Introduction
Colorectal cancer is the third most common diagnosis and cause of cancer-related death in both sexes in the United States.[1] Worldwide and in the United States, colon and rectal cancers are the second most common cause of cancer-related mortality.[2] Incidence rates have been decreasing in Western countries, mostly due to the widespread use of colonoscopy screening. However, the condition's incidence among younger adults is increasing.[3]
Most colon cancer is sporadic, and approximately 5 percent are due to an inherited genetic mutation, mostly due to Lynch syndrome (hereditary nonpolyposis colon cancer or HNPCC) and familial adenomatous polyposis (FAP). The transition from normal colon epithelium to invasive cancer takes several years and most commonly follows a sequence characterized by the accumulation of genetic mutations, adenoma formation, and subsequent carcinogenesis (adenoma-carcinoma sequence).[4][5][6] Certain cancers may follow alternative pathways, such as those involving DNA mismatch repair (MMR) and the BRAF gene.[7]
Colon cancer screening is recommended and may be performed using various modalities. Screening initiation and follow-up guidelines vary among organizations.[8] Colon cancer diagnosis requires a tissue biopsy, usually obtained via colonoscopy. All newly diagnosed colon cancers should be screened for common genetic mutations, and a complete colonoscopy and baseline carcinoembryonic antigen (CEA) should be performed. Most patients with invasive cancer require a baseline chest and abdominopelvic computed tomography (CT) scan.[9]
Surgical resection is the main modality for localized early-stage colon cancer. The most important prognostic indicator is the pathological stage. Staging dictates the need for further therapy, which may include chemotherapy, immunotherapy, or, rarely, radiation. Surveillance after treatment is crucial in detecting metastatic disease and local recurrence, which may be curable with multimodality therapy. Palliative systemic therapy is reserved for nonresectable or widely metastatic disease to improve quality of life and survival.[10][11][12]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Most colon cancer is sporadic (70%). Conditions with known inherited genetic mutations, such as HNPCC and FAP, account for 3% to 5% of cases. About 20% to 25% of patients have a strong colon cancer family history but no identifiable inherited mutation.
Risk factors for developing colon cancer include the following:
- Age: The median age of diagnosis in sporadic colon cancer is over 65 years.
- Family history: Colon cancer in a family member increases one's risk of developing the disease.
- Inherited colon cancer-related mutations: HNPCC, FAP, and Peutz-Jegher polyposis are genetic conditions that increase a person's colon cancer risk.[13][14]
- Adenomas on screening colonoscopy: The risk of cancer is most significant with villous adenomas and sessile serrated polyps.
- History of inflammatory bowel disease (IBD): Ulcerative colitis has an estimated annual incidence of 0.5% within the first 10 to 20 years following the diagnosis of IBD, increasing to 1% per year after that. Crohn disease may increase cancer risk if present in the ileocolic region.[15]
- Environment and Lifestyle factors: Alcohol consumption, cigarette smoking, obesity, diets rich in processed red meat, insulin resistance, history of prior radiation, and immunosuppression all increase the risk of this malignancy.[16][17]
Factors protective against colon cancer development have been studied in extensive population-based studies and include the following:
- Physical activity
- Diets rich in fruits, vegetables, fiber, resistant starch, and fish
- Supplementation with folate, folic acid, pyridoxine, calcium, vitamin D, and magnesium
- Garlic
- Coffee
- Medications, particularly aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs), hormonal replacement therapy in postmenopausal, statins, bisphosphonates, and angiotensin inhibitors
The data to support these factors are of variable quality.[18]
Epidemiology
Colorectal cancer is the third most common cancer in the United States. From the Surveillance, Epidemiology, and End Results (SEER) database estimates, over 153,000 new colorectal cancers were diagnosed in 2023, accounting for 7.8% of all newly diagnosed cancers. Roughly 70% of these malignancies are colonic, while rectal tumors comprise the rest. Men are more likely to develop colon cancer (53%) and at a younger age than women (68 vs. 72 years). The condition's incidence continuously decreases, but the rate of decline has slowed recently. An increasing proportion of newly diagnosed cancers occur in individuals younger than age 55.[19] The proportion of right-sided colon cancers and rectal cancers has also increased.
Racial and socioeconomic disparities contribute significantly to both the incidence and prognosis, with the highest rates noted among Alaskan natives and African-Americans. Global rates of colorectal cancer vary significantly by population, race, and socioeconomic status, with the highest rates reported among the more developed countries.
Pathophysiology
The transition from normal colonic epithelium to dysplasia involves genetic changes that accumulate over time, eventually leading to carcinoma. Colon cancer can develop through 3 main genetic pathways: chromosomal instability (CIN), MMR, and the CpG island methylator phenotype (CIMP).[20] These pathways are not mutually exclusive but significantly overlap.
