Introduction
Congenital and maternal syphilis is caused by the transmission of the spirochete Treponema pallidum from the mother to the fetus, resulting in a multitude of clinical presentations ranging from asymptomatic healthy babies to premature infants with a wide array of clinical signs and symptoms, at times resulting in stillbirth and perinatal death.[1] The incidence of this disease is rising, with 2022 having the most cases since 1997.[2][3] Congenital and maternal syphilis is a nationally reportable disease, with each case being detailed to the Centers for Disease Control (CDC) by all 50 states. Appropriate prenatal care and early treatment are vital to avoiding the manifestations of congenital syphilis.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The causative organism of syphilis was first identified by Fritz Schaudinn and Erich Hoffmann in 1905.[4] Congenital syphilis, like all other forms of the disease, is caused by Treponema pallidum (T Pallidum), which is a motile spirochete that is a helically coiled, corkscrew-shaped bacterium 6 to 15 μm long and 0.1 to 0.2 μm wide.[5] The first recorded outbreak of syphilis was in Europe in 1494 in modern-day Naples, Italy.[6] It is thought to have been brought to Naples by Columbus on his return from the New World. Subsequently, the disease spread throughout Europe.[6] Treponemal bacteria have only recently (2017) been successfully cultured in vitro using rabbit epithelial cells, but humans remain the only known host for T pallidum.[7][8][9]
Missed public health opportunities for reducing maternal syphilis include lack of proper antibiotic treatment despite an early diagnosis (30% to 40%), lack of prenatal care (20% to 30%), failure to perform prenatal syphilis testing (10% to 15%), and late identification of positive serology (10%).[10][11][12][13] As many as two-thirds of patients are lost to follow-up in some parts of the US, leading to preventable complications.[14][12][15][16][17][18]
Adding repeat prenatal syphilis testing early in the third trimester (at or around week 28 of gestation) has proven effective in reducing the incidence of congenital syphilis, especially since women can be infected or reinfected during pregnancy.[19][20] Besides early detection and adequate treatment of both the mother and infant, sexual partners of the mother should be tested and properly treated as well. Syphilis screening is part of the routine standard of care for all pregnant women in the United States, which has been shown to decrease rates of congenital syphilis significantly.[21] Most cases of the disease in neonates are seen in women without proper prenatal care or in those who received inadequate treatment.
Epidemiology
Researchers estimate that congenital syphilis is a complicating factor in about one million pregnancies every year worldwide.[22] Congenital syphilis has largely contributed to infant death and is responsible for 305,000 perinatal deaths around the world annually, making it the second leading cause of global perinatal mortality (prematurity being first).[10][23][24] As syphilis remains an easily treatable disease, most cases of congenital syphilis are seen in women who did not receive proper prenatal care or who received improper treatment.
In the United States, the rate of congenital syphilis peaked at roughly 100 cases per 100,000 live births in 1991, then declined dramatically with 334 total annual cases (8.4 cases per 100,000 live births) in 2012, and since then has steadily increased.[11][25] Since 2012, the incidence of congenital syphilis has been on the rise.[2][3][10][11] Between 2017 and 2022, the incidence rates of maternal and congenital syphilis increased in the US by over 2.5 times (from 87.2 to 280.4 per 100,000 live births, according to the National Center for Health Statistics (NCHS). The annual incidence in the US has significantly increased, with 5,726 cases reported and an annual rate of 77.9 cases per 100,000 live births in 2023.
The incidence of congenital syphilis in the US has increased over 10-fold between 2012 and 2022, with the fastest rate of increase in maternal syphilis being found in women younger than 24 years of age, according to the CDC.[3] This corresponds to a 40% decrease in federal funding in the US for preventive programs for sexually transmitted infections since 2000.[10] This is mirrored by a similar rise in Canada, where the incidence more than doubled between 2016 and 2020.[26][27] Regional distribution of congenital syphilis in the United States varies, with the highest incidence in the South, followed by the West and Midwest, with the lowest incidence in the Northeast.[11]
Significant racial disparity is seen in the incidence of congenital syphilis in the US. In 2014, the CDC reported that congenital syphilis was 10 times more prevalent in mothers who were Black compared to mothers who were White (38.2 versus 3.7 cases per 100,000 births).[28] They also reported a 3-fold higher prevalence in Black mothers compared to Hispanic mothers (38.2 versus 12.2 cases per 100,000 live births).[11] The NCHS reported that the ethnic groups with the greatest rise in incidence of maternal syphilis between 2017 and 2022 were Native Americans, while the lowest increase was in White and Asian Americans.[3]
Globally, the World Health Organization (WHO) has estimated that 7 in 1000 pregnant women have a maternal syphilis infection. This has caused over 200,000 annual neonatal deaths and 1.5 million cases of infected newborns reported worldwide in 2023, with almost two-thirds of all cases being found in Africa.[6][10][26] Without early diagnosis and treatment, up to 40% of pregnant women with syphilis will give birth to stillborn neonates, and 33% will deliver infants of low birth weight.[29][30] Untreated maternal and congenital syphilis costs nearly $310 million yearly.[10][14]
Overall, the worldwide incidence of congenital syphilis increased by 500% between 2011 and 2020.[31] The global rate of congenital syphilis was 473 cases per 100,000 live births in 2020 and 661,000 total cases worldwide, as estimated by the WHO.[32][33] These include 355,000 adverse birth outcomes (clinical or symptomatic congenital syphilis, early fetal demise, low birth weight, neonatal mortality, prematurity, or stillbirths).[33] Despite increasing worldwide prevalence, some countries have essentially eliminated congenital syphilis, including Cuba, Malaysia, Maldives, Sri Lanka, and Thailand.[10][26]
Pathophysiology
In adults, syphilis initiates an infection after local infiltration through subcutaneous tissues to cause a local immune response and establish an initial ulcerative lesion.[6] As the bacteria easily cross the placental barrier, the fetus of an infected mother is likely also to become infected. This may occur at any time during the pregnancy but most often during the third trimester.[10][34]
Congenital syphilis differs from adult disease in that T pallidum is released straight into the bloodstream of the fetus, causing spirochetemia with early fetal spread to most organs, including the bones, kidneys, spleen, liver, and heart. This leads to widespread inflammation throughout these organ systems, resulting in various clinical manifestations.
The placentas in fetuses with maternal syphilis tend to become significantly enlarged beyond normal due to a localized inflammatory response.[35][36][37] In addition to its large size and a general pallor, histological examination of the placenta may exhibit signs of enlarged hypercellular villi, necrotizing funisitis ("barber's pole" appearance), proliferative vascular changes, and acute and chronic villitis.[35]
Over 75% of the neonates born with a placenta heavier than the 90th percentile for the infant's birth weight have been found to have congenital syphilis. This finding is so common that serological testing for syphilis is suggested for all mothers and neonates when placental weight reaches the 90th percentile or higher.[38]
Histopathology
Treponema pallidum is 6 to 15 μm long and 0.1 to 0.2 μm wide.[5] Because of its limited width, it cannot be easily seen by direct microscopic examination and requires darkfield microscopy for visualization.[39] Darkfield microscopy allows visualization of live treponemes, which are active, thin, corkscrew spiral-shaped organisms enveloped by a cytoplasmic membrane and a loosely associated outer membrane that may appear to be moving forward and backward.[39][40] The overall sensitivity of darkfield microscopy in primary syphilis is about 80%.[40] The main advantage of dark-field microscopy is that it can allow a diagnosis even before serological testing becomes positive or where such testing is unavailable.[40][41]
Treponema pallidum may also be visualized using immunofluorescent staining and special silver stains, which also demonstrate the characteristic corkscrew-shaped spiral organisms.[42]
History and Physical
High-risk factors for congenital syphilis include maternal drug abuse, homelessness, incarceration, domestic violence, lack of health insurance, poverty, lower education levels, having sex with multiple partners, as well as failure to receive prenatal care, STD testing, or treatment during pregnancies.[10][28][43][44][45] Mental health issues, cultural factors, social stigma associated with sexually transmitted infections, and lack of monitoring after testing also contribute to an increased risk.[10][46]
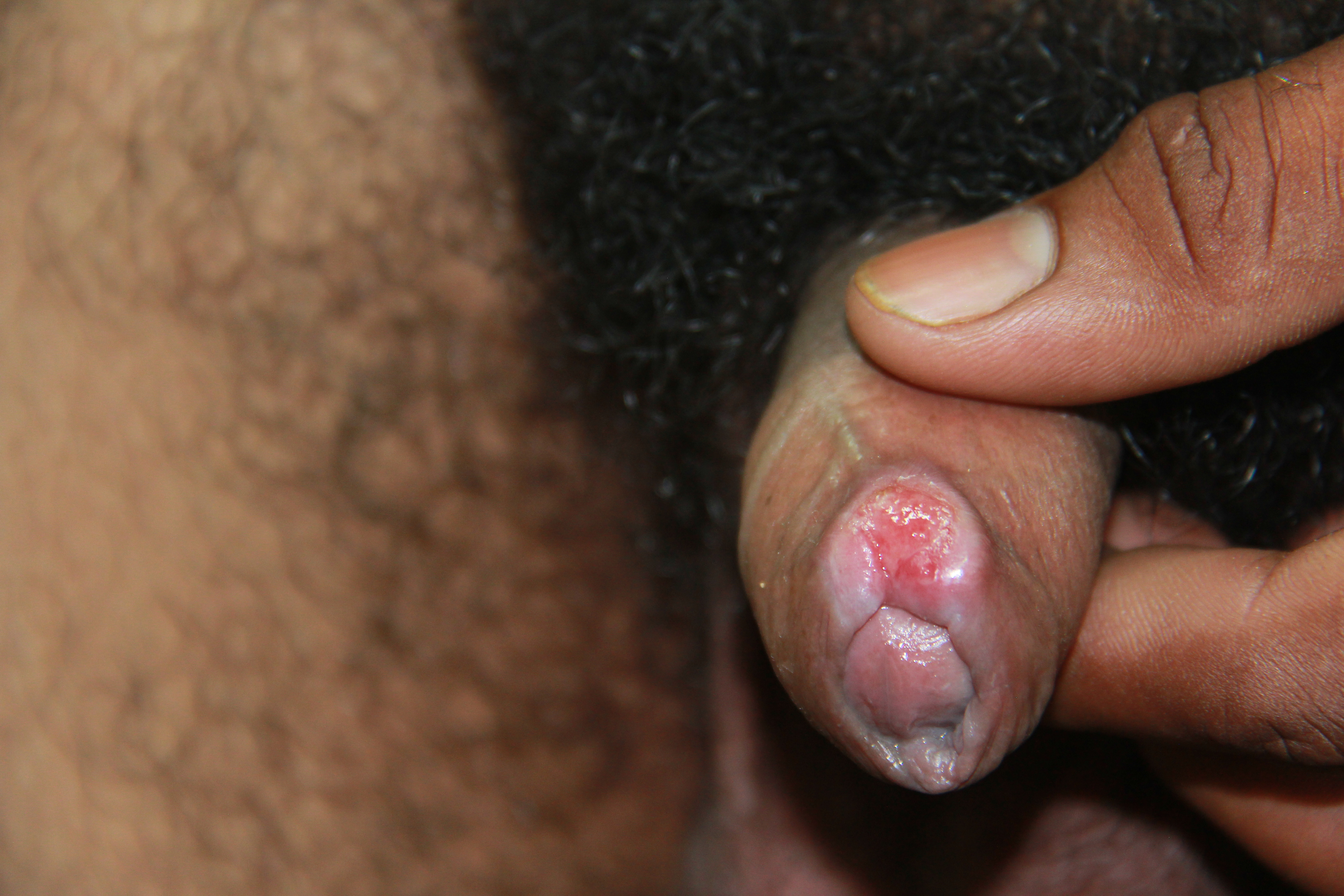
Physical findings of maternal syphilis are the same as for any woman of similar age.[14] One of the most common findings is a painless indurated chancre at the site of inoculation (see Image. Primary Syphilis Chancre). Other common findings on physical examination include lymphadenopathy and a maculopapular rash that affects the palms of the hands, the soles of the feet, and possibly the buccal mucosa (see Image. Keratotic Lesions on the Palms).[6] These signs will disappear even without treatment and lead to latent syphilis in 70% of cases but will progress to tertiary syphilis in the remainder.[6][14][44] See StatPearls' companion reference, "Syphilis," for more information.[6]
If a mother tests serologically positive for syphilis, the newborn should receive a careful physical examination looking for any early signs or symptoms of congenital disease as well as a nontreponemal (Venereal Disease Research Laboratory [VDRL] or Rapid Plasma Reagin [RPR]) assay.[14][44] A follow-up examination of the neonate in 3 months is also recommended.[14][44]
While congenital syphilis can cause severe illness and fetal demise, most neonates (70%) born with the disease are asymptomatic at birth.[10][47] If the fetus becomes infected during the second or third trimester, the neonate is typically born without symptoms and may appear healthy.[48] Worse outcomes (prematurity, spontaneous abortion, stillbirths, and perinatal demise) in neonates with congenital syphilis are associated with early transmission of T pallidum to the fetus from the mother during the first trimester.[48]
Symptoms of early congenital syphilis usually will be seen within the first 3 months after birth but may present up to 2 years of age.[10][48] Untreated infants typically begin to show clinical symptoms by 3 months of age. Late congenital syphilis would include those cases that only appear after the infant is 2 years old.[48]
Early symptoms of congenital syphilis in a neonate or infant will most commonly include 1 or more of the following: [6][14][44][49]
- Anemia and thrombocytopenia: These will be found in about 37% of cases. The anemia may be hemolytic.
