Introduction
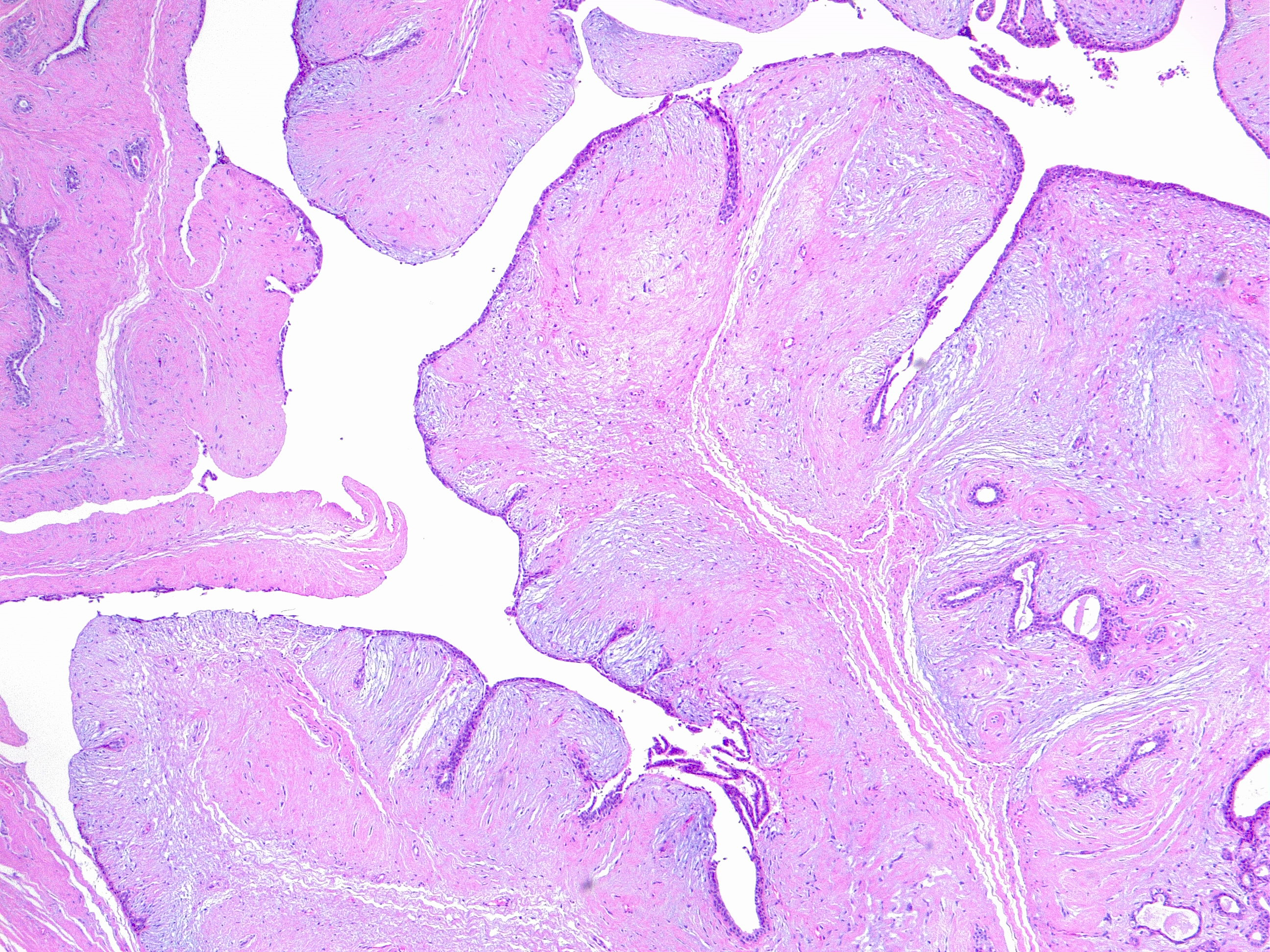
Phyllodes tumor of the breast is an infrequently encountered fibroepithelial neoplasm, which accounts for 0.3% to 1% of all tumors.[1] Phyllodes tumor presents a morphologic continuum from benign to malignant (see Image. Phyllodes Tumor of the Breast, Benign. H/E 4×). Based on histologic features, including nuclear atypia, stromal cellularity, mitotic activity, tumor margin appearance, and stromal overgrowth, the World Health Organization classifies Phyllodes tumors as benign, borderline, and malignant.[1] Phyllodes tumors have an inherent recurrence or metastatic potential, which varies based on histologic grade. Their diagnosis is mainly established based on histopathological examination. However, the differential diagnosis between benign phyllodes tumors and fibroadenoma remains challenging, especially on core biopsy specimens.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Genetic risk factors of phyllodes tumors are largely unknown, but the literature describes phyllodes tumors in Li-Fraumeni syndrome patients and a mother and daughter pair.[2] Rare cases of phyllodes tumors in men are often associated with gynecomastia, suggesting a role for hormonal imbalance.[3] Researchers have postulated that stromal induction of phyllodes tumors can occur due to growth factors produced by the breast epithelium. Trauma, pregnancy, increased estrogen activity, and lactation are occasionally implicated as factors stimulating tumor growth. The nature of these factors is not well understood, but endothelin-1, a stimulator of breast fibroblast growth, might be a contributory factor.[4]
Epidemiology
