Introduction
Calcium is the most abundant mineral in the human body. Although most calcium is found in teeth and bone, approximately 1% is dissolved in the bloodstream. As the human body ages, calcium is deposited in various body parts. Arterial calcification is closely related to vascular injury, inflammation, and repair. Calcification occurs very early in atherosclerosis; it is only detectable through imaging modalities when it accumulates in tissue and vasculature. This accumulation typically occurs after age 40, and most individuals older than 60 have diffuse calcification.[1]
A close relationship exists between coronary calcium burden and atherosclerosis despite not all plaques being calcified.[1] The presence and extent of coronary artery calcification (CAC) provides direct evidence of coronary artery disease. Unstable angina is characterized by lesions with smaller calcium deposits described as spotty or speckled; fewer, larger calcium deposits often characterize stable angina.[2][3] Lesions without calcium are usually nonocclusive (<25% stenosis). CAC independently predicts future major adverse cardiovascular events (MACE) more than other noninvasive modalities. [4] Scoring CAC can also guide management strategies for primary prevention in patients unsure about statin use. Computed tomography (CT) angiography is useful for imaging CAC as a surrogate for clinically significant atherosclerosis.[2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of CAC is multifactorial and involves both genetic and environmental factors. CAC occurs as part of the progression of atherosclerosis or, less commonly, in conditions that promote medial artery calcification. Medial calcification causes peripheral arterial disease and is associated with renal failure, hypercalcemia, hyperphosphatemia, and hyperparathyroidism. Intimal calcification is associated with coronary artery disease and atherosclerosis. Major causes and contributing risk factors for CAC include:
Atherosclerosis is the most common type of CAC. Risk factors contributing to atherosclerosis include hyperlipidemia, hypertension, diabetes mellitus, aging, and smoking.
Chronic kidney disease is a significant cause of medical CAC. The kidneys have impaired phosphate excretion, leading to hyperphosphatemia. This excess phosphate combines with calcium to form deposits in the walls of arteries, including the coronary arteries. Additionally, chronic kidney disease (CKD) leads to elevated levels of parathyroid hormone (PTH) and fibroblast growth factor-23 (FGF-23), both of which promote vascular calcification. Deficiency of calcification inhibitors, such as fetuin-A and matrix Gla protein (MGP), is also common in CKD patients, further promoting calcification.
Diabetes accelerates both intimal and medial CAC through the combined effects of hyperglycemia, oxidative stress, and inflammation. Advanced glycation end-products contribute to endothelial dysfunction, inflammation, and vascular smooth muscle cell transformation into osteoblast-like cells, promoting calcification.
Genetic predisposition is associated with mutations affecting regulators of vascular calcification, such as MGP or ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1), which can predispose individuals to accelerated arterial calcification.
Metabolic syndrome is caused by insulin resistance, which promotes endothelial dysfunction, increases inflammation, and alters calcium-phosphate homeostasis, all of which favor vascular calcification.
Chronic inflammation, such as rheumatoid arthritis and systemic lupus erythematosus, is associated with increased inflammatory cytokines that activate vascular smooth muscle cells and osteogenic pathways, increasing calcium deposition in arterial walls.
Aging is an independent risk factor for CAC. Chronic low-grade inflammation, endothelial dysfunction, and a decline in the activity of natural inhibitors of calcification, such as MGP and fetuin-A, cumulatively cause CAC.
Postmenopausal women have reduced estrogen, which increases the likelihood of developing atherosclerosis and CAC. Estrogen is thought to protect the vascular system by promoting endothelial function and reducing oxidative stress.
Hyperparathyroidism is associated with excess circulating PTH, which promotes bone resorption and increases serum calcium and phosphate levels, which can then deposit in the arteries and promote calcification.
Lifestyle factors influence CAC. A sedentary lifestyle, a diet high in saturated fats, cholesterol, and refined sugars, and excessive alcohol consumption contribute to the development of risk factors like hypertension, dyslipidemia, and obesity, which accelerate CAC.[5]
Epidemiology
The presence of coronary artery calcification is age- and gender-dependent. This calcification is present in 90% of men and 67% of women older than 70; the difference is attributed to the protective effect of estrogen in premenopausal women. The Women's Health Initiative demonstrated a lower mean CAC in the estrogen-treated group than the placebo group.[5] Additionally, CAC is 3 times higher in postmenopausal women than premenopausal women. In an autopsy study of individuals who died of sudden cardiac death, the extent of calcification was greater in men than women up to the sixth decade of life, but the difference was not significant after the seventh decade.[5][6][7]
Growing evidence indicates ethnic differences in CAC and clinical outcomes. In the Multi-Ethnic Study of Atherosclerosis, after adjustment for traditional risk factors, statistically significant differences in CAC across 4 ethnicities (White, Black, Hispanic, and Chinese) were evidenced. White participants had the highest CAC, followed in descending order by Chinese, Hispanic, and Black participants. The results were consistent across both sexes.[8]
Patients with diabetes tend to have higher CAC levels, which predicts adverse clinical outcomes. Higher HbA1C levels are associated with both incident and progressive CAC.[9] Other factors associated with an increased risk of CAC are metabolic syndrome, dyslipidemia, tobacco use, hypertension, CKD, and a high baseline serum C-reactive protein.[10]
Pathophysiology
The 2 distinct types of CAC are atherosclerotic calcification and calcification of the tunica media. Atherosclerotic CAC mainly affects the tunica intima. The main steps of intimal CAC include endothelial dysfunction, lipid accumulation and inflammation, plaque formation, smooth muscle cell activation, osteogenic differentiation, and calcium deposition. In medial CAC, significant steps include vascular smooth muscle cell differentiation, matrix vesicle release, disruption in calcium-phosphate homeostasis, extracellular matrix remodeling, chronic low-grade inflammation, and arterial stiffness.
