 Dermoscopy Overview and Extradiagnostic Applications
Dermoscopy Overview and Extradiagnostic Applications
Introduction
Cutaneous diagnosis is often, but not always, visually based. Dermatologists tend to encounter situations where the possibility of multiple differentials complicates the diagnosis and mandates investigations for confirmation. Methods commonly employed for cutaneous diagnosis may be invasive (skin and scalp biopsy), semi-invasive (slit skin smears, trichogram, etc.) or non-invasive (e.g., KOH smear, nail clipping, hair count for hair loss).[1] Dermoscopy, also known as epiluminescence microscopy, or skin surface microscopy, is a non-invasive, in-vivo technique, which has traditionally found use in the evaluation and differentiation of suspicious melanocytic lesions from dysplastic lesions and melanomas, as well as keratinocyte skin cancers such as basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).[2]
Over the last several years, the use of dermoscopy has been increasing in the context of general dermatological disorders including inflammatory dermatosis, pigmentary dermatosis, infectious dermatosis, and disorders of the hair, scalp, and nails. Some terms are used to describe specific indications: pigmentaroscopy for pigmented lesions, trichoscopy of the scalp and hair, onychoscopy of the nails, inflammoscopy for inflammatory dermatosis and lesions, as well as entomodermoscopy of skin infestations and infections [3][4]. The role of dermoscopy in diagnosing disorders of general dermatology has undergone elaborate discussion [4]. In this chapter, we shall review the plethora of extra-diagnostic indications of this technique and highlight technical aspects worth considering.
Equipment
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Equipment
A dermatoscope functionally simulates a magnifying lens, with the added features of much higher magnification, and an adjustable inbuilt illuminating system. A hand-lens, even with in-built illumination, cannot allow visualization beyond the surface of the skin because of the reflection and scattering of light from the stratum corneum. A dermatoscope can assess structures to the depth of reticular dermis, and record images for future comparison. The basic principle of dermoscopy is transillumination of a lesion in order to study it with high magnification to visualize subtle features. Light incident on a surface like the skin may be reflected, refracted, diffracted and/or absorbed [Figure 1A]. The physical properties of the skin influence these phenomena. Most light incident on dry, scaly skin is reflected, but smooth, oily skin allows light to pass through to reach the deeper dermis. Application of a linkage or immersion fluid (like mineral oil, liquid paraffin, ultrasound gel, or 70% alcohol-based commercial solutions) over the skin, enhances translucency and improves visibility of subsurface skin structures of the lesion under investigation. The essential components of a dermatoscope include: 1) a set of achromatic lenses with magnification starting from 10× up to 200× or even higher, 2) an inbuilt illuminating system composed of halogen lamps placed within the handheld piece, and 3) a source of power supply such as rechargeable or replaceable batteries or rechargeable handles.
The newest generation of dermatoscopes include inbuilt crossed-polarizers, which filter out scattered light from the periphery, reduce glare, and permit visualization of substratal structures without the need of a linkage fluid. Figure 1B demonstrates the basic working of the modern dermatoscopes schematically. Some dermatoscopes have an inbuilt photography system with supporting software for the capture and storage of images. For those dermatoscopes without inbuilt systems, special adapters are available to connect to digital cameras. Advanced devices have whole body mapping systems for detailed analysis and follow up of skin lesions over time. Newer handheld units can attach to smart-phones for easier image capture and documentation [5].
Technique or Treatment
Dermatoscopes may be with or without an in-built image capturing facilities. A simple hand-held dermatoscope looks like a broader version of an otoscope and lacks an inbuilt camera. Image-capture dermatoscopes have a special lens, which mounts onto a conventional or a digital camera. United serial bus (USB) video-dermatoscopes have a high-resolution camera fitted to the handpiece that allows visualization of the image on the computer screen as well as capturing videos. Advanced dermatoscopes have an analytical capability in addition to image-capture.
