Introduction
Dermatofibroma, also known as fibrous histiocytoma, is a common, benign, cutaneous soft-tissue lesion characterized by firm subcutaneous nodules. The term "fibrous histiocytoma" primarily describes the morphologic appearance of the cell populations forming these lesions rather than solely indicating the cellular lineage. Clinically, these mesenchymal cell lesions of the dermis are commonly found on the extremities and are relatively small, typically measuring 1 cm or less in diameter. Dermatofibromas are prevalent across all age groups; however, they are more frequently observed in individuals aged 20 to 50, especially among females.
Dermatofibromas are typically asymptomatic but may sometimes cause pain, tenderness, or itchiness. The lesions represent a benign dermal proliferation of fibroblasts. Despite the unknown pathogenesis, sometimes dermatofibroma may occur due to insect bites, trauma, minor injuries, or superficial puncture wounds. Although dermatofibromas are benign tumors, cases of local recurrence and, even more rarely, distant metastases have been reported. Therefore, when considering the differential diagnosis of these lesions, it is crucial to distinguish dermatofibroma from dermatofibrosarcoma protuberans (DFSP)—a similar-appearing but more aggressive cutaneous neoplasm.[1][2][3][4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The nature of dermatofibroma as either a reactive process or a true neoplasm remains unclear. Although the exact cause of the condition remains unknown, it is common for patients presenting with a dermatofibroma to report a history of local trauma at the site, such as from an insect bite, minor injuries, a rose thorn injury, or a superficial puncture wound, but not consistently. While patients diagnosed with dermatofibroma often report a history of local trauma, suggesting a reactive process, such trauma is not consistently reported and is found in only about one-fifth of cases. More often, dermatofibromas develop spontaneously without any specific inciting event or trauma. Spontaneous regression is rare, which may argue against a primarily reactive process and instead support a clonal or neoplastic model.
Dermatofibroma lesions consist of proliferating fibroblasts, often with the involvement of histiocytes. Some studies have detected clonal markers in dermatofibroma cells through analysis of X chromosome inactivation, suggesting a monoclonal pattern and potentially supporting a neoplastic origin of development. Conversely, others suggest a heterogeneous derivation of dermatofibroma, positing that it represents both reactive and neoplastic processes within the same lesion. In this model, the histiocytoid component may be neoplastic, while the fibrous portion may arise from reactive fibroblastic proliferation.[4][5][6]