- Bony abnormalities: Long osteolytic bone lesions that appear moth-eaten are pathognomonic for congenital syphilis.
- Osteochondritis and periostitis are commonly seen (75%).
- Pain may cause the pseudoparalysis of a limb (Pseudoparalysis of Parrot)
- Hepatomegaly and hepatobiliary dysfunction: This is the single most common finding in congenital syphilis and may occur with splenomegaly.
- A biopsy of the liver followed by darkfield microscopy may reveal the spirochete.
- Jaundice may or may not be present, depending on the extent of liver injury.
- Liver function tests may be abnormal, signifying inflammation.
- Lymphadenopathy: Generalized, nontender lymphadenopathy is a common finding.
- Rhinitis that is refractory or continuous: Significant rhinitis with a constant and intractable nasal discharge is often among the earliest clinical presentations of congenital syphilis, usually in the first week of life.[2]
- The rhinitis produces a copious, persistent white discharge, which is continuous, unresponsive to treatment, and persistent.
- The highly contagious discharge contains active spirochetes that can be visualized under darkfield microscopy.
- Rash: A rash usually appears 1 to 2 weeks after rhinitis in about 40% of cases.
- Small red or pink-colored maculopapular lesions may be commonly seen on the back, buttocks, posterior thigh, and especially the palms and the soles of the feet.
- The rash generally progresses to desquamation and crusting.
If congenital syphilis is not diagnosed early, persistent inflammation may lead to scarring and gumma formation, as well as many other possible symptoms.[50]
Additional clinical findings in congenital syphilis may include any of the following: [6][7][14][44][49][50][51][52][53][54][55][56][57][58][59][60]
- Cataracts
- Clavicle enlargement (Higoumenakis sign is the enlargement of the sternoclavicular region of the clavicle.)
- Cranial nerve palsies
- Chorioretinitis
- Condyloma lata
- Destruction of the nasal cartilage (saddle nose)
- Eye involvement may include unexplained changes in vision, interstitial keratitis, secondary glaucoma, chorioretinitis, uveitis, and corneal scarring.
- Eyebrow loss
- Diabetes insipidus
- Facial changes might include frontal bossing (Olympian brown), saddle nose, and short maxilla.
- Failure to thrive without any other explanation
- Fetal hydrops
- Fever
- Gastrointestinal disorders (malabsorption, rectal bleeding, ileitis, and necrotizing enterocolitis)
- Glaucoma
- Hydrocephalus
- Hutchinson teeth (Hypoplastic notched or peg-shaped permanent teeth, usually of the upper central incisors)
- Hutchinson triad (Hutchinson teeth, interstitial keratitis, and sensorineural hearing loss) is relatively specific for congenital syphilis.
- Juvenile general paresis
- Low birth weight
- Myocarditis
- Nephrotic syndrome
- Neurodevelopmental delay
- Pancreatitis
- Perioral fissures or scarring
- Pneumonia alba (X-ray findings may include classic, complete opacification of both lungs or a generalized diffuse, fluffy infiltrate.)
- Protuberant mandible
- Sawtooth metaphyseal serrations (Wegener sign)
- Seizures
- Sensorineural hearing loss
- Sepsis
- Skin and mucous membrane gummas
- Sterile joint effusion (Clutton joints)
- Thrombocytopenia
- Tibial deformities (Anterior bowing or "saber shins" and localized demineralization of the proximal medial tibia [Wimberger sign])
Congenital syphilis that is untreated or undiagnosed during the neonatal period may progress during the first 4 years following birth to a state similar to a severe form of secondary syphilis in adults. Bulging fontanels, cranial nerve palsies, condyloma lata, hepatosplenomegaly, rashes, seizures, and unexplained fevers are typical findings in late congenital syphilis.[6] Untreated neonates may also develop a latent form of the disease. As adults, these patients can progress to develop symptoms of tertiary or neurosyphilis such as nerve-related deafness (otosyphilis), ocular syphilis with visual disturbances possibly resulting in blindness, syphilitic paresis, and tabes dorsalis.[6][53][61][62][63]
Evaluation
Congenital syphilis can be difficult to diagnose at birth as most infants born with the disease are asymptomatic at the time of delivery.[6][44] Maternal IgG antibodies are transferred to the fetus until delivery, which interferes with serological testing of the neonate and makes interpretation of treponemal tests on neonatal serum difficult.[14][44] This effect can persist for 15 months or more, during which time specific treponemal serological testing on the infant is not recommended.[44]
Nontreponemal tests (VDRL or RPR) may be performed on the neonate instead, as they utilize IgM antibodies, which do not cross the placenta.[14][44][64][65] Nontreponemal IgA and IgG maternal antibodies may passively cross the placental barrier, which reduces their sensitivity, but they are still clinically useful.[14][66] If the results are positive, the neonate should be followed by serial nontreponemal testing every 2 to 3 months. Most neonates with elevated titers at birth who are uninfected will normalize their serology by the age of 6 months.[14][44] Persistently elevated nontreponemal titers suggest infection or inadequate treatment.[14][44]
Initial serological testing is focused on the mother. It includes nontreponemal serum assays (RPR or VDRL), which can be used for disease tracking, and treponemal antibody tests—fluorescent treponemal antibody absorption assay (FTA-ABS), T pallidum particle agglutination assay (TPPA), T pallidum hemagglutination assay (TPHA)—which are considered confirmatory.[6][44][53][67]
Maternal screening for syphilis in early pregnancy at the first prenatal visit is of prime importance in preventing congenital syphilis.[6][14][44][68] Standard nontreponemal screening assays for maternal syphilis (VDRL, RPR) require confirmation with an antibody treponemal-specific test, but rapid point-of-care screening tests (see below) can be used to allow immediate treatment for high-risk populations who test positive where follow-up is uncertain and resources are limited.[6][44][53][68][69][70][71][72][73][74]
Routine screening for maternal syphilis with a nontreponemal test (VDRL, RPR) is recommended by the CDC, the American Academy of Pediatrics, the American Association of Family Physicians, the US Preventive Services Task Force, the World Health Organization (WHO), and the American College of Obstetrics and Gynecology during the first prenatal visit.[6][14][44][68]
Patients at higher risk, such as those who live in a geographic area with a high incidence of syphilis (defined by the WHO as an endemic prevalence of 5% or higher), should have additional testing sometime during the pregnancy (28 weeks of gestation is suggested) and at the time of delivery. This is especially important as many women can be infected with syphilis during pregnancy.[6][14][44][75]
Reverse sequence screening for syphilis is now being performed by some laboratories. Standard serological testing has traditionally begun with a nontreponemal test (VDRL, RPR), followed by a treponemal antibody test for confirmation if positive. However, automated treponemal immunoassays have made it reasonable for some laboratories to perform the treponemal test first, followed by the nontreponemal assay for confirmation and quantification. Either method is considered acceptable by the CDC. Which protocol to follow depends on laboratory resources, logistics, and local standards of care.[76]
Rapid point-of-care screening tests for syphilis that provide results in less than 20 minutes are now commercially available, allowing for immediate treatment of pregnant women at risk at their first prenatal visit.[6][77] Most rapid point-of-care tests are antibody-based (treponemal) tests and optimally require confirmation with a nontreponemal assay, although a few combine the two, which is preferred.[6][69][70][71][72][73][74][77]
Rapid screening tests are not intended as a replacement for standard laboratory testing, but they allow for immediate therapy and are suggested for global use in high-risk populations, as well as where the syphilis prevalence rate is elevated, healthcare resources are stretched thin, follow-up is unreliable, and where it is cost-effective.[44][77][78][79][80][81][82][83][84][85][86][87]
The FDA has approved an over-the-counter treponemal antibody test for syphilis that can be performed at home in the US. This is a treponemal antibody test (with a control) on a test strip designed to use just a few drops of blood, and the results are available in about 15 minutes. In clinical trials, this test showed a negative predictive value of 99.5% and a positive predictive value of 93.4%. Like other treponemal antibody tests, once the test becomes positive, it remains that way for life, so patients will require additional nontreponemal testing for confirmation.[88] See the companion StatPearls' reference article on "Point-of-Care Testing."[77]
Ultrasonography may be able to identify intrauterine fetal syphilis starting at 18 weeks of gestational age.[14] Findings include hepatomegaly (80% of cases), anemia (33%), placental enlargement (27%), polyhydramnios (12%), and hydrops fetalis (10%).[14][89][90][91][92][93][94][95][96][97] Various gastrointestinal abnormalities, fetal growth restriction, and increased middle cerebral artery peak systolic velocity (suggesting fetal hydrops) may also be found on prenatal fetal ultrasonography in affected fetuses.[98] Aggressive therapy with IM benzathine penicillin G and IV aqueous penicillin G for 10 to 14 days has been suggested, with some successes reported, but no established standardized protocol exists.[99][100][101] The lack of any abnormal ultrasonographic findings does not preclude a fetal treponemal infection, as 12% to 15% of neonates born with congenital syphilis will have normal prenatal ultrasounds.[14][89][90]
Evaluation of an infant or neonate suspected of possible congenital syphilis is primarily based on the finding of material syphilis (positive serology in the mother) or suspicious clinical findings. Initial workup for an infant born to a woman with reactive nontreponemal and treponemal testing should follow the recommendations of the CDC or the American Academy of Pediatrics.[44][78]
The key diagnostic test is a quantitative nontreponemal serological assay (RPR or VDRL) on the serum of any newborn infant where the mother tests positive for syphilis or where the child is otherwise considered suspicious for having the disease.[44][102] The same nontreponemal test, and preferably the same laboratory, should be used for testing both mother and child sera.[44] The umbilical cord blood should not be used for serological testing for syphilis. Any newborn at risk for congenital syphilis should also be tested for HIV.[44]
The laboratory, histologic, and radiographic abnormalities in a newborn can aid in the diagnosis of congenital syphilis as follows: [6][49][98]
- Chest x-ray, which may show diffuse opacification of both lung fields.
- CSF abnormalities, including reactive VDRL titers, increased leukocyte count, and elevated protein.
- CSF PCR for detection of treponemal DNA.
- Darkfield microscopic examination of the nasal discharge or suspicious lesions, if present.
- Exceptionally high placental weight.
- Histologic examination of the placenta and cord for the typical pathological changes and presence of spirochetes.