The CIN pathway, initially described as the classical adenoma-carcinoma sequence, is characterized by a mutation gain, resulting in an imbalance between oncogene and tumor suppressors. Common mutations included APC, KRAS, and TP53. APC mutations often incite carcinogenesis and are found in roughly 60% of colon cancers. Normal APC binds to β-catenin and controls the Wnt-signalling pathway. APC mutations cause aberrations in this pathway, deregulating growth, apoptosis, and cell differentiation. KRAS and BRAF mutations are also seen in CIN tumors but are not exclusive to this pathway.[21]
MMR is characterized by MLH1, MSH2, MSH6, or PMS2 gene mutations, resulting in DNA replication error accumulation. Microsatellite instability, an MMR byproduct, may be identified in tissue specimens. Germline MMR mutations are characteristic of HNPCC. However, most microsatellite instability-high (MSI-high) tumors are sporadic. MSI-high lesions are often right-sided, have poorly differentiated or mucinous histology, and are relatively 5-FU resistant. These malignancies are more sensitive to immunotherapy than microsatellite-stable tumors.
The CIMP pathway is implicated in roughly 15% of colon cancers and is characterized by CpG island hypermethylation. CIMP-related tumors tend to arise in serrated polyps rather than classical adenomas and are often associated with KRAS and BRAF mutations. When seen with MSI, the prognosis is significantly better than hypermethylated non-MSI-high tumors.[22]