Epidemiology
According to a study, dermatofibroma is a prevalent skin lesion found across almost all populations, constituting approximately 3% of all dermatopathology laboratory specimens. Determining its global incidence is challenging as most patients are asymptomatic and may not seek medical attention. These lesions commonly manifest in individuals aged from their 20s to their 40s. Although most studies suggest a female predominance, the incidence varies from equivocal to slightly higher, potentially twice as frequent in females as males.[4][5][6]
Histopathology
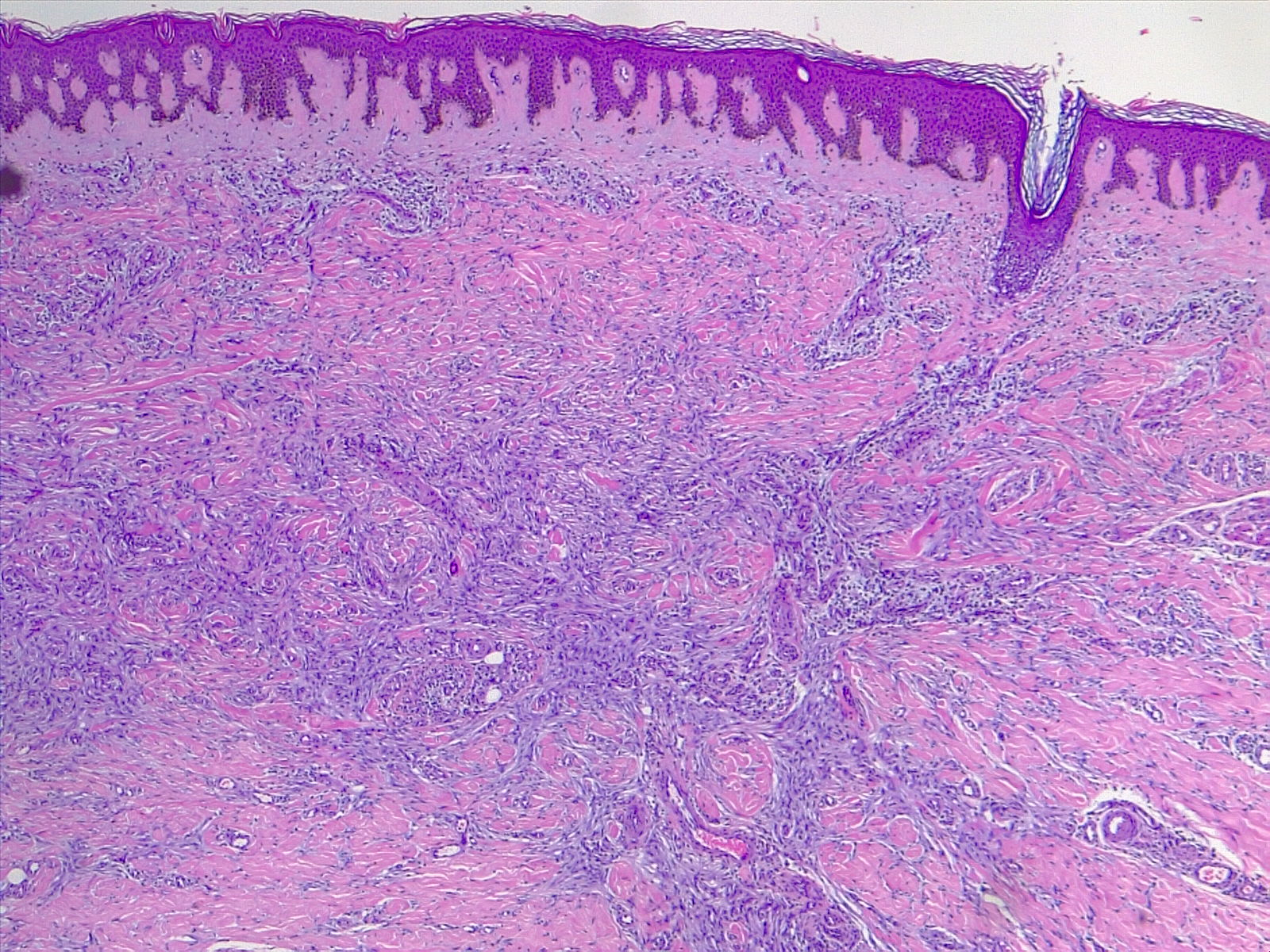
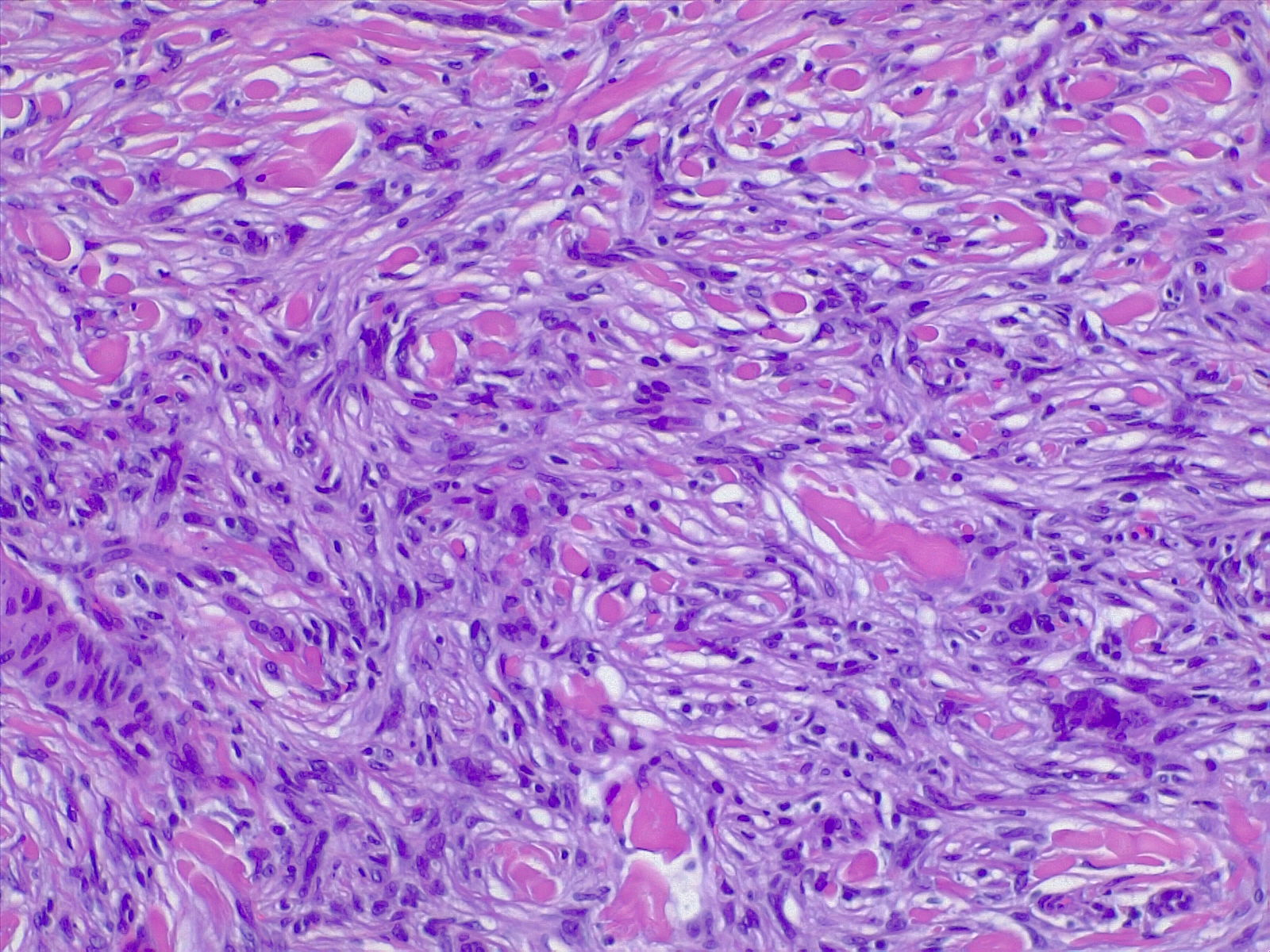
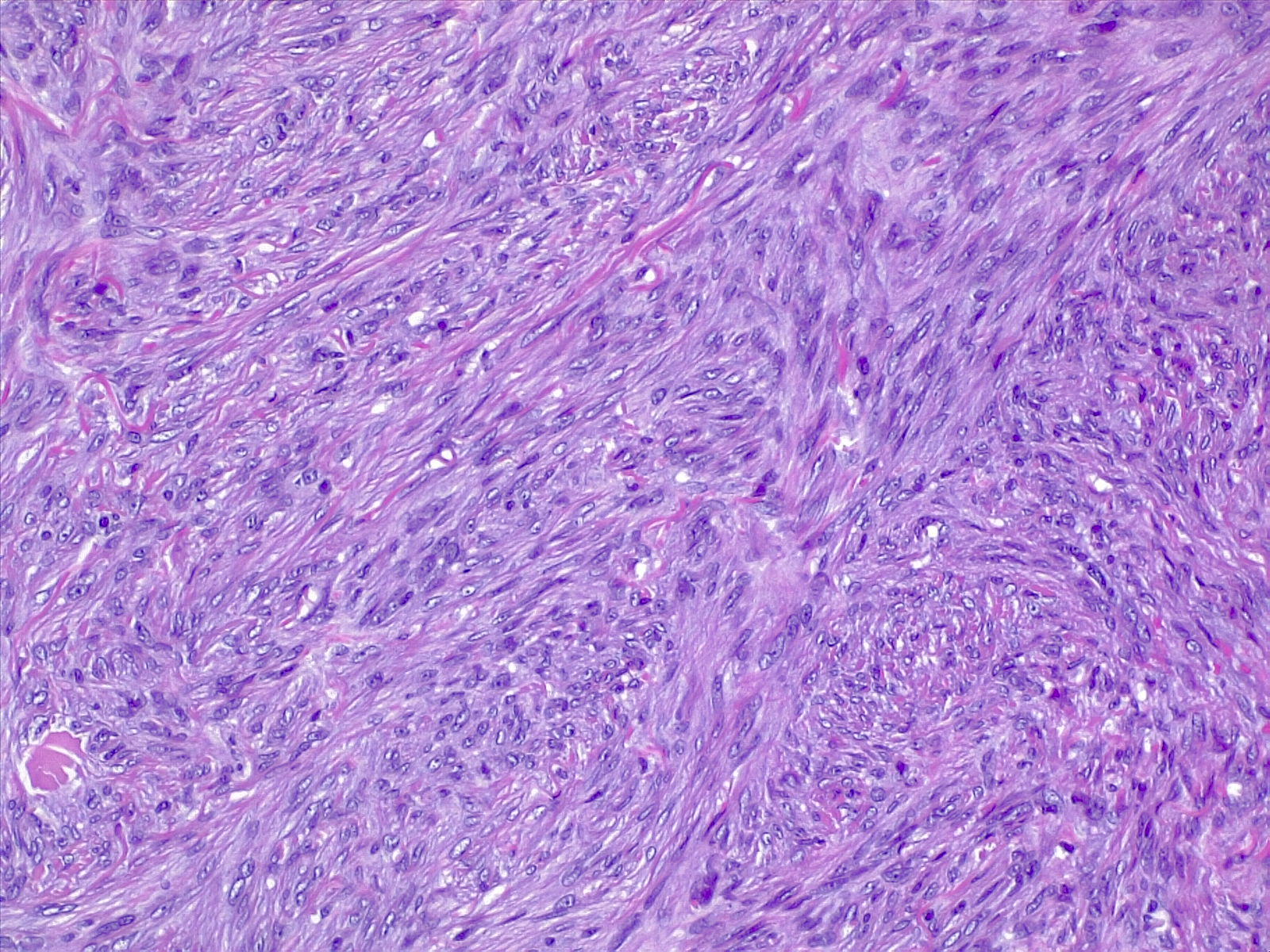
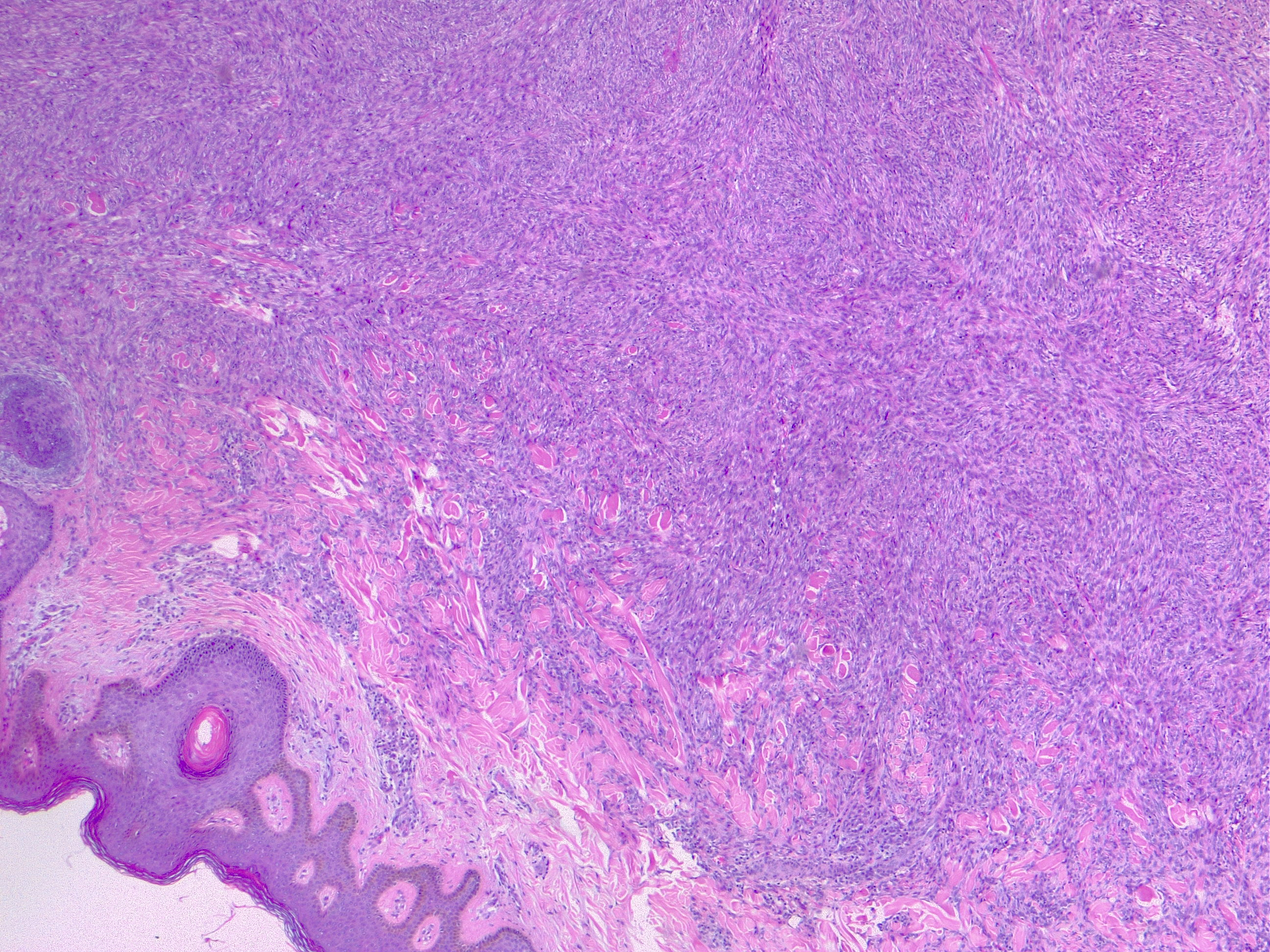
The most commonly diagnosed histologic variant of dermatofibroma is the common fibrous histiocytoma, also known as common dermatofibroma. Histopathologically, common dermatofibroma is characterized by a localized proliferation of spindle-shaped fibrous cells mixed with histiocytoid cells within the dermis. Typically nodular in appearance, these proliferations exhibit spiculated but moderately defined borders that may appear to push against surrounding tissue. The spindle cells may form a focal storiform pattern characterized by a multicentric whorling appearance of elongated nuclei. Intermixed capillaries, lymphocytes, or multinucleated giant cells may also be present. While these proliferations typically remain within the dermis, it is common to observe a small portion of the lesion extending into the subcutaneous tissue along septal lines.
A distinguishing characteristic of dermatofibroma is the presence of trapped collagen bundles or collagen balls within and between the fascicles of spindled fibrous cells. These entrapped collagen collections are frequently located at the periphery of the lesion. A delineated and unaffected zone typically separates the overlying epidermis—the Grenz zone. Reactive changes in the epidermis commonly include hyperkeratosis and acanthosis. Additionally, the epidermis often displays elongated rete ridges extending into the dermis, with hyperpigmented basal keratinocytes, a feature referred to as the "dirty-feet sign."
Arguably, the most crucial distinction is between dermatofibroma and the more concerning DFSP, which bears a similar appearance. DFSP lesions are typically more cellular, exhibiting pronounced storiform patterns and deep invasion into the subcutis, often entangling fat as it proliferates along subcutaneous septae.[7][8][9] Dermatofibroma stains positive for factor XIIIa and vimentin but is negative for CD34.
Types of Dermatofibromas
Various uncommon histologic variants of dermatofibroma are mentioned below.
