Introduction
Congenital melanocytic nevi (CMN) are one of the most frequent skin lesions encountered at birth. They are composed of collections of melanocytes (pigment-forming cells) that show distinct clinical and histopathological features. They also present a different biological behavior to acquired melanocytic nevi.[1]
Their clinical relevance relies on their association with the development of malignant melanoma and with central nervous system (CNS) involvement (neurocutaneous melanosis), which are greatly dependent on their size.[2]
This article will discuss the etiology, clinical features, diagnosis, and management of CMN.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Congenital melanocytic nevi occur as a result of in-utero somatic mutations concerning genes that play a role in the mitogen-activated protein kinase (MAPK) pathway (mainly NRAS and BRAF). These mutations lead to the development of cutaneous (and sometimes extracutaneous) mosaicisms.[3]
Epidemiology
The incidence in congenital melanocytic nevi varies greatly depending on the study (0.2% to 6%). The incidence is lower in studies where the diagnosis is histologically confirmed, whereas it tends to be more frequent when clinical diagnostic criteria are used.[4][5]
Giant congenital nevi are rare, their incidence is approximately 0.005%.[6]
Pathophysiology
In utero, stem cells from the neuroectoderm migrate to the skin as melanoblasts, which later on differentiate into melanocytes. Mutations arising in these cells can lead to mosaicisms. When these mutations take place early on in the embryogenesis, multipotent progenitor cells can be affected, leading to the presence of multiple CMN and extracutaneous involvement. The same mutations have been found to be present in different lesions of a single patient.[7][8]
Histopathology
It is not always possible to differentiate congenital melanocytic nevi from acquired melanocytic nevi solely on a histologic basis. CMN are composed of melanocytes that are arranged in nests and which can be located in the dermo-epidermal junction, dermis, or they may be compound. They usually present a V-shaped configuration on lower magnification, and they often involve deep areas of the dermis, sometimes reaching the subcutaneous fat or the fascia. They tend to surround appendageal structures such as sweat glands and follicles, as well as blood vessels and nerves. Cytological atypia is generally minimal or absent. Melanocytes are commonly larger in the superficial area of the lesion, diminishing in size as we go deeper. This is referred to as the "maturation gradient."[1]
Proliferative nodules (PN) constitute nodular lesions arising within CMN, which are made up of polymorphic cells. They usually have a pushing border which can deform surrounding structures. There is usually no cytological atypia or increased proliferative rate. Necrosis is usually absent. These are all useful factors in differentiating PN from melanoma.[9]
Occasionally, CMN may exhibit "neuroid differentiation," presenting areas of spindle cells with myxoid stroma. The latter may resemble neurofibromas.[1]