Recently, several gene-expression-based classification systems were analyzed and categorized into 4 major molecular subtypes.[23]
- CMS1 (MSI-immune, 14%), hypermutated burden, dMMR, microsatellite unstable and immune solid activation
- CMS2 (canonical, 37%), high chromosomal instability, epithelial, marked WNT and MYC signaling activation
- CMS3 (metabolic, 13%), epithelial and evident metabolic dysregulation, KRAS mutation
- CMS4 (mesenchymal, 23%), CpG hypermethylation, prominent transforming growth factor-β activation, stromal invasion, and angiogenesis.
This classification allows an elegant understanding of interconnected gene expression but is not currently widely used in clinical practice.
Histopathology
Colon adenocarcinoma is the predominant colonic malignancy (>90%), with neuroendocrine and gastrointestinal stromal tumors and lymphomas making up most of the rest. The pathologic diagnosis is often made on biopsy and further refined based on final pathology if the patient undergoes definitive resection. Synoptic colon cancer pathology reporting is the currently accepted standard by most pathologists, and several of the most important features are listed below.[24]
- Specimen integrity: Intact specimens facilitate a definitive pathological diagnosis, particularly regarding margins and invasion depth. Piecemeal or fragmented tissue is less likely to contain this information. Complete surgical resection (R0) with all negative circumferential resection margins (CRM) must be achieved to avoid local and distant recurrence (38% CRM+ versus 10% CRM-) and increase survival.