Dermoscopy is performable by either the non-contact or the contact technique. In the contact technique, the glass plate of the instrument touches the lesion through the linkage fluid. In the non-contact technique, the cross-polarized lens absorbs all the scattered light and hence allows only light in a single plane to pass through it without contact of the lens with the skin. The contact technique gives better illumination and resolution. The advantage of the non-contact technique is the prevention of inter-patient infections. Avoidance of cross-infection in the case of contact dermoscopy is by using a barrier like a cling film or adhesive tape over the lesional skin.[2]
Although the advent of high-quality dermatoscopes with polarizers has rendered the use of linkage fluids and contact dermoscopy almost redundant; it is worthwhile to be cognizant of the concept. The linkage fluid enhances the translucency of stratum corneum facilitating imaging of deeper structures. Many substances can function in this capacity including mineral oil, ethanol, liquid paraffin, and ECG/USG gel. The latter remains the most commonly used linkage fluid in the current era, especially for onychoscopy.
The recent improvements in the manufacturing of dermatoscopes include - reduction in dimensions and bulk of the device, wi-fi connectivity for USB dermatoscopes, digital image analysis, and attempts to incorporate artificial intelligence to create an automated diagnostic unit.
Complications
As a non-invasive technique, dermoscopy is essentially free of complications. The only issue is the minimal possibility of cross-infection between patients, especially with contact dermoscopy. Many tricks can obviate the possibility of cross-infection:
1) Use of polarized non-contact dermoscopy.
2) Disinfection of the lens (in case of contact dermoscopy) or the rim of the USB video-dermatoscope with isopropyl alcohol after examining each patient.
3) Use of disposable transparent lens shielding material like a cling film or soft plastic caps over the device; the latter are now being provided complimentary with most of the high-quality dermatoscopes - for use in both hand-held and USB video-dermatoscopes.
Minor issues worth consideration
1) One should be aware of artifacts of dermoscopy that may suffer from incorrect interpretation.[6] Common artifacts include vermillion powder, colored powders, dust particles, hair dye, henna, hair fibers minoxidil crystals, hair styling gel, etc. in trichoscopy, dust particles, topical applications especially sunscreen and make-up ingredients during dermoscopy of the face, and nail paint and varnish in onychoscopy. The area to be scoped should be thoroughly cleaned with alcohol first to remove these artifacts.
2) Inter-device color difference in images: Different dermatoscopes tend to give images with a mildly skewed color balance. One must be aware of that and interpret results accordingly
3) Differences in different Fitzpatrick skin types: It is now amply clear that many features that are easy to appreciate in Fitzpatrick types I-II skin, are either not seen or are obscured in darker skin types. Honeycombing in the scalp is considered suggestive of androgenetic alopecia (AGA) in Fitzpatrick skin phototypes I to III but is a normal finding in the scalp of individuals with darker skin types. The colors (black, brown, gray, and blue) that have their basis in the histological level are neither easy to observe nor interpret in dark skin. Brown pigmented structures are often seen on dermoscopy of various disorders in ethnic skin due to the propensity of post-inflammatory hyperpigmentation, and thus warrant careful interpretation.
4) Lack of 'dermoscopic nomograms': To master the interpretation of histopathology, one must be thoroughly conversant with the normal histology, taking into account expected physiological variations due to a particular part of the human body, age, and gender. For example, the normal mucoscopic images from buccal mucosa reveal plentiful vessels, which should not be confused as a pathological feature. There is an urgent need to have an image bank of such site-specific, and skin type-specific dermoscopic nomograms to minimize errors in the interpretation of dermoscopic structures.
Clinical Significance
Dermoscopy may result in confirmation of clinical diagnosis, often avoiding the need for a skin biopsy. Although a skin biopsy and clinicopathological correlation (CPC) remain the gold standard for cutaneous diagnosis, dermoscopy often helps tilt the clinical differential in instances where it identifies a distinct pattern. Its use has been most popular for differentiation between melanocytic nevi and melanomas in the West. However, the indications of dermoscopy are continually expanding and include an evaluation of hair disorders, general pigmentary disorders, appendageal tumors, inflammatory disorders like psoriasis and lichen planus, and for pre- and post-evaluation of therapeutic procedures. In the current scheme of things, we are gradually moving from CPC to clinico-dermoscopic-pathological correlation (CDPC).[7]
Dermoscopic patterns (mainly based on pigment and vascular patterns) for conditions like melanoma and NMSCs have been well established, leading to the development of dermoscopic algorithms for early diagnosis and triage.[8][9] Documentation of dermoscopy patterns for general dermatological conditions, other than tumors, has also improved significantly over the past few years.[4] Work is also being done to establish differences in these patterns, especially in patients with a skin of color (SOC).