History and Physical
Congenital melanocytic nevi can be present at birth, or they can develop during the first 2 to 3 years of life. The latter is usually referred to as tardive congenital nevi.[10]
They are classified according to their estimated adult size as small (less than 1.5 cm), medium (1.5 to 20 cm), large (20 to 40 cm), and giant (>40 cm). This classification is essential as the size of nevi is one of the main risk factors of melanoma development and neurological involvement.[11]
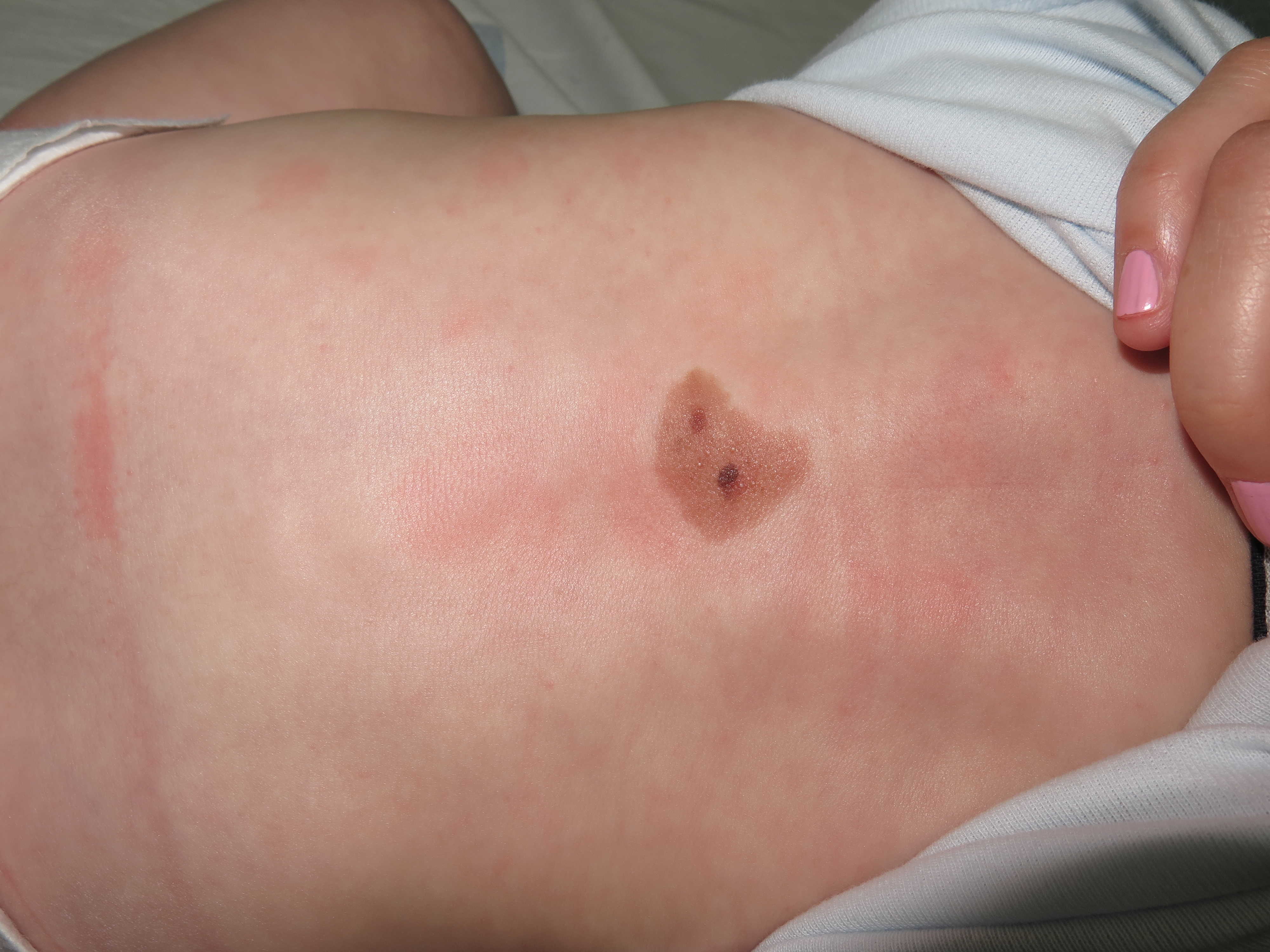
At birth, they usually present as pigmented macules or slightly raised oval papules or plaques, which can be initially mistaken for café-au-lait macules. They usually show color darkening over time and tend to end up having a well-defined edge. They can also become raised or verrucous. They can display a wide variety of colors ranging from light brown to black. [figure1] Hypertrichosis is a common finding and is usually accompanied by perifollicular hypopigmentation. CMN usually grows proportionally with the child, covering the same area of skin in adulthood as they did at birth. [10]
PN can be found within CMN. They can be present at birth or develop during childhood. They are generally round or oval soft papules or nodules with a well-defined border. They are normally less pigmented than their environment.[8]
Pruritus and eczematization may be occasionally associated.[10]
Giant CMNs frequently present with a garment appearance (in a bathing trunk, in coat sleeve, etcetera). They occasionally show satellite lesions, which resemble small or medium CMN.[12] [figure 2]
Neurocutaneous melanosis is a disorder where CMNs are associated with melanocyte proliferation in the CNS. Those with larger CMN and satellite lesions are especially at risk. They may be asymptomatic, or they may have neurological symptoms, including seizures, cranial nerve dysfunction, or signs and symptoms of increased intracranial pressure. Symptomatic patients usually have an ominous prognosis.[13]
Evaluation
Dermoscopy is vital in their evaluation, as it is a cheap, quick, non-invasive method that can provide the physician with very useful information in distinguishing CMN from other cutaneous lesions and in diagnosing early malignant transformation. Globular, cobblestone, and reticular patterns are the most frequently encountered. Useful features in diagnosing CMN include haloed and target globules, blotches, and perifollicular hypopigmentation.[14]
On acral skin, CMN frequently combine a parallel furrow pattern (similar to the one seen in acquired melanocytic nevi) with dots on the crista, presenting a "peas-in-a-pod" appearance.[15]
Whenever there is clinical suspicion of melanoma, an urgent biopsy with histopathological examination must be performed.[8]
Patients with large or giant CMN, especially when satellitosis is present, should undergo prompt evaluation (within the first 4 to 6 months of life) with gadolinium-enhanced magnetic resonance imaging (MRI) to rule out neurological involvement. MRI can detect melanocytic deposits, which manifest as hyperintensities on T1-weighted images and hypointensities on T2-weighted images. The temporal lobe (especially the amygdala) is the most commonly affected brain region. Leptomeningeal involvement is also frequent.[16]