Phyllodes tumors occur predominantly in middle-aged women (average age at presentation, 40 to 50 years), about 15 to 20 years later than fibroadenomas.[5] Phyllodes tumor occurs mainly in women, although there are reports of some cases in men.[6] Data analysis from the Surveillance, Epidemiology, and End Results Program data registry from 2000 to 2004 reported that 500 women are diagnosed with malignant phyllodes tumors in the US annually.[7]
Pathophysiology
Unlike breast carcinoma, phyllodes tumors start outside the lobules and ducts in the breast’s connective tissue, called the stroma, including the ligaments and fatty tissue surrounding the lobules, ducts, lymph, and blood vessels in the breast. In addition to epithelial cells from the ducts and lobules, phyllodes tumors can also contain stromal cells.[8] They most likely develop de novo, although there have been reports of progression of fibroadenoma to phyllodes tumor.[9]
Molecular Features
Recent studies have focused on defining a molecular classification of phyllodes tumors. Comparative genomic hybridization studies showed recurrent chromosome imbalances, including +1q, −6q, −13q, −9p, −10p, and +5p. Although currently no chromosomal aberrations were found to be specific to phyllodes tumors, some authors reported that low-grade and high-grade (borderline/malignant) phyllodes tumors segregate in 2 genetic groups based on genomic alterations, with high-grade phyllodes tumors consistently showing 1q gain and 13q loss and low-grade phyllodes tumors showing few or no alterations.[10] Preliminary data from array comparative genomic hybridization demonstrate interstitial deletion 9p21 involving the CDKN2A locus and 9p deletion in malignant and some borderline phyllodes tumors.[11]
Histopathology
Macroscopic Examination
