Indications
Deferoxamine (DFO or DFOA) is FDA approved to treat iron overload, either acute or chronic. The definition of iron overload is serial ferritin levels above 800 to 3000 ng/mL.[1] The FDA has not approved deferoxamine as first-line therapy for hereditary hemochromatosis unless there is a contraindication to phlebotomy. Clinicians can also use deferoxamine as an off-label treatment for aluminum toxicity in chronic kidney disease (CKD) patients.
Transfusion-related iron overload occurs in patients that require frequent transfusions throughout their life. These patients include those affected by Thalassemia, Sickle cell disease, myelodysplastic syndromes, ineffective hematopoiesis, and other inherited anemic disorders. In this population, chelation should begin two years after transfusions start, serum ferritin levels greater than 1000 mcg/L, or when liver iron concentration (LIC) is greater than 3 mg Fe/g.[2][3][4]
Another indication for iron chelation is a cardiac T2* <20 milliseconds found on cardiac magnetic resonance.[2][3][4] Cardiac MRIs can measure proton relaxation times (T2*) in the cardiac nuclei. Excess iron within these cells speeds up the rate of proton relaxation. This reduced cardiac T2* time is associated with cardiomyopathy, dramatically increasing mortality.[5] Chelation therapy improves overall survival in these patients and should not be delayed.[6][7][8]
The use of deferoxamine in acute ingestion of iron is an indication when patients present with systemic toxicity, hemodynamic instability, lethargy, persistent vomiting, metabolic acidosis, or toxic serum iron levels.[9] Iron levels >500 mcg/dL are considered hazardous, and chelation should be initiated.[10] Signs and symptoms of systemic toxicity include coagulopathy, cardiomyopathy, and hepatic and renal failure.[11] Acute iron toxicity progresses through five clinical stages, with the most deadly consequences occurring within the first four days.[10] Thus, rapid progression through these stages is another indicator that chelation may be necessary.
Aluminum toxicity is an off-label use for deferoxamine chelation therapy. Aluminum toxicity can occur in patients with chronic kidney disease who undergo bladder irrigation with aluminum-containing products, use phosphate binders that contain aluminum, or receive hemodialysis with a water source contaminated with aluminum. Its use is indicated in patients with signs and symptoms associated with chronic aluminum levels greater than >20 mcg/L, such as osteomalacia, anemia, hypercalcemia, and dialysis dementia.[12][13] It is also an indicated use in acute toxicity, which results from exposure to >200 mcg/L of aluminum resulting in acute encephalopathy.[14]
Mechanism of Action
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Mechanism of Action
Iron is an essential part of human physiology. It is a vital element in proteins such as hemoglobin, myoglobin, and cytochrome and also functions as a cofactor for many enzymes. It is stored by ferritin and transferred throughout the serum by transferrin. No physiological mechanism exists to excrete iron. Instead, humans regulate GI uptake by altering hepcidin levels.[15] When iron storing proteins become saturated, free iron species accumulate in the plasma. These include non-transferable bound iron (NTBI) and labile plasma iron (LBI).[16]
Cells also take up free iron to form labile iron pools (LIP) within their cell membranes.[16] This excess iron within cells catalyzes the production of free radicals via the Fenton reaction.[17] Free radicals lead to DNA destruction and cellular damage. Free iron also precipitates acidosis by inhibiting oxidative phosphorylation in mitochondria.[17] Deferoxamine is a molecule produced by the fermentation of Streptomyces pilosus.[18] It binds free plasma iron and excess iron within cells. deferoxamine is a hexadentate molecule and is able to bind iron at a 1-to-1 ratio.[19]
The bound form of deferoxamine is then excreted via the urine or bile.[19] Deferoxamine chelates non-transferrin bound iron (free iron), iron in transit between transferrin and ferritin (labile chelating iron pool), hemosiderin, and ferritin. Although deferoxamine can directly bind and remove iron from myocardial cells, it will not bind iron already bound to molecules such as transferrin, hemoglobin, or cytochromes.[19] Thus, only a small amount of iron is available for chelation at any given time. Although this is a small fraction of total body iron, it has a profound effect. When bound, the resultant ferrioxamine is very water-soluble. If chelation occurs in hepatocytes, the compound will be excreted in bile, and when chelation occurs with free iron in plasma or other tissues, it is excreted by the kidneys.
Deferoxamine can also bind aluminum within the plasma to form aluminoxane, which is renally excreted. In the case of CKD/ESRD patients, the product is dialyzable using a high-flux membrane.[20] Deferoxamine can draw aluminum deposited in tissues into the plasma.[20] For this reason, patients with a measured serum aluminum concentration >200 mcg/L should not be treated with deferoxamine as it may lead to severely high levels of aluminum and fatal neurotoxicity. The recommended administration will be discussed in the following section.