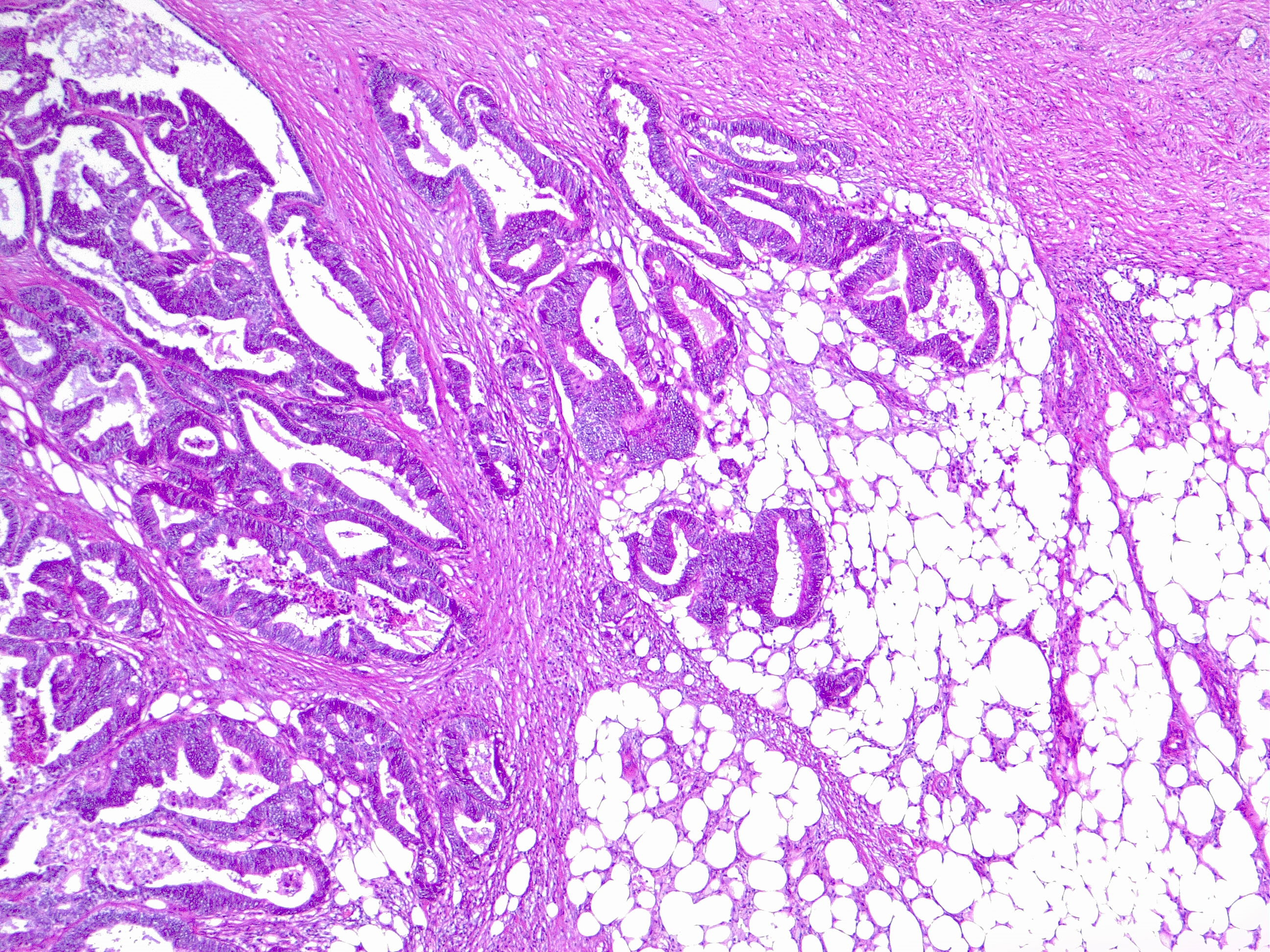
- Histologic subtype: Adenocarcinomas may be further differentiated histologically by the predominant cell morphology (see Image. Colon Adenocarcinoma). Variants include cribriform comedo-type, medullary, micropapillary, serrated, mucinous, and signet-ring cells.
- Histologic grade: Adenocarcinomas are graded by the degree of gland formation into well (> 95%), moderately (> 50%), and poorly (< 49%) differentiated tumors. Undifferentiated tumors show no evidence of any discernible gland formation or squamous differentiation. Cytokeratin 20 (CK20) and caudal-type homeobox 2 (CDX2) immunohistochemistry (IHC) can accurately identify cells of colonic origin, except medullary-type carcinomas.
- Tumor extent: This parameter describes the degree of invasion into the colonic wall and allows T stage calculation.
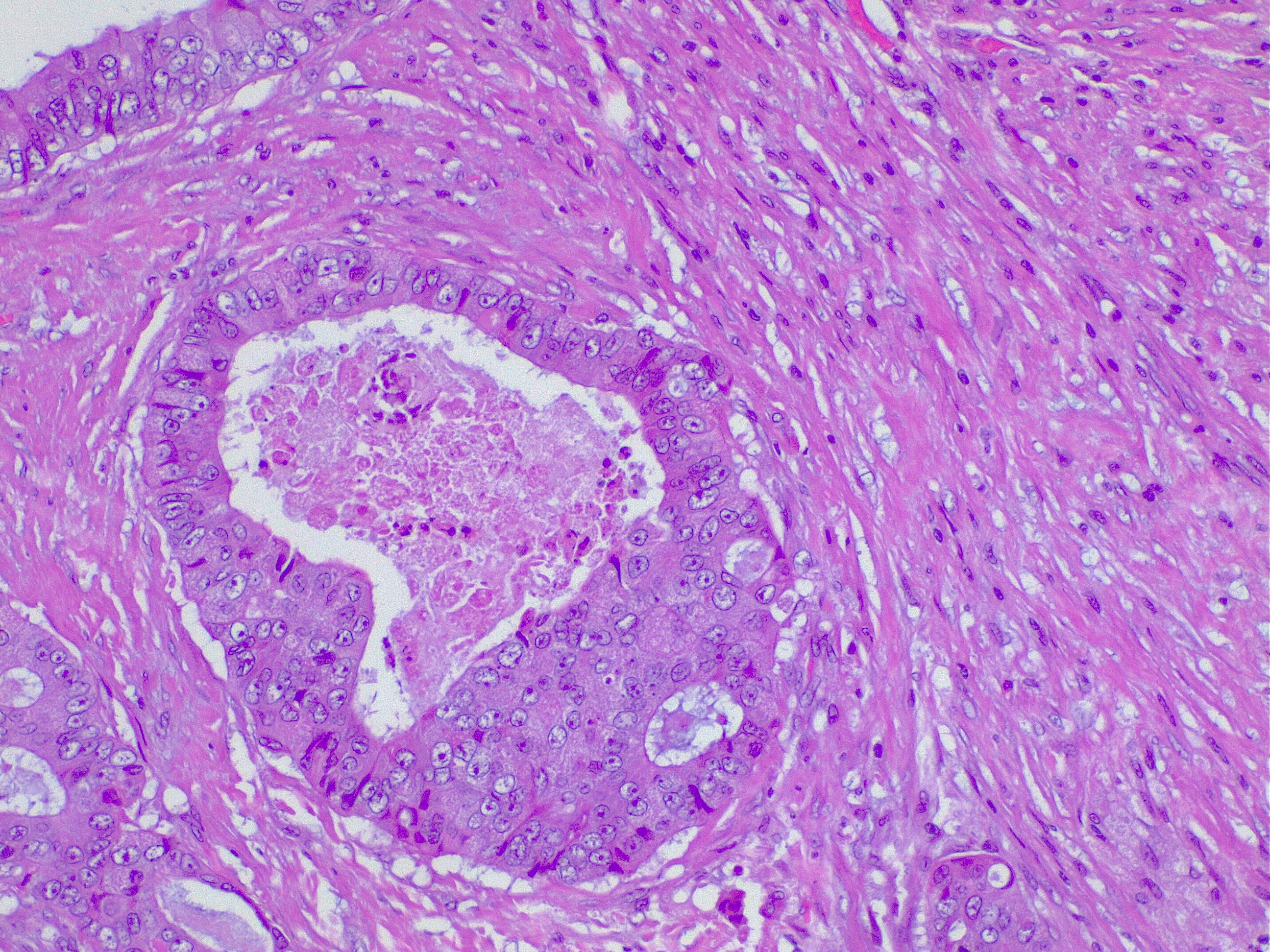
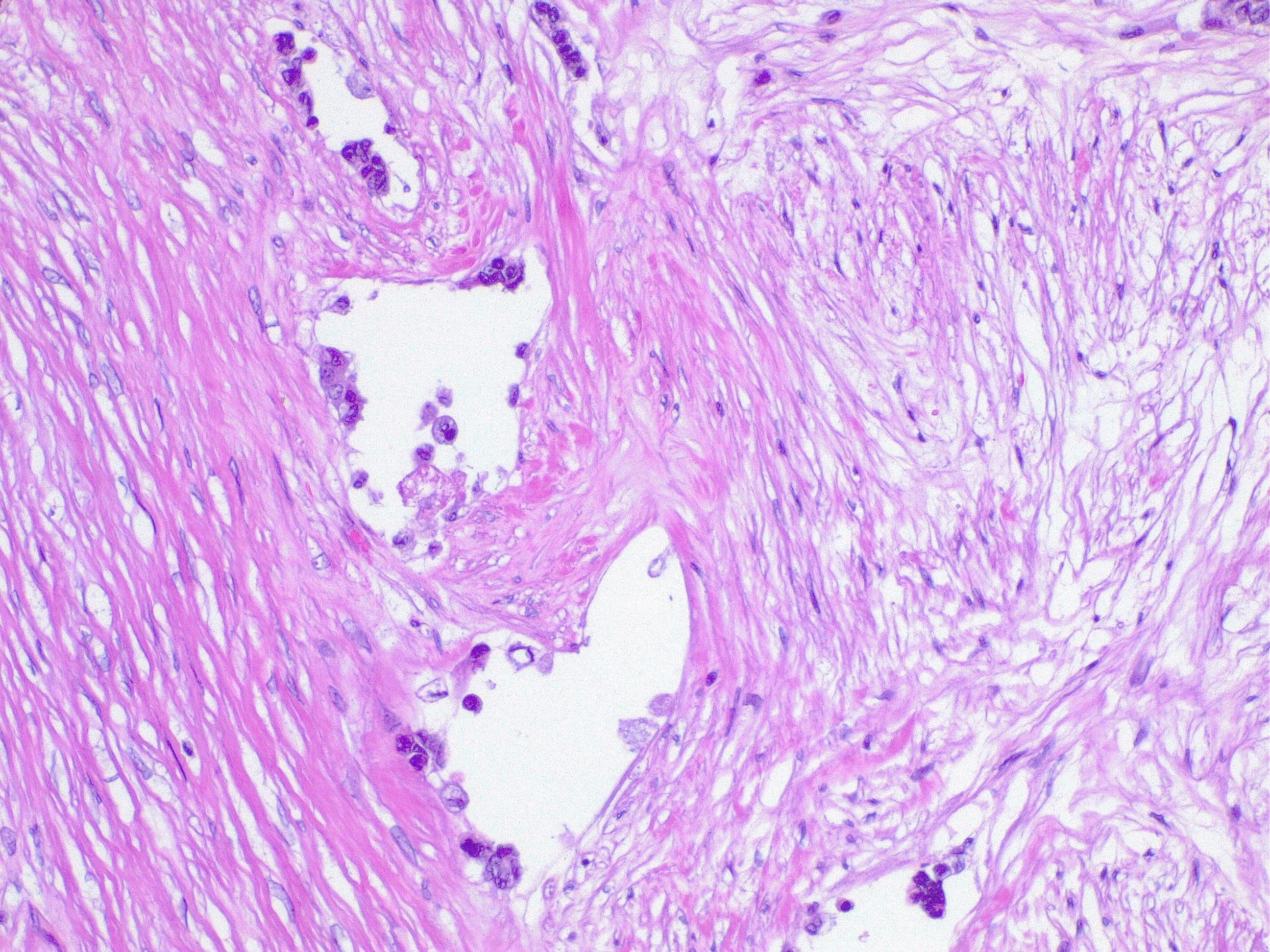
- Angiolymphatic invasion: Tumor cells invading blood vessels or lymphatics are an independent poor prognostic factor (see Image. Lymphatic Invasion in Colorectal Cancer). Angiolymphatic invasion in the polypectomy specimen may strengthen the recommendation of an oncologic resection. This condition may be a factor that encourages adjuvant chemotherapy if found in early-stage colon cancer.