Phyllodes tumors form well-circumscribed, firm, protruding masses. When cut, the surface is tan or pink to grey and may present as fleshy and mucoid. The characteristic whorled pattern with curved clefts resembling leaf buds is most evident in large lesions, but smaller lesions may have a homogeneous appearance. Hemorrhage or necrosis may be present in large lesions.[1]
Microscopic Examination
Phyllodes tumors exhibit an enhanced intra-canalicular growth pattern with leaf-like projections into variably dilated elongated lumina. The epithelial component consists of luminal epithelial and myoepithelial cells stretched into arc-like clefts surmounting stromal fronds.[1]
Grading
There have been proposals for several grading systems, but using the 3-tiered system, including benign, borderline, and malignant phyllodes tumors, is preferred because this approach leads to greater certainty at the ends of the spectrum of these fibroepithelial lesions.[1]
Benign Phyllodes Tumors
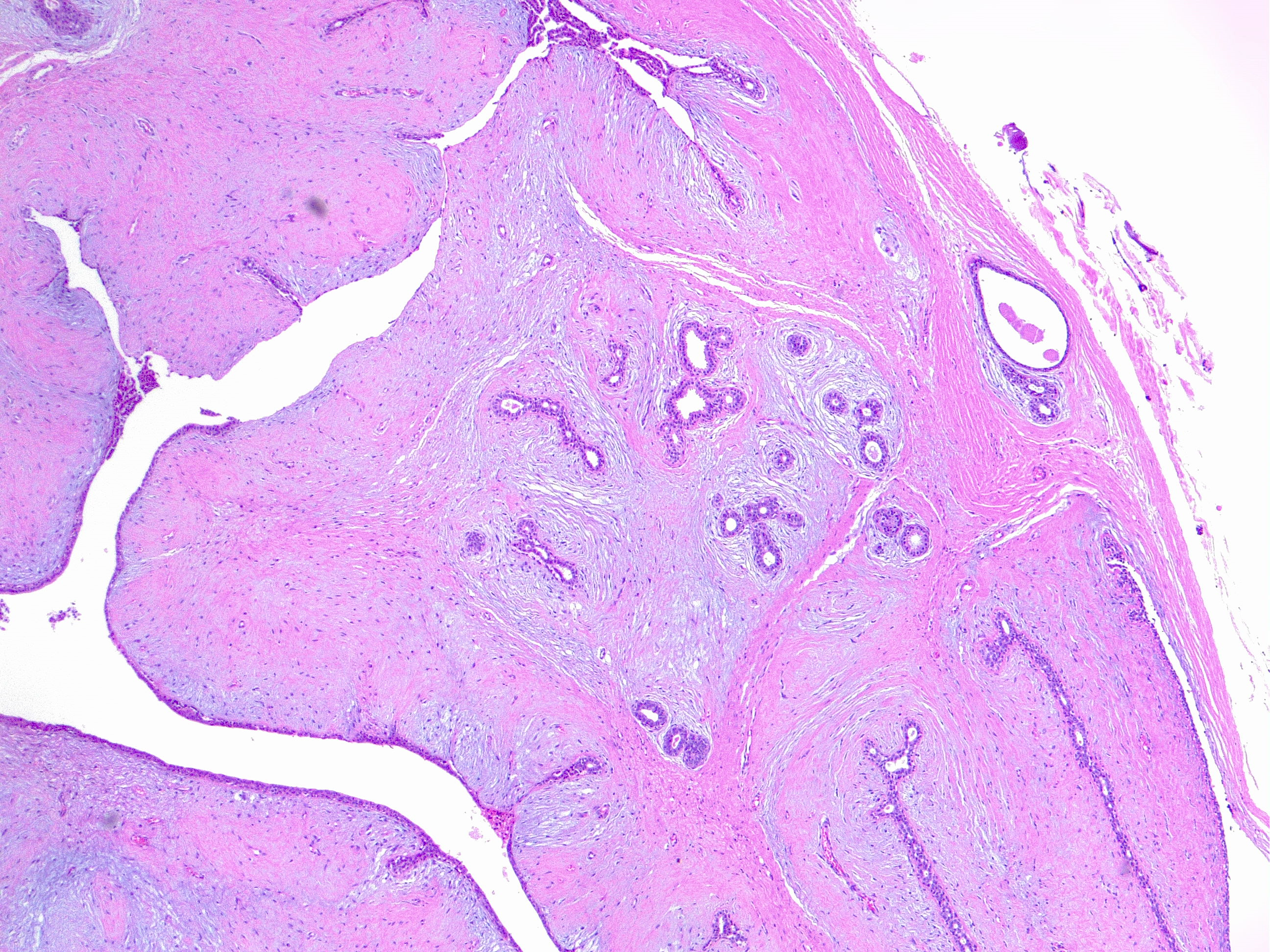
This variety comprises 60% to 75% of all phyllodes tumors (see Image. Benign Phyllodes Tumor of the Breast. H/E 4×). In benign phyllodes tumors, the stroma is usually more cellular than in fibroadenomas. The spindle-cell stromal nuclei are uniform, and mitoses are rare, generally less than 5 per 10 high-power fields. Areas of sparse stromal cellularity, hyalinization, or myxoid changes are not uncommon, reflecting stromal heterogeneity. The margins are usually well-delimited and pushing.[1]
Borderline Phyllodes Tumors
These tumors are diagnosed when the mass does not possess all the adverse histological characteristics of malignant phyllodes tumors. Borderline phyllodes tumors may have frequent mitoses (5 to 9 per 10 HPF), moderate stromal cellularity, a circumscribed or focally invasive border, and stromal atypia. Stromal overgrowth is often absent.