- Long bone radiographs may show findings of pathologic fractures, metaphyseal serration, localized demineralization, and osseous destruction. Moth-eaten lesions in the long bones due to demineralization are highly characteristic of congenital syphilis.[49]
- Nontreponemal tests: VDRL or RPR of serum from the neonate.
- Specific serological treponemal tests include fluorescent treponemal antibody absorption (FTA-ABS), hemagglutination test for antibodies to T pallidum (TPHA), or T pallidum particle agglutination assay (TPPA).
Polymerase chain reaction (PCR) assays, darkfield microscopic examinations, and direct fluorescence antibody testing are not readily available in the US or many other parts of the world, and silver staining is not considered reliable.[6][14][31][103] Nucleic acid amplification tests (NAATs) are available with a sensitivity of 75% to 100%.[14] These tests can be clinically useful, but the FDA currently approves none for use in the US.[14][44] This means that serological testing is the primary diagnostic testing modality for both maternal and congenital syphilis.[6][31][44][53]
The CDC recommends that any woman who has had a fetal demise beyond 20 weeks of gestation receive a serological test for syphilis. They also recommend that no new mother or infant should be permitted to leave the hospital without at least 1 documented serological test result for syphilis recorded for the mother.[44]
Neurosyphilis in neonates and infants is usually asymptomatic. However, up to 60% of neonates born with symptoms of congenital syphilis will also have neurosyphilis.[14][98][104][105]
The diagnosis is made by laboratory evaluation of the cerebrospinal fluid (CSF) in high-risk neonates. These findings include increased protein (>0.15 g/L), elevated cell count (>25 cell/mm3), and a positive VDRL of the CSF.[1][106] CSF VDRL is highly specific (90% specificity) but has a sensitivity of only 50%.[14][98][104][105] For these reasons, some experts have suggested omitting the CSF examination, making the diagnosis based on other evidence, and treating the infant for neurosyphilis regardless.[107] PCR testing is only marginally sensitive (about the same as VDRL), but with a 97% specificity, it may be useful where it is available in the laboratory evaluation of the CSF to help diagnose neurosyphilis.[108][109][110]
Symptomatic neurosyphilis in infants is rare except for patients with untreated congenital syphilis.[7] Symptoms may include bulging fontanelles, cerebral infarctions, cranial nerve palsies, fever, hydrocephalus (progressive), increased cranial circumference, neurodevelopmental delay or regression, papilledema, seizures, visual and optical problems, vomiting, and widening of the cranial suture lines. Pituitary abnormalities may also occur, which would include diabetes insipidus and hypoglycemia.[111][112]
Treatment / Management
Maternal syphilis (syphilis infection in pregnant women) is treatable with 2.4 million units of benzathine penicillin G, which is extremely effective in eliminating congenital syphilis in the fetus, but the optimal dosing regimen and timing have not been definitively established.[11][44][113] A single IM dose of 2.4 million units of benzathine penicillin G administered before the 28th week of gestation has been effective in Africa.[114][115][116][117] However, there is evidence that a second equivalent IM dose of benzathine penicillin G given 1 week later is beneficial, especially if there is any evidence of fetal involvement.[44][115][118][119][120] In general, maternal syphilis should be treated based on the stage of the disease as outlined by the CDC and summarized in StatPearls' companion reference on "Syphilis."[6][44](A1)
Factors that interfere with the early diagnosis and treatment of maternal syphilis include the lack of timely prenatal care, lack of rapid point-of-care tests, inadequate antibiotic supplies, drug dispensing failures, poor monitoring of pregnant women and their partners by the regional healthcare system, logistical delays in receiving the first penicillin dose, poor postnatal care especially within 1 month of delivery, and the low treatment compliance of sexual partners.[10][121][122][123][124] More than a third of maternal syphilis patients with resultant stillbirths will have received no prenatal care.[125](B2)
A fourfold reduction in nontreponemal titers compared to baseline six months following antibiotic therapy for syphilis generally indicates a good response.[126] However, during pregnancy, the lack of such a response by the date of delivery in patients who received antibiotic treatment between 16 and 22 weeks gestation is not associated with any adverse outcomes for the newborn.[126]
In high-risk areas where medical follow-up is uncertain—such as in Tanzania, where 6% of pregnant women have syphilis—it is recommended that pregnant patients who test positive on a rapid point-of-care screening test immediately receive benzathine penicillin G without waiting for any confirmatory testing.[114][115][116][117] Treatment of maternal syphilis with benzathine penicillin is 98% effective at preventing congenital syphilis.[11][114][115][116][127](B2)
For latent infections or when the duration of the syphilitic infection is unknown, 3 weekly IM doses of 2.4 million units of benzathine penicillin G are recommended.[14][44] Any delay in therapy of 10 days or more requires complete retreatment.[14][44]
In cases of penicillin-allergic mothers, testing to determine the extent of the allergy and desensitization is recommended, as it is the most effective treatment for maternal syphilis.[44] Many patients labeled as penicillin allergic may have only a mild intolerance.
Maternal reinfection or therapeutic failure is suggested by a fourfold or greater increase in a nontreponemal (VDRL, RPR) assay titer maintained for at least 2 weeks after antibiotic treatment.[44] As these nontreponemal titers tend to increase right after antibiotics are given, a follow-up serological assay should wait until about 2 months after therapy is completed.[44]
The chances of inadequate neonatal treatment are increased if therapy was given <1 month before giving birth, the mother had clinical signs of infection at the time of delivery, or the maternal nontreponemal serum assay titer is more than 4-fold higher than the pretreatment level.[44]
Treatment of the neonate with congenital syphilis depends on the diagnostic likelihood of the disease. Various healthcare organizations and medical societies may have slightly different recommendations.
A summary of the 2021 guidelines from the CDC is as follows: [1][44]
Proven or Highly Likely Neonatal Disease
This applies to infants where the mother is positive serologically with both a treponemal test and a nontreponemal assay, PLUS any of the following:
- The neonate has an abnormal physical examination suggestive of congenital syphilis.
- The neonate's serum has 4 times or greater nontreponemal titer (VDRL or RPR) than the mother's serum level at delivery.
- Positive identification of T pallidum by darkfield microscopy, silver staining, or PCR of bodily fluids, lesions, or the placenta.
Additional Recommended Tests:
- Complete blood count (CBC) and differential and platelet count
- CSF analysis for VDRL, WBC count, and protein
- Long-bone x-rays
- Other optional tests as clinically indicated:
- Auditory brain stem response
- Chest x-ray
- Liver function
- Neurological imaging
- Ophthalmology (ocular) exam
Recommended Treatment:
- Aqueous crystalline penicillin G 50,000 units/kg body weight is given intravenously every 12 hours during the first week after birth and then every 8 hours for a total of 10 days.
OR
- Procaine penicillin G 50,000 units/kg body weight IM daily for 10 days (may be unavailable in the US due to discontinuation by the manufacturer)
If a day of treatment is missed, the entire regimen must start over from the beginning. If any agent other than penicillin is used, close follow-up serological testing of the neonate will be necessary. As many as 60% of symptomatic neonates will test positive for neurosyphilis.[14] The VDRL test of the CSF in neonates has good specificity at 90% but only 50% sensitivity.[14][62][104]
The standard recommended antibiotic protocol listed above for a neonate requires hospitalization for a lengthy course of IV treatment, which is expensive, onerous, and demanding on the infant and family. A careful review of the efficacy and safety of the various antibiotics used in neonates has suggested that parenteral ceftriaxone and oral amoxicillin are potential alternative antimicrobials for use in congenital syphilis.[128] Other potentially useful antibiotics would be ampicillin, cefixime, cefotaxime, doxycycline, and linezolid.[128](B3)
Probable or Likely Neonatal Disease:
Applies to infants with all of the following:
- The mother is positive serologically with both a treponemal test and a nontreponemal assay.
- The mother did not receive any treatment, received incomplete treatment, was not treated with penicillin, or treatment was undocumented or began less than 30 days before delivery.
- The neonate has a normal physical examination.
- The neonate's serum is no more than 4 times the nontreponemal (VDRL or RPR) titer of the mother's serum at delivery.
Additional Recommended Tests (optional and may be omitted if the neonate receives a full ten-day course of parenteral penicillin therapy but not benzathine penicillin G.)
- Complete blood count (CBC) and differential and platelet count.
- CSF analysis for VDRL, WBC count, and protein.
- Long-bone X-rays.
Recommended Treatment:
- Aqueous crystalline penicillin G 50,000 units/kg body weight is given intravenously every 12 hours during the first week after birth and then every 8 hours for a total of 10 days.
OR
- Procaine penicillin G 50,000 units/kg body weight IM daily for 10 days. (may be unavailable in the US due to discontinuation by the manufacturer)
OR
- Benzathine penicillin G 50,000 units/kg body weight IM as a single dose
If the mother's risk of untreated maternal syphilis is low and the neonate has a nonreactive nontreponemal serum test, benzathine penicillin G may be used without further testing or evaluation.
Neonates whose mothers had untreated early syphilis should have a full 10-day course of IV penicillin G even if the neonate's nontreponemal assay is negative.
Possible Neonatal Disease:
Applies to infants with all of the following:
- The mother is positive serologically with both a treponemal test and a nontreponemal assay.
- The mother received appropriate treatment during the pregnancy, which began more than 30 days before delivery.
- The mother shows no evidence of a relapse or reinfection.
- The neonate has a normal physical examination.
- The neonate's serum is no more than 4 times the nontreponemal (VDRL or RPR) titer of the mother's serum at delivery.
Additional Recommended Tests: None
Recommended Treatment:
- Benzathine penicillin G 50,000 units/kg body weight IM as a single dose.
OR
- In cases where follow-up is certain and reliable, close serological follow-up of the neonate every 2 to 3 months for 6 months if the mother's nontreponemal titer dropped by at least 75% after appropriate syphilis therapy or has remained stable at a low-level (VDRL <1:2 or RPR <1:4)
Unlikely Neonatal Disease:
Applies to infants with all of the following:
- The mother was positive serologically with both a treponemal test and a nontreponemal assay.
- The mother received appropriate treatment for syphilis before becoming pregnant.
- The mother's nontreponemal titer is low and has remained stable throughout the pregnancy and at delivery (VDRL ≤1:2 or RPR ≤1:4).
- The neonate has normal physical examination findings.
- The neonate's serum is no more than 4 times the nontreponemal (VDRL or RPR) titer of the mother's serum at delivery.
Additional Recommended Tests: None
Recommended Treatment:
- No treatment is required, but consider benzathine penicillin G 50,000 units/kg body weight IM as a single dose. (optional, but suggested if follow-up is uncertain or unreliable or the neonate had a reactive nontreponemal assay test)
Follow-up
All neonates with a reactive nontreponemal test should be followed serologically to monitor its resolution. If the nontreponemal test is nonreactive by the age of 6 months, no further evaluation or treatment is needed. If the neonate still has a reactive nontreponemal test, he is likely to be infected and should receive treatment.[10][44]
Neonates with a negative nontreponemal titer at birth but are considered suspicious or high-risk or whose mothers tested positive at the time of delivery should be retested with another nontreponemal test when they reach 3 months of age.[44] If follow-up is uncertain or unlikely, consideration should be given to treating the infant with a 10-day course of penicillin G.
Neonates who received adequate treatment but have persistently reactive nontreponemal tests by 6 to 12 months of age should have a CSF examination and be considered for a full retreatment protocol with a 10-day course of penicillin G.[44]
A penicillin allergy can be problematic as there is no acceptable substitute for penicillin in neonates or their pregnant mothers. Many patients labeled as such do not have a true penicillin allergy. Patients with a true allergy, including infants, may require penicillin desensitization therapy.[6][44][129][130][131][132][133] See StatPearls' companion reference, "Penicillin Allergy," for more information.