Intimal CAC begins in the atherosclerotic core. The death of macrophages in the atheromatous core serves as a nucleating site for the formation of calcium deposits. The dying macrophages or smooth muscle cells release extracellular vesicles, which provide the scaffolding for developing calcium deposits. Reduced expression of mineralization inhibitors, otherwise produced by intact macrophages and vascular smooth muscle cells, occurs. Calcium-phosphate metabolism control is lost. Calcium-phosphate concentration in autophagosomes rises and forms calcium-phosphate crystals, which can be seen through electron microscopy. Macroscopic CAC evolves from these foci.[11]
Calcium is deposited in the extracellular matrix of the intimal plaque. Calcification can occur in 2 forms. Microcalcifications, associated with plaque instability, appear as small, granular deposits often found in the early calcification stages. Macrocalcifications are larger, sheet-like deposits that occur in more advanced stages of atherosclerosis. Macrocalcifications are often associated with stable plaques but may contribute to arterial stiffness. The calcium nodules contribute to the distortion of the endothelial lining and can contribute to acute luminal thrombosis. Calcified nodules contribute to 2% to 7% of coronary artery thrombosis.[3]
Intimal CAC resembles endochondral bone formation. In intimal CAC, most chondrocyte-like cells originate from the bone marrow with a minimal contribution from the differentiation of local smooth muscle cells into chondrocytes. In contrast, medial CAC is driven mainly by medial smooth muscle cells undergoing osteochondrogenic differentiation. Inflammatory mediators and mechanical stress drive the transition of vascular smooth muscle cells into osteoblast-like cells. These cells express osteogenic markers like bone morphogenetic proteins (BMPs) and Runx2, which are involved in bone formation. This osteogenic process leads to the formation of hydroxyapatite crystals, the same material found in bones, within the atherosclerotic plaque. The lesion progresses with the cytokines produced by the chondrocyte-like cells.[11]
CAC causes reduced myocardial perfusion, abnormal vasomotor response, and impaired vascular compliance. Calcification in the coronary arteries can occur as early as the second decade of life, immediately after fatty streak formation. Laboratory analysis of lesions of young adults has demonstrated aggregation of crystalline calcium among lipid particles. Furthermore, calcific deposits are found in greater quantities in older adults and complex lesions.[12][13][14] CAC is hypothesized to be a protective mechanism to strengthen atherosclerotic plaques, as calcified areas are less likely to rupture. Plaques with calcified caps are less prone to rupture than unstable plaques with a necrotic lipid core.[15] Dense CAC (measuring >400 HU) is less prone to plaque rupture. In contrast, spotty microcalcification more commonly accompanies unstable plaques.
Risk Factors for Coronary Artery Calcification
Risk factors can contribute more to intimal versus medial CAC. The risk factors for intimal CAC include advanced age, diabetes, hypertension, dyslipidemia, male gender, cigarette smoking, and hyperphosphatemia. The risk factors for medical CAC include advanced age, diabetes, renal dysfunction, hypercalcemia, hyperphosphatemia, and duration of dialysis.[16]
Histopathology
Histologically, CAC starts with microcalcifications as small as 0.5 to 15 μ, which are thought to originate from smooth muscle apoptosis. The microcalcifications progress to larger punctuate and fragmented lesions, which become sheets of calcium deposits measuring greater than 3 mm. The sheets of calcium can break off into nodules, forming intraluminal thromboses.[5]
History and Physical
Although CAC has no specific clinical manifestations, it has significant prognostic implications. The calcification can independently predict future cardiovascular events and reclassify patients into more accurate and clinically relevant categories. As noted above, the history may include diabetes, hypertension, hyperlipidemia, metabolic disease, renal disease, or known coronary artery disease. Patients may report chest pain.
Physical examination findings may include hypertension, heart murmur, decreased peripheral pulses, or skin changes associated with peripheral vascular disease, such as shiny skin, skin discoloration, hair loss, or nonhealing lower extremity ulcers.