Malignant Phyllodes Tumors
Malignant phyllode tumors show a combination of marked nuclear pleomorphism of stromal cells, stromal overgrowth defined as the absence of epithelial elements in 1 low-power microscopic field containing only stroma, increased mitoses (greater than or equal to 10 per 10 HPF), increased stromal cellularity, which is usually diffuse, and infiltrative borders.[1]
Immunohistochemistry
Multiple immunohistochemistry markers have undergone a study to improve the classification of PT and predict their outcomes. Studies demonstrate that p53, Ki67, CD117, EGFR, p16, and VEGF (the lowest in benign phyllodes tumors and the highest in malignant phyllodes tumors) are associated with histologic grades of phyllodes tumors. Still, none has been proven to be clinically useful.[12][13][14][15] Among these markers, p53 expression and Ki67 index were reported in some studies to be significantly associated with disease-free and overall survival. Still, other studies found no association with recurrence or clinical behavior.[16][17] PAX3 and SIX1 expression by immunohistochemistry and gene expression analysis have recently been identified in borderline and malignant phyllodes tumors and correlate with a poor clinical outcome.[18]
History and Physical
Clinically, phyllodes tumors tend to present as unilateral firm, enlarging painless breast masses that stretch the overlying skin with striking distension of superficial veins. The size may range from 1 to 45 cm and occupy the entire breast. Bloody nipple discharge caused by spontaneous infarction of the tumor has been described. Ulceration and nipple retraction are uncommon. Although axillary lymphadenopathy is common, nodal metastases are the exception.[19]
Evaluation
The imaging findings on mammography are typically round-lobulated dense masses with partially indistinct or circumscribed margins. Calcifications within the mass are rare, but they can be large.[20] On ultrasound, phyllodes tumor presents as a hypoechoic, partially indistinct, or partially circumscribed mass with frequent posterior enhancement. A cystic component is more typical in malignant phyllodes tumors. Frequently, phyllodes tumors show increased vascularity on color or power Doppler.[21] MRI evaluation may be beneficial for chest wall invasion in malignant phyllodes tumor. The characteristics present on MRI are typically heterogeneously low signal on T1-weighted images, although areas of T1-hyperintense hemorrhage may be visible. T2-weighted images usually show a lobulated mass with hyperintense fluid in slit-like spaces and sometimes hyperintensity in the surrounding tissues.[22]
Treatment / Management
Complete wide local excision with greater than 1 cm margins is often curative and reduces the risk of local recurrence.[23] Large tumors require a mastectomy. Despite the higher incidence of local recurrence secondary to breast-conserving surgery, studies have shown no differences between breast-conserving surgery versus mastectomy regarding metastasis-free survival or overall survival. Adjuvant therapy options mainly include radiation therapy, which has been found to reduce local recurrence. Research has not shown a clinical benefit of giving adjuvant chemotherapy.[24](B2)
Differential Diagnosis
The differential diagnosis for phyllodes tumor of the breast is as follows:
- Fibroadenoma: The principal differential diagnosis for benign phyllodes tumor is fibroadenoma, which has a pronounced intracanalicular pattern.[25]
- Sarcomas: malignant phyllodes tumor may be confused with pure breast sarcomas.[26]
- Periductal stromal tumor: the periductal stromal tumor is an entity that histologically overlaps with malignant phyllodes tumor, the main difference being the absence of leaf-like processes. It is non-circumscribed, consisting of spindle-cell proliferation localized around open tubules.[27]
- Metaplastic carcinoma: carcinoma is also in the differential diagnosis of malignant phyllodes tumor, but the immunohistochemical demonstration of epithelial differentiation helps resolve the problem.