Alternative antibiotics generally cannot be used in pregnancy or remain of unproven benefit.[44] Doxycycline and tetracycline cannot be used in pregnant women during the second and third trimesters. Azithromycin and erythromycin must be avoided as they are inadequate for the treatment of the fetus, and they are not adequate therapy for maternal syphilis.[44][113] There is promising but insufficient data on the use of ceftriaxone as an alternative antibiotic for treating maternal or congenital syphilis.[44][133][134][135][136](A1)
Inadequate supplies of penicillin are also an issue in many locations. For example, the manufacturer has discontinued procaine penicillin production in the US. In such situations, pregnant women who test positive for syphilis should be given the top priority for any available supplies. Ceftriaxone may be substituted at 50 mg/kg to 75 mg/kg body weight intravenously daily for 10 days in the neonate, but ceftriaxone is not considered equivalent to penicillin and requires careful follow-up clinical evaluations and serological testing.[43][44][133][134][135][136][137](A1)
Jarisch-Herxheimer Reaction
As many as 40% of maternal syphilis patients who receive treatment will develop a Jarisch-Herxheimer reaction.[14] The reaction is caused by the release of cytotoxins from the dying spirochetes.[138][139][140] Typical symptoms include cramping, fever, general malaise, headache, muscle aches, rash, and tachycardia.[6][138][139][140] The symptoms begin just a few hours after the administration of penicillin and usually resolve spontaneously within 24 hours.[6][138] Treatment is mainly supportive.[138](B3)
Continuous fetal heart monitoring for up to 24 hours is suggested for maternal syphilis patients who develop a Jarisch-Herxheimer reaction and for those at high risk.[14] The most severely infected individuals (highest VDRL or RPR titers) are the most likely to develop a Jarisch-Herxheimer reaction.[6][138] Patients should be warned about a possible reaction before being given treatment, and this would not indicate a penicillin allergy. See StatPearls' companion reference, "Jarisch-Herxheimer Reaction," for more information.
Differential Diagnosis
Congenital syphilis may present similarly to quite a large number of other disease processes, including the following:
- Anthrax
- Bejel
- Chorioretinitis
- Gonorrhea
- Granuloma inguinale
- Lyme Disease
- Lymphogranuloma venereum
- Neonatal hepatitis
- Neonatal sepsis
- Parvovirus B19 Infection
- Pediatric acropustulosis
- Pediatric enteroviral Infections
- Pediatric erythema toxicum
- Pediatric HIV
- Pediatric tuberculosis
- Pinta
- Rat-bite fever
- Relapsing fever
- Sepsis
- TORCH infections (Toxoplasmosis, Rubella, Cytomegalovirus, and Herpes simplex)
- Venereal warts
- Yaws
While congenital syphilis is the most common cause of increased placental weight, other etiologies to consider in patients who serologically test negative for syphilis and have increased placental weight include the following: [6]
- Beckwith-Weidemann syndrome
- Cerebral palsy
- Cytomegalovirus infection
- Hydrops fetalis
- Maternal diabetes
Prognosis
The prognosis is excellent if diagnosed and treated appropriately in a timely fashion. Syphilis is easily treated with penicillin. However, there is an increased risk of worse outcomes and possible death in the following cases:
- Premature infants
- Those with a significant delay in starting therapy or who do not otherwise receive proper treatment
- Patients who display an extensive spread of the disease with multiple organ failure
- Infants with a severe Jarisch-Herxheimer reaction upon treatment [138]
The WHO has set worldwide target goals to reduce maternal and congenital syphilis. It will require a significant worldwide commitment to public health, political and financial resources, as well as patient and public education, to reach those goals, which include the following: [10]
- At least 90% of pregnant women are tested for syphilis (preferably at their first prenatal visit).
- At least 90% of serologically positive pregnant women receive adequate treatment.
Complications
About 40% of the babies of women with syphilis who are not treated for the disease will either be stillborn or die shortly after birth.[29][30]
Delayed diagnosis and treatment can lead to late, persistent clinical features of intellectual disability, skin gummas or rashes, scarring, hearing and visual deficits, severe anemia, meningitis, jaundice, liver failure, splenomegaly, skeletal abnormalities, and tertiary syphilis. Initiation of treatment in some infants can lead to a Jarisch–Herxheimer reaction, resulting in fevers, chills, hypotension, and possibly even fetal death as a result of the inflammatory response caused by the dying spirochetes.
Untreated patients who survived congenital syphilis as neonates may show signs of tertiary syphilis or neurosyphilis as adults.
Deterrence and Patient Education
The surveillance of all pregnant women for syphilis and timely treatment of the disease during pregnancy is the most effective means of eliminating congenital syphilis.
Considerations that may lead to decreased rates of congenital syphilis are as follows:
- Dealing with barriers to treatment, such as fear of injections and "loss of dignity"
- Education on safe sexual practices and the use of condoms to decrease the spread of syphilis
- Eliminating the fear of legal action or child custody issues
- Elimination of penicillin supply shortages
- Expansion of basic prenatal care to all pregnant women for early detection of syphilis in pregnancy, starting with the first prenatal visit
- Expansion of free STD clinics for early diagnosis and treatment
- Implementing automatic testing (opt-out screenings) in ERs and urgent care centers
- Improved availability of rapid point-of-care testing kits with immediate antibiotic treatment of high-risk individuals
- Improving testing and treatment of the sexual partners of maternal syphilis patients
- Including syphilis testing with the initial pregnancy test
Congenital syphilis is a preventable disease, which makes its growing prevalence so frustrating. Public health measures so far have not been able to reduce the rapid growth and prevalence of the disease, and it remains a significant, growing global public health epidemic with significant morbidity and mortality.[141] Improved public health programs to promote prenatal screening and treatment of syphilis in pregnancy, better access to prenatal care, the expanded use of rapid point-of-care testing with immediate antibiotic therapy in high-risk populations, and increased therapy of sexual partners are proven means to reduce the rising epidemic of congenital syphilis around the world.[142][143] It is estimated that such programs could cut syphilis-related stillbirths and perinatal deaths by at least 50%.[142][144]
Pearls and Other Issues
Pertinent clinical pearls of congenital and maternal syphilis include the following:
- Maternal serological testing for syphilis should start at the first prenatal visit, at about 28 weeks of gestation, and again at delivery, particularly for higher-risk mothers.
- Maternal syphilis treatment is with 2.4 million units IM of benzathine penicillin G, but to be considered adequate, it must be given more than 30 days before delivery. Some experts recommend a second IM injection 1 week after the first.
- Neonatal serological testing in high-risk situations should be at delivery and again at 3 months.
- HIV testing should be performed on both the mother and infant when serological testing for syphilis is positive.
- When maternal syphilis cannot be ruled out by serological testing or prenatal care is deficient or even nonexistent, the neonate should be carefully examined and definitive serological testing performed.
- In borderline or questionable situations, it is generally better to give the neonate a 10-day course of penicillin rather than risk the infant receiving inadequate treatment.
- Breastfeeding is allowed in neonatal syphilis as long as there are no active or open lesions on the breast.[10][145] Diagnosis and treatment of syphilis can sometimes be complex. Local health departments and infectious disease specialists can be consulted for assistance.
- Positive syphilis testing in a young child suggests undiagnosed maternal syphilis or possible child sexual abuse.
Enhancing Healthcare Team Outcomes
Congenital syphilis is a serious infection with the potential for long-term complications. In the United States, each case of congenital syphilis case must be reported to the public health department. Even among women who receive prenatal care, the detection and treatment of maternal syphilis is often too late for optimal results. Treating congenital syphilis involves a team approach, including primary care physicians, infectious disease specialists, neonatologists, pediatricians, obstetricians, advanced care practitioners, nurses, pharmacists, and laboratory technicians.[146]
Obstetricians have the important task of testing and treating pregnant women for syphilis, which significantly decreases the incidence of congenital syphilis in the neonate. Infectious disease specialists, pediatricians, and neonatologists are typically involved early in the acute treatment of the disease. Given the overwhelming preponderance of high-quality evidence, physicians should stringently follow the published screening and treatment guidelines.
Pediatricians also follow up on the successful eradication of the disease and monitor for complications. Nurses play a vital role in the management of congenital syphilis by providing essential prenatal education and offering ongoing support to mothers and their newborns. Pharmacists have become even more critical in the treatment of maternal and congenital syphilis as supplies of penicillin have sometimes reached critical levels. Maternal syphilis patients should receive the highest priority if penicillin supplies are low.[44]
Care coordination plays a pivotal role in ensuring that the patient's journey from diagnosis to treatment and follow-up is well-managed, minimizing errors and enhancing patient safety. By embracing these principles of skill, strategy, ethics, responsibilities, interprofessional communication, and care coordination, healthcare professionals can deliver patient-centered care, ultimately improving patient outcomes and enhancing interprofessional team performance in the management of congenital syphilis.
Media
(Click Image to Enlarge)
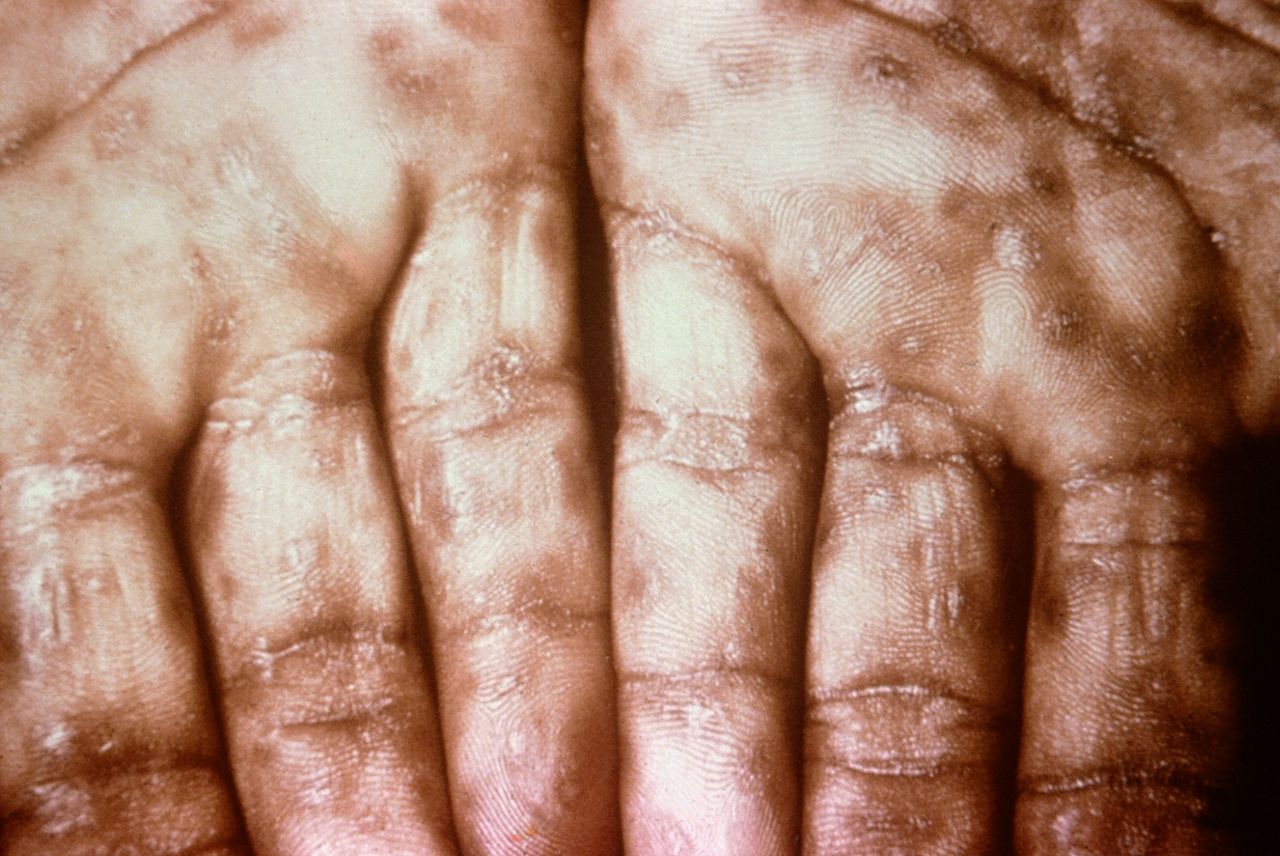
Keratotic Lesions on the Palms. This photograph shows a close-up view of keratotic lesions on the palms of a patient’s hands due to a secondary syphilitic infection. Syphilis is a complex sexually transmitted disease (STD) caused by the bacterium Treponema pallidum. It has often been called “the great imitator” because many signs and symptoms are indistinguishable from those of other diseases.