Administration
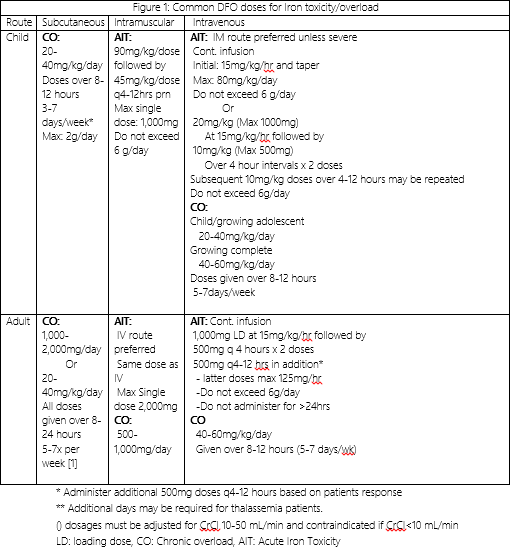
Deferoxamine is poorly absorbed from the GI tract when taken orally. For this reason, it must be given intramuscularly, subcutaneously, or intravenously.[3][21] The subcutaneous route is preferable for those patients with chronic iron overload. Intravenous deferoxamine is reserved for those with acute ingestion and life-threatening symptoms.[9] Figure 1 summarizes recommended dosages and administration times for deferoxamine in iron toxicity.
A 25 gauge or smaller butterfly needle is used for the SQ route. The abdomen is generally the safest and most common area to avoid important vessels and nerves. A 10% deferoxamine solution is administered subcutaneously over 8 to 12 hours using a slow infusion pump.[22] The dose is dependent on the patient's age and weight. Approximately 40 to 60 mg/kg/day is given for 4 to 5 days per week.[22] The total dose should not exceed 2.5g daily.[23]
Intravenous administration is reserved for those patients with severe acute iron toxicity with Iron levels >500 mcg/dL, severe cardiac disease (dysrhythmias, LV dysfunction, severe heart iron loading (T2*<6 ms on MRI)), or those who cannot tolerate the subcutaneous infusion.[10][24][25] The standard dose of 50 to 60 mg/kg/day or 5 to 15 mg/kg/h is given as a 24-hour infusion using an indwelling catheter.[26][27] Patients should not receive deferoxamine for more than 24 hours intravenously as this can increase the risk of developing ARDS and other complications.[28][29]
The dose of deferoxamine may be reduced or increased by using clinical judgment, decreasing liver ion concentrations, or calculating the therapeutic index, which divides the daily dose of deferoxamine by the serum ferritin levels. The therapeutic index should be <.025 at all times to avoid serious adverse effects.[30]
Vitamin C can potentiate the therapeutic effect of deferoxamine by mobilizing iron stores, subsequently increasing the concentration of chelatable iron.[5] On the other hand, this increase in free iron can potentiate iron toxicity leading to impaired cardiac function and worsening overload. For this reason, the FDA advises that supplemental vitamin C should be avoided in patients with cardiac failure and should only start after an initial one month of standard deferoxamine treatment has been completed. Furthermore, its use is only indicated in those patients receiving regular deferoxamine therapy and should not exceed 200 mg/day. Close evaluation of the patient's cardiac function is of the utmost importance when using this combined therapy.
In aluminum toxicity, the appropriate dosing and therapy duration of the drug is uncertain and should be tailored by serum aluminum levels, symptoms, and response. The National Kidney Foundation has listed specific guidelines for the use of deferoxamine in aluminum toxicity, which this article will cover. A proposed mechanism by the Kidney Disease Outcomes Quality Initiative states that in symptomatic patients with serum aluminum levels >60 μg/L but <200 μg/L or a rise in aluminum after DFO >50 μg/L, deferoxamine should be given to treat the aluminum overload. Deferoxamine's ability to draw aluminum out of tissues and into the plasma makes its use very dangerous in those patients with serum aluminum levels >200 μg/L. To avoid DFO-induced neurotoxicity in these patients, its use should delay until the completion of intensive dialysis (6 days per week) with a high-flux dialysis membrane and a dialysate aluminum level of <5 μg/L and until the pre-dialysis serum aluminum level has been reduced to <200 μg/L.