Prognosis
The prognosis of phyllodes tumor is good, with an 87% 10-year survival. The recurrence risk varies with tumor size and surgical approach. Most phyllodes tumors behave benignly, with local recurrences occurring in a small proportion of cases. Very rarely, the tumor may metastasize, most commonly in malignant-grade tumors. Malignant phyllodes tumors carry a poor prognosis.
Complications
Local Recurrence
Local recurrences can occur in all phyllodes tumors at an overall rate of 21%, with ranges of 10% to 17%, 14% to 25%, and 23% to 30% for benign, borderline malignant, and phyllodes tumors, respectively. Local recurrences generally develop within 2 to 3 years.[28]
Distant Metastases
Distant metastases are almost exclusively a feature of malignant phyllodes tumors. The lungs (66%), the bones (28%), and the brain (9%) are the most common sites of spread.[28] Rarely, metastases can involve the liver and heart.
Consultations
Consultations that are typically requested for patients with phyllodes tumor of the breast include the following:
- Surgical oncologist
- Oncologist
Deterrence and Patient Education
Patients might be instructed to self-examine their breasts regularly and consult their doctors if any abnormality is detected.
Enhancing Healthcare Team Outcomes
Accurate preoperative pathological diagnosis of phyllodes tumors enables adequate surgical planning and avoidance of reoperation. The distinction between phyllodes tumor and fibroadenoma is important but can be difficult on core biopsy. Management of phyllodes tumors needs an interprofessional team consisting of a surgical oncologist, an oncologist, a pathologist, a radiologist, and oncology specialty-trained nurses, all working together as an interprofessional team to deliver the best clinical results. The primary care provider must refer patients with suspected breast masses to an oncologist for further workup. Treatment can be either wide local excision or mastectomy to achieve histologically clear margins. After treating the phyllodes tumor, long-term follow-up by the primary care providers is necessary to detect local and distant relapse.
Media
References
Zhang Y, Kleer CG. Phyllodes Tumor of the Breast: Histopathologic Features, Differential Diagnosis, and Molecular/Genetic Updates. Archives of pathology & laboratory medicine. 2016 Jul:140(7):665-71. doi: 10.5858/arpa.2016-0042-RA. Epub [PubMed PMID: 27362571]
Foucar CE, Hardy A, Siziopikou KP, Wang L, Parini V, Hansen N, Jeruss JS. A mother and daughter with phyllodes tumors of the breast. Clinical breast cancer. 2012 Oct:12(5):373-7. doi: 10.1016/j.clbc.2012.07.011. Epub 2012 Sep 14 [PubMed PMID: 22981939]
Level 3 (low-level) evidenceNielsen VT, Andreasen C. Phyllodes tumour of the male breast. Histopathology. 1987 Jul:11(7):761-2 [PubMed PMID: 3040567]
Level 3 (low-level) evidenceMishra SP, Tiwary SK, Mishra M, Khanna AK. Phyllodes tumor of breast: a review article. ISRN surgery. 2013:2013():361469. doi: 10.1155/2013/361469. Epub 2013 Mar 20 [PubMed PMID: 23577269]
Jing P, Wei B, Yang X. Phyllodes tumor of the breast with nipple discharge: A case report. Medicine. 2018 Dec:97(52):e13767. doi: 10.1097/MD.0000000000013767. Epub [PubMed PMID: 30593154]
Level 3 (low-level) evidenceAnsah-Boateng Y, Tavassoli FA. Fibroadenoma and cystosarcoma phyllodes of the male breast. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1992 Mar:5(2):114-6 [PubMed PMID: 1315437]