- Perineural invasion: The presence of tumor cells invading the nerve sheath is also a poor prognostic factor.[25]
- Tumor budding: Tumor budding is defined as the presence of malignant cells at the tumor edge. This phenomenon has been increasingly found to correlate with local tumor recurrence, lymph node spread, and distant metastases.[26]
Histopathological evaluation may provide insights into the tumor's response to specific treatments.
History and Physical
With the advent of screening colonoscopy, most colon cancers are now caught asymptomatic. When symptomatic, the manifestations and their severity depend on the tumor's location and size. Symptoms that trigger diagnostic colonoscopies are rectal bleeding (37%), abdominal pain (34%), and anemia (23%). Right-sided tumors often present with anemia, while left-sided lesions cause defecation disturbances. Patients can present with acute surgical emergencies, usually due to tumor-related obstruction, perforation, or bleeding. Advanced disease may present with various symptoms, depending on the organ of spread.
A general physical exam should focus on signs of metastatic disease in all patients, with exams tailored to the patient's presentation. A focused abdominal exam should be performed to evaluate for tenderness, palpable masses, hernias, previous scars, and organomegaly. A rectal exam is critical and must not be missed in all patients with suspected gastrointestinal malignancy. In addition to examining for signs of malignancy, rectal exams are invaluable in providing details about sphincter tone and continence.
Evaluation
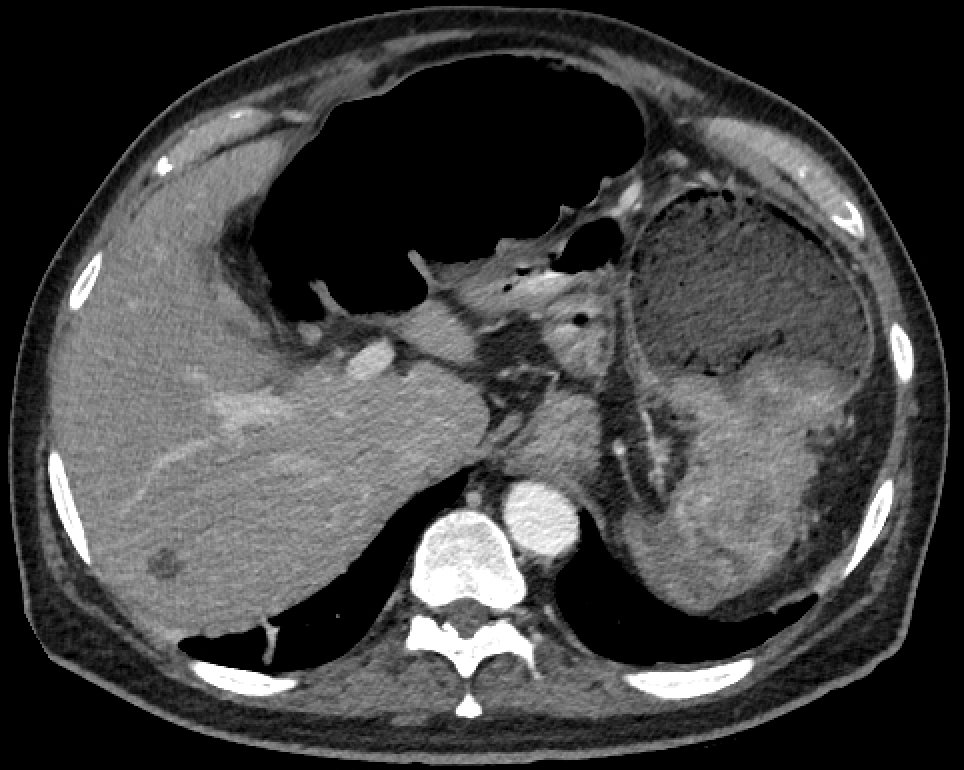
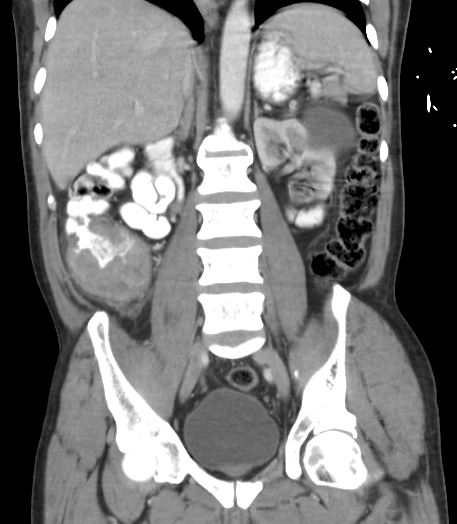
Initial evaluation may involve barium enema or CT colonography (see Images. Colon Cancer and Right-Sided Colon Cancer on Computed Tomography). Ultimately, a colonoscopy is required for a tissue diagnosis.[27][28][29] Colonoscopy sensitivity is about 94.7% if performed by an experienced operator and with good bowel preparation. (95% CI 90% to 97%). Around 2% to 6% of lesions may be missed, especially right-sided, sessile, and flat lesions. Colonoscopy should include multiple biopsies of the suspected lesion and tattooing of the peritumoral colon to facilitate intraoperative identification. Routine laboratory workups should include complete blood count, iron studies, a basic metabolic panel, liver function tests, and coagulation tests. Iron deficiency anemia is sometimes seen due to chronic tumoral bleeding. CEA is the most commonly used tumor marker for colon cancer and should be measured at baseline. High CEA levels correlate with poor prognosis, and the CEA levels after treatment are useful in detecting disease recurrence.