Adverse Effects
Chronic deferoxamine therapy can lead to sensorineural hearing loss and retinopathy. Though the mechanism of ocular injury is not well understood, it appears to partially occur due to damage to the retinal pigment epithelium, which can lead to decreased visual acuity, visual field defects, and color vision defects.[31] Hearing and vision loss can be reversible if the patient discontinues the drug early in the course.[23] Growth retardation can also occur in children receiving deferoxamine treatment, and clinicians should monitor patients for appropriate growth velocity over time.[32]
Administering less than 2.5 g of deferoxamine per day and monitoring the therapeutic index is the best means to avoid such complications. Acute side effects can include GI complaints, anaphylaxis, skin discoloration, skin irritation, and anaphylaxis. Chelation of iron and formation of the water-soluble compound ferioxamine may lead to rose-colored urine. Deferoxamine can increase the risk of infection by specific pathogens and invasive fungi such as mucormycosis, Yersinia, and Vibrio.[33][34] ARDS is another potential and rare complication that occurs most often when giving the drug via intravenous infusion for more than 24 hours.[28][29]
Contraindications
Deferoxamine is relatively safe and well-tolerated by patients. Its use is contraindicated in patients with previous hypersensitivity reactions to the drug and those with renal disease or anuria. Deferoxamine is a pregnancy category C drug and is usually reserved for women at high risk of cardiac disease or severe symptoms from acute ingestion. Although there is no evidence to indicate that the drug is a teratogen, animal studies have shown adverse fetal effects. Clinicians should be cautious in using during pregnancy, and the risks vs. benefits must merit consideration in each case. It is unknown if deferoxamine is excreted in breast milk.
Monitoring
The therapeutic index is a crucial measurement for deferoxamine therapy, and the provider should calculate it regularly. It can be calculated by dividing the mean daily dose over seven days by the measured ferritin levels.[30] Complication risk can be mitigated while using deferoxamine by keeping the therapeutic index below 0.025, and the patient's daily dose requires adjustment for the alternating ferritin levels.[30] Besides monitoring the patient's iron stores, regular screening for adverse effects is also advisable. A screening hearing exam should be performed in the clinic every six months and a formal audiogram every 12 months.[23]
An evaluation by an ophthalmologist should take place in children every six months and annually in adults. Since the kidneys excrete most of the chelation byproduct ferrioxamine, it is essential to monitor the patient's renal function. The patient's chemistry, BUN/Cr, and urine protein/Cr ratios should be measured at least four times a year, and the clinicians should reduce the deferoxamine dose with worsening renal function.
Toxicity
Patients tolerate deferoxamine well, and there is no specific antidote for the medication. The precautions and dose reductions are described elsewhere in this paper.
Enhancing Healthcare Team Outcomes
Deferoxamine treatment can be a tedious and painful process, with common local skin reactions. The patient will require strong supportive relationships with several providers, nurses, and family to maximize compliance. To provide safe chelation therapy, a patient must comply with their primary care doctor, ophthalmologist, endocrinologist, nephrologist, and hematologist. These interprofessional teams are vital to successful outcomes that decrease mortality and complications. Many of those who require chelation begin at a young age due to hereditary disease. Compliance in this age group is usually high compared to others due to parental support.[35]
Compliance with the strict regimen can become problematic in adolescence or when life burdens become too cumbersome for a patient to manage. One multicenter study in Germany found that patients had more misery from chelation treatment than the disease requiring it.[35]
Patient involvement, education, and behavioral support are of utmost importance. A systematic review from the Agency for Healthcare Research and Quality on interventions to improve adherence to self-administered medications found that reduced out-of-pocket expenses, case management, and patient education with behavioral support improved medication adherence.[36] Shared decision-making also has an important role. There are a variety of chelators available for Iron overdose, and providers should seek the option with the lowest burden to the patient. Allowing patients to change the chelator for various reasons helped increase compliance with the regimen.[37] We must remember that chelation will be a life-long therapy for most of these patients. It is thus critical for providers to be empathetic, educational, and inspiring.