Adesoye T, Neuman HB, Wilke LG, Schumacher JR, Steiman J, Greenberg CC. Current Trends in the Management of Phyllodes Tumors of the Breast. Annals of surgical oncology. 2016 Oct:23(10):3199-205. doi: 10.1245/s10434-016-5314-0. Epub 2016 Jun 22 [PubMed PMID: 27334214]
Mitus JW, Blecharz P, Jakubowicz J, Reinfuss M, Walasek T, Wysocki W. Phyllodes tumors of the breast. The treatment results for 340 patients from a single cancer centre. Breast (Edinburgh, Scotland). 2019 Feb:43():85-90. doi: 10.1016/j.breast.2018.11.009. Epub 2018 Nov 26 [PubMed PMID: 30521986]
Faridi SH, Siddiqui B, Ahmad SS, Aslam M. Progression of Fibroadenoma to Malignant Phyllodes Tumour in a 14-Year Female. Journal of the College of Physicians and Surgeons--Pakistan : JCPSP. 2018 Jan:28(1):69-71. doi: 10.29271/jcpsp.2018.01.69. Epub [PubMed PMID: 29290198]
Laé M, Vincent-Salomon A, Savignoni A, Huon I, Fréneaux P, Sigal-Zafrani B, Aurias A, Sastre-Garau X, Couturier J. Phyllodes tumors of the breast segregate in two groups according to genetic criteria. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2007 Apr:20(4):435-44 [PubMed PMID: 17334353]
Jones AM, Mitter R, Springall R, Graham T, Winter E, Gillett C, Hanby AM, Tomlinson IP, Sawyer EJ, Phyllodes Tumour Consortium. A comprehensive genetic profile of phyllodes tumours of the breast detects important mutations, intra-tumoral genetic heterogeneity and new genetic changes on recurrence. The Journal of pathology. 2008 Apr:214(5):533-44. doi: 10.1002/path.2320. Epub [PubMed PMID: 18288784]
Noronha Y, Raza A, Hutchins B, Chase D, Garberoglio C, Chu P, Weiss L, Wang J. CD34, CD117, and Ki-67 expression in phyllodes tumor of the breast: an immunohistochemical study of 33 cases. International journal of surgical pathology. 2011 Apr:19(2):152-8. doi: 10.1177/1066896910382009. Epub 2010 Nov 17 [PubMed PMID: 21087983]
Level 3 (low-level) evidenceTan PH, Jayabaskar T, Yip G, Tan Y, Hilmy M, Selvarajan S, Bay BH. p53 and c-kit (CD117) protein expression as prognostic indicators in breast phyllodes tumors: a tissue microarray study. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2005 Dec:18(12):1527-34 [PubMed PMID: 16258510]
Tse GM, Lui PC, Vong JS, Lau KM, Putti TC, Karim R, Scolyer RA, Lee CS, Yu AM, Ng DC, Tse AK, Tan PH. Increased epidermal growth factor receptor (EGFR) expression in malignant mammary phyllodes tumors. Breast cancer research and treatment. 2009 Apr:114(3):441-8. doi: 10.1007/s10549-008-0030-5. Epub 2008 Apr 29 [PubMed PMID: 18443904]
Karim RZ, Gerega SK, Yang YH, Spillane A, Carmalt H, Scolyer RA, Lee CS. p16 and pRb immunohistochemical expression increases with increasing tumour grade in mammary phyllodes tumours. Histopathology. 2010 Jun:56(7):868-75. doi: 10.1111/j.1365-2559.2010.03562.x. Epub 2010 May 19 [PubMed PMID: 20497245]
Yonemori K, Hasegawa T, Shimizu C, Shibata T, Matsumoto K, Kouno T, Ando M, Katsumata N, Fujiwara Y. Correlation of p53 and MIB-1 expression with both the systemic recurrence and survival in cases of phyllodes tumors of the breast. Pathology, research and practice. 2006:202(10):705-12 [PubMed PMID: 16889904]