The National Comprehensive Cancer Network (NCCN) recommends that all patients diagnosed with colon cancer should undergo universal testing for MMR/MSI status, which impacts approximately 15% to 20% of sporadic colorectal cancer cases. KRAS, NRAS, HER2, and BRAF mutation testing is recommended for patients with nonresectable metastatic colon cancer. Chest, abdomen, and pelvic CT scans are recommended for all patients diagnosed with colon cancer. Magnetic resonance imaging (MRI) is typically used only if liver metastases are suspected or if the patient is allergic to iodinated contrast. A positron emission tomography-CT scan is not routinely indicated.
Treatment / Management
Surgical resection is the standard of care in resectable colon cancer. The resection type, lymphadenectomy extent, and specific techniques depend on the location and stage of the tumor. Adjuvant chemotherapy is indicated in a subset of more advanced colon cancers. Patients with metastatic disease are managed with systemic therapy. Radiation is seldom used in colon cancer. In some cases, surgical procedures may be employed to provide palliative relief for patients with advanced colon cancer.
Differential Diagnosis
The differential diagnosis of colon cancer includes the following conditions:
- Arteriovenous malformation: can cause rectal bleeding
- Carcinoid tumors: may develop from colonic mucosal neuroendocrine cells, varying in terms of size, location, and aggressiveness. Carcinoid colonic tumors often present with nonspecific symptoms, such as abdominal pain, changes in bowel habits, rectal bleeding, or signs of bowel obstruction. However, some patients may remain asymptomatic, and the tumors may be incidentally discovered during colonoscopy.
- Crohn disease: can manifest with bowel inflammation and strictures resembling malignancy
- Gastrointestinal lymphoma: may present with bowel obstruction or mass lesions
- Ischemic bowel: can cause abdominal pain and bloody stools
- Ileus: characterized by bowel obstruction and lack of peristalsis and may produce symptoms similar to those seen in advanced colon cancer
- Small intestine carcinomas: can present with nonspecific symptoms, such as abdominal pain and bowel obstruction, similar to colon cancer
- Small intestine diverticulosis: can cause abdominal pain and bleeding and is associated with changes in bowel habits
- Ulcerative colitis: symptoms may overlap with those of malignancy
A thorough clinical evaluation and diagnostic workup can differentiate colon cancer from these conditions.
Surgical Oncology
Surgical interventions are pivotal in colon cancer management, addressing both diagnostic and therapeutic aspects of the disease. The primary goal of surgical procedures for colon cancer is to achieve complete tumor removal while preserving optimal bowel function and minimizing complications. Emergent operations like fecal diversion or stent placement may be necessary to provide obstruction relief before definitive resection.
Endoscopic Resection
Endoscopic resection is typically performed by colonoscopy with options including polypectomy, endoscopic mucosal resection, and endoscopic submucosal dissection.[30] Endoscopy is usually reserved for pedunculated polyps, where any of these techniques may be used to excise the entire polyp intact and with a margin of healthy tissue. Often, the final pathology determines the necessity of further operations. Tumors with greater invasion than T1, large size, or poor histological features should have an oncologic surgical resection with a lymphadenectomy for final staging. Patients undergoing polypectomy should also be agreeable to close surveillance. Major complications of endoscopic techniques are perforation (6.5%) and bleeding (1.5%). However, mortality is extremely low at 0.8%.[31] All lesions should be tattooed to allow site identification on further required steps regardless of the procedure[32]
