Introduction
Dandy-Walker malformation (DWM) or syndrome is a posterior fossa anomaly characterized by:
- Agenesis or hypoplasia of the vermis
- Cystic enlargement of the fourth ventricle with communication to a large cystic dilated posterior fossa
- Upward displacement of tentorium and torcula (torcular-lambdoid inversion)
- Enlargement of the posterior fossa [1][2][3]
The first description of DWM dates back to 1887 by Sutton. Dandy and Blackfan described this deformity in 1914, and Taggart and Walker expanded on it in 1942. Bender first described the condition as DWM in 1954.[4]
The "Dandy-Walker complex" and "Dandy-Walker spectrum" are 2 of the most often used radiological entities proposed by Barkovich et al. in 1989.[5] Most patients have hydrocephalus at the time of diagnosis.[6] DWM is the most common posterior fossa malformation, and it typically occurs sporadically.[7]
Keeping the development of cerebellar vermis as the standard, the Dandy-Walker spectrum can be divided into:
- Dandy-Walker malformation
- Dandy-Walker variant
- Simple posterior fossa cistern widening
Dandy-Walker variant is characterized by cerebellar vermian hypoplasia, cystic fourth ventricular dilatation, and normal posterior fossa volume. This terminology is currently going out of favor as it generates a lot of confusion. Rather, individual physical abnormalities should be mentioned.[8]
Many patients remain clinically asymptomatic for years, while others may present with a variety of comorbidities leading to earlier diagnosis.[9] Treatment is generally focused on alleviating hydrocephalus and posterior fossa symptoms, often including surgical interventions like ventriculoperitoneal and cystoperitoneal shunting.[6][10]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Historically, DWM was believed to be caused by atresia of the Luschka and Magendie foramina, leading to enlargement of the fourth ventricle and vermian hypoplasia. However, recent evidence suggests that DWM results from developmental abnormalities affecting the roof of the rhombencephalon, leading to variable degrees of vermian hypoplasia and cystic enlargement. This complex malformation may be initiated by 2 different pathophysiological mechanisms: the arrest of vermian development and the failure of fourth ventricle foramina fenestration, leading to an enlarged Blake’s pouch and causing compression of the vermis.[11]
DWM may be isolated or associated with chromosomal abnormalities, Mendelian disorders, syndromic malformations, congenital infections, and various other comorbidities.[11] Central nervous system (CNS) disorders related to DWM include malformations of cortical development, holoprosencephaly, dysgenesis of the corpus callosum, and neural tube defects.[3]
Rare mutations have been described in some genes including FOXC1 (in locus 6p25.3), ZIC1, and ZIC4 (in locus 3q24), FGF17, LAMC1, and NID1.[7]
Epidemiology
DWM and related variants have a prevalence of 1 in 350,000 live births in the United States. These malformations account for approximately 1% to 4% of hydrocephalus cases.[1][3] Most DWM patients present with signs and symptoms from increased intracranial pressure, most commonly related to hydrocephalus and the posterior fossa cyst. For this reason, therapy generally aims to control intracranial pressure, usually through surgery.
Some institutions have an estimated mortality rate of 12% to 50%.[1] Treatment of hydrocephalus with shunting substantially improves mortality.[12] Fetal mortality directly correlates with the presence of extra-CNS abnormalities.[13]
Pathophysiology
The majority of cases are sporadic. However, some may result from chromosomal aneuploidy, Mendelian disorders, and environmental exposures, including congenital rubella and fetal alcohol exposure. This condition underscores the intricate interplay between genetic and environmental factors in brain development.[9]
Several conditions have been reported to be associated with DWM:
- Mendelian disorders:
- Warburg syndrome
- Coffin-Siris syndrome
- Fraser cryptophthalmos
- Joubert–Boltshauser syndrome
- Meckel–Gruber syndrome
- Aicardi syndrome
- Neurofibromatosis type 1[14]
- Chromosomal aberrations:
- Duplication syndromes, including those involving 5q; 8p; 8q; 17q
- Trisomies 9, 13, and 18
- Triploidy
- Deletion at 6(p24–p25)
- 9ph+ heteromorphism
- Environmental agents:
- Prenatal exposure to rubella, cytomegalovirus, toxoplasmosis, coumadin, alcohol
- Maternal diabetes
- Sporadic syndromes:
- Klippel–Feil syndrome
- Goldenhar syndrome
- Cornelia de Lange syndrome
Concept of the Midline as a Developmental Field (Opitz and Gilbert)
An insult to this field can result in pleiotropic sequelae on midline structures- the central nervous system (CNS), heart, palate, midface, vertebrae, genitalia, and sacrococcygeal region. DWM should be considered a complex developmental disturbance of the midline of marked genetic and etiological heterogeneity.