Uses of dermoscopy beyond diagnosis
The dermatoscope is an immensely versatile tool, with many more uses beyond diagnosis.[7] Its uses beyond diagnosis include:
Disease activity evaluation: Dermoscopy can confidently predict disease activity, e.g., active alopecia areata (AA) shows black dots, 'exclamation-mark' hairs, broken hairs, yellow dots and clustered short vellus hairs, whereas, in a treatment-responsive patch of AA, black dots tend to disappear, pig-tail, and upright regrowing hairs appear and yellow dots persist.[10] Dermoscopy has similarly been found useful for assessing vitiligo stability, which is an essential criterion for surgical intervention.[11] For evaluation of vitiligo, perifollicular depigmentation (PFD), marginal hyperpigmentation, and presence of leucotrichia are suggestive of lesional stability, while an altered pigment pattern, perifollicular pigment retention (PFP), and features such as star-burst appearance, comet tail sign, and ‘tapioca sago’ appearance are suggestive of disease activity. Forf rontal fibrosing alopecia (FFA), presence of background erythema is suggestive of disease activity [12]. Dermoscopic features have also been reported to predict the presence of residual disease [residual disease-associated dermoscopic criteria (RDADC)] in skin tumors such as basal cell carcinoma [13].
Early comparison of pre- and post-treatment: Appreciation of the post-treatment effect often precedes clinical improvement; this is especially true of chronic relapsing, recalcitrant dermatoses like melasma, lichen planus pigmentosus (LPP), vitiligo, alopecias, etc.[14][15] The dermoscopic images not only offer confidence to the treating dermatologist about the appropriateness of the therapeutic protocol; they provide reassurance to the patient about the effectiveness of the treatment and increase patient compliance.
Ex-Vivo Dermoscopy (EVD) to improve histopathological evaluation: Dermoscopy-guided histological sectioning of the biopsy tissue can improve upon the sectioning of melanocytic neoplasms.[16] Combining EVD with derm-dotting (DD) in which a dermoscopically suspicious area of the biopsy specimen gets marked with nail varnish has been reported to allow more accurate and less time-consuming histopathologic diagnosis of skin tumors.[17]
Better doctor-patient communication: Dermoscopy improves the doctor-patient communication regarding all aspects of skin disease. Explaining the nature of the disorder becomes easier by showing lesional dermoscopic images to patients. Patients who warrant a skin biopsy but refuse can be convinced for the same after having been shown dermoscopic images, a concept popular as 'dermoscopy-induced biopsy.'[7] Dermoscopy also aids in the selection of the optimum site for biopsy. Dermoscopy-guided skin biopsy is useful in various pigmentary disorders, scalp conditions, vasculitis, and skin tumours.[18][19][16]
Dermoscopic images as an evolving tool for clinical studies: Since dermoscopy has proven a role as an early, objective and reliable measure of pre- and post-treatment comparison, it is now increasingly being used as an adjuvant tool for efficacy evaluation of therapeutic modalities in clinical studies.[20][21][22][23][24] Gaining more experience and evidence of dermoscopy as the marker of therapeutic outcome may eventually obviate the need for repeat biopsy for response evaluation, rendering the study less and less invasive.
