PubMed PMID: 11759645]
Level 1 (high-level) evidenceKonstam MA, Neaton JD, Dickstein K, Drexler H, Komajda M, Martinez FA, Riegger GA, Malbecq W, Smith RD, Guptha S, Poole-Wilson PA, HEAAL Investigators. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet (London, England). 2009 Nov 28:374(9704):1840-8. doi: 10.1016/S0140-6736(09)61913-9. Epub 2009 Nov 16 [PubMed PMID: 19922995]
Level 1 (high-level) evidencePacker M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F, EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. The New England journal of medicine. 2020 Oct 8:383(15):1413-1424. doi: 10.1056/NEJMoa2022190. Epub 2020 Aug 28 [PubMed PMID: 32865377]
Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet (London, England). 2020 Sep 19:396(10254):819-829. doi: 10.1016/S0140-6736(20)31824-9. Epub 2020 Aug 30 [PubMed PMID: 32877652]
Level 1 (high-level) evidenceBhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B, SOLOIST-WHF Trial Investigators. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. The New England journal of medicine. 2021 Jan 14:384(2):117-128. doi: 10.1056/NEJMoa2030183. Epub 2020 Nov 16 [PubMed PMID: 33200892]
Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, Komajda M, Miller A, Pehrson S, Teerlink JR, Schnaidt S, Zeller C, Schnee JM, Anker SD. Effect of Empagliflozin on Worsening Heart Failure Events in Patients With Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial. Circulation. 2021 Oct 19:144(16):1284-1294. doi: 10.1161/CIRCULATIONAHA.121.056824. Epub 2021 Aug 29 [PubMed PMID: 34459213]
Peikert A, Martinez FA, Vaduganathan M, Claggett BL, Kulac IJ, Desai AS, Jhund PS, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Shah SJ, Katova T, Merkely B, Vardeny O, Wilderäng U, Lindholm D, Petersson M, Langkilde AM, McMurray JJV, Solomon SD. Efficacy and Safety of Dapagliflozin in Heart Failure With Mildly Reduced or Preserved Ejection Fraction According to Age: The DELIVER Trial. Circulation. Heart failure. 2022 Oct:15(10):e010080. doi: 10.1161/CIRCHEARTFAILURE.122.010080. Epub 2022 Aug 27 [PubMed PMID: 36029467]
Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. The New England journal of medicine. 2011 Jan 6:364(1):11-21. doi: 10.1056/NEJMoa1009492. Epub 2010 Nov 14 [PubMed PMID: 21073363]
Level 1 (high-level) evidenceSzabo B, Benson L, Savarese G, Hage C, Fudim M, Devore A, Pitt B, Lund LH. Previous heart failure hospitalization, spironolactone, and outcomes in heart failure with preserved ejection fraction - a secondary analysis of TOPCAT. American heart journal. 2024 May:271():136-147. doi: 10.1016/j.ahj.2024.02.021. Epub 2024 Feb 25 [PubMed PMID: 38412897]
Taylor AL, Ziesche S, Yancy C, Carson P, D'Agostino R Jr, Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M, Cohn JN, African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. The New England journal of medicine. 2004 Nov 11:351(20):2049-57 [PubMed PMID: 15533851]
Level 1 (high-level) evidenceSwedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L, SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet (London, England). 2010 Sep 11:376(9744):875-85. doi: 10.1016/S0140-6736(10)61198-1. Epub [PubMed PMID: 20801500]
Level 1 (high-level) evidenceArmstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O'Connor CM, VICTORIA Study Group. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. The New England journal of medicine. 2020 May 14:382(20):1883-1893. doi: 10.1056/NEJMoa1915928. Epub 2020 Mar 28 [PubMed PMID: 32222134]
Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. The New England journal of medicine. 1997 Feb 20:336(8):525-33 [PubMed PMID: 9036306]
Level 1 (high-level) evidenceMarchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F, GISSI-Prevenzione Investigators. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002 Apr 23:105(16):1897-903 [PubMed PMID: 11997274]
Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ, PEARL-HF Investigators. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. European heart journal. 2011 Apr:32(7):820-8. doi: 10.1093/eurheartj/ehq502. Epub 2011 Jan 5 [PubMed PMID: 21208974]
Level 1 (high-level) evidenceFaselis C, Arundel C, Patel S, Lam PH, Gottlieb SS, Zile MR, Deedwania P, Filippatos G, Sheriff HM, Zeng Q, Morgan CJ, Wopperer S, Nguyen T, Allman RM, Fonarow GC, Ahmed A. Loop Diuretic Prescription and 30-Day Outcomes in Older Patients With Heart Failure. Journal of the American College of Cardiology. 2020 Aug 11:76(6):669-679. doi: 10.1016/j.jacc.2020.06.022. Epub [PubMed PMID: 32762901]
Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O'Connor CM, NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. The New England journal of medicine. 2011 Mar 3:364(9):797-805. doi: 10.1056/NEJMoa1005419. Epub [PubMed PMID: 21366472]
Level 1 (high-level) evidenceMullens W, Dauw J, Martens P, Meekers E, Nijst P, Verbrugge FH, Chenot F, Moubayed S, Dierckx R, Blouard P, Derthoo D, Smolders W, Ector B, Hulselmans M, Lochy S, Raes D, Van Craenenbroeck E, Vandekerckhove H, Hofkens PJ, Goossens K, Pouleur AC, De Ceuninck M, Gabriel L, Timmermans P, Prihadi EA, Van Durme F, Depauw M, Vervloet D, Viaene E, Vachiery JL, Tartaglia K, Ter Maaten JM, Bruckers L, Droogne W, Troisfontaines P, Damman K, Lassus J, Mebazaa A, Filippatos G, Ruschitzka F, Dupont M. Acetazolamide in Decompensated Heart Failure with Volume Overload trial (ADVOR): baseline characteristics. European journal of heart failure. 2022 Sep:24(9):1601-1610. doi: 10.1002/ejhf.2587. Epub 2022 Jul 12 [PubMed PMID: 35733283]
Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Göhring UM, Keren A, Khintibidze I, Kragten H, Martinez FA, Metra M, Milicic D, Nicolau JC, Ohlsson M, Parkhomenko A, Pascual-Figal DA, Ruschitzka F, Sim D, Skouri H, van der Meer P, Lewis BS, Comin-Colet J, von Haehling S, Cohen-Solal A, Danchin N, Doehner W, Dargie HJ, Motro M, Butler J, Friede T, Jensen KH, Pocock S, Jankowska EA, AFFIRM-AHF investigators. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet (London, England). 2020 Dec 12:396(10266):1895-1904. doi: 10.1016/S0140-6736(20)32339-4. Epub 2020 Nov 13 [PubMed PMID: 33197395]
Level 1 (high-level) evidenceKittleson MM, Panjrath GS, Amancherla K, Davis LL, Deswal A, Dixon DL, Januzzi JL Jr, Yancy CW. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. Journal of the American College of Cardiology. 2023 May 9:81(18):1835-1878. doi: 10.1016/j.jacc.2023.03.393. Epub 2023 Apr 19 [PubMed PMID: 37137593]
Level 3 (low-level) evidenceWRITING COMMITTEE MEMBERS, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013 Oct 15:128(16):e240-327. doi: 10.1161/CIR.0b013e31829e8776. Epub 2013 Jun 5 [PubMed PMID: 23741058]
Level 3 (low-level) evidencePonikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European heart journal. 2016 Jul 14:37(27):2129-2200. doi: 10.1093/eurheartj/ehw128. Epub 2016 May 20 [PubMed PMID: 27206819]
Golla MSG, Brown KN, Gupta N. Percutaneous Transluminal Coronary Arteriography. StatPearls. 2024 Jan:(): [PubMed PMID: 30844185]