Embryology
The cerebellar vermis develops from top to bottom at 17 to 18 weeks gestation.[15] Due to vermian developmental arrest or compression of the vermis brought on by a failure of fourth ventricle foramina fenestration and consequent growing of Blake's pouch, the roof of the rhombencephalon does not form correctly.
History and Physical
The clinical presentation is nonspecific, subject to multiple factors, including the severity of hydrocephalus, intracranial hypertension, and associated comorbidities.[1] Most patients will present in their first year of life with signs and symptoms of increased intracranial pressure.[6][7][12] The most common manifestation is macrocephaly, affecting 90% to 100% of patients during their first months of life.[7] The syndromic form of DWM may also have malformations of the heart, face, limbs, and gastrointestinal or genitourinary system that could draw initial medical attention.[9] Almost 75% of cases will have hydrocephalus by the age of 3 months, and eventually, almost 90% of the patients will have hydrocephalus.
A case series of 42 patients with DWM showed hydrocephaly in all patients at the time of diagnosis, vermian hypoplasia in 88%, and cerebellar hypoplasia in 59%.[6]
Evaluation
Ultrasound is typically the first imaging modality in assessing the fetal brain. CNS structures evaluated through this modality should include head size and shape, choroid plexus, thalami, cerebellum, cavum septum pellucidum, lateral ventricles, nuchal fold, cisterna magna, and spine. Imaging may also demonstrate other CNS abnormalities that commonly correlate with DWM.
Measurement of the cisterna magna forms part of the ultrasound evaluation of the fetal brain. A case series showed an upper limit of normal of 10 mm and a mean size of 5 plus or minus 3 mm at 15 or more gestational weeks. A prominent cisterna magna during prenatal fetal ultrasound evaluation may raise concern for congenital posterior fossa abnormalities. However, as an isolated finding, a prominent cisterna magna is unlikely to be clinically significant if the patient presents no other abnormalities.[16]
Radiological Criteria for Diagnosis
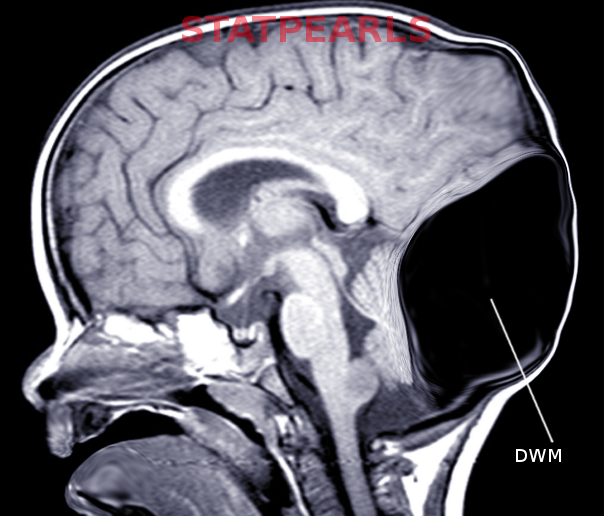
The classical radiological triad of DWM includes hypoplastic vermis, fourth ventricle enlargement, and torcular elevation.[17]
However, Klein et al. modified the classification by Barkovich et al. in 2003 to identify the radiological criteria for the diagnosis of DWM:
- Large, median posterior fossa cyst widely communicating with the fourth ventricle (see Image. Occipital Meningocele With Dandy-Walker Cyst)
- Absence of the lower portion of the vermis at different degrees (lower 3/4, lower half, lower 1/4)
- Hypoplasia, anterior rotation, and upward displacement of the remnant of the vermis
- Absence or flattening of the angle of the fastigium
- Large bossing posterior fossa with an elevation of the torcula
- Anterolateral displacement of normal or hypoplastic cerebellar hemispheres [18]
Like ultrasound, magnetic resonance imaging (MRI) is more helpful after the 20th week of gestation, and it may aid in evaluating CNS malformations not satisfactorily described by ultrasound and search for commonly associated abnormalities.[3][19][20]
Prenatal diagnosis by ultrasound is possible after the 18th week of gestation when the cerebellar vermis has completely developed. Diagnosis may be confirmed by magnetic resonance.[19][21] For patients with the Dandy-Walker variant (DWV), discrepancies may be found in prenatal and postnatal imaging based on the variation of vermian development.[20] Magnetic resonance will also help distinguish DWM from other posterior fossa lesions (see Image. Dandy-Walker Malformation).[3]
Karyotype and postnatal imaging should be offered for all patients with prenatal imaging findings consistent with DWM to confirm findings and search for other possibly associated abnormalities.[13][20]
Measurement of the brainstem-vermian (BV) angle and brainstem-tentorium (BT) angle substantially help in the differential diagnosis of patients with an increased size of the cisterna magna. The BV angle increases with the severity of the condition. Angles <18 degrees are normal, while 18 to 30 degrees suggest Blake's pouch malformation. The BT angle ranges typically between 21 to 44 degrees. Both angles >45 degrees are strongly suggestive of DWM.[22]
Associated CNS abnormalities include occipital encephalocele, polymicrogyria, agenesis, or dysgenesis of the corpus callosum, and heterotopia.