Surgical Resection
The goal of surgery is the diseased colonic segment’s en bloc removal with 5-cm margins and lymphadenectomy (mesocolic excision). A minimum of 12 lymph nodes is required for adequate staging.[9] The tumor must be precisely located before surgery, as location dictates the type and extent of colonic resection. Colonoscopic determination of the involved colonic segment is sometimes tricky. Lesion tattooing and preoperative imaging can help intraoperative localization. A primary intestinal anastomosis usually follows elective colon resection. Fecal diversion is generally reserved for emergent operations. Open, laparoscopic, or robotic-assisted surgery are accepted treatment options based on patient factors and surgeon experience. Regardless of the technique, the same oncologic principles apply.[33]
Choice of surgery by anatomic tumor location
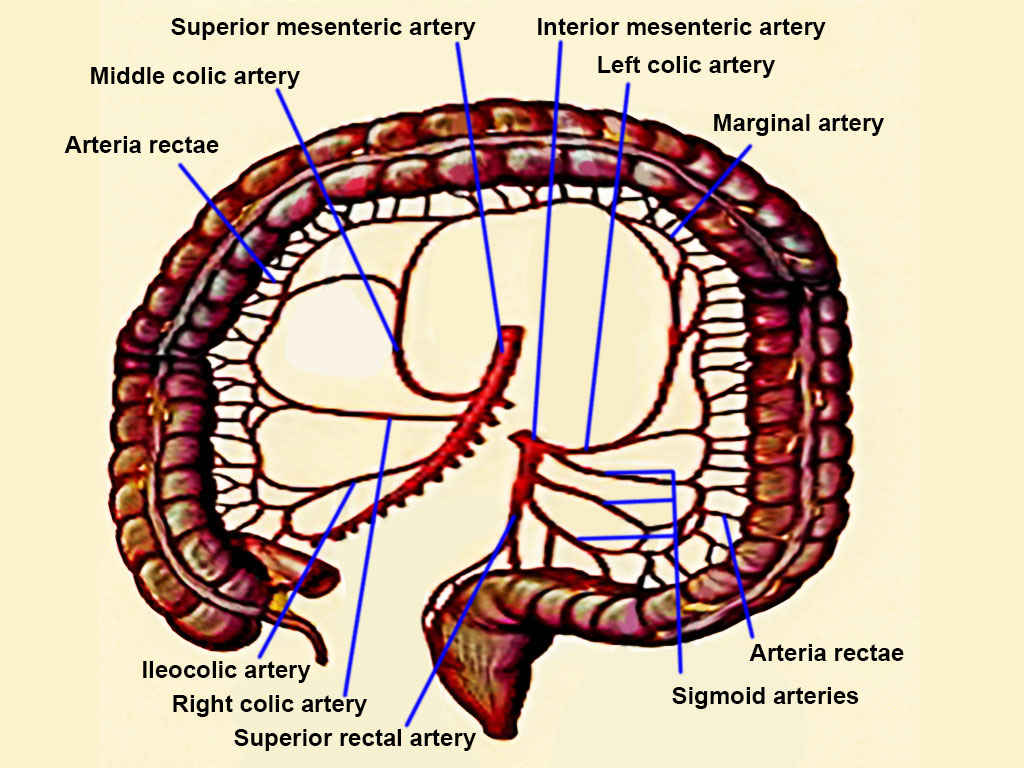
- Sigmoid colon: Sigmoid colon cancers are treated with sigmoid colectomy. Low sigmoid or rectosigmoid lesions may require upper rectal third excision. High inferior mesenteric artery ligation or selective ligation of the superior hemorrhoidal or left colic artery after branching off may be performed based on the lesion’s characteristics and the surgeon's preference (see Image. Colon Arteries). Proximal vessel ligation aims to obtain an adequate lymph node yield. This procedure risks ureteral and gonadal vessel injury. Preoperative ureteral stenting may help identify the ureter in cases where the dissection is expected to be more complex. Anastomosis is created between the proximal and distal ends of the colon after tumor resection using a side-to-side or end-to-end technique. Mobilization of the colon’s splenic flexure may be required to facilitate tension-free anastomosis.
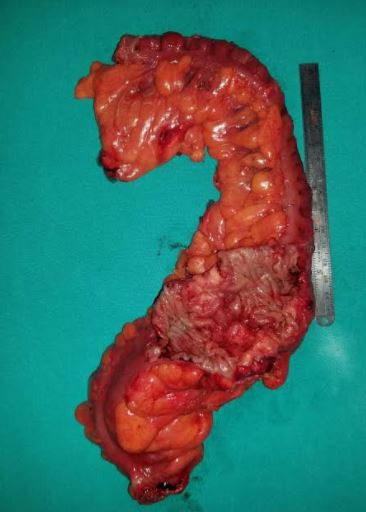
- Splenic flexure and left colon lesions: Lesions in these segments are best managed with a left colectomy (see Image. Left Hemicolectomy Specimen). The left colic artery is ligated at its origin from the inferior mesenteric artery. Occasionally, the inferior mesenteric artery might be ligated at its root, with the sigmoid colon also often excised. The middle colic artery's left branch is typically ligated proximally, followed by creating an anastomosis between the transverse and sigmoid colon. In most cases, the inferior mesenteric vein is also ligated at the Treitz ligament.
- Transverse colon lesions: Midtansverse colon tumors are typically managed with a transverse colectomy, involving ligation of the middle colic artery at its root and creating an anastomosis between the ascending and descending colon. However, this procedure can be challenging if the transverse colon lacks redundancy, requiring mobilization of both the ascending and descending colon. For proximal or distal transverse colon cancers, extended right or left colectomy is often preferred. The underlying duodenum and pancreas are at risk of injury with these procedures, especially if the tumor is bulky.
- Right colon and hepatic flexure tumors: Lesions in these segments are typically managed with a right or extended colectomy. The ileocolic pedicle, right colic artery, and middle colic artery’s right branch are often ligated at their origins. The proximal end of the resection is at the terminal ileum, usually 5 to 10 cm proximal to the ileocecal valve. The middle colic artery may be sacrificed at its origin for bulkier hepatic flexure lesions, necessitating an ileum-to-distal transverse colon or left colon anastomosis.