Treatment / Management
Patients should be evaluated on an individual basis. The main factors that should be taken into account in deciding whether congenital melanocytic nevi should be excised are the risk of developing melanoma and cosmetic disfigurement and derived psychological impact.
The risk of developing melanoma in individual small and medium lesions is extremely low. Therefore, routine monitoring in this group does not seem necessary.[8]
When a giant or multiple congenital melanocytic nevi are present, follow-up is recommended. When a large number of nevi are present, mole mapping with high-quality photography and dermoscopy is ideal. Excision surgery is recommended when malignancy is suspected, or when it can improve cosmetic outcome.[17]
When CMN are removed for cosmetic reasons, dermabrasion, various laser modalities, and curettage are sometimes used.[18] These techniques do not remove the lesions completely, and should not be performed for medical reasons since they do not reduce the risk of melanoma development. Besides, some of these patients will present with recurrence of the lesions.
A pediatric neurologist should evaluate patients with neurocutaneous melanosis. They can sometimes benefit from surgery and/or chemotherapy. Symptomatic medical treatment (i.e., anticonvulsants, dexamethasone) should be used whenever necessary.[13]
Differential Diagnosis
Congenital melanocytic nevi can sometimes be very hard to differentiate from acquired atypical nevi or melanoma. When this is the case, a biopsy should be performed in order to rule out the latter. Ideally, it should be excisional. Other differential diagnoses include smooth muscle hamartomas, Becker melanosis, and plexiform neurofibromas.
Surgical Oncology
Smaller congenital melanocytic nevi can be excised by primary excision, whereas larger lesions may require more complex surgery, including serial excision, tissue expansion surgery, skin grafts or flaps.[19]
Radiation Oncology
Radiotherapy can be used as a palliative treatment in leptomeningeal melanoma and in late-stage cutaneous melanoma.[8]
Pertinent Studies and Ongoing Trials
Giant congenital melanocytic nevi are caused by mutations resulting in the activation of the MAPK pathway. The same mutations are found in their associated neurological melanocytic proliferations. Recent case reports have shown promising results using drugs that block this pathway trough MEK (MAP/ERK kinase) inhibition in neurocutaneous melanosis. Larger trials are needed in order to validate their use.[20][21][22]
Medical Oncology
Different chemotherapy regimens have been tried in severe neurocutaneous melanosis with limited success.[23] However, the nature of these therapies exceeds the purpose of this work and will not be discussed here.
Prognosis
The prognosis of these patients depends on the risk of malignancy and the presence of neurocutaneous involvement.