Treatment / Management
Treatment consists of treating the manifestations and associated comorbidities. Most patients present with signs and symptoms from increased intracranial pressure, most commonly related to hydrocephalus and posterior fossa cyst. For this reason, therapy generally aims to control intracranial pressure, usually through surgery.[3]
Surgical treatment may include ventriculoperitoneal (VP) or cystoperitoneal (CP) shunts. Other patients may be candidates for endoscopic procedures, including endoscopic third ventriculostomy (ETV).[23](B2)
Differential Diagnosis
Differential diagnosis includes posterior fossa abnormalities that may be associated with hydrocephalus sometimes, which commonly may present in a similar way to DWM; these include retrocerebellar arachnoid cysts, cystic hygroma, Blake's pouch cyst, mega cisterna magna, and vermian hypoplasia. Additionally, several syndromes correlate with DWM, such as Aase-Smith, cerebro-oculo-muscular syndrome, Coffin-Siris, Cornelia de Lange, and Aicardi.[3][9]
The other cystic malformations of the posterior fossa include persistent Blake's pouch, mega cisterna magna, and arachnoid cyst.
The non-cystic malformations include Joubert syndrome, rhombencephalosynapsis, tectocerebellar dysraphia, and neocerebellar dysgenesis.[5]
Retrocerebellar arachnoid cysts may be large enough to cause compression of cerebellar hemispheres and the fourth ventricle.[3]
Blake's cyst is a retrocerebellar fluid collection with a medial line communication to the fourth ventricle.[3]
Mega cisterna manga refers to a fluid collection located posteroinferior to a normally developed cerebellum.[3]
Several comorbidities may also correlate with DWM, including syndromic and non-syndromic, CNS and non-CNS anomalies, chromosomal abnormalities, cardiovascular conditions, mental illness, and severe intellectual disability.[9]
Prognosis
The prognosis for patients with DWM is highly variable and dependent on several factors, including the severity of the malformation, the timeliness of diagnosis and intervention, and the presence of associated complications. In milder cases, where the cerebellar vermis is less affected and with minimal intracranial pressure, individuals may experience fewer neurological deficits and lead relatively normal lives with appropriate management and support. However, in more severe cases with significant cerebellar and posterior fossa abnormalities, patients are at a higher risk of experiencing developmental delays, intellectual disabilities, motor coordination issues, and hydrocephalus.
Fifty percent of children with untreated hydrocephalus die before age 3, and only approximately 20% to 23% will reach adult life. Most of the patients with untreated hydrocephalus who reach adult life will have motor, visual, and auditory deficits.[24]
The diameter of the fetal lateral ventricle measured by obstetric ultrasound may have substantial prognostic value. As described in a previous study, lateral ventricles measuring between 11 mm to 15 mm correlate with a 21% risk of developmental delay. If the diameter measures >15 mm, the risk of developmental delay is above 50%.[25]
Most case series of patients with nontumoral hydrocephalus present an overall risk for epilepsy of approximately 30%.[24]
Functional outcome is subject to several factors, which include other structural brain abnormalities, extra-CNS manifestations, epilepsy, motor, visual, or hearing impairment, and other congenital abnormalities.[26][27]
Complications
Complications in DWM can encompass a broad spectrum of neurological and developmental challenges. One of the primary complications is hydrocephalus, leading to increased intracranial pressure. This increased pressure can lead to symptoms such as headaches, vomiting, and cognitive impairments, making timely intervention essential. The most common complications associated with shunting are infection and shunt malfunction.[12]
Patients with DWM may experience various motor and coordination deficits due to the cerebellar abnormalities, affecting their daily functioning and quality of life. Cognitive and intellectual disabilities, speech and language impairments, and behavioral problems are common as well, adding to the complexity of managing this condition. Furthermore, the associated structural anomalies may result in additional complications, including the risk of syringomyelia or other spinal cord abnormalities.