Robert Sumpter; Public Health Image Library, Public Domain, Centers for Disease Control and Prevention
References
Arnold SR, Ford-Jones EL. Congenital syphilis: A guide to diagnosis and management. Paediatrics & child health. 2000 Nov:5(8):463-9 [PubMed PMID: 20177559]
The Lancet. Congenital syphilis in the USA. Lancet (London, England). 2018 Oct 6:392(10154):1168. doi: 10.1016/S0140-6736(18)32360-2. Epub [PubMed PMID: 30319097]
Gregory ECW, Ely DM. Trends and Characteristics in Maternal Syphilis Rates During Pregnancy: United States, 2016-2022. NCHS data brief. 2024 Feb:(496):1-8 [PubMed PMID: 38358322]
Franzen C. Syphilis in composers and musicians--Mozart, Beethoven, Paganini, Schubert, Schumann, Smetana. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2008 Dec:27(12):1151-7. doi: 10.1007/s10096-008-0571-x. Epub 2008 Jul 1 [PubMed PMID: 18592279]
Baron S, Radolf JD. Treponema. Medical Microbiology. 1996:(): [PubMed PMID: 21413263]
Tudor ME, Al Aboud AM, Leslie SW, Gossman W. Syphilis. StatPearls. 2024 Jan:(): [PubMed PMID: 30521201]
Woods CR. Syphilis in children: congenital and acquired. Seminars in pediatric infectious diseases. 2005 Oct:16(4):245-57 [PubMed PMID: 16210105]
Cox DL. Culture of Treponema pallidum. Methods in enzymology. 1994:236():390-405 [PubMed PMID: 7968624]
Level 3 (low-level) evidenceEdmondson DG, Norris SJ. In Vitro Cultivation of the Syphilis Spirochete Treponema pallidum. Current protocols. 2021 Feb:1(2):e44. doi: 10.1002/cpz1.44. Epub [PubMed PMID: 33599121]
Sankaran D, Partridge E, Lakshminrusimha S. Congenital Syphilis-An Illustrative Review. Children (Basel, Switzerland). 2023 Jul 29:10(8):. doi: 10.3390/children10081310. Epub 2023 Jul 29 [PubMed PMID: 37628309]
Bowen V, Su J, Torrone E, Kidd S, Weinstock H. Increase in incidence of congenital syphilis - United States, 2012-2014. MMWR. Morbidity and mortality weekly report. 2015 Nov 13:64(44):1241-5. doi: 10.15585/mmwr.mm6444a3. Epub 2015 Nov 13 [PubMed PMID: 26562206]
Kimball A, Torrone E, Miele K, Bachmann L, Thorpe P, Weinstock H, Bowen V. Missed Opportunities for Prevention of Congenital Syphilis - United States, 2018. MMWR. Morbidity and mortality weekly report. 2020 Jun 5:69(22):661-665. doi: 10.15585/mmwr.mm6922a1. Epub 2020 Jun 5 [PubMed PMID: 32497029]
Neblett Fanfair R, Tao G, Owusu-Edusei K, Gift TL, Bernstein KT. Suboptimal Prenatal Syphilis Testing Among Commercially Insured Women in the United States, 2013. Sexually transmitted diseases. 2017 Apr:44(4):219-221. doi: 10.1097/OLQ.0000000000000569. Epub [PubMed PMID: 28282647]
Stafford IA, Workowski KA, Bachmann LH. Syphilis Complicating Pregnancy and Congenital Syphilis. The New England journal of medicine. 2024 Jan 18:390(3):242-253. doi: 10.1056/NEJMra2202762. Epub [PubMed PMID: 38231625]
Stafford IA, Berra A, Minard CG, Fontenot V, Kopkin RH, Rodrigue E, Roitsch CM, Rac MW, Hill JB. Challenges in the Contemporary Management of Syphilis among Pregnant Women in New Orleans, LA. Infectious diseases in obstetrics and gynecology. 2019:2019():2613962. doi: 10.1155/2019/2613962. Epub 2019 Feb 13 [PubMed PMID: 30894787]
Patel SJ, Klinger EJ, OʼToole D, Schillinger JA. Missed opportunities for preventing congenital syphilis infection in New York City. Obstetrics and gynecology. 2012 Oct:120(4):882-8 [PubMed PMID: 22996106]
Kachikis A, Schiff MA, Moore K, Chapple-McGruder T, Arluck J, Hitti J. Risk Factors Associated with Congenital Syphilis, Georgia, 2008-2015. Infectious diseases in obstetrics and gynecology. 2023:2023():3958406. doi: 10.1155/2023/3958406. Epub 2023 Nov 8 [PubMed PMID: 38026087]
McDonald R, O'Callaghan K, Torrone E, Barbee L, Grey J, Jackson D, Woodworth K, Olsen E, Ludovic J, Mayes N, Chen S, Wingard R, Johnson Jones M, Drame F, Bachmann L, Romaguera R, Mena L. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis - United States, 2022. MMWR. Morbidity and mortality weekly report. 2023 Nov 17:72(46):1269-1274. doi: 10.15585/mmwr.mm7246e1. Epub 2023 Nov 17 [PubMed PMID: 37971936]
Matthias JM, Rahman MM, Newman DR, Peterman TA. Effectiveness of Prenatal Screening and Treatment to Prevent Congenital Syphilis, Louisiana and Florida, 2013-2014. Sexually transmitted diseases. 2017 Aug:44(8):498-502. doi: 10.1097/OLQ.0000000000000638. Epub [PubMed PMID: 28703731]
Hersh AR, Megli CJ, Caughey AB. Repeat Screening for Syphilis in the Third Trimester of Pregnancy: A Cost-Effectiveness Analysis. Obstetrics and gynecology. 2018 Sep:132(3):699-707. doi: 10.1097/AOG.0000000000002795. Epub [PubMed PMID: 30095767]
Lin JS, Eder M, Bean S. Screening for Syphilis Infection in Pregnant Women: A Reaffirmation Evidence Update for the U.S. Preventive Services Task Force. 2018 Sep:(): [PubMed PMID: 30234936]
Walker DG, Walker GJ. Prevention of congenital syphilis--time for action. Bulletin of the World Health Organization. 2004 Jun:82(6):401 [PubMed PMID: 15356930]
Cerqueira LRP, Monteiro DLM, Taquette SR, Rodrigues NCP, Trajano AJB, Souza FM, Araújo BM. The magnitude of syphilis: from prevalence to vertical transmission. Revista do Instituto de Medicina Tropical de Sao Paulo. 2017 Dec 21:59():e78. doi: 10.1590/S1678-9946201759078. Epub 2017 Dec 21 [PubMed PMID: 29267586]
Salomè S, Cambriglia MD, Scarano SM, Capone E, Betts I, Pacella D, Sansone M, Mazzarelli LL, Lo Vecchio A, Ranucci G, Marinosci GZ, Capasso L, Salvatore P, Raimondi F. Congenital syphilis in the twenty-first century: an area-based study. European journal of pediatrics. 2023 Jan:182(1):41-51. doi: 10.1007/s00431-022-04703-5. Epub 2022 Nov 14 [PubMed PMID: 36376519]
Centers for Disease Control and Prevention (CDC). Congenital syphilis - United States, 2003-2008. MMWR. Morbidity and mortality weekly report. 2010 Apr 16:59(14):413-7 [PubMed PMID: 20395934]
Gilmour LS, Walls T. Congenital Syphilis: a Review of Global Epidemiology. Clinical microbiology reviews. 2023 Jun 21:36(2):e0012622. doi: 10.1128/cmr.00126-22. Epub 2023 Mar 15 [PubMed PMID: 36920205]
Benoit P, Tennenhouse L, Lapple A, Hill-Carroll G, Shaw S, Bullard J, Plourde P. Congenital syphilis re-emergence in Winnipeg, Manitoba. Canada communicable disease report = Releve des maladies transmissibles au Canada. 2022 Feb 24:48(2-3):89-94. doi: 10.14745/ccdr.v48i23a06. Epub 2022 Feb 24 [PubMed PMID: 35342366]
Smullin C, Wagman J, Mehta S, Klausner JD. A Narrative Review of the Epidemiology of Congenital Syphilis in the United States From 1980 to 2019. Sexually transmitted diseases. 2021 Feb 1:48(2):71-78. doi: 10.1097/OLQ.0000000000001277. Epub [PubMed PMID: 32925597]
Level 3 (low-level) evidenceWatson-Jones D, Changalucha J, Gumodoka B, Weiss H, Rusizoka M, Ndeki L, Whitehouse A, Balira R, Todd J, Ngeleja D, Ross D, Buvé A, Hayes R, Mabey D. Syphilis in pregnancy in Tanzania. I. Impact of maternal syphilis on outcome of pregnancy. The Journal of infectious diseases. 2002 Oct 1:186(7):940-7 [PubMed PMID: 12232834]
Level 2 (mid-level) evidenceCenters for Disease Control and Prevention (CDC). Congenital syphilis--United States, 1998. MMWR. Morbidity and mortality weekly report. 1999 Sep 3:48(34):757-61 [PubMed PMID: 10499788]
Whiting C, Schwartzman G, Khachemoune A. Syphilis in Dermatology: Recognition and Management. American journal of clinical dermatology. 2023 Mar:24(2):287-297. doi: 10.1007/s40257-022-00755-3. Epub 2023 Jan 23 [PubMed PMID: 36689103]
Moseley P, Bamford A, Eisen S, Lyall H, Kingston M, Thorne C, Piñera C, Rabie H, Prendergast AJ, Kadambari S. Resurgence of congenital syphilis: new strategies against an old foe. The Lancet. Infectious diseases. 2024 Jan:24(1):e24-e35. doi: 10.1016/S1473-3099(23)00314-6. Epub 2023 Aug 18 [PubMed PMID: 37604180]
Salomè S, Cambriglia MD, Montesano G, Capasso L, Raimondi F. Congenital Syphilis: A Re-Emerging but Preventable Infection. Pathogens (Basel, Switzerland). 2024 Jun 6:13(6):. doi: 10.3390/pathogens13060481. Epub 2024 Jun 6 [PubMed PMID: 38921779]
Penner J, Hernstadt H, Burns JE, Randell P, Lyall H. Stop, think SCORTCH: rethinking the traditional 'TORCH' screen in an era of re-emerging syphilis. Archives of disease in childhood. 2021 Feb:106(2):117-124. doi: 10.1136/archdischild-2020-318841. Epub 2020 Jun 25 [PubMed PMID: 32586930]
Genest DR, Choi-Hong SR, Tate JE, Qureshi F, Jacques SM, Crum C. Diagnosis of congenital syphilis from placental examination: comparison of histopathology, Steiner stain, and polymerase chain reaction for Treponema pallidum DNA. Human pathology. 1996 Apr:27(4):366-72 [PubMed PMID: 8617480]
Berman SM. Maternal syphilis: pathophysiology and treatment. Bulletin of the World Health Organization. 2004 Jun:82(6):433-8 [PubMed PMID: 15356936]
Malan AF, Woods DL, Van der Elst CW, Meyer MP. Relative placental weight in congenital syphilis. Placenta. 1990 Jan-Feb:11(1):3-6 [PubMed PMID: 2326235]
Pillay S, Horn AR, Tooke L. Placental weights of neonates born with symptomatic congenital syphilis. Frontiers in pediatrics. 2023:11():1215387. doi: 10.3389/fped.2023.1215387. Epub 2023 Oct 6 [PubMed PMID: 37868268]
Larsen SA. Syphilis. Clinics in laboratory medicine. 1989 Sep:9(3):545-57 [PubMed PMID: 2676323]
Pierce EF, Katz KA. Darkfield microscopy for point-of-care syphilis diagnosis. MLO: medical laboratory observer. 2011 Jan:43(1):30-1 [PubMed PMID: 21309351]
Lejarraga-Cañas C, Ayerdi-Aguirrebengoa O, Menéndez-Prieto B, Tello-Romero E, Rodríguez-Martín C, Del Romero-Guerrero J. Is dark-field microscopy still useful for the primary syphilis diagnosis in the 21(ST) century? Enfermedades infecciosas y microbiologia clinica (English ed.). 2022 Jan:40(1):32-34. doi: 10.1016/j.eimce.2021.10.001. Epub 2021 Oct 31 [PubMed PMID: 34732343]