Evaluation
Diagnostic Methods for Measuring Coronary Artery Calcification
Computed tomography: Coronary artery calcium is primarily evaluated by noncontrast, electrocardiographic (ECG)-gated cardiac electron beam computed tomography (EBCT) or multislice detector computed tomography (MDCT). A coronary calcium score is associated with plaque burden; it is not a marker of plaque vulnerability. Nonetheless, it gives an insight into the patient’s level of cardiovascular disease risk and helps guide interventions or prevent coronary artery disease.[17][18][19]
The detection of CAC via CT was made possible in the 1980s after EBCT's development; EBCT's significantly superior speed allows the detection of CAC despite heart motion. MDCT allows even faster image acquisition. MDCT is used more commonly than EBCT due to increased accuracy and image quality.[20][21] Newer cardiac CT angiography (CTA) developments can also reveal characteristics such as plaque volume and density.
The evaluation of CAC scoring via CT offers a fast, reproducible, and relatively inexpensive modality to determine the extent and presence of coronary calcification. CT does not require intravenous access or specific patient preparation. Scans are typically obtained with prospective electrocardiogram triggering during diastole. After imaging is acquired, the extent of calcification is quantified using the Agatston score. The Agatston score is obtained by multiplying the area of calcification by the corresponding density factor (1-4) in Hounsfield units (HU) as follows:
- 130-199 HU: 1
- 200-299 HU: 2
- 300-399 HU: 3
- 400+ HU: 4
For example, for a calcification area measuring 7 mm² and a HU of 400, the Agatston score is 7 x 4 = 28. The score is obtained using a slice thickness of 2.5 mm to 3 mm. The Agatston score is the most validated method of CAC quantification. The total CAC is calculated by summing individual calcification speck scores. Other methods of CAC quantification include calcium volume score, visual assessment, calcium density score, calcium mass score, and segment involvement score.
Currently, the American College of Cardiology/American Heart Association gives class IIa indication for coronary CTA in asymptomatic patients with intermediate-risk (10%-20%) of cardiac events over 10 years based on the Framingham risk score, as well as for asymptomatic individuals 40 years and older with diabetes. CAC measurement is generally not recommended for patients at low (<10%) or high (>20%) 10-year risk of cardiac events based on the Framingham risk score.
The following definitions are used to quantify coronary artery calcium score and coronary plaque burden:
- 0: No identifiable disease
- 1 to 99: Mild disease
- 100 to 399: Moderate disease
- Greater than 400: Severe Disease
Although CAC can help predict the presence or absence of coronary artery stenosis, it is generally a better marker for the extent of coronary atherosclerosis rather than the degree of stenosis. In early atherosclerosis, the arteries have compensatory enlargement to accommodate the plaque. Therefore, although extensive plaque burden may be present, there may not be any clinically relevant stenosis. Severe coronary calcification (Agatston score >1000) is associated with advanced obstructive coronary disease.
CAC may also be found incidentally on CT performed for other indications. The presence and extent of CAC should be reported on all noncontrast chest CTs. Nongated scans usually do not have a slice thickness of 2.5 mm to 3 mm, and hence, formal Agatston scoring may not be possible. However, a significant correlation between the gated Agatston and nongated ordinal scores is evidenced (see Image. CT Coronary Angiogram).[22]
The effective radiation exposure with EBCT is approximately 0.7 mSv to 1.0 mSv in men and 0.9 mSv to 1.3 mSv in women. MDCT has a slightly higher radiation dose of 1.0 mSv to 1.5 mSv in men and 1.1 mSv to 1.9 mSv in women. The average annual background radiation in the United States is 3.0 mSv to 3.6 mSv.
A zero Agatston score is the most powerful negative predictive risk factor in asymptomatic patients compared to a within reference-range level of hs-CRP or lack of carotid plaque.[23]
Coronary angiography: Compared with intravascular ultrasound (IVUS) and CT, coronary angiography has a low-moderate sensitivity and high specificity for CAC. The angiographic classification of CAC is primarily based on visual assessment during coronary angiography. Mild calcification is typically spotted as faint, localized, or linear opacities within the vessel wall and is visible only during the cardiac cycle's later phases. Moderate calcification appears as dense, linear opacities during systole and diastole before contrast injection. Severe calcification is observed without cardiac motion before contrast injection; it appears as dense, radiopaque calcium deposits visible in both systole and diastole and may extend around the entire circumference of the vessel ("tram-track" appearance).