Sixteen percent of patients with isolated DWM have chromosomal abnormalities.[28] DWM may be associated with malformations of the face, limbs, heart, and genitourinary or gastrointestinal system.
Deterrence and Patient Education
The cerebellar vermis development varies between individuals and usually completes its formation late in the first half of pregnancy. Some fetuses may achieve vermis development around gestational week 18. Therefore, the diagnosis of DWM based on imaging performed before weeks 16 to 18 of development may be premature and erroneous. Additionally, the cisterna magna has not reached its final anatomy during the first half of pregnancy. A relatively wide opening in the cerebellomedullary cistern may not indicate vermian dysgenesis at that stage of development. Therefore, the cerebellum should be reevaluated at 20 to 22 weeks gestation to rule out vermian abnormalities.[29]
Karyotype and comprehensive fetal ultrasound should be offered for patients with features of the Dandy-Walker complex.[20]
In the absence of other lesions in the posterior fossa, an isolated prominent cisterna magna may not be clinically significant.[16]
Enhancing Healthcare Team Outcomes
In managing patients with DWM, a multidisciplinary healthcare team comprised of physicians, advanced practitioners, nurses, pharmacists, and other health professionals plays a pivotal role in enhancing patient-centered care, outcomes, patient safety, and team performance. Pediatric healthcare providers should be familiar with the broad spectrum of congenital posterior fossa abnormalities to provide an accurate diagnosis, optimal therapy, and genetic counseling.[29] Additionally, posterior fossa imaging findings will change based on the gestational age; as such, findings suggestive of disease in earlier weeks should be reevaluated at weeks 20 to 22 gestation.[16]
Skillful expertise, honed through continuous education and training, is essential for correctly diagnosing and treating this complex neurological condition. A well-structured strategy involving the collaboration of various professionals ensures that each aspect of the patient's care plan is addressed comprehensively. Timely consultation should be obtained from the pediatric neurosurgeon/neurologist.
Ethical considerations, such as informed consent and respect for patient autonomy, guide decision-making and promote trust within the interprofessional team. Responsibilities are distributed efficiently, with clear roles and accountabilities to optimize patient care. Effective interprofessional communication fosters the exchange of critical information and the seamless coordination of care, helping to minimize errors and improve patient safety. Care coordination, facilitated by healthcare professionals, enables a holistic approach to patient management, offering the best chance for positive outcomes in the challenging realm of DWM.
Media
(Click Image to Enlarge)
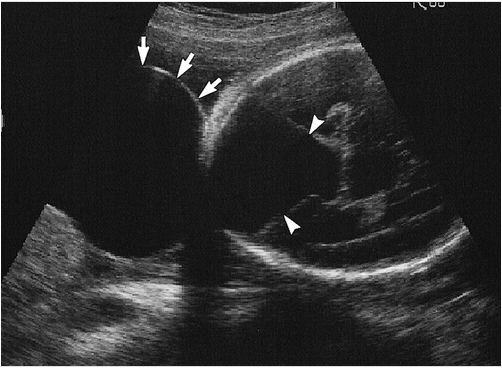
Occipital Meningocele With Dandy-Walker Cyst. A transverse image through the fetal head shows a large cystic structure outside the calvaria in the occipital region (arrows). A large cystic structure is also seen in the posterior fossa, consistent with a Dandy-Walker cyst (arrowheads). A small defect is seen in the cranium at the base of the cyst.