Rawstron SA, Vetrano J, Tannis G, Bromberg K. Congenital syphilis: detection of Treponema pallidum in stillborns. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1997 Jan:24(1):24-7 [PubMed PMID: 8994751]
Fang J, Silva RM, Tancredi DJ, Pinkerton KE, Sankaran D. Examining associations in congenital syphilis infection and socioeconomic factors between California's small-to-medium and large metro counties. Journal of perinatology : official journal of the California Perinatal Association. 2022 Nov:42(11):1434-1439. doi: 10.1038/s41372-022-01445-y. Epub 2022 Jun 23 [PubMed PMID: 35739308]
Dalby J, Stoner BP. Sexually Transmitted Infections: Updates From the 2021 CDC Guidelines. American family physician. 2022 May 1:105(5):514-520 [PubMed PMID: 35559639]
DiOrio D, Kroeger K, Ross A. Social Vulnerability in Congenital Syphilis Case Mothers: Qualitative Assessment of Cases in Indiana, 2014 to 2016. Sexually transmitted diseases. 2018 Jul:45(7):447-451. doi: 10.1097/OLQ.0000000000000783. Epub [PubMed PMID: 29465662]
Level 2 (mid-level) evidenceLuo Z, Ding Y, Yuan J, Wu Q, Tian L, Zhang L, Li B, Mou J. Predictors of Seronegative Conversion After Centralized Management of Syphilis Patients in Shenzhen, China. Frontiers in public health. 2021:9():755037. doi: 10.3389/fpubh.2021.755037. Epub 2021 Nov 25 [PubMed PMID: 34900903]
Ortiz-Lopez N, Diez M, Diaz O, Simon F, Diaz A. Epidemiological surveillance of congenital syphilis in Spain, 2000-2010. The Pediatric infectious disease journal. 2012 Sep:31(9):988-90 [PubMed PMID: 22572752]
Level 2 (mid-level) evidenceArrieta AC, Singh J. Congenital Syphilis. The New England journal of medicine. 2019 Nov 28:381(22):2157. doi: 10.1056/NEJMicm1904420. Epub [PubMed PMID: 31774960]
Rasool MN, Govender S. The skeletal manifestations of congenital syphilis. A review of 197 cases. The Journal of bone and joint surgery. British volume. 1989 Nov:71(5):752-5 [PubMed PMID: 2584243]
Level 2 (mid-level) evidenceChakraborty R, Luck S. Syphilis is on the increase: the implications for child health. Archives of disease in childhood. 2008 Feb:93(2):105-9. doi: 10.1136/adc.2006.103515. Epub [PubMed PMID: 18208988]
Dorfman DH, Glaser JH. Congenital syphilis presenting in infants after the newborn period. The New England journal of medicine. 1990 Nov 8:323(19):1299-302 [PubMed PMID: 2215616]
Fiumara NJ, Lessell S. Manifestations of late congenital syphilis. An analysis of 271 patients. Archives of dermatology. 1970 Jul:102(1):78-83 [PubMed PMID: 4993238]
Peeling RW, Mabey D, Chen XS, Garcia PJ. Syphilis. Lancet (London, England). 2023 Jul 22:402(10398):336-346. doi: 10.1016/S0140-6736(22)02348-0. Epub [PubMed PMID: 37481272]
Braunstein GD, Lewis EJ, Galvanek EG, Hamilton A, Bell WR. The nephrotic syndrome associated with secondary syphilis. An immune deposit disease. The American journal of medicine. 1970 May:48(5):643-8 [PubMed PMID: 4912935]
Gamble CN, Reardan JB. Immunopathogenesis of syphilitic glomerulonephritis. Elution of antitreponemal antibody from glomerular immune-complex deposits. The New England journal of medicine. 1975 Feb 27:292(9):449-54 [PubMed PMID: 1089888]
Lago EG, Vaccari A, Fiori RM. Clinical features and follow-up of congenital syphilis. Sexually transmitted diseases. 2013 Feb:40(2):85-94. doi: 10.1097/OLQ.0b013e31827bd688. Epub [PubMed PMID: 23324972]
Rathbun KC. Congenital syphilis. Sexually transmitted diseases. 1983 Apr-Jun:10(2):93-9 [PubMed PMID: 6318372]
Ricci JM, Fojaco RM, O'Sullivan MJ. Congenital syphilis: the University of Miami/Jackson Memorial Medical Center experience, 1986-1988. Obstetrics and gynecology. 1989 Nov:74(5):687-93 [PubMed PMID: 2812644]
Tsan K, Mulcahy B, Malhotra A. Long bone radiographic abnormalities of congenital syphilis in a preterm infant. Archives of disease in childhood. Fetal and neonatal edition. 2023 Sep:108(5):451. doi: 10.1136/archdischild-2021-323134. Epub 2022 Jan 25 [PubMed PMID: 35078780]
Iskandar W, Permatagalih V, Primadi A, Gamayani U. Congenital neurosyphilis presenting as neonatal sepsis. Journal of infection in developing countries. 2022 Jun 30:16(6):1113-1117. doi: 10.3855/jidc.15662. Epub 2022 Jun 30 [PubMed PMID: 35797308]
Koundanya VV, Tripathy K. Syphilis Ocular Manifestations. StatPearls. 2024 Jan:(): [PubMed PMID: 32644383]
Ha T, Tadi P, Leslie SW, Dubensky L. Neurosyphilis. StatPearls. 2024 Jan:(): [PubMed PMID: 31082023]
Bhandari J, Thada PK, Leslie SW, Ratzan RM. Tabes Dorsalis. StatPearls. 2024 Jan:(): [PubMed PMID: 32491814]
Herremans T, Kortbeek L, Notermans DW. A review of diagnostic tests for congenital syphilis in newborns. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2010 May:29(5):495-501. doi: 10.1007/s10096-010-0900-8. Epub 2010 Mar 25 [PubMed PMID: 20336337]
Herremans M, Notermans DW, Mommers M, Kortbeek LM. Comparison of a Treponema pallidum IgM immunoblot with a 19S fluorescent treponemal antibody absorption test for the diagnosis of congenital syphilis. Diagnostic microbiology and infectious disease. 2007 Sep:59(1):61-6 [PubMed PMID: 17662551]
Satyaputra F, Hendry S, Braddick M, Sivabalan P, Norton R. The Laboratory Diagnosis of Syphilis. Journal of clinical microbiology. 2021 Sep 20:59(10):e0010021. doi: 10.1128/JCM.00100-21. Epub 2021 May 12 [PubMed PMID: 33980644]
Park IU, Tran A, Pereira L, Fakile Y. Sensitivity and Specificity of Treponemal-specific Tests for the Diagnosis of Syphilis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020 Jun 24:71(Suppl 1):S13-S20. doi: 10.1093/cid/ciaa349. Epub [PubMed PMID: 32578866]
US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Kubik M, Kurth AE, Landefeld CS, Mangione CM, Phipps MG, Silverstein M, Simon MA, Tseng CW, Wong JB. Screening for Syphilis Infection in Pregnant Women: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA. 2018 Sep 4:320(9):911-917. doi: 10.1001/jama.2018.11785. Epub [PubMed PMID: 30193283]
Naidu P, Tsang RS. Canadian Public Health Laboratory Network guidelines for the use of point-of-care tests for Treponema pallidum in Canada. Journal of the Association of Medical Microbiology and Infectious Disease Canada = Journal officiel de l'Association pour la microbiologie medicale et l'infectiologie Canada. 2022 Jun:7(2):85-96. doi: 10.3138/jammi-2021-0021. Epub 2022 Jun 3 [PubMed PMID: 36337357]
Taylor MM, Peeling RW, Toskin I, Ghinidelli M. Role of dual HIV/syphilis test kits in expanding syphilis screening. Sexually transmitted infections. 2017 Nov:93(7):458-459. doi: 10.1136/sextrans-2017-053301. Epub 2017 Aug 4 [PubMed PMID: 28778981]
Gliddon HD, Peeling RW, Kamb ML, Toskin I, Wi TE, Taylor MM. A systematic review and meta-analysis of studies evaluating the performance and operational characteristics of dual point-of-care tests for HIV and syphilis. Sexually transmitted infections. 2017 Dec:93(S4):S3-S15. doi: 10.1136/sextrans-2016-053069. Epub 2017 Jul 26 [PubMed PMID: 28747410]
Level 1 (high-level) evidencePham MD, Ong JJ, Anderson DA, Drummer HE, Stoové M. Point-of-Care Diagnostics for Diagnosis of Active Syphilis Infection: Needs, Challenges and the Way Forward. International journal of environmental research and public health. 2022 Jul 4:19(13):. doi: 10.3390/ijerph19138172. Epub 2022 Jul 4 [PubMed PMID: 35805831]
Arai C, Lemos-Machado JA, Aun MV, Bonet-Bub C, Santos LD, Miranda AE, Avelino-Silva VI. Sensitivity and specificity of a syphilis rapid diagnostic test in blood donors' samples. The Brazilian journal of infectious diseases : an official publication of the Brazilian Society of Infectious Diseases. 2023 Nov-Dec:27(6):103689. doi: 10.1016/j.bjid.2023.103689. Epub 2023 Nov 14 [PubMed PMID: 37972650]
Singh AE, Ives N, Gratrix J, Vetland C, Ferron L, Crawford M, Hale-Balla L, Dong K, Meyer G, Smyczek P, Galli R, Rourke SB, Fonseca K, PoSH Study Team (Rabia Ahmed, Sheila Braun, Nicole Cardinal, Jenny Doherty, Rebecca Rosenblum, Tom Wong, Wadieh Yacoub). Sensitivity and specificity of two investigational Point of care tests for Syphilis and HIV (PoSH Study) for the diagnosis and treatment of infectious syphilis in Canada: a cross-sectional study. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2023 Jul:29(7):940.e1-940.e7. doi: 10.1016/j.cmi.2023.02.015. Epub 2023 Mar 2 [PubMed PMID: 36868357]
Level 2 (mid-level) evidenceO'Connor NP, Gonzalez BE, Esper FP, Tamburro J, Kadkhoda K, Foster CB. Congenital syphilis: Missed opportunities and the case for rescreening during pregnancy and at delivery. IDCases. 2020:22():e00964. doi: 10.1016/j.idcr.2020.e00964. Epub 2020 Sep 23 [PubMed PMID: 33024697]
Level 3 (low-level) evidencePapp JR, Park IU, Fakile Y, Pereira L, Pillay A, Bolan GA. CDC Laboratory Recommendations for Syphilis Testing, United States, 2024. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2024 Feb 8:73(1):1-32. doi: 10.15585/mmwr.rr7301a1. Epub 2024 Feb 8 [PubMed PMID: 38319847]
Larkins MC, Thombare A. Point-of-Care Testing. StatPearls. 2024 Jan:(): [PubMed PMID: 37276307]
Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2015 Jun 5:64(RR-03):1-137 [PubMed PMID: 26042815]
Pereira LE, McCormick J, Dorji T, Kang J, Sun Y, Shukla M, Hopkins A, Deutsch J, Kersh EN, Bernstein K, Fakile YF. Laboratory Evaluation of a Commercially Available Rapid Syphilis Test. Journal of clinical microbiology. 2018 Oct:56(10):. doi: 10.1128/JCM.00832-18. Epub 2018 Sep 25 [PubMed PMID: 30021825]