Atypical dermatofibroma: Atypical dermatofibroma, also known as atypical fibrous histiocytoma or dermatofibroma with monster cells, is a rare variant of dermatofibroma. This variant is characterized by a mixture of pleomorphic cells with large, bizarre, hyperchromatic nuclei within a background of typical spindle cells arranged around thick collagen bundles.[10]
Cellular benign dermatofibroma: Cellular benign dermatofibroma, also known as cellular fibrous histiocytoma, is characterized by a dense proliferation of fibrohistiocytic cells that may extend into the subcutaneous tissue and exhibit normal mitotic figures. Lesions excised with involved margins have been reported to have a recurrence rate of 10%.[11]
Epithelioid dermatofibroma: Epithelioid dermatofibroma comprises large, angulated epithelioid cells resembling the cells found in the intradermal Spitz nevus.
Aneurysmal dermatofibroma: Aneurysmal dermatofibroma is a rare type of mesenchymal tumor originating from fibroblast and histiocytic cells within the dermal layer of the skin. This variant typically presents as blue, red, or purple papules commonly found on the lower extremities. These lesions are often clinically misdiagnosed as vascular lesions. Histologically, they consist of spindle-like cells with interspersed collagen bundles and typically exhibit blood-filled tissue spaces along with hemosiderin deposition in the surrounding cells.
Hemosiderotic dermatofibroma: Hemosiderotic dermatofibroma, also known as hemosiderotic fibrous histiocytomas, may be associated with aneurysmal dermatofibroma. These lesions are characterized by the presence of small blood vessels and extravasated red cells with hemosiderin deposition. They may be mistaken for melanoma or other pigmented lesions due to their appearance.[12]
In patients with 2 or more simultaneously occurring dermatofibromas, the lesions typically appear histopathologically similar, despite the existence of multiple histologic benign variants of dermatofibroma.
History and Physical
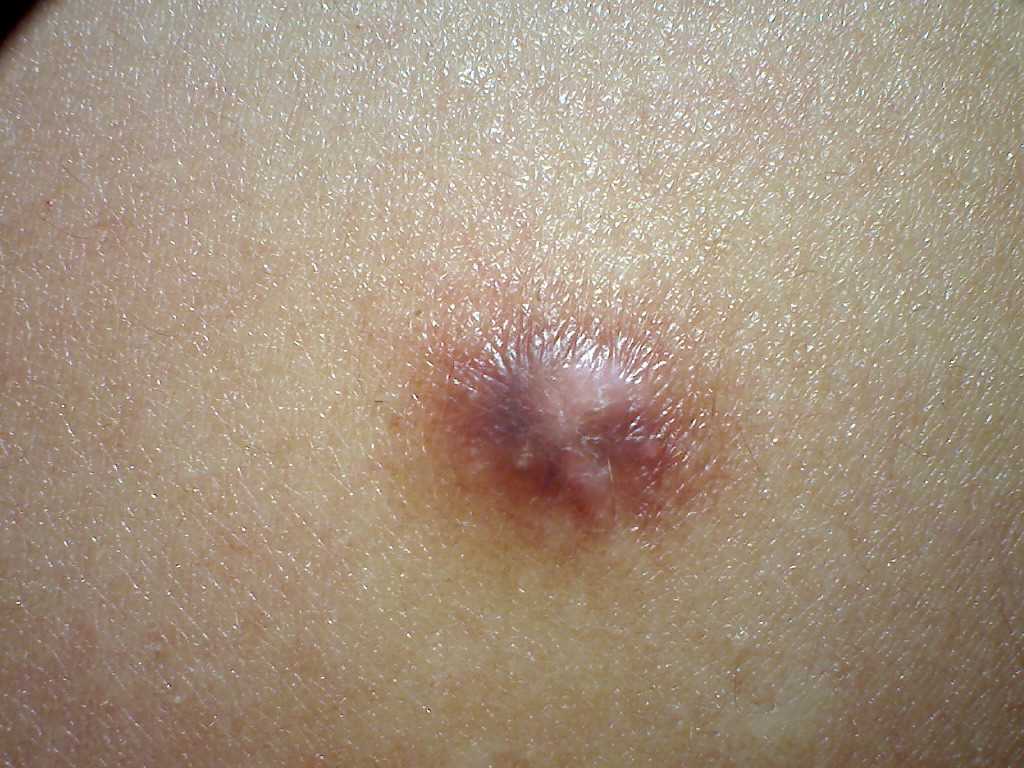
Dermatofibromas are slow-growing lesions that can develop on any part of the body, although they commonly affect the extremities. Clinically, they present as firm, nontender cutaneous nodules with or without accompanying skin changes, such as tan-pink to reddish-brown discoloration, which may vary depending on the age of the lesion. Typically, dermatofibromas measure 1 cm or less in diameter, but larger lesions exceeding 3 cm in diameter have been documented.