The risk of melanoma in patients with congenital melanocytic nevi has historically been overestimated. Prognosis in small and medium-sized single CMN is usually excellent, and their lifetime risk of developing melanoma is estimated to be around 1%, being extremely rare before puberty. In patients with large or giant CMN, the risk is estimated to be around 5%, being greater as the size increases. In the latter, melanomas tend to arise earlier on in life (during childhood).[24] Even when apparently complete excisions are performed, these patients appear to be at an increased risk of developing melanoma when compared to the normal population. This is probably due to the fact that melanomas do not necessarily arise on the cutaneous lesion but can be derived from melanocytes elsewhere in the body (i.e., CNS). The presence of neurological abnormalities in MRI is also a risk factor for the development of melanoma. The mortality rate of melanoma arising in the CNS is virtually 100%.[8]
The other associated condition which has a huge impact on morbidity and mortality is neurocutaneous melanosis. The estimated risk in big and giant CMN ranges between 10% to 33% and, as with melanomas, is greatly dependent on size and satellitosis. The prognosis is much worse when neurological signs and/or symptoms are present.[13]
Complications
Some patients tend to develop pruritus and eczema at the site of the lesion. This can usually be managed with symptomatic treatments (i.e., topical steroids).[25]
Psychological problems, including depression, anxiety, and self-isolation, can result from body disfigurement secondary to congenital melanocytic nevi. Therapeutic strategies focused on cosmetic improvement should be discussed with the patients and their families, and psychological advice should be sought if necessary.[26]
Melanomas arising from CMN are highly aggressive. They usually arise in the deep dermis or subcutis, presenting as papules or nodules, sometimes with ulceration. It is, therefore, difficult to detect them early on in their development. They can also develop in the CNS or other organs. Lymphadenopathy due to lymph node metastasis is frequently present at the time of diagnosis.[8]
The presence of neurological involvement can be asymptomatic (noted on MRI), manifest as developmental delay, seizures, psychiatric symptoms, or result in increased intracranial pressure. The latter can present as irritability, lethargy, increased head circumference, cranial nerve palsies, etc. The prognosis in these patients is ominous, and they tend to die at a very early age.[13][27]
Deterrence and Patient Education
Patients have to be instructed to perform routine self-examinations, consulting with their doctors whenever any changes in their lesions take place. In individual small or medium-sized nevi, patients should be reassured of the benign nature of their condition to avoid unnecessary psychological distress.
They should be given realistic expectations before any procedure aiming to provide a cosmetic improvement is performed.
Pearls and Other Issues
- Congenital melanocytic nevi are pigmented lesions that are usually present at birth but which can actually become apparent in the first two years of life (tardive CMN).
- They are often polychromatic and can present hypertrichosis and a verrucous surface.
- Patients with single small or medium CMN have a very low risk of developing melanoma.
- The presence of large, especially multiple lesions, is the main risk factor for melanoma in pediatric populations.
- Individuals with multiple large or giant CMN are also at a higher risk of presenting neurological involvement and should have an MRI performed in the first year of life (ideally in the first 6 months) to rule out neurocutaneous melanosis.
- Melanomas arising from CMN usually present as subcutaneous nodules and tend to be very aggressive.
- A new understanding of the genetics of CMN is leading to better understanding and managing this condition.
Enhancing Healthcare Team Outcomes
Primary care providers should coordinate the management of these patients. They must be aware of the nature of these lesions and follow them up accordingly. A referral to a dermatologist should be done whenever cutaneous malignancy is suspected or when pediatricians of family physicians do not feel comfortable managing these patients due to insufficient experience, short consultation times, lack of equipment, etcetera.