Intravascular ultrasound: IVUS provides high-resolution, cross-sectional coronary artery images, allowing for a better assessment of calcified plaques than angiography alone. IVUS provides the calcification's depth, extent, and distribution and helps distinguish between superficial and deeper calcification. IVUS can be used to characterize calcified lesions before stent deployment further. Superficial calcification is more likely to impede stent expansion, requiring specific treatment strategies, such as atherectomy or intravascular lithotripsy. Following stent deployment, IVUS can confirm whether the stent is fully expanded and well-apposed, even in heavily calcified vessels. On IVUS, calcified plaques typically appear as bright, hyperechoic areas with acoustic shadowing. This distinctive shadowing is a hallmark of calcification. [16]
Optical coherence tomography: Optical coherence tomography (OCT) provides a much higher resolution (10–20 μ) than IVUS, allowing for precise visualization of calcified plaques and associated structures. OCT has limited penetration depth compared to IVUS (1–2 mm vs. up to 10 mm for IVUS). OCT can accurately define the surface characteristics of calcified plaques, including the presence of calcific nodules or irregularities, which are often difficult to detect with angiography or IVUS. OCT can precisely measure the arc (in degrees) and length of calcification. If the calcium arc is more than 180 degrees, as defined by involving more than half the vessel circumference, more aggressive intervention may be required to modify the plaque before stent placement. Thicker calcium (usually ≥0.5 mm) may indicate a need for atherectomy or other plaque-modifying techniques before attempting stent expansion.[16]
Treatment / Management
The overall incidence of adverse cardiac events is lower than expected in most large-scale studies, possibly due to aggressively treating the risk factors of study patients, such as blood pressure, lipids, and smoking. Controlling diabetes and CKD is also important. Lifestyle modifications, risk factor control, screening, and monitoring are the cornerstones for managing CAC.
Use of CAC in primary prevention of atherosclerotic cardiovascular diseases: The clinical practice guidelines have recognized the utility of CAC in the primary prevention of atherosclerotic disease. A positive CAC score can help guide decisions about statin use. If the CAC score is 0 and smoking, diabetes, and a family history of premature CAD are absent, then no statin therapy is indicated. Initiation of a statin is indicated for patients with a CAC score of 1-99 if they are older than 55. For patients with a CAC score of 1-99 but younger than 55, statins can be withheld, and the patient can be reassessed for candidacy in 3 to 5 years. For patients with a CAC score over 100, initiation of a statin is recommended.[24] Aspirin may be useful in primary prevention for patients younger than 70 with a CAC score over 100.[25] Serial CAC testing to assess treatment efficacy is not recommended.
Coronary artery calcification in coronary intervention: Advances in treating coronary artery calcification have occurred. The Disrupt CAD III study researched intravascular lithotripsy for modifying severe CAC.[26] In addition, the presence of CAC makes a percutaneous coronary intervention during cardiac catheterization more challenging. Techniques that can be utilized during cardiac catheterization in addition to drug-eluting or bare-metal stent placement include rotational, orbital, or laser atherectomy and cutting balloons.[27][28][29](B3)
Differential Diagnosis
The differential diagnosis of CAC includes artifacts that can appear as calcification, such as increased background noise misinterpreted as microcalcifications, pericardial calcification near the epicardial vessels, movement-related noise, and perhaps most significantly, tunica media calcification, which can be misinterpreted as intimal calcification but has a different etiology and prognosis.
Prognosis
Coronary artery calcification has been shown in several large observational studies to predict future cardiovascular events. Furthermore, when added to commonly used risk prediction models, CAC significantly improves risk prediction and stratification compared to other biomarkers. CAC scores can accurately classify patients into low-risk and high-risk categories. Patients have an extremely low risk of cardiovascular disease and events if no coronary calcification is detected (ie, CAC score of 0).
Adding CAC to Framingham risk factors leads to improved prediction of MACE.[30] Noninvasive assessment of CAC is reasonable in asymptomatic individuals with intermediate risk.[24] For example, in patients classified as low-risk due to risk factors present or Framingham risk score assessment, a CAC of 100 indicated an estimated 10-year all-coronary heart disease event rate of nearly 10%. However, in the same high-risk patients, a CAC score of 0 is associated with a 10-year all-coronary heart disease event risk of only 3%. A CAC score over 400 is associated with worsened clinical outcomes. These scenarios illustrate the ability of CAC scoring to help reclassify the risk of many patients and estimate future cardiovascular events.
The PROMISE trial is a randomized control trial assigning patients with stable chest pain and suspected coronary artery disease into functional testing (exercise electrocardiography, nuclear stress testing, or stress echocardiography) and CTA groups. The primary outcome of all-cause mortality, myocardial infarction, unstable angina, or major complications from a cardiovascular procedure was similar between the 2 groups. The CTA group had a higher rate of cardiac catheterization than the functional stress test (FST) group but lower rates of nonobstructive CAD. Therefore, a low pretest probability combined with a negative CTA has a high negative predictive value for CAD.[31] Although both the functional testing and CTA groups overall had similar rates of major adverse cardiac events, in subgroup analysis, patients with diabetes had significantly fewer events in the CTA group than those randomized to FST. This is likely due to different symptom presentations and suggests that in patients with diabetes and stable angina, CTA should be the initial test.[32]
Another large randomized control trial, SCOT-HEART, also addressed the utility of CTA, but in this study, CTA was performed after FST. SCOT-HEART also demonstrated the safety and efficacy of CTA, and results suggest a trend toward reducing major cardiac events with both CTA and FST compared to FST alone.[31]
The benefit of CTA over FST is that CTA is an anatomical study and can directly visualize the coronary vessels. Both the PROMISE and SCOT-HEART showed that after 5-year follow-up, nonobstructive lesions were associated with as many adverse cardiac events as obstructive lesions; however, CTA negative for calcification maintained its negative predictive value.[33] As noted above, certain characteristics of coronary calcifications are considered high risk, including plaque stenosis greater than 70%, thin-cap fibroatheromas, and plaques with a large necrotic lipid core. Although overall adverse cardiac events were not significantly associated with high-risk plaques, there was a positive association in the subgroups of younger patients and women.[34]
Complications
The risks of an increased CAC are angina, myocardial infarction, and increased coronary artery stiffness. The risks of evaluating CAC include the complications of coronary interventions such as coronary catheterization, percutaneous stenting, and coronary artery bypass grafting.