Lesions are typically asymptomatic but may occasionally be pruritic and tender. Approximately 1 out of 5 cases involve a history of local trauma at the site of the lesion, such as vaccination or an insect bite. A thorough full-body skin examination is crucial as these lesions may be subtle, with multiple lesions present in 10% of cases. A characteristic finding upon examination is the "dimple sign," where lateral inward digital pressure on the skin produces central dimpling over the lesion due to its fixation to the subcutaneous tissue.[8][9]
Multiple "eruptive" dermatofibromas have been observed in various clinical contexts, including patients with autoimmune diseases receiving immunosuppressive therapies, individuals with HIV infection, and pregnant women.[13][14][15] In addition, rare familial cases inherited in an autosomal dominant pattern have also been reported.[16]
Diagnosis of dermatofibromas is typically based on clinical appearance and history. Longstanding lesions should not exhibit a history of rapid change. Metastasizing benign dermatofibromas are exceedingly rare. They are generally larger in size (>3 cm) than common dermatofibromas. These dermatofibromas may morphologically display features of cellular, aneurysmal, or atypical dermatofibroma, along with an increased number of mitotic figures and extension into the subcutaneous tissue.[17][18]
Multiple clustered dermatofibroma is an exceedingly rare variant of dermatofibroma, which manifests as a plaque consisting of numerous (>15) individual reddish to hyperpigmented papules, typically found on the lower extremities.[19] This variant is commonly observed in children and young adults and may be either congenital or eruptive.[20] The differential diagnosis includes DFSP and atypical fibroxanthoma.
Evaluation
Dermoscopic evaluation of dermatofibroma typically reveals a central white patch surrounded by a peripheral pigmented network. These lesions are reliably diagnosed through histopathological examination, complemented by the correlated clinical examination. When evaluated before biopsy using sonography, dermatofibromas should appear as avascular lesions within the dermis, often with superficial subcutis involvement and spiculated margins that are not well defined. The size and margins of these lesions typically correlate well on histopathological examination with sonographic findings.[21][22][23]
Treatment / Management
Treatment for dermatofibromas is generally unnecessary unless the lesion is causing symptoms. Excision for histopathological examination is indicated when the lesion shows signs of malignancy, such as changes in appearance or bleeding. Atypical variants are more prone to recurrence and, rarely, may metastasize. Therefore, complete excision with clear margins is recommended.[10] Patients considering excision for cosmetic reasons should be informed that the resulting scar may be more noticeable than the initial lesion, particularly on the lower extremities. Cryotherapy may serve as an alternative treatment option for lesions that protrude above the skin surface and become irritated due to repeated trauma.[24](B3)
Differential Diagnosis
Differentiating benign dermatofibromas from more advanced and aggressive neoplasms that may mimic their appearance is crucial.
Dermatofibrosarcoma protuberans: DFSP is a locally aggressive neoplasm of low-to-intermediate grade that can resemble a benign dermatofibroma. An early lesion of DFSP may manifest as an indurated skin-colored nodule that gradually enlarges over months to years. A histopathological examination is essential for accurate diagnosis, as several distinguishing features exist.
DFSP is characteristically more cellular and tends to involve a greater extent of the subcutis, often displaying a "honeycomb" entrapment of subcutaneous fat. Immunohistochemical staining can be particularly helpful in challenging cases, as DFSP typically stains positive for CD34 and negative for factor XIIIa. In contrast, benign dermatofibromas will stain strongly positive for factor XIIIa but negative for CD34. Additionally, dermatofibromas may exhibit higher positive staining for D2-40. Ki-67 staining should indicate a much higher proliferation index in DFSP, and the mitotic rate should also be significantly higher in DFSP compared to dermatofibroma.
Kaposi sarcoma: Kaposi sarcoma is characterized by spindled cell proliferation within the dermis and may be confused with the aneurysmal variant of dermatofibroma. Kaposi sarcoma typically exhibits red blood cells between spindled cellular areas and vascularity. However, Kaposi sarcoma should test positive for human herpesvirus-8 (HHV-8) and may also stain positive for CD31, CD34, and D2-40.
Intradermal nevi: Intradermal nevi typically have a softer consistency than dermatofibromas and do not exhibit dimpling upon pinching.
Keratoacanthomas: Keratoacanthomas are characterized by a rapid growth rate and often display a central keratinous plug.
Basal cell carcinomas: Basal cell carcinomas can resemble dermatofibromas, especially when they exhibit overlying follicular induction, characterized by epidermal hyperplasia such as basaloid proliferation. Differentiating between these lesions can be challenging, particularly in superficial biopsies that may miss the underlying dermatofibroma. A characteristic feature of basal cell carcinomas is that they exhibit peripheral palisading. However, important distinguishing factors include the presence of CK20-positive Merkel cells in follicular induction. Signs favoring dermatofibroma may include clear cell hyperplasia, the absence of nuclear atypia, and less crowding.[7][25][26][27]
Prognosis
Dermatofibromas are benign lesions with an excellent prognosis. Some lesions may even undergo spontaneous regression, resulting in hypopigmented skin. Most dermatofibromas remain stable for years without significant changes. With thorough excision, these lesions rarely recur, and only the most aggressive variants show local recurrence in approximately 20% of patients. Consequently, in excisional biopsies involving cellular or atypical variants, re-excision may be recommended to ensure clear margins due to the documented, albeit low, rate of local recurrence. Although extremely rare, metastases have been reported.[3][27][28]
Complications
Complications associated with dermatofibroma primarily arise from surgical removal and may include bleeding, infection, scarring or disfigurement, and the necessity for additional procedures.