Contributed From the Departments of Radiology (J.R.M., R.L., J.F.) and Obstetrics and Gynecology (P.M.), McMaster University Medical Centre, 1200 Main St W, Hamilton, Ontario, Canada L8N 3Z5. Presented as a scientific exhibit at the 1998 RSNA scientific assembly. Received February 11, 1999; revisions requested March 4 and received March 31; accepted April 5. Address reprint requests to J.R.M. (Open Access)
References
Treviño Alanís MG, González Cantú N, Montes Cruz JV, García Flores JB, Martínez Menchaca HR, Rivera Silva G. [Dandy Walker malformation]. Archivos argentinos de pediatria. 2014 Feb:112(1):103-4 [PubMed PMID: 24566791]
Level 3 (low-level) evidenceJurcă MC, Kozma K, Petcheşi CD, Bembea M, Pop OL, MuŢiu G, Coroi MC, Jurcă AD, Dobjanschi L. Anatomic variants in Dandy-Walker complex. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie. 2017:58(3):1051-1055 [PubMed PMID: 29250689]
Correa GG, Amaral LF, Vedolin LM. Neuroimaging of Dandy-Walker malformation: new concepts. Topics in magnetic resonance imaging : TMRI. 2011 Dec:22(6):303-12. doi: 10.1097/RMR.0b013e3182a2ca77. Epub [PubMed PMID: 24132069]
Reith W, Haussmann A. [Dandy-Walker malformation]. Der Radiologe. 2018 Jul:58(7):629-635. doi: 10.1007/s00117-018-0403-7. Epub [PubMed PMID: 29797040]
Di Nora A, Costanza G, Pizzo F, Di Mari A, Sapuppo A, Basile A, Fiumara A, Pavone P. Dandy-Walker malformation and variants: clinical features and associated anomalies in 28 affected children-a single retrospective study and a review of the literature. Acta neurologica Belgica. 2023 Jun:123(3):903-909. doi: 10.1007/s13760-022-02059-z. Epub 2022 Sep 6 [PubMed PMID: 36068432]
Level 2 (mid-level) evidenceKumar R, Jain MK, Chhabra DK. Dandy-Walker syndrome: different modalities of treatment and outcome in 42 cases. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2001 May:17(6):348-52 [PubMed PMID: 11417415]
Level 3 (low-level) evidenceBosemani T, Orman G, Boltshauser E, Tekes A, Huisman TA, Poretti A. Congenital abnormalities of the posterior fossa. Radiographics : a review publication of the Radiological Society of North America, Inc. 2015 Jan-Feb:35(1):200-20. doi: 10.1148/rg.351140038. Epub [PubMed PMID: 25590398]
Alves CAPF, Sidpra J, Manteghinejad A, Sudhakar S, Massey FV, Aldinger KA, Haldipur P, Lucato LT, Ferraciolli SF, Teixeira SR, Öztekin Ö, Bhattacharya D, Taranath A, Prabhu SP, Mirsky DM, Andronikou S, Millen KJ, Barkovich AJ, Boltshauser E, Dobyns WB, Barkovich MJ, Whitehead MT, Mankad K. Dandy-Walker Phenotype with Brainstem Involvement: 2 Distinct Subgroups with Different Prognosis. AJNR. American journal of neuroradiology. 2023 Oct:44(10):1201-1207. doi: 10.3174/ajnr.A7967. Epub 2023 Aug 17 [PubMed PMID: 37591769]
Stambolliu E, Ioakeim-Ioannidou M, Kontokostas K, Dakoutrou M, Kousoulis AA. The Most Common Comorbidities in Dandy-Walker Syndrome Patients: A Systematic Review of Case Reports. Journal of child neurology. 2017 Sep:32(10):886-902. doi: 10.1177/0883073817712589. Epub 2017 Jun 21 [PubMed PMID: 28635420]
Level 3 (low-level) evidenceOsenbach RK, Menezes AH. Diagnosis and management of the Dandy-Walker malformation: 30 years of experience. Pediatric neurosurgery. 1992:18(4):179-89 [PubMed PMID: 1472430]
Level 3 (low-level) evidenceRobinson AJ. Inferior vermian hypoplasia--preconception, misconception. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2014 Feb:43(2):123-36. doi: 10.1002/uog.13296. Epub [PubMed PMID: 24497418]
Bokhari I, Rehman L, Hassan S, Hashim MS. Dandy-Walker Malformation: A Clinical and Surgical Outcome Analysis. Journal of the College of Physicians and Surgeons--Pakistan : JCPSP. 2015 Jun:25(6):431-3 [PubMed PMID: 26100996]
Nyberg DA, Cyr DR, Mack LA, Fitzsimmons J, Hickok D, Mahony BS. The Dandy-Walker malformation prenatal sonographic diagnosis and its clinical significance. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 1988 Feb:7(2):65-71 [PubMed PMID: 3279226]