Media
References
Ballas SK, Zeidan AM, Duong VH, DeVeaux M, Heeney MM. The effect of iron chelation therapy on overall survival in sickle cell disease and β-thalassemia: A systematic review. American journal of hematology. 2018 Jul:93(7):943-952. doi: 10.1002/ajh.25103. Epub 2018 Apr 28 [PubMed PMID: 29635754]
Level 1 (high-level) evidenceAydinok Y, Kattamis A, Viprakasit V. Current approach to iron chelation in children. British journal of haematology. 2014 Jun:165(6):745-55. doi: 10.1111/bjh.12825. Epub 2014 Mar 20 [PubMed PMID: 24646011]
Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997 Feb 1:89(3):739-61 [PubMed PMID: 9028304]
Level 3 (low-level) evidenceMaggio A. Light and shadows in the iron chelation treatment of haematological diseases. British journal of haematology. 2007 Aug:138(4):407-21 [PubMed PMID: 17659052]
He T. Cardiovascular magnetic resonance T2* for tissue iron assessment in the heart. Quantitative imaging in medicine and surgery. 2014 Oct:4(5):407-12. doi: 10.3978/j.issn.2223-4292.2014.10.05. Epub [PubMed PMID: 25392825]
Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet (London, England). 2000 Jun 10:355(9220):2051-2 [PubMed PMID: 10885361]
Level 3 (low-level) evidenceDelea TE, Edelsberg J, Sofrygin O, Thomas SK, Baladi JF, Phatak PD, Coates TD. Consequences and costs of noncompliance with iron chelation therapy in patients with transfusion-dependent thalassemia: a literature review. Transfusion. 2007 Oct:47(10):1919-29 [PubMed PMID: 17880620]
Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, Romeo MA, Forni GL, Gamberini MR, Ghilardi R, Piga A, Cnaan A. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004 Oct:89(10):1187-93 [PubMed PMID: 15477202]
Level 2 (mid-level) evidenceMadiwale T, Liebelt E. Iron: not a benign therapeutic drug. Current opinion in pediatrics. 2006 Apr:18(2):174-9 [PubMed PMID: 16601499]
Level 3 (low-level) evidenceKlein-Schwartz W, Oderda GM, Gorman RL, Favin F, Rose SR. Assessment of management guidelines. Acute iron ingestion. Clinical pediatrics. 1990 Jun:29(6):316-21 [PubMed PMID: 2361339]
Level 2 (mid-level) evidenceMills KC, Curry SC. Acute iron poisoning. Emergency medicine clinics of North America. 1994 May:12(2):397-413 [PubMed PMID: 8187690]
Delmez JA, Slatopolsky E. Hyperphosphatemia: its consequences and treatment in patients with chronic renal disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1992 Apr:19(4):303-17 [PubMed PMID: 1562018]
Bansal VK, Bansal S. Nervous system disorders in dialysis patients. Handbook of clinical neurology. 2014:119():395-404. doi: 10.1016/B978-0-7020-4086-3.00025-4. Epub [PubMed PMID: 24365308]
Berend K, van der Voet G, Boer WH. Acute aluminum encephalopathy in a dialysis center caused by a cement mortar water distribution pipe. Kidney international. 2001 Feb:59(2):746-53 [PubMed PMID: 11168958]
Origa R, Galanello R, Ganz T, Giagu N, Maccioni L, Faa G, Nemeth E. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007 May:92(5):583-8 [PubMed PMID: 17488680]
Fleming RE, Ponka P. Iron overload in human disease. The New England journal of medicine. 2012 Jan 26:366(4):348-59. doi: 10.1056/NEJMra1004967. Epub [PubMed PMID: 22276824]
Hebbel RP. Auto-oxidation and a membrane-associated 'Fenton reagent': a possible explanation for development of membrane lesions in sickle erythrocytes. Clinics in haematology. 1985 Feb:14(1):129-40 [PubMed PMID: 2985310]
Barona-Gómez F, Wong U, Giannakopulos AE, Derrick PJ, Challis GL. Identification of a cluster of genes that directs desferrioxamine biosynthesis in Streptomyces coelicolor M145. Journal of the American Chemical Society. 2004 Dec 22:126(50):16282-3 [PubMed PMID: 15600304]
Hershko C, Konijn AM, Nick HP, Breuer W, Cabantchik ZI, Link G. ICL670A: a new synthetic oral chelator: evaluation in hypertransfused rats with selective radioiron probes of hepatocellular and reticuloendothelial iron stores and in iron-loaded rat heart cells in culture. Blood. 2001 Feb 15:97(4):1115-22 [PubMed PMID: 11159545]
Level 3 (low-level) evidenceMolitoris BA, Alfrey AC, Alfrey PS, Miller NL. Rapid removal of DFO-chelated aluminum during hemodialysis using polysulfone dialyzers. Kidney international. 1988 Jul:34(1):98-101 [PubMed PMID: 3172641]