Deterrence and Patient Education
Patients should be informed that dermatofibromas are benign entities and do not necessarily require excision. Although clinical history, histopathological examination, and imaging studies may suggest a dermatofibroma, a conclusive diagnosis can only be established through pathological examination. Moreover, patient preferences and desires are critical in the decision to excise these lesions versus opting for surveillance.
Enhancing Healthcare Team Outcomes
Patients should be informed that dermatofibromas are benign entities and do not necessarily require excision. Although clinical history, histopathological examination, and imaging studies may suggest a dermatofibroma, a definitive diagnosis can only be made following a pathological examination. Patients' preferences may also impact the decision to excise these lesions or opt for surveillance. If surveillance is chosen, any abrupt or unusual change in the behavior of any skin lesion should prompt a referral to a competent dermatologist for evaluation.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Chen TC, Kuo T, Chan HL. Dermatofibroma is a clonal proliferative disease. Journal of cutaneous pathology. 2000 Jan:27(1):36-9 [PubMed PMID: 10660130]
Massoumi R, Podda M, Fässler R, Paus R. Cylindroma as tumor of hair follicle origin. The Journal of investigative dermatology. 2006 May:126(5):1182-4 [PubMed PMID: 16484982]
Level 3 (low-level) evidenceLuzar B, Calonje E. Cutaneous fibrohistiocytic tumours - an update. Histopathology. 2010 Jan:56(1):148-65. doi: 10.1111/j.1365-2559.2009.03447.x. Epub [PubMed PMID: 20055912]
Buehler D, Weisman P. Soft Tissue Tumors of Uncertain Histogenesis: A Review for Dermatopathologists. Clinics in laboratory medicine. 2017 Sep:37(3):647-671. doi: 10.1016/j.cll.2017.06.005. Epub [PubMed PMID: 28802505]
Hui P, Glusac EJ, Sinard JH, Perkins AS. Clonal analysis of cutaneous fibrous histiocytoma (dermatofibroma). Journal of cutaneous pathology. 2002 Aug:29(7):385-9 [PubMed PMID: 12139632]
Bandyopadhyay MR, Besra M, Dutta S, Sarkar S. Dermatofibroma: Atypical Presentations. Indian journal of dermatology. 2016 Jan-Feb:61(1):121. doi: 10.4103/0019-5154.174131. Epub [PubMed PMID: 26955137]
Agarwal A, Gopinath A, Tetzlaff MT, Prieto VG. Phosphohistone-H3 and Ki67: Useful Markers in Differentiating Dermatofibroma From Dermatofibrosarcoma Protuberans and Atypical Fibrohistiocytic Lesions. The American Journal of dermatopathology. 2017 Jul:39(7):504-507. doi: 10.1097/DAD.0000000000000690. Epub [PubMed PMID: 28609344]
Lee WJ, Jung JM, Won CH, Chang SE, Choi JH, Moon KC, Lee MW. Clinical and histological patterns of dermatofibroma without gross skin surface change: A comparative study with conventional dermatofibroma. Indian journal of dermatology, venereology and leprology. 2015 May-Jun:81(3):263-9. doi: 10.4103/0378-6323.154795. Epub [PubMed PMID: 25851763]
Level 2 (mid-level) evidenceMentzel T, Wiesner T, Cerroni L, Hantschke M, Kutzner H, Rütten A, Häberle M, Bisceglia M, Chibon F, Coindre JM. Malignant dermatofibroma: clinicopathological, immunohistochemical, and molecular analysis of seven cases. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2013 Feb:26(2):256-67. doi: 10.1038/modpathol.2012.157. Epub 2012 Sep 21 [PubMed PMID: 22996372]
Level 3 (low-level) evidenceKaddu S, McMenamin ME, Fletcher CD. Atypical fibrous histiocytoma of the skin: clinicopathologic analysis of 59 cases with evidence of infrequent metastasis. The American journal of surgical pathology. 2002 Jan:26(1):35-46 [PubMed PMID: 11756767]
Level 3 (low-level) evidenceGaufin M, Michaelis T, Duffy K. Cellular Dermatofibroma: Clinicopathologic Review of 218 Cases of Cellular Dermatofibroma to Determine the Clinical Recurrence Rate. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2019 Nov:45(11):1359-1364. doi: 10.1097/DSS.0000000000001833. Epub [PubMed PMID: 30741794]
Level 3 (low-level) evidenceAlves JV, Matos DM, Barreiros HF, Bártolo EA. Variants of dermatofibroma--a histopathological study. Anais brasileiros de dermatologia. 2014 May-Jun:89(3):472-7 [PubMed PMID: 24937822]