Dermoscopy in Dermatosurgery & Aesthetics: Dermoscopy has been reported to facilitate the prompt identification and removal of retained sutures in crusted wounds. Dermoscopy can also facilitate the identification and removal of a foreign body impacted in the skin.[25] Dermoscopy also finds its utility in certain cosmetic/aesthetic procedures, e.g., the dermoscopic photoaging scale (DPAS), has been recently conceived and validated for quantitative evaluation of photoaged facial skin.[26]
Dermoscopic evaluation of peri-ocular hyperpigmentation can aid in identifying the predominant abnormality (skin pigmentation, abnormal vasculature, skin laxity) thereby help in the development of customized treatment protocol.[27]
Dermoscopy-based imaging system has recently been reported to be instrumental in the evaluation and categorization of ‘enlarged’ facial open pores.[28][29]
Dermoscopy plays a significant role in optimizing the outcome of laser hair reduction (LHR) and has been reported to be useful in monitoring the response to LHR in women.[30] Evaluation of the scattering and thickness of hairs before laser session has been especially utile in dark-skinned patients with hirsutism. Laser parameters, especially pulse duration (that depends on the thickness of the hair being targeted) can be fine-tuned.
Hair transplant surgeons are increasingly using trichoscopy. It allows recording of the number of existing follicular units, the number of hair per follicular unit, the size of hair follicles, and other parameters at the donor and recipient sites. Trichoscopy aids in early differential diagnosis of post-transplant complications such as folliculitis and secondary lichen planopilaris. It is also useful in optimizing outcome of ancillary trichology procedures such as scalp micro pigmentation (SMP).[31]
Miscellaneous Uses: Dermoscopy has been reported to aid in confirmation of pathergy test in patients with Behcet’s disease.[32] On similar principles, dermoscopic imaging can aid in confirmation of a patch test reaction being a true positive or irritant, especially in dark-skinned individuals. Dermoscopy has also proved useful in mapping and better ablation of 'cosmetic warts,' especially verruca plana in the shaving region of men.[7]
Teledermoscopy: The acquisition and storage of digital dermoscopic images are referred to as digital dermoscopy, while teledermoscopy (TDD) refers to transferring a digitalized dermoscopic image (visual data) for diagnosis, education, consultation or followup.[33] Mobile TDD uses a smartphone to deliver the same type of service. Teledermoscopy can greatly reduce the number of unnecessary referrals, wait times, and the cost of providing and receiving skin care. Although the role of TDD in improving the reliability of telediagnosis and prognostication of patients with melanoma is well-established,[34] the concept of TDD also has potential implications for delivery of better skin care services in the developing world, where primary care physicians (PCPs) who are non-dermatologists cater to a large proportion of patients with cutaneous disorders.[35]
Enhancing Healthcare Team Outcomes
Although dermoscopy is an excellent tool for triage, it needs to be combined with the macro clinical picture and histopathology to be conclusive. The role of the dermatopathologist is vital in this regard. From what used to a clinicopathological correlation, we are moving towards a clinical-dermoscopic-pathological correlation.
As far as levels of evidence are concerned, a recently published Cochrane meta-analysis sums it up aptly-although evidence-based is limited, the conclusion is that when specialists use dermoscopy, it is a better tool for the diagnosis of melanoma as compared to simple visual examination. Also, dermoscopy is more effective when interpreted with the actual patient, rather than with a dermoscopy image.[36]
Dermoscopy should not be considered as an 'ancillary' or 'optional' tool now, at least for a dermatologist.
Dermoscopy is not only for dermatologists, rather the skill should be acquired and customized by other specialists too, especially general practitioners/family physicians, pediatricians, and dermatosurgeons. Pediatricians in particular must get acquainted with dermoscopy as the non-invasive and visually engaging property of dermoscopy and its images make pediatricians' interaction with an anxious child much more convenient.[7]
The use of dermoscopy by general physicians is very low. The impact of subspecialization and dermatoscopy use on the accuracy of melanoma diagnosis among primary care doctors in Australia.[37] With respect to general/family physicians, regrettably, many barriers have resulted in extremely low usage of dermoscopy. Some of these barriers include - costs of dermoscopy—both the equipment cost and the relatively inadequate reimbursement for its use in practice, the need for dermoscopy training, lack of information about learning resources and the unwillingness to invest time, both for training and to use dermoscopy in practice.