Peterman TA, Fakile YF. What Is the Use of Rapid Syphilis Tests in the United States? Sexually transmitted diseases. 2016 Mar:43(3):201-3. doi: 10.1097/OLQ.0000000000000413. Epub [PubMed PMID: 26859809]
Swartzendruber A, Steiner RJ, Adler MR, Kamb ML, Newman LM. Introduction of rapid syphilis testing in antenatal care: A systematic review of the impact on HIV and syphilis testing uptake and coverage. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2015 Jun:130 Suppl 1(Suppl 1):S15-21. doi: 10.1016/j.ijgo.2015.04.008. Epub 2015 Apr 29 [PubMed PMID: 26001704]
Level 1 (high-level) evidenceTowns JM, Tieosapjaroen W, Mello MB, Baggaley RC, Johnson CC, Jamil MS, Rowley J, Barr-DiChiara M, Terris-Prestholt F, Chen MY, Chow EPF, Fairley CK, Zhang L, Ong JJ. The role of syphilis self-testing as an additional syphilis testing approach in key populations: a systematic review and meta-analysis. The Lancet. Public health. 2023 Sep:8(9):e726-e734. doi: 10.1016/S2468-2667(23)00128-7. Epub 2023 Jul 21 [PubMed PMID: 37482070]
Level 1 (high-level) evidenceMabey DC, Sollis KA, Kelly HA, Benzaken AS, Bitarakwate E, Changalucha J, Chen XS, Yin YP, Garcia PJ, Strasser S, Chintu N, Pang T, Terris-Prestholt F, Sweeney S, Peeling RW. Point-of-care tests to strengthen health systems and save newborn lives: the case of syphilis. PLoS medicine. 2012:9(6):e1001233. doi: 10.1371/journal.pmed.1001233. Epub 2012 Jun 12 [PubMed PMID: 22719229]
Level 3 (low-level) evidenceHackett C, Frank L, Heldt-Werle L, Loosier PS. Provider-Reported Barriers in Sexual Health Care Services for Women With Upstream Barriers: The Case of Syphilis and Congenital Syphilis in Southern Colorado, 2022. Sexually transmitted diseases. 2024 May 1:51(5):337-341. doi: 10.1097/OLQ.0000000000001936. Epub 2024 Jan 23 [PubMed PMID: 38301636]
Level 3 (low-level) evidenceValencia J, Vázquez L, Lazarus JV, Cuevas G, Torres-Macho J, Domingorena J, Castrillo M, Ryan P. On-site testing and treatment of sexually transmitted infections among female sex workers using molecular point-of-care testing integrated into harm reduction services in Madrid, Spain. The International journal on drug policy. 2024 Jan:123():104281. doi: 10.1016/j.drugpo.2023.104281. Epub 2023 Dec 5 [PubMed PMID: 38056222]
Chitneni P, Owembabazi M, Muyindike W, Asiimwe S, Masete G, Mbalibulha Y, Nakku-Joloba E, Manabe YC, Haberer JE, Matthews LT, Van Der Pol B. Sexually Transmitted Infection Point-of-Care Testing in Resource-Limited Settings: A Narrative Review Guided by an Implementation Framework. Sexually transmitted diseases. 2023 Oct 1:50(10):e11-e16. doi: 10.1097/OLQ.0000000000001848. Epub 2023 Jul 4 [PubMed PMID: 37433000]
Level 3 (low-level) evidenceRomero CP, Marinho DS, Castro R, de Aguiar Pereira CC, Silva E, Caetano R, Silva Elias FT, Chilcott J, Dixon S. Cost-Effectiveness Analysis of Point-of-Care Rapid Testing Versus Laboratory-Based Testing for Antenatal Screening of Syphilis in Brazil. Value in health regional issues. 2020 Dec:23():61-69. doi: 10.1016/j.vhri.2020.03.004. Epub 2020 Aug 22 [PubMed PMID: 32841902]
Amerson EH, Castillo Valladares HB, Leslie KS. Resurgence of Syphilis in the US-USPSTF Reaffirms Screening Guidelines. JAMA dermatology. 2022 Nov 1:158(11):1241-1243. doi: 10.1001/jamadermatol.2022.3499. Epub [PubMed PMID: 36166232]
Rac MW, Bryant SN, McIntire DD, Cantey JB, Twickler DM, Wendel GD Jr, Sheffield JS. Progression of ultrasound findings of fetal syphilis after maternal treatment. American journal of obstetrics and gynecology. 2014 Oct:211(4):426.e1-6. doi: 10.1016/j.ajog.2014.05.049. Epub 2014 Jun 4 [PubMed PMID: 24907700]
Sheffield JS, Sánchez PJ, Morris G, Maberry M, Zeray F, McIntire DD, Wendel GD Jr. Congenital syphilis after maternal treatment for syphilis during pregnancy. American journal of obstetrics and gynecology. 2002 Mar:186(3):569-73 [PubMed PMID: 11904625]
Mari G, Deter RL, Carpenter RL, Rahman F, Zimmerman R, Moise KJ Jr, Dorman KF, Ludomirsky A, Gonzalez R, Gomez R, Oz U, Detti L, Copel JA, Bahado-Singh R, Berry S, Martinez-Poyer J, Blackwell SC. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses. The New England journal of medicine. 2000 Jan 6:342(1):9-14 [PubMed PMID: 10620643]
Hernandez-Andrade E, Scheier M, Dezerega V, Carmo A, Nicolaides KH. Fetal middle cerebral artery peak systolic velocity in the investigation of non-immune hydrops. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2004 May:23(5):442-5 [PubMed PMID: 15133792]
Level 2 (mid-level) evidenceDelle Chiaie L, Buck G, Grab D, Terinde R. Prediction of fetal anemia with Doppler measurement of the middle cerebral artery peak systolic velocity in pregnancies complicated by maternal blood group alloimmunization or parvovirus B19 infection. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2001 Sep:18(3):232-6 [PubMed PMID: 11555452]
Society for Maternal-Fetal Medicine (SMFM), Norton ME, Chauhan SP, Dashe JS. Society for maternal-fetal medicine (SMFM) clinical guideline #7: nonimmune hydrops fetalis. American journal of obstetrics and gynecology. 2015 Feb:212(2):127-39. doi: 10.1016/j.ajog.2014.12.018. Epub 2014 Dec 31 [PubMed PMID: 25557883]
Level 1 (high-level) evidenceHoddick WK, Mahony BS, Callen PW, Filly RA. Placental thickness. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 1985 Sep:4(9):479-82 [PubMed PMID: 3903201]
Vintzileos AM, Neckles S, Campbell WA, Andreoli JW Jr, Kaplan BM, Nochimson DJ. Fetal liver ultrasound measurements during normal pregnancy. Obstetrics and gynecology. 1985 Oct:66(4):477-80 [PubMed PMID: 3900834]
Park J, Ondusko DS, Chang BH, Edwards EA, Doan S, Gatter K, Hajjali I, Kim A. Fetal Hepatomegaly. NeoReviews. 2022 Dec 1:23(12):e861-e868. doi: 10.1542/neo.23-12-e861. Epub [PubMed PMID: 36450648]
David M, Hcini N, Mandelbrot L, Sibiude J, Picone O. Fetal and neonatal abnormalities due to congenital syphilis: A literature review. Prenatal diagnosis. 2022 May:42(5):643-655. doi: 10.1002/pd.6135. Epub 2022 Apr 9 [PubMed PMID: 35352829]
Rosenthal M, Poliquin V. Exploring management of antenatally diagnosed fetal syphilis infection. Canada communicable disease report = Releve des maladies transmissibles au Canada. 2022 Feb 24:48(2-3):111-114. doi: 10.14745/ccdr.v48i23a09. Epub 2022 Feb 24 [PubMed PMID: 35342369]
Galan HL, Yandell PM, Knight AB. Intravenous penicillin for antenatal syphilotherapy. Infectious diseases in obstetrics and gynecology. 1993:1(1):7-11 [PubMed PMID: 18476198]
Chen I, Chandra S, Singh A, Kumar M, Jain V, Turnell R. Successful outcome with intrauterine transfusion in non-immune hydrops fetalis secondary to congenital syphilis. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstetrique et gynecologie du Canada : JOGC. 2010 Sep:32(9):861-865. doi: 10.1016/S1701-2163(16)34658-8. Epub [PubMed PMID: 21050519]
Kimball A, Bowen VB, Miele K, Weinstock H, Thorpe P, Bachmann L, McDonald R, Machefsky A, Torrone E. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep:148(3):. doi: 10.1542/peds.2020-049080. Epub [PubMed PMID: 34465590]
Grange PA, Gressier L, Dion PL, Farhi D, Benhaddou N, Gerhardt P, Morini JP, Deleuze J, Pantoja C, Bianchi A, Lassau F, Avril MF, Janier M, Dupin N. Evaluation of a PCR test for detection of treponema pallidum in swabs and blood. Journal of clinical microbiology. 2012 Mar:50(3):546-52. doi: 10.1128/JCM.00702-11. Epub 2012 Jan 4 [PubMed PMID: 22219306]
Ropper AH. Neurosyphilis. The New England journal of medicine. 2019 Oct 3:381(14):1358-1363. doi: 10.1056/NEJMra1906228. Epub [PubMed PMID: 31577877]
Medoro AK, Sánchez PJ. Syphilis in Neonates and Infants. Clinics in perinatology. 2021 Jun:48(2):293-309. doi: 10.1016/j.clp.2021.03.005. Epub [PubMed PMID: 34030815]
Risser WL, Hwang LY. Problems in the current case definitions of congenital syphilis. The Journal of pediatrics. 1996 Oct:129(4):499-505 [PubMed PMID: 8859255]
Level 3 (low-level) evidenceBeeram MR, Chopde N, Dawood Y, Siriboe S, Abedin M. Lumbar puncture in the evaluation of possible asymptomatic congenital syphilis in neonates. The Journal of pediatrics. 1996 Jan:128(1):125-9 [PubMed PMID: 8551402]
Gao ZX, Gou Y, Liu XQ, Peng LW. Advances in laboratory diagnostic methods for cerebrospinal fluid testing for neurosyphilis. Frontiers in public health. 2022:10():1030480. doi: 10.3389/fpubh.2022.1030480. Epub 2022 Nov 14 [PubMed PMID: 36452956]
Level 3 (low-level) evidenceVanhaecke C, Grange P, Benhaddou N, Blanche P, Salmon D, Parize P, Lortholary O, Caumes E, Pelloux I, Epaulard O, Guinard J, Dupin N, Neurosyphilis Network, Neurosyphilis network. Clinical and Biological Characteristics of 40 Patients With Neurosyphilis and Evaluation of Treponema pallidum Nested Polymerase Chain Reaction in Cerebrospinal Fluid Samples. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 Nov 1:63(9):1180-1186 [PubMed PMID: 27585981]
Marks M, Lawrence D, Kositz C, Mabey D. Diagnostic performance of PCR assays for the diagnosis of neurosyphilis: a systematic review. Sexually transmitted infections. 2018 Dec:94(8):585-588. doi: 10.1136/sextrans-2018-053666. Epub 2018 Jul 30 [PubMed PMID: 30061192]
Level 1 (high-level) evidenceDaaboul JJ, Kartchner W, Jones KL. Neonatal hypoglycemia caused by hypopituitarism in infants with congenital syphilis. The Journal of pediatrics. 1993 Dec:123(6):983-5 [PubMed PMID: 8229534]
Nolt D, Saad R, Kouatli A, Moritz ML, Menon RK, Michaels MG. Survival with hypopituitarism from congenital syphilis. Pediatrics. 2002 Apr:109(4):e63 [PubMed PMID: 11927736]
Walker GJ. Antibiotics for syphilis diagnosed during pregnancy. The Cochrane database of systematic reviews. 2001:2001(3):CD001143 [PubMed PMID: 11686978]