Deterrence and Patient Education
CAC occurs when calcium deposits build up in the walls of the coronary arteries, which supply blood to the heart. Over time, these calcium deposits can harden and narrow the arteries, a condition known as atherosclerosis. CAC is a key indicator of heart health. The amount of calcification in the arteries can predict your risk of heart disease. A CAC score (obtained through a particular type of CT scan) helps assess your risk of heart attack or other serious cardiovascular events. A higher CAC score indicates a higher risk. While CAC cannot be entirely reversed, progression can be slowed or even halted with lifestyle changes and medical management. Lifestyle changes include regular physical activity, a heart-healthy diet, quitting smoking, and managing stress. A clinician can prescribe certain medications to manage CAC. Periodic monitoring is essential for patients with CAC.
Pearls and Other Issues
Additional key facts to keep in mind regarding CAC:
- Coronary lesions with hemodynamically significant stenosis have some degree of calcification.
- Functional stress testing and cardiac CT angiography are the 2 most commonly used noninvasive tests to evaluate the presence of possible coronary ischemia. CT angiography relies on CAC.
- Plaque formation starts with a lipid streak or core (which can become necrotic over time) and undergoes a calcification process similar to bone calcification.
- Plaques demonstrating "spottiness" or "speckling" are usually associated with unstable angina or infarction. Plaques with fewer, more significant areas of calcification are associated with stable angina.
- A plaque with a necrotic core and thin fibrous cap is considered a high risk for embolization.
- A negative CAC is highly predictive against major adverse cardiac events.
Enhancing Healthcare Team Outcomes
Healthcare workers, especially primary care and emergency department practitioners, frequently see patients with coronary artery disease. These patients are often referred to a cardiologist, who performs imaging studies to determine the degree of calcification in the coronary vessels.
In several extensive observational studies, coronary artery calcification has been shown to predict future cardiovascular events. Furthermore, when added to commonly used risk prediction models, CAC significantly improves risk prediction and stratification compared to other biomarkers. It can accurately classify patients into low-risk and high-risk categories. Patients have an extremely low risk of major adverse cardiac events if they have no coronary calcification detected (CAC score of 0). Significant evidence reveals that testing for CAC can contribute to overall healthcare savings and morbidity by avoiding invasive testing in low-risk patients.[1]
Care coordination is pivotal in ensuring seamless and efficient patient care when measuring CAC. Physicians, advanced practitioners, nurses, pharmacists, and other healthcare professionals must work together to streamline the patient's journey, from diagnosis through treatment and follow-up. This coordination minimizes errors, reduces delays, and enhances patient safety, ultimately leading to improved outcomes and patient-centered care that prioritizes the well-being and satisfaction of patients.