Media
(Click Image to Enlarge)
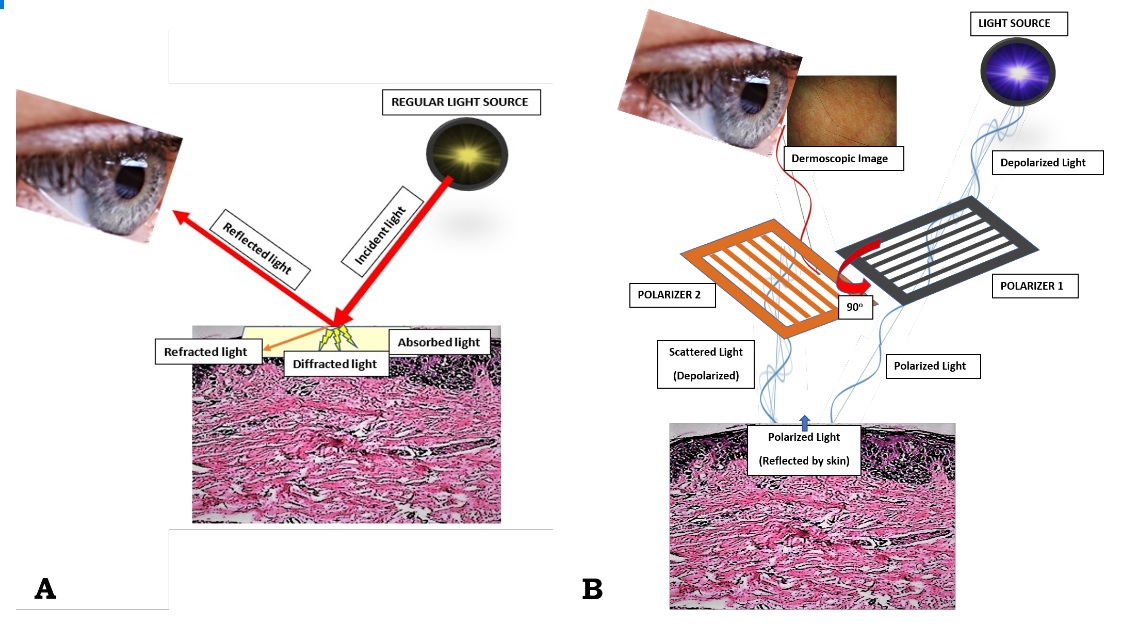
Figure 1. (A) When light is incident on the skin (thick red arrow), most of it tends to get reflected back (thin red arrow), while the remaining gets refracted (oblique orange arrow), diffracted (yellow shooting arrows) or absorbed (crimson area). On looking directly at the skin by unassisted eyes, one sees the external image of the skin formed by the reflected light; (B) Working principle of a modern dermoscope. In the polarized mode, the light from the dermoscope gets polarized by two cross-polarizers, cutting out the scattered light reflected from the skin, and allowing an image formation with visualization of sub-stratal structures. Contributed by Sidharth Sonthalia, MD, DNB, MNAMS
References
Sgouros D, Apalla Z, Ioannides D, Katoulis A, Rigopoulos D, Sotiriou E, Stratigos A, Vakirlis E, Lallas A. Dermoscopy of Common Inflammatory Disorders. Dermatologic clinics. 2018 Oct:36(4):359-368. doi: 10.1016/j.det.2018.05.003. Epub 2018 Jul 31 [PubMed PMID: 30201145]
Kaliyadan F. The scope of the dermoscope. Indian dermatology online journal. 2016 Sep-Oct:7(5):359-363 [PubMed PMID: 27730030]
Rosendahl C. Dermatoscopy in general practice. The British journal of dermatology. 2016 Oct:175(4):673-4. doi: 10.1111/bjd.14609. Epub [PubMed PMID: 27650743]
Errichetti E,Stinco G, Dermoscopy in General Dermatology: A Practical Overview. Dermatology and therapy. 2016 Dec; [PubMed PMID: 27613297]
Level 3 (low-level) evidenceCampos-do-Carmo G, Ramos-e-Silva M. Dermoscopy: basic concepts. International journal of dermatology. 2008 Jul:47(7):712-9. doi: 10.1111/j.1365-4632.2008.03556.x. Epub [PubMed PMID: 18613881]
Sonthalia S, Tiwary P. Colored dots on trichoscopy-beware of artifacts. Journal of the American Academy of Dermatology. 2019 Jun:80(6):e143-e144. doi: 10.1016/j.jaad.2018.11.052. Epub 2018 Dec 6 [PubMed PMID: 30529542]
Sonthalia S, Errichetti E. Dermoscopy - Not just for diagnosis and not just for Dermatologists ! Kathmandu University medical journal (KUMJ). 2017 Jan.-Mar.:15(57):1-2 [PubMed PMID: 29446353]
Carli P, De Giorgi V, Massi D, Giannotti B. The role of pattern analysis and the ABCD rule of dermoscopy in the detection of histological atypia in melanocytic naevi. The British journal of dermatology. 2000 Aug:143(2):290-7 [PubMed PMID: 10951135]
Ferrante di Ruffano L, Takwoingi Y, Dinnes J, Chuchu N, Bayliss SE, Davenport C, Matin RN, Godfrey K, O'Sullivan C, Gulati A, Chan SA, Durack A, O'Connell S, Gardiner MD, Bamber J, Deeks JJ, Williams HC, Cochrane Skin Cancer Diagnostic Test Accuracy Group. Computer-assisted diagnosis techniques (dermoscopy and spectroscopy-based) for diagnosing skin cancer in adults. The Cochrane database of systematic reviews. 2018 Dec 4:12(12):CD013186. doi: 10.1002/14651858.CD013186. Epub 2018 Dec 4 [PubMed PMID: 30521691]