Level 2 (mid-level) evidenceBeatrous SV, Riahi RR, Grisoli SB, Cohen PR. Associated conditions in patients with multiple dermatofibromas: Case reports and literature review. Dermatology online journal. 2017 Sep 22:23(9):. pii: 13030/qt8zv852d8. Epub 2017 Sep 22 [PubMed PMID: 29469716]
Level 3 (low-level) evidenceZaccaria E, Rebora A, Rongioletti F. Multiple eruptive dermatofibromas and immunosuppression: report of two cases and review of the literature. International journal of dermatology. 2008 Jul:47(7):723-7. doi: 10.1111/j.1365-4632.2008.03575.x. Epub [PubMed PMID: 18613883]
Level 3 (low-level) evidenceQueirós C, Uva L, Soares de Almeida L, Filipe P. Multiple eruptive dermatofibromas associated with pregnancy- a case and literature review. Dermatology online journal. 2019 May 15:25(5):. pii: 13030/qt29d3q6p1. Epub 2019 May 15 [PubMed PMID: 31220902]
Level 3 (low-level) evidenceSupsrisunjai C, Hsu CK, Michael M, Duval C, Lee JYW, Yang HS, Huang HY, Chaikul T, Onoufriadis A, Steiner RA, Ariëns RAS, Sarig O, Sprecher E, Eskin-Schwartz M, Samlaska C, Simpson MA, Calonje E, Parsons M, McGrath JA. Coagulation Factor XIII-A Subunit Missense Mutation in the Pathobiology of Autosomal Dominant Multiple Dermatofibromas. The Journal of investigative dermatology. 2020 Mar:140(3):624-635.e7. doi: 10.1016/j.jid.2019.08.441. Epub 2019 Sep 4 [PubMed PMID: 31493396]
Mentzel T. Cutaneous mesenchymal tumours: an update. Pathology. 2014 Feb:46(2):149-59. doi: 10.1097/PAT.0000000000000046. Epub [PubMed PMID: 24378387]
Doyle LA, Fletcher CD. Metastasizing "benign" cutaneous fibrous histiocytoma: a clinicopathologic analysis of 16 cases. The American journal of surgical pathology. 2013 Apr:37(4):484-95. doi: 10.1097/PAS.0b013e31827070d4. Epub [PubMed PMID: 23426120]
Level 3 (low-level) evidenceGershtenson PC, Krunic AL, Chen HM. Multiple clustered dermatofibroma: case report and review of the literature. Journal of cutaneous pathology. 2010 Sep:37(9):e42-5. doi: 10.1111/j.1600-0560.2009.01325.x. Epub 2009 Jul 10 [PubMed PMID: 19614987]
Level 3 (low-level) evidenceFinch J, Berke A, McCusker M, Chang MW. Congenital multiple clustered dermatofibroma in a 12-year-old girl. Pediatric dermatology. 2014 Jan-Feb:31(1):105-6. doi: 10.1111/j.1525-1470.2011.01681.x. Epub 2011 Dec 30 [PubMed PMID: 22211625]
Level 3 (low-level) evidenceKelati A, Aqil N, Baybay H, Gallouj S, Mernissi FZ. Beyond classic dermoscopic patterns of dermatofibromas: a prospective research study. Journal of medical case reports. 2017 Sep 20:11(1):266. doi: 10.1186/s13256-017-1429-6. Epub 2017 Sep 20 [PubMed PMID: 28927449]
Level 2 (mid-level) evidenceMorariu SH, Suciu M, Vartolomei MD, Badea MA, Cotoi OS. Aneurysmal dermatofibroma mimicking both clinical and dermoscopic malignant melanoma and Kaposi's sarcoma. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie. 2014:55(3 Suppl):1221-4 [PubMed PMID: 25607410]
Level 3 (low-level) evidenceWon KY, Park SY, Jin W, Lew BL. Dermatofibroma: sonographic findings and pathologic correlation. Acta radiologica (Stockholm, Sweden : 1987). 2018 Apr:59(4):454-459. doi: 10.1177/0284185117721263. Epub 2017 Aug 9 [PubMed PMID: 28791883]
Lanigan SW, Robinson TW. Cryotherapy for dermatofibromas. Clinical and experimental dermatology. 1987 Mar:12(2):121-3 [PubMed PMID: 3677469]
Sadullahoğlu C, Dere Y, Atasever TR, Öztop MT, Karaaslan Ö. The Role of CD34 and D2-40 in the Differentiation of Dermatofibroma and Dermatofibrosarcoma Protuberans. Turk patoloji dergisi. 2017:1(1):223-227. doi: 10.5146/tjpath.2017.01402. Epub [PubMed PMID: 28832078]
Stanoszek LM, Wang GY, Harms PW. Histologic Mimics of Basal Cell Carcinoma. Archives of pathology & laboratory medicine. 2017 Nov:141(11):1490-1502. doi: 10.5858/arpa.2017-0222-RA. Epub [PubMed PMID: 29072946]
Romano RC, Fritchie KJ. Fibrohistiocytic Tumors. Clinics in laboratory medicine. 2017 Sep:37(3):603-631. doi: 10.1016/j.cll.2017.05.007. Epub 2017 Jun 15 [PubMed PMID: 28802503]
Parish LC, Yazdanian S, Lambert WC, Lambert PC. Dermatofibroma: a curious tumor. Skinmed. 2012 Sep-Oct:10(5):268-70 [PubMed PMID: 23163067]