Level 1 (high-level) evidenceBeris P. Introduction: management of thalassemia. Seminars in hematology. 1995 Oct:32(4):243 [PubMed PMID: 8560281]
Cappellini MD. Overcoming the challenge of patient compliance with iron chelation therapy. Seminars in hematology. 2005 Apr:42(2 Suppl 1):S19-21 [PubMed PMID: 15846581]
Olivieri NF, Buncic JR, Chew E, Gallant T, Harrison RV, Keenan N, Logan W, Mitchell D, Ricci G, Skarf B. Visual and auditory neurotoxicity in patients receiving subcutaneous deferoxamine infusions. The New England journal of medicine. 1986 Apr 3:314(14):869-73 [PubMed PMID: 3485251]
Porter JB, Abeysinghe RD, Marshall L, Hider RC, Singh S. Kinetics of removal and reappearance of non-transferrin-bound plasma iron with deferoxamine therapy. Blood. 1996 Jul 15:88(2):705-13 [PubMed PMID: 8695819]
Westwood MA, Anderson LJ, Maceira AM, Shah FT, Prescott E, Porter JB, Wonke B, Walker JM, Pennell DJ. Normalized left ventricular volumes and function in thalassemia major patients with normal myocardial iron. Journal of magnetic resonance imaging : JMRI. 2007 Jun:25(6):1147-51 [PubMed PMID: 17520718]
Level 2 (mid-level) evidenceDavis BA, O'Sullivan C, Jarritt PH, Porter JB. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood. 2004 Jul 1:104(1):263-9 [PubMed PMID: 15001468]
Davis BA, Porter JB. Long-term outcome of continuous 24-hour deferoxamine infusion via indwelling intravenous catheters in high-risk beta-thalassemia. Blood. 2000 Feb 15:95(4):1229-36 [PubMed PMID: 10666195]
Ioannides AS, Panisello JM. Acute respiratory distress syndrome in children with acute iron poisoning: the role of intravenous desferrioxamine. European journal of pediatrics. 2000 Mar:159(3):158-9 [PubMed PMID: 10664227]
Level 3 (low-level) evidenceTenenbein M, Kowalski S, Sienko A, Bowden DH, Adamson IY. Pulmonary toxic effects of continuous desferrioxamine administration in acute iron poisoning. Lancet (London, England). 1992 Mar 21:339(8795):699-701 [PubMed PMID: 1347583]
Level 3 (low-level) evidencePorter JB, Jaswon MS, Huehns ER, East CA, Hazell JW. Desferrioxamine ototoxicity: evaluation of risk factors in thalassaemic patients and guidelines for safe dosage. British journal of haematology. 1989 Nov:73(3):403-9 [PubMed PMID: 2605127]
Simon S, Athanasiov PA, Jain R, Raymond G, Gilhotra JS. Desferrioxamine-related ocular toxicity: a case report. Indian journal of ophthalmology. 2012 Jul:60(4):315-7. doi: 10.4103/0301-4738.98714. Epub [PubMed PMID: 22824603]
Level 3 (low-level) evidenceDe Virgiliis S, Congia M, Frau F, Argiolu F, Diana G, Cucca F, Varsi A, Sanna G, Podda G, Fodde M. Deferoxamine-induced growth retardation in patients with thalassemia major. The Journal of pediatrics. 1988 Oct:113(4):661-9 [PubMed PMID: 3171791]
Level 2 (mid-level) evidenceBoelaert JR, de Locht M, Van Cutsem J, Kerrels V, Cantinieaux B, Verdonck A, Van Landuyt HW, Schneider YJ. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. The Journal of clinical investigation. 1993 May:91(5):1979-86 [PubMed PMID: 8486769]
Level 3 (low-level) evidenceGreen NS. Yersinia infections in patients with homozygous beta-thalassemia associated with iron overload and its treatment. Pediatric hematology and oncology. 1992 Jul-Sep:9(3):247-54 [PubMed PMID: 1525003]
Level 3 (low-level) evidenceGoldbeck L, Baving A, Kohne E. [Psychosocial aspects of beta-thalassemia: distress, coping and adherence]. Klinische Padiatrie. 2000 Sep-Oct:212(5):254-9 [PubMed PMID: 11048284]
Viswanathan M, Golin CE, Jones CD, Ashok M, Blalock SJ, Wines RC, Coker-Schwimmer EJ, Rosen DL, Sista P, Lohr KN. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Annals of internal medicine. 2012 Dec 4:157(11):785-95 [PubMed PMID: 22964778]
Level 1 (high-level) evidenceTrachtenberg F, Vichinsky E, Haines D, Pakbaz Z, Mednick L, Sobota A, Kwiatkowski J, Thompson AA, Porter J, Coates T, Giardina PJ, Olivieri N, Yamashita R, Neufeld EJ, Thalassemia Clinical Research Network. Iron chelation adherence to deferoxamine and deferasirox in thalassemia. American journal of hematology. 2011 May:86(5):433-6. doi: 10.1002/ajh.21993. Epub [PubMed PMID: 21523808]
Level 2 (mid-level) evidence