Level 3 (low-level) evidenceNiezabitowski A, Lackowska B, Rys J, Kruczak A, Kowalska T, Mitus J, Reinfuss M, Markiewicz D. Prognostic evaluation of proliferative activity and DNA content in the phyllodes tumor of the breast: immunohistochemical and flow cytometric study of 118 cases. Breast cancer research and treatment. 2001 Jan:65(1):77-85 [PubMed PMID: 11245343]
Level 3 (low-level) evidenceTan WJ, Thike AA, Bay BH, Tan PH. Immunohistochemical expression of homeoproteins Six1 and Pax3 in breast phyllodes tumours correlates with histological grade and clinical outcome. Histopathology. 2014 May:64(6):807-17. doi: 10.1111/his.12329. Epub 2014 Jan 20 [PubMed PMID: 24438019]
Level 2 (mid-level) evidenceGullett NP, Rizzo M, Johnstone PA. National surgical patterns of care for primary surgery and axillary staging of phyllodes tumors. The breast journal. 2009 Jan-Feb:15(1):41-4. doi: 10.1111/j.1524-4741.2008.00669.x. Epub [PubMed PMID: 19141133]
Tan H, Zhang S, Liu H, Peng W, Li R, Gu Y, Wang X, Mao J, Shen X. Imaging findings in phyllodes tumors of the breast. European journal of radiology. 2012 Jan:81(1):e62-9. doi: 10.1016/j.ejrad.2011.01.085. Epub 2011 Feb 25 [PubMed PMID: 21353414]
Liberman L, Bonaccio E, Hamele-Bena D, Abramson AF, Cohen MA, Dershaw DD. Benign and malignant phyllodes tumors: mammographic and sonographic findings. Radiology. 1996 Jan:198(1):121-4 [PubMed PMID: 8539362]
Yoo JL, Woo OH, Kim YK, Cho KR, Yong HS, Seo BK, Kim A, Kang EY. Can MR Imaging contribute in characterizing well-circumscribed breast carcinomas? Radiographics : a review publication of the Radiological Society of North America, Inc. 2010 Oct:30(6):1689-702. doi: 10.1148/rg.306105511. Epub [PubMed PMID: 21071383]
Pezner RD, Schultheiss TE, Paz IB. Malignant phyllodes tumor of the breast: local control rates with surgery alone. International journal of radiation oncology, biology, physics. 2008 Jul 1:71(3):710-3. doi: 10.1016/j.ijrobp.2007.10.051. Epub 2008 Jan 30 [PubMed PMID: 18234448]
Level 2 (mid-level) evidenceRuiz-Flores L, Ebuoma LO, Benveniste MF, Nagi C, OrtizPerez T, Benveniste AP. Case Report: Metastatic Phyllodes Tumor. Seminars in ultrasound, CT, and MR. 2018 Feb:39(1):122-126. doi: 10.1053/j.sult.2017.05.011. Epub 2017 Jun 23 [PubMed PMID: 29317034]
Level 3 (low-level) evidenceYasir S, Nassar A, Jimenez RE, Jenkins SM, Hartmann LC, Degnim AC, Frost M, Visscher DW. Cellular fibroepithelial lesions of the breast: A long term follow up study. Annals of diagnostic pathology. 2018 Aug:35():85-91. doi: 10.1016/j.anndiagpath.2018.01.005. Epub 2018 Jan 10 [PubMed PMID: 30029048]
Goto W, Kashiwagi S, Takada K, Asano Y, Morisaki T, Noda S, Takashima T, Onoda N, Hirakawa K, Ohira M. [A Case of a Malignant Phyllodes Tumor That Was Difficult to Distinguish from Stromal Sarcoma]. Gan to kagaku ryoho. Cancer & chemotherapy. 2018 Dec:45(13):2429-2431 [PubMed PMID: 30692487]
Level 3 (low-level) evidenceBurga AM, Tavassoli FA. Periductal stromal tumor: a rare lesion with low-grade sarcomatous behavior. The American journal of surgical pathology. 2003 Mar:27(3):343-8 [PubMed PMID: 12604890]
Tan PH, Thike AA, Tan WJ, Thu MM, Busmanis I, Li H, Chay WY, Tan MH, Phyllodes Tumour Network Singapore. Predicting clinical behaviour of breast phyllodes tumours: a nomogram based on histological criteria and surgical margins. Journal of clinical pathology. 2012 Jan:65(1):69-76. doi: 10.1136/jclinpath-2011-200368. Epub 2011 Nov 2 [PubMed PMID: 22049216]