Level 1 (high-level) evidenceKibar M, Aktan Ş, Lebe B, Bilgin M. Trichoscopic findings in alopecia areata and their relation to disease activity, severity and clinical subtype in Turkish patients. The Australasian journal of dermatology. 2015 Feb:56(1):e1-6. doi: 10.1111/ajd.12102. Epub 2013 Aug 29 [PubMed PMID: 23991834]
Kumar Jha A, Sonthalia S, Lallas A, Chaudhary RKP. Dermoscopy in vitiligo: diagnosis and beyond. International journal of dermatology. 2018 Jan:57(1):50-54. doi: 10.1111/ijd.13795. Epub 2017 Oct 26 [PubMed PMID: 29076154]
Sonthalia S, Jha AK, Tiwary PK. A Dermoscopic Diagnosis and Activity Evaluation of Frontal Fibrosing Alopecia in an Indian Lady. Indian dermatology online journal. 2017 Mar-Apr:8(2):162-163. doi: 10.4103/idoj.IDOJ_307_16. Epub [PubMed PMID: 28405571]
Apalla Z, Lallas A, Tzellos T, Sidiropoulos T, Lefaki I, Trakatelli M, Sotiriou E, Lazaridou E, Evangelou G, Patsatsi A, Kyrgidis A, Stratigos A, Zalaudek I, Argenziano G, Ioannides D. Applicability of dermoscopy for evaluation of patients' response to nonablative therapies for the treatment of superficial basal cell carcinoma. The British journal of dermatology. 2014 Apr:170(4):809-15. doi: 10.1111/bjd.12749. Epub [PubMed PMID: 24283541]
Shetty VH, Goel S. Dermoscopic pre- and posttreatment evaluation in patients with androgenetic alopecia on platelet-rich plasma-A prospective study. Journal of cosmetic dermatology. 2019 Oct:18(5):1380-1388. doi: 10.1111/jocd.12845. Epub 2018 Dec 16 [PubMed PMID: 30556270]
Jha AK, Sonthalia S, Lallas A, Chaudhary RKP. Post-graft trichrome and Manchurian gravy signs on dermoscopy can predict disease activity in vitiligo lesions post-skin grafting. International journal of dermatology. 2018 Nov:57(11):e144-e145. doi: 10.1111/ijd.14181. Epub 2018 Aug 3 [PubMed PMID: 30074621]
Merkel EA, Amin SM, Lee CY, Rademaker AW, Yazdan P, Martini MC, Guitart J, Gerami P. The utility of dermoscopy-guided histologic sectioning for the diagnosis of melanocytic lesions: A case-control study. Journal of the American Academy of Dermatology. 2016 Jun:74(6):1107-13. doi: 10.1016/j.jaad.2016.01.002. Epub 2016 Jan 28 [PubMed PMID: 26826889]
Level 2 (mid-level) evidenceHaspeslagh M, Hoorens I, Degryse N, De Wispelaere I, Degroote A, Van Belle S, Verboven J, Vossaert K, Facchetti F, Van Dorpe J, De Schepper S, Brochez L. Pathologic Evaluation of Skin Tumors With Ex Vivo Dermoscopy With Derm Dotting. JAMA dermatology. 2017 Feb 1:153(2):154-161. doi: 10.1001/jamadermatol.2016.4444. Epub [PubMed PMID: 28030717]
Liu WC, Tey HL, Lee JS, Goh BK. Exogenous ochronosis in a Chinese patient: use of dermoscopy aids early diagnosis and selection of biopsy site. Singapore medical journal. 2014 Jan:55(1):e1-3 [PubMed PMID: 24452981]