Media
(Click Image to Enlarge)
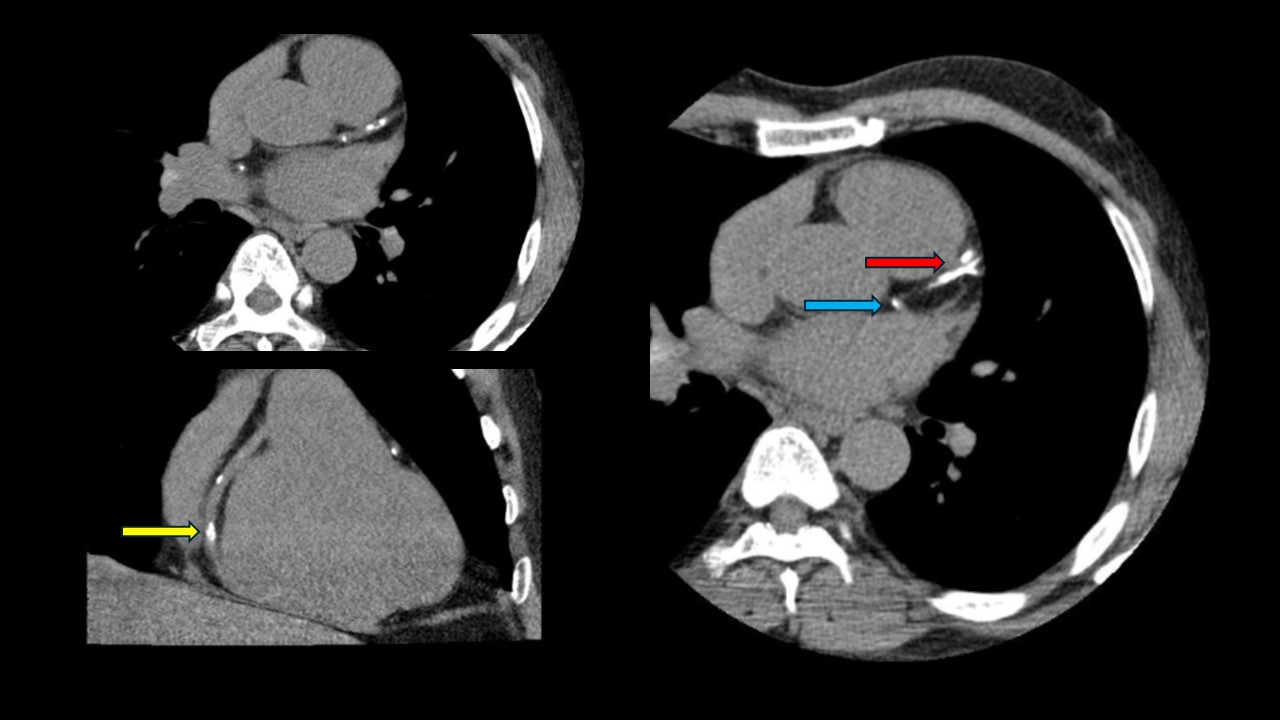
Computed Tomography Coronary Angiogram. This computed tomography coronary angiogram of a patient with an Agatston score of 828 reveals a heavily calcified left anterior descending coronary artery (red arrow). The right coronary artery (yellow arrow) and ostial left circumflex artery (blue arrow) also have calcified lesions.
Contributed by P Shams, MBBS, FCPS
References
Beverly J, Budoff MJ. Use of Coronary Computed Tomography for Calcium Screening of Atherosclerosis. Heart international. 2020:14(2):76-79. doi: 10.17925/HI.2020.14.2.76. Epub 2020 Dec 17 [PubMed PMID: 36276503]
Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008 Jun 3:117(22):2938-48. doi: 10.1161/CIRCULATIONAHA.107.743161. Epub [PubMed PMID: 18519861]
Jinnouchi H, Sato Y, Sakamoto A, Cornelissen A, Mori M, Kawakami R, Gadhoke NV, Kolodgie FD, Virmani R, Finn AV. Calcium deposition within coronary atherosclerotic lesion: Implications for plaque stability. Atherosclerosis. 2020 Aug:306():85-95. doi: 10.1016/j.atherosclerosis.2020.05.017. Epub 2020 Jun 14 [PubMed PMID: 32654790]
Onnis C, Virmani R, Kawai K, Nardi V, Lerman A, Cademartiri F, Scicolone R, Boi A, Congiu T, Faa G, Libby P, Saba L. Coronary Artery Calcification: Current Concepts and Clinical Implications. Circulation. 2024 Jan 16:149(3):251-266. doi: 10.1161/CIRCULATIONAHA.123.065657. Epub 2024 Jan 16 [PubMed PMID: 38227718]
Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary Artery Calcification and its Progression: What Does it Really Mean? JACC. Cardiovascular imaging. 2018 Jan:11(1):127-142. doi: 10.1016/j.jcmg.2017.10.012. Epub [PubMed PMID: 29301708]
Burke AP, Weber DK, Kolodgie FD, Farb A, Taylor AJ, Virmani R. Pathophysiology of calcium deposition in coronary arteries. Herz. 2001 Jun:26(4):239-44 [PubMed PMID: 11479935]
Burke AP, Farb A, Malcom G, Virmani R. Effect of menopause on plaque morphologic characteristics in coronary atherosclerosis. American heart journal. 2001 Feb:141(2 Suppl):S58-62 [PubMed PMID: 11174360]
Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005 Mar 15:111(10):1313-20 [PubMed PMID: 15769774]
Level 2 (mid-level) evidenceCarson AP, Steffes MW, Carr JJ, Kim Y, Gross MD, Carnethon MR, Reis JP, Loria CM, Jacobs DR Jr, Lewis CE. Hemoglobin a1c and the progression of coronary artery calcification among adults without diabetes. Diabetes care. 2015 Jan:38(1):66-71. doi: 10.2337/dc14-0360. Epub 2014 Oct 16 [PubMed PMID: 25325881]
Vervloet M, Cozzolino M. Vascular calcification in chronic kidney disease: different bricks in the wall? Kidney international. 2017 Apr:91(4):808-817. doi: 10.1016/j.kint.2016.09.024. Epub 2016 Nov 30 [PubMed PMID: 27914706]
Nakahara T, Dweck MR, Narula N, Pisapia D, Narula J, Strauss HW. Coronary Artery Calcification: From Mechanism to Molecular Imaging. JACC. Cardiovascular imaging. 2017 May:10(5):582-593. doi: 10.1016/j.jcmg.2017.03.005. Epub [PubMed PMID: 28473100]
Lemanowicz A, Białecki M, Leszczyński W, Hawrył M. Coronary age, based on coronary calcium measurement, is increased in patients with morbid obesity. Polish journal of radiology. 2018:83():e415-e420. doi: 10.5114/pjr.2018.78624. Epub 2018 Sep 5 [PubMed PMID: 30655919]
Kowall B, Lehmann N, Mahabadi AA, Moebus S, Erbel R, Jöckel KH, Stang A. Associations of metabolically healthy obesity with prevalence and progression of coronary artery calcification: Results from the Heinz Nixdorf Recall Cohort Study. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2019 Mar:29(3):228-235. doi: 10.1016/j.numecd.2018.11.002. Epub 2018 Nov 15 [PubMed PMID: 30648599]