Level 2 (mid-level) evidenceShah SP, Gohil JA, Kesavapisharady K, Easwer HV. Revisiting Dandy-Walker Malformation with Associated Neurofibromatosis. Asian journal of neurosurgery. 2021 Oct-Dec:16(4):850-853. doi: 10.4103/ajns.AJNS_468_20. Epub 2021 Dec 18 [PubMed PMID: 35071091]
Ecker JL, Shipp TD, Bromley B, Benacerraf B. The sonographic diagnosis of Dandy-Walker and Dandy-Walker variant: associated findings and outcomes. Prenatal diagnosis. 2000 Apr:20(4):328-32 [PubMed PMID: 10740206]
Mahony BS, Callen PW, Filly RA, Hoddick WK. The fetal cisterna magna. Radiology. 1984 Dec:153(3):773-6 [PubMed PMID: 6387792]
Beaman MM, Guidugli L, Hammer M, Barrows C, Gregor A, Lee S, Deak KL, McDonald MT, Jensen C, Zaki MS, Masri AT, Hobbs CA, Gleeson JG, Cohen JL. Novel association of Dandy-Walker malformation with CAPN15 variants expands the phenotype of oculogastrointestinal neurodevelopmental syndrome. American journal of medical genetics. Part A. 2023 Nov:191(11):2757-2767. doi: 10.1002/ajmg.a.63363. Epub 2023 Aug 19 [PubMed PMID: 37596828]
Klein O, Pierre-Kahn A, Boddaert N, Parisot D, Brunelle F. Dandy-Walker malformation: prenatal diagnosis and prognosis. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2003 Aug:19(7-8):484-9 [PubMed PMID: 12879343]
Kline-Fath BM, Calvo-Garcia MA. Prenatal imaging of congenital malformations of the brain. Seminars in ultrasound, CT, and MR. 2011 Jun:32(3):167-88. doi: 10.1053/j.sult.2011.02.010. Epub [PubMed PMID: 21596274]
Harper T, Fordham LA, Wolfe HM. The fetal dandy walker complex: associated anomalies, perinatal outcome and postnatal imaging. Fetal diagnosis and therapy. 2007:22(4):277-81 [PubMed PMID: 17369695]
Level 2 (mid-level) evidenceYu F, Fu L, Xu C, Wang J, Huang X, Zhang G, Zhang H. Prenatal Magnetic Resonance Imaging helps Discover Cerebellar Dysplasia or Malformations in Foetuses. Current medical imaging. 2023 Oct 31:():. doi: 10.2174/0115734056256514231020103822. Epub 2023 Oct 31 [PubMed PMID: 37921151]
Volpe P, Contro E, De Musso F, Ghi T, Farina A, Tempesta A, Volpe G, Rizzo N, Pilu G. Brainstem-vermis and brainstem-tentorium angles allow accurate categorization of fetal upward rotation of cerebellar vermis. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2012 Jun:39(6):632-5. doi: 10.1002/uog.11101. Epub 2012 May 14 [PubMed PMID: 22253138]
Mohanty A, Biswas A, Satish S, Praharaj SS, Sastry KV. Treatment options for Dandy-Walker malformation. Journal of neurosurgery. 2006 Nov:105(5 Suppl):348-56 [PubMed PMID: 17328256]
Level 2 (mid-level) evidenceChumas P, Tyagi A, Livingston J. Hydrocephalus--what's new? Archives of disease in childhood. Fetal and neonatal edition. 2001 Nov:85(3):F149-54 [PubMed PMID: 11668153]
Yashon D, Jane JA, Sugar O. The course of severe untreated infantile hydrocephalus. Prognostic significance of the cerebral mantle. Journal of neurosurgery. 1965 Nov:23(5):509-16 [PubMed PMID: 5295268]
Donders J, Canady AI, Rourke BP. Psychometric intelligence after infantile hydrocephalus. A critical review and reinterpretation. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1990 May:6(3):148-54 [PubMed PMID: 2357712]
Dennis M, Fitz CR, Netley CT, Sugar J, Harwood-Nash DC, Hendrick EB, Hoffman HJ, Humphreys RP. The intelligence of hydrocephalic children. Archives of neurology. 1981 Oct:38(10):607-15 [PubMed PMID: 6975094]
Grinberg I, Northrup H, Ardinger H, Prasad C, Dobyns WB, Millen KJ. Heterozygous deletion of the linked genes ZIC1 and ZIC4 is involved in Dandy-Walker malformation. Nature genetics. 2004 Oct:36(10):1053-5 [PubMed PMID: 15338008]
Level 3 (low-level) evidenceBromley B, Nadel AS, Pauker S, Estroff JA, Benacerraf BR. Closure of the cerebellar vermis: evaluation with second trimester US. Radiology. 1994 Dec:193(3):761-3 [PubMed PMID: 7972820]