Heart Failure Society of America, Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. HFSA 2010 Comprehensive Heart Failure Practice Guideline. Journal of cardiac failure. 2010 Jun:16(6):e1-194. doi: 10.1016/j.cardfail.2010.04.004. Epub [PubMed PMID: 20610207]
Level 1 (high-level) evidenceLund LH, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, Dobbels F, Goldfarb SB, Levvey BJ, Meiser B, Yusen RD, Stehlik J, International Society of Heart and Lung Transplantation. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report--2014; focus theme: retransplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2014 Oct:33(10):996-1008. doi: 10.1016/j.healun.2014.08.003. Epub 2014 Aug 14 [PubMed PMID: 25242124]
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members, Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European journal of heart failure. 2016 Aug:18(8):891-975. doi: 10.1002/ejhf.592. Epub 2016 May 20 [PubMed PMID: 27207191]
Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, Rogers JG, Naka Y, Mancini D, Miller LW. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007 Jul 31:116(5):497-505 [PubMed PMID: 17638928]
Level 2 (mid-level) evidenceAbraham WT, Fonarow GC, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB, OPTIMIZE-HF Investigators and Coordinators. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Journal of the American College of Cardiology. 2008 Jul 29:52(5):347-56. doi: 10.1016/j.jacc.2008.04.028. Epub [PubMed PMID: 18652942]
Xiao YF. Cardiac arrhythmia and heart failure: From bench to bedside. Journal of geriatric cardiology : JGC. 2011 Sep:8(3):131-2. doi: 10.3724/SP.J.1263.2011.00131. Epub [PubMed PMID: 22783298]
Haeusler KG, Laufs U, Endres M. Chronic heart failure and ischemic stroke. Stroke. 2011 Oct:42(10):2977-82. doi: 10.1161/STROKEAHA.111.628479. Epub 2011 Sep 8 [PubMed PMID: 21903953]
Divani AA, Vazquez G, Asadollahi M, Qureshi AI, Pullicino P. Nationwide frequency and association of heart failure on stroke outcomes in the United States. Journal of cardiac failure. 2009 Feb:15(1):11-6. doi: 10.1016/j.cardfail.2008.09.001. Epub 2008 Dec 25 [PubMed PMID: 19181288]
Kim W, Kim EJ. Heart Failure as a Risk Factor for Stroke. Journal of stroke. 2018 Jan:20(1):33-45. doi: 10.5853/jos.2017.02810. Epub 2018 Jan 31 [PubMed PMID: 29402070]
Beemath A, Stein PD, Skaf E, Al Sibae MR, Alesh I. Risk of venous thromboembolism in patients hospitalized with heart failure. The American journal of cardiology. 2006 Sep 15:98(6):793-5 [PubMed PMID: 16950187]
Sandek A, Swidsinski A, Schroedl W, Watson A, Valentova M, Herrmann R, Scherbakov N, Cramer L, Rauchhaus M, Grosse-Herrenthey A, Krueger M, von Haehling S, Doehner W, Anker SD, Bauditz J. Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. Journal of the American College of Cardiology. 2014 Sep 16:64(11):1092-102. doi: 10.1016/j.jacc.2014.06.1179. Epub [PubMed PMID: 25212642]
Udani SM, Koyner JL. The effects of heart failure on renal function. Cardiology clinics. 2010 Aug:28(3):453-65. doi: 10.1016/j.ccl.2010.04.004. Epub [PubMed PMID: 20621250]
Level 3 (low-level) evidenceDeWalt DA, Schillinger D, Ruo B, Bibbins-Domingo K, Baker DW, Holmes GM, Weinberger M, Macabasco-O'Connell A, Broucksou K, Hawk V, Grady KL, Erman B, Sueta CA, Chang PP, Cene CW, Wu JR, Jones CD, Pignone M. Multisite randomized trial of a single-session versus multisession literacy-sensitive self-care intervention for patients with heart failure. Circulation. 2012 Jun 12:125(23):2854-62. doi: 10.1161/CIRCULATIONAHA.111.081745. Epub 2012 May 9 [PubMed PMID: 22572916]
Level 1 (high-level) evidence