Gheorghe AG, Jacobsen C, Thomsen R, Linnet K, Lynnerup N, Andersen CB, Fuchs A, Kofoed KF, Banner J. Coronary artery CT calcium score assessed by direct calcium quantification using atomic absorption spectroscopy and compared to macroscopic and histological assessments. International journal of legal medicine. 2019 Sep:133(5):1485-1496. doi: 10.1007/s00414-018-01998-8. Epub 2019 Jan 4 [PubMed PMID: 30610447]
Wexler L, Brundage B, Crouse J, Detrano R, Fuster V, Maddahi J, Rumberger J, Stanford W, White R, Taubert K. Coronary artery calcification: pathophysiology, epidemiology, imaging methods, and clinical implications. A statement for health professionals from the American Heart Association. Writing Group. Circulation. 1996 Sep 1:94(5):1175-92 [PubMed PMID: 8790070]
Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Généreux P. Coronary artery calcification: pathogenesis and prognostic implications. Journal of the American College of Cardiology. 2014 May 6:63(17):1703-14. doi: 10.1016/j.jacc.2014.01.017. Epub 2014 Feb 12 [PubMed PMID: 24530667]
Chen Y, Hu Z, Li M, Jia Y, He T, Liu Z, Wei D, Yu Y. Comparison of Nongated Chest CT and Dedicated Calcium Scoring CT for Coronary Calcium Quantification Using a 256-Dector Row CT Scanner. Academic radiology. 2019 Oct:26(10):e267-e274. doi: 10.1016/j.acra.2018.12.005. Epub 2019 Jan 23 [PubMed PMID: 30685312]
Sharma SK, Bolduan RW, Patel MR, Martinsen BJ, Azemi T, Giugliano G, Resar JR, Mehran R, Cohen DJ, Popma JJ, Waksman R. Impact of calcification on percutaneous coronary intervention: MACE-Trial 1-year results. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2019 Aug 1:94(2):187-194. doi: 10.1002/ccd.28099. Epub 2019 Jan 25 [PubMed PMID: 30681262]
Williams MC, Moss AJ, Dweck M, Adamson PD, Alam S, Hunter A, Shah ASV, Pawade T, Weir-McCall JR, Roditi G, van Beek EJR, Newby DE, Nicol ED. Coronary Artery Plaque Characteristics Associated With Adverse Outcomes in the SCOT-HEART Study. Journal of the American College of Cardiology. 2019 Jan 29:73(3):291-301. doi: 10.1016/j.jacc.2018.10.066. Epub [PubMed PMID: 30678759]
Lembcke A, Hein PA, Dohmen PM, Klessen C, Wiese TH, Hoffmann U, Hamm B, Enzweiler CN. Pictorial review: electron beam computed tomography and multislice spiral computed tomography for cardiac imaging. European journal of radiology. 2006 Mar:57(3):356-67 [PubMed PMID: 16427236]
Level 3 (low-level) evidenceDisthabanchong S, Boongird S. Role of different imaging modalities of vascular calcification in predicting outcomes in chronic kidney disease. World journal of nephrology. 2017 May 6:6(3):100-110. doi: 10.5527/wjn.v6.i3.100. Epub [PubMed PMID: 28540199]
Hecht HS, Cronin P, Blaha MJ, Budoff MJ, Kazerooni EA, Narula J, Yankelevitz D, Abbara S. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: A report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. Journal of cardiovascular computed tomography. 2017 Jan-Feb:11(1):74-84. doi: 10.1016/j.jcct.2016.11.003. Epub 2016 Nov 10 [PubMed PMID: 27916431]
Nasir K, Cainzos-Achirica M. Role of coronary artery calcium score in the primary prevention of cardiovascular disease. BMJ (Clinical research ed.). 2021 May 4:373():n776. doi: 10.1136/bmj.n776. Epub 2021 May 4 [PubMed PMID: 33947652]
Taron J, Lyass A, Mahoney TF, Ehrbar RQ, Vasan RS, D'Agostino RB Sr, Hoffmann U, Massaro JM, Lu MT. Coronary Artery Calcium Score-Directed Primary Prevention With Statins on the Basis of the 2018 American College of Cardiology/American Heart Association/Multisociety Cholesterol Guidelines. Journal of the American Heart Association. 2021 Jan 5:10(1):e018342. doi: 10.1161/JAHA.120.018342. Epub 2020 Dec 22 [PubMed PMID: 33348999]