Brain MRI should be performed in patients with risk factors (multiple lesions, large diameter, neurological symptoms) in the first 6 months of life to rule out neurocutaneous melanosis.[Level 3][8]
An interprofessional approach with communication between pediatricians, dermatologists, pediatric neurologists, plastic surgeons, and psychologists is essential in the appropriate management of these patients.[10]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Zayour M, Lazova R. Congenital melanocytic nevi. Clinics in laboratory medicine. 2011 Jun:31(2):267-80. doi: 10.1016/j.cll.2011.03.004. Epub [PubMed PMID: 21549240]
Levy R, Lara-Corrales I. Melanocytic Nevi in Children: A Review. Pediatric annals. 2016 Aug 1:45(8):e293-8. doi: 10.3928/19382359-20160720-07. Epub [PubMed PMID: 27517357]
Price HN. Congenital melanocytic nevi: update in genetics and management. Current opinion in pediatrics. 2016 Aug:28(4):476-82. doi: 10.1097/MOP.0000000000000384. Epub [PubMed PMID: 27307047]
Level 3 (low-level) evidenceIngordo V, Gentile C, Iannazzone SS, Cusano F, Naldi L. Congenital melanocytic nevus: an epidemiologic study in Italy. Dermatology (Basel, Switzerland). 2007:214(3):227-30 [PubMed PMID: 17377384]
Alper JC, Holmes LB. The incidence and significance of birthmarks in a cohort of 4,641 newborns. Pediatric dermatology. 1983 Jul:1(1):58-68 [PubMed PMID: 6679890]
Castilla EE, da Graça Dutra M, Orioli-Parreiras IM. Epidemiology of congenital pigmented naevi: I. Incidence rates and relative frequencies. The British journal of dermatology. 1981 Mar:104(3):307-15 [PubMed PMID: 7213564]
Schaffer JV. Update on melanocytic nevi in children. Clinics in dermatology. 2015 May-Jun:33(3):368-86. doi: 10.1016/j.clindermatol.2014.12.015. Epub 2014 Dec 8 [PubMed PMID: 25889140]
Kinsler VA, O'Hare P, Bulstrode N, Calonje JE, Chong WK, Hargrave D, Jacques T, Lomas D, Sebire NJ, Slater O. Melanoma in congenital melanocytic naevi. The British journal of dermatology. 2017 May:176(5):1131-1143. doi: 10.1111/bjd.15301. Epub 2017 Apr 4 [PubMed PMID: 28078671]
Vergier B, Laharanne E, Prochazkova-Carlotti M, de la Fouchardière A, Merlio JP, Kadlub N, Avril MF, Bodemer C, Lacoste C, Boralevi F, Taieb A, Ezzedine K, Fraitag S. Proliferative Nodules vs Melanoma Arising in Giant Congenital Melanocytic Nevi During Childhood. JAMA dermatology. 2016 Oct 1:152(10):1147-1151. doi: 10.1001/jamadermatol.2016.2667. Epub [PubMed PMID: 27486690]
Lyon VB. Congenital melanocytic nevi. Pediatric clinics of North America. 2010 Oct:57(5):1155-76. doi: 10.1016/j.pcl.2010.07.005. Epub [PubMed PMID: 20888464]
Krengel S, Scope A, Dusza SW, Vonthein R, Marghoob AA. New recommendations for the categorization of cutaneous features of congenital melanocytic nevi. Journal of the American Academy of Dermatology. 2013 Mar:68(3):441-51. doi: 10.1016/j.jaad.2012.05.043. Epub 2012 Sep 13 [PubMed PMID: 22982004]
Viana AC, Gontijo B, Bittencourt FV. Giant congenital melanocytic nevus. Anais brasileiros de dermatologia. 2013 Nov-Dec:88(6):863-78. doi: 10.1590/abd1806-4841.20132233. Epub [PubMed PMID: 24474093]
Flores-Sarnat L. Neurocutaneous melanocytosis. Handbook of clinical neurology. 2013:111():369-88. doi: 10.1016/B978-0-444-52891-9.00042-7. Epub [PubMed PMID: 23622187]
Stefanaki C, Soura E, Stergiopoulou A, Kontochristopoulos G, Katsarou A, Potouridou I, Rigopoulos D, Antoniou C, Stratigos A. Clinical and dermoscopic characteristics of congenital melanocytic naevi. Journal of the European Academy of Dermatology and Venereology : JEADV. 2018 Oct:32(10):1674-1680. doi: 10.1111/jdv.14988. Epub 2018 May 18 [PubMed PMID: 29633355]