Level 3 (low-level) evidenceChoo JY, Bae JM, Lee JH, Lee JY, Park YM. Blue-gray blotch: A helpful dermoscopic finding in optimal biopsy site selection for true vasculitis. Journal of the American Academy of Dermatology. 2016 Oct:75(4):836-838. doi: 10.1016/j.jaad.2016.05.022. Epub [PubMed PMID: 27646743]
Carbotti M, Coppola R, Zanframundo S, Devirgiliis V, Panasiti V. Efficacy of Ingenol Mebutate in the Treatment of Actinic Keratoses: A Pre- and Posttreatment Dermoscopic Comparative Analysis. BioMed research international. 2018:2018():4381019. doi: 10.1155/2018/4381019. Epub 2018 Aug 29 [PubMed PMID: 30246021]
Level 2 (mid-level) evidenceJha AK, Udayan UK, Roy PK, Amar AKJ, Chaudhary RKP. Original article: Platelet-rich plasma with microneedling in androgenetic alopecia along with dermoscopic pre- and post-treatment evaluation. Journal of cosmetic dermatology. 2018 Jun:17(3):313-318. doi: 10.1111/jocd.12394. Epub 2017 Aug 3 [PubMed PMID: 28771982]
Mun JH, Park JM, Song M, Jwa SW, Kim HS, Ko HC, Kim BS, Kim MB. The use of dermatoscopy to monitor therapeutic response of Bowen disease: a dermatoscopic pathological study. The British journal of dermatology. 2012 Dec:167(6):1382-5. doi: 10.1111/j.1365-2133.2012.11124.x. Epub [PubMed PMID: 22759263]
Micali G, Lacarrubba F, Tedeschi A. Videodermatoscopy enhances the ability to monitor efficacy of scabies treatment and allows optimal timing of drug application. Journal of the European Academy of Dermatology and Venereology : JEADV. 2004 Mar:18(2):153-4 [PubMed PMID: 15009292]
Corazza M, Maietti E, Toni G, Virgili A, Borghi A. Combining topical tretinoin with mometasone furoate in the treatment of vulvar lichen sclerosus: Results of dermoscopic assessment. Dermatologic therapy. 2018 Nov:31(6):e12735. doi: 10.1111/dth.12735. Epub 2018 Oct 17 [PubMed PMID: 30334327]
Sonthalia S, Jha AK, Kaliyadan F. Dermoscopy for the detection and safe extraction of an intracutaneous foreign body. Journal of the American Academy of Dermatology. 2018 Aug:79(2):e19-e20. doi: 10.1016/j.jaad.2018.03.003. Epub 2018 Mar 10 [PubMed PMID: 29535034]
Isik B, Gurel MS, Erdemir AT, Kesmezacar O. Development of skin aging scale by using dermoscopy. Skin research and technology : official journal of International Society for Bioengineering and the Skin (ISBS) [and] International Society for Digital Imaging of Skin (ISDIS) [and] International Society for Skin Imaging (ISSI). 2013 May:19(2):69-74. doi: 10.1111/srt.12033. Epub 2013 Jan 20 [PubMed PMID: 23331299]
Mostafa WZ, Kadry DM, Mohamed EF. The effects of normobaric oxygen therapy on patients with periorbital darkening: An open, uncontrolled trial. Indian journal of dermatology, venereology and leprology. 2015 Jul-Aug:81(4):427-9. doi: 10.4103/0378-6323.159946. Epub [PubMed PMID: 26144854]
Uhoda E, Piérard-Franchimont C, Petit L, Piérard GE. The conundrum of skin pores in dermocosmetology. Dermatology (Basel, Switzerland). 2005:210(1):3-7 [PubMed PMID: 15604536]