Cainzos-Achirica M, Miedema MD, McEvoy JW, Al Rifai M, Greenland P, Dardari Z, Budoff M, Blumenthal RS, Yeboah J, Duprez DA, Mortensen MB, Dzaye O, Hong J, Nasir K, Blaha MJ. Coronary Artery Calcium for Personalized Allocation of Aspirin in Primary Prevention of Cardiovascular Disease in 2019: The MESA Study (Multi-Ethnic Study of Atherosclerosis). Circulation. 2020 May 12:141(19):1541-1553. doi: 10.1161/CIRCULATIONAHA.119.045010. Epub 2020 Apr 1 [PubMed PMID: 32233663]
Hill JM, Kereiakes DJ, Shlofmitz RA, Klein AJ, Riley RF, Price MJ, Herrmann HC, Bachinsky W, Waksman R, Stone GW, Disrupt CAD III Investigators. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Artery Disease. Journal of the American College of Cardiology. 2020 Dec 1:76(22):2635-2646. doi: 10.1016/j.jacc.2020.09.603. Epub 2020 Oct 15 [PubMed PMID: 33069849]
Shavadia JS, Vo MN, Bainey KR. Challenges With Severe Coronary Artery Calcification in Percutaneous Coronary Intervention: A Narrative Review of Therapeutic Options. The Canadian journal of cardiology. 2018 Dec:34(12):1564-1572. doi: 10.1016/j.cjca.2018.07.482. Epub 2018 Aug 14 [PubMed PMID: 30527144]
Level 3 (low-level) evidenceCano-Megías M, Bouarich H, Guisado-Vasco P, Pérez Fernández M, de Arriba-de la Fuente G, Álvarez-Sanz C, Rodríguez-Puyol D. Coronary artery calcification in patients with diabetes mellitus and advanced chronic kidney disease. Endocrinologia, diabetes y nutricion. 2019 May:66(5):297-304. doi: 10.1016/j.endinu.2018.09.003. Epub 2018 Nov 30 [PubMed PMID: 30509882]
Guo J, Nunley KA, Costacou T, Miller RG, Rosano C, Edmundowicz D, Orchard TJ. Greater progression of coronary artery calcification is associated with clinically relevant cognitive impairment in type 1 diabetes. Atherosclerosis. 2019 Jan:280():58-65. doi: 10.1016/j.atherosclerosis.2018.11.003. Epub 2018 Nov 8 [PubMed PMID: 30471556]
Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010 Apr 28:303(16):1610-6. doi: 10.1001/jama.2010.461. Epub [PubMed PMID: 20424251]
Fordyce CB, Newby DE, Douglas PS. Diagnostic Strategies for the Evaluation of Chest Pain: Clinical Implications From SCOT-HEART and PROMISE. Journal of the American College of Cardiology. 2016 Feb 23:67(7):843-52. doi: 10.1016/j.jacc.2015.11.055. Epub [PubMed PMID: 26892420]
Sharma A, Coles A, Sekaran NK, Pagidipati NJ, Lu MT, Mark DB, Lee KL, Al-Khalidi HR, Hoffmann U, Douglas PS. Stress Testing Versus CT Angiography in Patients With Diabetes and Suspected Coronary Artery Disease. Journal of the American College of Cardiology. 2019 Mar 5:73(8):893-902. doi: 10.1016/j.jacc.2018.11.056. Epub [PubMed PMID: 30819356]
Serruys PW, Hara H, Garg S, Kawashima H, Nørgaard BL, Dweck MR, Bax JJ, Knuuti J, Nieman K, Leipsic JA, Mushtaq S, Andreini D, Onuma Y. Coronary Computed Tomographic Angiography for Complete Assessment of Coronary Artery Disease: JACC State-of-the-Art Review. Journal of the American College of Cardiology. 2021 Aug 17:78(7):713-736. doi: 10.1016/j.jacc.2021.06.019. Epub [PubMed PMID: 34384554]
Ferencik M, Mayrhofer T, Bittner DO, Emami H, Puchner SB, Lu MT, Meyersohn NM, Ivanov AV, Adami EC, Patel MR, Mark DB, Udelson JE, Lee KL, Douglas PS, Hoffmann U. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients With Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA cardiology. 2018 Feb 1:3(2):144-152. doi: 10.1001/jamacardio.2017.4973. Epub [PubMed PMID: 29322167]
Level 1 (high-level) evidence