Minagawa A, Koga H, Saida T. Dermoscopic characteristics of congenital melanocytic nevi affecting acral volar skin. Archives of dermatology. 2011 Jul:147(7):809-13. doi: 10.1001/archdermatol.2011.150. Epub [PubMed PMID: 21768479]
Level 2 (mid-level) evidenceJakchairoongruang K, Khakoo Y, Beckwith M, Barkovich AJ. New insights into neurocutaneous melanosis. Pediatric radiology. 2018 Nov:48(12):1786-1796. doi: 10.1007/s00247-018-4205-x. Epub 2018 Aug 3 [PubMed PMID: 30074086]
Arad E, Zuker RM. The shifting paradigm in the management of giant congenital melanocytic nevi: review and clinical applications. Plastic and reconstructive surgery. 2014 Feb:133(2):367-376. doi: 10.1097/01.prs.0000436852.32527.8a. Epub [PubMed PMID: 24469170]
Marghoob AA, Borrego JP, Halpern AC. Congenital melanocytic nevi: treatment modalities and management options. Seminars in cutaneous medicine and surgery. 2007 Dec:26(4):231-40. doi: 10.1016/j.sder.2008.03.007. Epub [PubMed PMID: 18395671]
Arneja JS, Gosain AK. Giant congenital melanocytic nevi. Plastic and reconstructive surgery. 2009 Jul:124(1 Suppl):1e-13e. doi: 10.1097/PRS.0b013e3181ab11be. Epub [PubMed PMID: 19568135]
Kinsler VA, O'Hare P, Jacques T, Hargrave D, Slater O. MEK inhibition appears to improve symptom control in primary NRAS-driven CNS melanoma in children. British journal of cancer. 2017 Apr 11:116(8):990-993. doi: 10.1038/bjc.2017.49. Epub 2017 Mar 2 [PubMed PMID: 28253523]
Küsters-Vandevelde HV, Willemsen AE, Groenen PJ, Küsters B, Lammens M, Wesseling P, Djafarihamedani M, Rijntjes J, Delye H, Willemsen MA, van Herpen CM, Blokx WA. Experimental treatment of NRAS-mutated neurocutaneous melanocytosis with MEK162, a MEK-inhibitor. Acta neuropathologica communications. 2014 Apr 8:2():41. doi: 10.1186/2051-5960-2-41. Epub 2014 Apr 8 [PubMed PMID: 24713450]
Level 3 (low-level) evidenceMir A, Agim NG, Kane AA, Josephs SC, Park JY, Ludwig K. Giant Congenital Melanocytic Nevus Treated With Trametinib. Pediatrics. 2019 Mar:143(3):. pii: e20182469. doi: 10.1542/peds.2018-2469. Epub [PubMed PMID: 30792255]
Chu WC, Lee V, Chan YL, Shing MM, Chik KW, Li CK, Ma KC. Neurocutaneous melanomatosis with a rapidly deteriorating course. AJNR. American journal of neuroradiology. 2003 Feb:24(2):287-90 [PubMed PMID: 12591651]
Level 3 (low-level) evidenceKrengel S, Hauschild A, Schäfer T. Melanoma risk in congenital melanocytic naevi: a systematic review. The British journal of dermatology. 2006 Jul:155(1):1-8 [PubMed PMID: 16792745]
Level 1 (high-level) evidenceRolland S,Kokta V,Marcoux D, Meyerson phenomenon in children: observation in five cases of congenital melanocytic nevi. Pediatric dermatology. 2009 May-Jun; [PubMed PMID: 19706090]
Level 3 (low-level) evidenceVivar KL, Kruse L. The impact of pediatric skin disease on self-esteem. International journal of women's dermatology. 2018 Mar:4(1):27-31. doi: 10.1016/j.ijwd.2017.11.002. Epub 2017 Dec 12 [PubMed PMID: 29872673]
Waelchli R, Aylett SE, Atherton D, Thompson DJ, Chong WK, Kinsler VA. Classification of neurological abnormalities in children with congenital melanocytic naevus syndrome identifies magnetic resonance imaging as the best predictor of clinical outcome. The British journal of dermatology. 2015 Sep:173(3):739-50. doi: 10.1111/bjd.13898. Epub 2015 Aug 27 [PubMed PMID: 25966033]
Level 2 (mid-level) evidence