Kim BY, Choi JW, Park KC, Youn SW. Sebum, acne, skin elasticity, and gender difference - which is the major influencing factor for facial pores? Skin research and technology : official journal of International Society for Bioengineering and the Skin (ISBS) [and] International Society for Digital Imaging of Skin (ISDIS) [and] International Society for Skin Imaging (ISSI). 2013 Feb:19(1):e45-53. doi: 10.1111/j.1600-0846.2011.00605.x. Epub 2011 Dec 28 [PubMed PMID: 22211382]
Mohamed EE, Ahmed AM, Tawfik KM, Ibrahim SM. Trichoscopic changes in hair during treatment of hirsutism with 1064-nm neodymium:yttrium-aluminum-garnet laser. Journal of cosmetic dermatology. 2016 Mar:15(1):31-5. doi: 10.1111/jocd.12164. Epub 2015 Jul 29 [PubMed PMID: 26223429]
Dhurat RS, Shanshanwal SJS, Dandale AL. Standardization of SMP Procedure and Its Impact On Outcome. Journal of cutaneous and aesthetic surgery. 2017 Jul-Sep:10(3):145-149. doi: 10.4103/JCAS.JCAS_116_16. Epub [PubMed PMID: 29403185]
Scherrer MA, de Castro LP, Rocha VB, Pacheco L. [The dermatoscopy in the skin pathergy testing: case series in patients with suspected Behçet's Disease]. Revista brasileira de reumatologia. 2014 Nov-Dec:54(6):494-8. doi: 10.1016/j.rbr.2014.06.003. Epub 2014 Sep 28 [PubMed PMID: 25445631]
Level 2 (mid-level) evidenceLee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatology practical & conceptual. 2018 Jul:8(3):214-223. doi: 10.5826/dpc.0803a13. Epub 2018 Jul 31 [PubMed PMID: 30116667]
Ferrándiz L, Ruiz-de-Casas A, Martin-Gutierrez FJ, Peral-Rubio F, Mendez-Abad C, Rios-Martin JJ, Moreno-Ramirez D. Effect of teledermatology on the prognosis of patients with cutaneous melanoma. Archives of dermatology. 2012 Sep:148(9):1025-8. doi: 10.1001/archdermatol.2012.778. Epub [PubMed PMID: 22986852]
Sonthalia S, Agrawal M, Goldust M, Das S, Bhattacharya SN. Antifungal therapeutic failures in India: an important issue being overlooked. The Lancet. Infectious diseases. 2018 Nov:18(11):1181-1182. doi: 10.1016/S1473-3099(18)30593-0. Epub 2018 Oct 24 [PubMed PMID: 30507401]
Dinnes J, Deeks JJ, Chuchu N, Ferrante di Ruffano L, Matin RN, Thomson DR, Wong KY, Aldridge RB, Abbott R, Fawzy M, Bayliss SE, Grainge MJ, Takwoingi Y, Davenport C, Godfrey K, Walter FM, Williams HC, Cochrane Skin Cancer Diagnostic Test Accuracy Group. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. The Cochrane database of systematic reviews. 2018 Dec 4:12(12):CD011902. doi: 10.1002/14651858.CD011902.pub2. Epub 2018 Dec 4 [PubMed PMID: 30521682]
Level 1 (high-level) evidenceRosendahl C, Williams G, Eley D, Wilson T, Canning G, Keir J, McColl I, Wilkinson D. The impact of subspecialization and dermatoscopy use on accuracy of melanoma diagnosis among primary care doctors in Australia. Journal of the American Academy of Dermatology. 2012 Nov:67(5):846-52. doi: 10.1016/j.jaad.2011.12.030. Epub 2012 Feb 9 [PubMed PMID: 22325462]