Level 1 (high-level) evidenceWatson-Jones D, Gumodoka B, Weiss H, Changalucha J, Todd J, Mugeye K, Buvé A, Kanga Z, Ndeki L, Rusizoka M, Ross D, Marealle J, Balira R, Mabey D, Hayes R. Syphilis in pregnancy in Tanzania. II. The effectiveness of antenatal syphilis screening and single-dose benzathine penicillin treatment for the prevention of adverse pregnancy outcomes. The Journal of infectious diseases. 2002 Oct 1:186(7):948-57 [PubMed PMID: 12232835]
Level 2 (mid-level) evidenceWatson-Jones D, Oliff M, Terris-Prestholt F, Changalucha J, Gumodoka B, Mayaud P, Semakafu AM, Kumaranayake L, Gavyole A, Mabey D, Hayes R. Antenatal syphilis screening in sub-Saharan Africa: lessons learned from Tanzania. Tropical medicine & international health : TM & IH. 2005 Sep:10(9):934-43 [PubMed PMID: 16135202]
Level 2 (mid-level) evidenceTerris-Prestholt F, Watson-Jones D, Mugeye K, Kumaranayake L, Ndeki L, Weiss H, Changalucha J, Todd J, Lisekie F, Gumodoka B, Mabey D, Hayes R. Is antenatal syphilis screening still cost effective in sub-Saharan Africa. Sexually transmitted infections. 2003 Oct:79(5):375-81 [PubMed PMID: 14573832]
Level 2 (mid-level) evidenceTerris-Prestholt F, Vickerman P, Torres-Rueda S, Santesso N, Sweeney S, Mallma P, Shelley KD, Garcia PJ, Bronzan R, Gill MM, Broutet N, Wi T, Watts C, Mabey D, Peeling RW, Newman L. The cost-effectiveness of 10 antenatal syphilis screening and treatment approaches in Peru, Tanzania, and Zambia. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2015 Jun:130 Suppl 1(Suppl 1):S73-80. doi: 10.1016/j.ijgo.2015.04.007. Epub 2015 Apr 29 [PubMed PMID: 25963907]
Wendel GD Jr, Sheffield JS, Hollier LM, Hill JB, Ramsey PS, Sánchez PJ. Treatment of syphilis in pregnancy and prevention of congenital syphilis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002 Oct 15:35(Suppl 2):S200-9 [PubMed PMID: 12353207]
Zhu L, Qin M, Du L, Xie RH, Wong T, Wen SW. Maternal and congenital syphilis in Shanghai, China, 2002 to 2006. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2010 Sep:14 Suppl 3():e45-8. doi: 10.1016/j.ijid.2009.09.009. Epub 2010 Feb 6 [PubMed PMID: 20137991]
Level 2 (mid-level) evidenceAdhikari EH. Syphilis in Pregnancy. Obstetrics and gynecology. 2020 May:135(5):1121-1135. doi: 10.1097/AOG.0000000000003788. Epub [PubMed PMID: 32282589]
Torres PMA, Reis ARP, Santos ASTD, Negrinho NBDS, Menegueti MG, Gir E. Factors associated with inadequate treatment of syphilis during pregnancy: an integrative review. Revista brasileira de enfermagem. 2022:75(6):e20210965. doi: 10.1590/0034-7167-2021-0965. Epub 2022 Sep 9 [PubMed PMID: 36102473]
Biswas HH, Chew Ng RA, Murray EL, Chow JM, Stoltey JE, Watt JP, Bauer HM. Characteristics Associated With Delivery of an Infant With Congenital Syphilis and Missed Opportunities for Prevention-California, 2012 to 2014. Sexually transmitted diseases. 2018 Jul:45(7):435-441. doi: 10.1097/OLQ.0000000000000782. Epub [PubMed PMID: 29465666]
Slutsker JS, Hennessy RR, Schillinger JA. Factors Contributing to Congenital Syphilis Cases - New York City, 2010-2016. MMWR. Morbidity and mortality weekly report. 2018 Oct 5:67(39):1088-1093. doi: 10.15585/mmwr.mm6739a3. Epub 2018 Oct 5 [PubMed PMID: 30286056]
Level 3 (low-level) evidenceHakizimana T, Muhumuza J, Selamo FM, Ishimwe MPS, Kajabwangu R, Jelle OM, Muhumuza J, Kiyaka SM, Nyakato S, Fajardo Y. Prevalence and Factors Associated with Syphilis among Mothers with Missed Opportunities for Antenatal Syphilis Testing in Rural Western Uganda: A Cross-Sectional Study. International journal of reproductive medicine. 2023:2023():2971065. doi: 10.1155/2023/2971065. Epub 2023 Aug 24 [PubMed PMID: 37664641]
Level 2 (mid-level) evidenceMachefsky A, Hufstetler K, Bachmann L, Barbee L, Miele K, O'Callaghan K. Rising Stillbirth Rates Related to Congenital Syphilis in the United States From 2016 to 2022. Obstetrics and gynecology. 2024 Aug 15:():. doi: 10.1097/AOG.0000000000005700. Epub 2024 Aug 15 [PubMed PMID: 39146539]
Kaminiów K, Kotlarz A, Kiołbasa M, Pastuszczak M. Lack of Serological Response by Delivery to Syphilis Treatment Does Not Impact Pregnancy Outcomes. Journal of clinical medicine. 2024 Jul 10:13(14):. doi: 10.3390/jcm13144031. Epub 2024 Jul 10 [PubMed PMID: 39064071]
Alexander JM, Sheffield JS, Sanchez PJ, Mayfield J, Wendel GD Jr. Efficacy of treatment for syphilis in pregnancy. Obstetrics and gynecology. 1999 Jan:93(1):5-8 [PubMed PMID: 9916946]
Villarreal DD, Le J, Klausner JD. Congenital Syphilis - Comprehensive Narrative Review of Alternative Antibiotic Treatment for Use in Neonates. Sexually transmitted diseases. 2024 Jul 24:():. doi: 10.1097/OLQ.0000000000002057. Epub 2024 Jul 24 [PubMed PMID: 39046152]
Level 3 (low-level) evidenceCaimmi S, Caffarelli C, Saretta F, Liotti L, Crisafulli G, Cardinale F, Bottau P, Mori F, Franceschini F, Bernardini R, Marseglia GL. Drug desensitization in allergic children. Acta bio-medica : Atenei Parmensis. 2019 Jan 28:90(3-S):20-29. doi: 10.23750/abm.v90i3-S.8158. Epub 2019 Jan 28 [PubMed PMID: 30830058]
Chastain DB, Hutzley VJ, Parekh J, Alegro JVG. Antimicrobial Desensitization: A Review of Published Protocols. Pharmacy (Basel, Switzerland). 2019 Aug 9:7(3):. doi: 10.3390/pharmacy7030112. Epub 2019 Aug 9 [PubMed PMID: 31405062]
Wendel GD Jr, Stark BJ, Jamison RB, Molina RD, Sullivan TJ. Penicillin allergy and desensitization in serious infections during pregnancy. The New England journal of medicine. 1985 May 9:312(19):1229-32 [PubMed PMID: 3921835]
Pham MN, Ho HE, Desai M. Penicillin desensitization: Treatment of syphilis in pregnancy in penicillin-allergic patients. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2017 May:118(5):537-541. doi: 10.1016/j.anai.2017.03.013. Epub [PubMed PMID: 28477786]
Roberts CP, Raich A, Stafylis C, Klausner JD. Alternative Treatments for Syphilis During Pregnancy. Sexually transmitted diseases. 2019 Oct:46(10):637-640. doi: 10.1097/OLQ.0000000000001050. Epub [PubMed PMID: 31517802]
Liang Z, Chen YP, Yang CS, Guo W, Jiang XX, Xu XF, Feng SX, Liu YQ, Jiang G. Meta-analysis of ceftriaxone compared with penicillin for the treatment of syphilis. International journal of antimicrobial agents. 2016 Jan:47(1):6-11. doi: 10.1016/j.ijantimicag.2015.10.020. Epub 2015 Nov 23 [PubMed PMID: 26724187]
Level 1 (high-level) evidenceKatanami Y, Hashimoto T, Takaya S, Yamamoto K, Kutsuna S, Takeshita N, Hayakawa K, Kanagawa S, Ohmagari N. Amoxicillin and Ceftriaxone as Treatment Alternatives to Penicillin for Maternal Syphilis. Emerging infectious diseases. 2017 May:23(5):827-829. doi: 10.3201/eid2305.161936. Epub [PubMed PMID: 28418316]
Zhou P, Gu Z, Xu J, Wang X, Liao K. A study evaluating ceftriaxone as a treatment agent for primary and secondary syphilis in pregnancy. Sexually transmitted diseases. 2005 Aug:32(8):495-8 [PubMed PMID: 16041252]
Coyle M, Depcinski S, Thirumoorthi M. Prevention of congenital syphilis using ceftriaxone in a woman with Stevens-Johnson syndrome reaction to penicillin: A case report. Case reports in women's health. 2022 Oct:36():e00446. doi: 10.1016/j.crwh.2022.e00446. Epub 2022 Aug 20 [PubMed PMID: 36072694]
Level 3 (low-level) evidenceDhakal A, Sbar E. Jarisch-Herxheimer Reaction. StatPearls. 2024 Jan:(): [PubMed PMID: 32491752]
Gautam M, Sethi S, Nadkarni NJ. Jarisch‒Herxheimer reaction. Indian journal of sexually transmitted diseases and AIDS. 2023 Jan-Jun:44(1):79-81. doi: 10.4103/ijstd.ijstd_107_22. Epub 2023 Jun 6 [PubMed PMID: 37457541]
Butler T. The Jarisch-Herxheimer Reaction After Antibiotic Treatment of Spirochetal Infections: A Review of Recent Cases and Our Understanding of Pathogenesis. The American journal of tropical medicine and hygiene. 2017 Jan 11:96(1):46-52. doi: 10.4269/ajtmh.16-0434. Epub 2016 Oct 24 [PubMed PMID: 28077740]
Level 3 (low-level) evidenceFoles AI, Eiras Dias M, Figueiredo M, Marçal M. Congenital syphilis: the re-emergence of a forgotten disease. BMJ case reports. 2024 Jan 16:17(1):. doi: 10.1136/bcr-2023-257694. Epub 2024 Jan 16 [PubMed PMID: 38233003]
Level 3 (low-level) evidenceSwayze EJ, Cambou MC, Melo M, Segura ER, Raney J, Santos BR, Lira R, Pinto RB, Dos Santos Varella IR, Nielsen-Saines K. Ineffective penicillin treatment and absence of partner treatment may drive the congenital syphilis epidemic in Brazil. AJOG global reports. 2022 May:2(2):. pii: 100050. doi: 10.1016/j.xagr.2022.100050. Epub 2022 Feb 4 [PubMed PMID: 36081843]
Rac MW, Revell PA, Eppes CS. Syphilis during pregnancy: a preventable threat to maternal-fetal health. American journal of obstetrics and gynecology. 2017 Apr:216(4):352-363. doi: 10.1016/j.ajog.2016.11.1052. Epub 2016 Dec 9 [PubMed PMID: 27956203]
Hawkes S, Matin N, Broutet N, Low N. Effectiveness of interventions to improve screening for syphilis in pregnancy: a systematic review and meta-analysis. The Lancet. Infectious diseases. 2011 Sep:11(9):684-91. doi: 10.1016/S1473-3099(11)70104-9. Epub 2011 Jun 15 [PubMed PMID: 21683653]
Level 1 (high-level) evidenceLawrence RM, Lawrence RA. Breast milk and infection. Clinics in perinatology. 2004 Sep:31(3):501-28 [PubMed PMID: 15325535]
. Congenital Syphilis: A Discussion of Epidemiology, Diagnosis, Management, and Nurses' Role in Early Identification and Treatment. Advances in neonatal care : official journal of the National Association of Neonatal Nurses. 2018 Dec:18(6):E1-E2. doi: 10.1097/ANC.0000000000000563. Epub [PubMed PMID: 30499828]
Level 3 (low-level) evidence