- Emergent operations: Urgent or emergent surgical intervention may be required for obstruction, bleeding, or volvulus. In such situations, oncologic resection should be performed at the initial operation if possible. An anastomosis is often avoided, especially if the patient has intraabdominal fecal contamination, sepsis signs, malnutrition, or a high risk of poor wound healing. These cases require either a proximal diverting ileostomy or an end colostomy based on the tumor’s location. An alternative for patients with obstructing colon cancer is placing a temporary intraluminal stent, allowing for colonic decompression and a complete colonoscopy before a semielective colon resection.
Alternative surgical approaches may be required for individuals with hereditary polyposis syndromes, which are discussed at more length in the individual chapters.
Medical Oncology
Adjuvant therapy is crucial when managing colon cancer, reducing recurrence risk and improving overall survival rates. Interventional strategies are tailored to the disease stage and patient risk factors. Close monitoring and treatment adherence are essential to optimize outcomes and minimize potential side effects.
Adjuvant Therapy
Adjuvant chemotherapy has no role in stage I colon cancer management. Patients with low-risk stage II colon cancer can opt for adjuvant therapy-free surveillance, individualized medical oncology discussions, or clinical trial enrollment. Current guidelines from the American Society of Clinical Oncology (ASCO), European Society for Medical Oncology (ESMO), and NCCN strongly recommend adjuvant therapy for selected stage II cancers with high-risk features, ie, those with proficient DNA mismatch repair or microsatellite stability (pMMR/MSI-S) disease, and all stage III (node-positive) tumors.
For colon cancer adjuvant therapy, 3-year disease-free survival (DFS) is an acceptable surrogate marker for 5-year overall survival recognized by the FDA based on data from the Adjuvant Colon Cancer End Points (ACCENT) study group. Guidelines advocate delivering adjuvant chemotherapy within 6 to 8 weeks of surgical resection pending the patient’s recovery.
A meta-analysis revealed that extending the time to adjuvant chemotherapy beyond 4 weeks was linked to a notable decrease in both overall survival (hazard ratio 1.14; 95% confidence interval 1.10-1.17) and DFS (hazard ratio 1.14; 95% confidence interval 1.10-1.18). In terms of chemotherapy regimens, an oxaliplatin-based combination is preferred over monotherapy, whether it be 5-fluorouracil/leucovorin (5-FU/LV), as demonstrated in the Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) and the National Surgical Adjuvant Breast and Bowel Project (NSABP) C-07 trials, or capecitabine, as shown in the Xeloda in Adjuvant Colon Cancer Therapy (XELOXA) trial.
The standard duration of adjuvant therapy remains 6 months, although ongoing investigation in the International Duration Evaluation of Adjuvant Chemotherapy (IDEA) trial is exploring the potential noninferiority of a 3-month regimen for low-risk patients or those experiencing oxaliplatin’s dose-limiting neurotoxicity. Administering a full weight-based chemotherapy dosing is recommended, especially in cases where the intention is curative.[34][35][36]
Adjuvant fluorouracil-based chemotherapy became the standard treatment for stage III colon cancer in the early 1990s following the groundbreaking NSABP C-01 trial. This trial was the first randomized clinical trial to demonstrate a significant improvement in DFS and overall survival. Subsequent evidence from the NSABP C-03 trial showed that 6 months of 5-FU/LV therapy resulted in even better DFS and overall survival at 3 years compared to the NSABP C-01 regimen of lomustine, vincristine, and 5-FU. For over a decade, 5-FU/LV remained the standard adjuvant therapy. However, the landscape shifted when combinations containing oxaliplatin, irinotecan, and capecitabine (an oral fluorouracil prodrug) were introduced for the treatment of advanced colorectal cancer.
Oxaliplatin was approved as part of adjuvant treatment for stage III colon cancer after the 2004 MOSAIC trial. Investigators randomly assigned patients who had undergone curative colon resection for stage II (40%) or III (60%) to receive 5-FU/LV alone (leucovorin 200 mg/m as a 2-hour infusion, followed by a 5-FU bolus 400 mg/m, and then a 22-hour infusion of 5-FU 600 mg/m, every 2 weeks) or with oxaliplatin (5-FU/LV with 85 mg/m day 1 every 14 days, a regimen termed Fluorouracil, Leucovorin, and Oxaliplatin regimen version 4 or “FOLFOX4”) for 6 months.
FOLFOX4 therapy significantly improved 5-year DFS rates to 73.3% compared to 67.4% in the 5-FU/LV group (hazard ratio = 0.80; 95% confidence interval, 0.68 to 0.93; P = .003) and 6-year overall survival rates were 78.5% and 76.0% in the FOLFOX4 and 5-FU/LV groups, respectively (hazard ratio = 0.84; 95% confidence interval, 0.71 to 1.00; P = .046). Stratified, 6-year overall survival rates for patients with stage III disease were 72.9% and 68.7%, respectively (hazard ratio = 0.80; 95% confidence interval, 0.65 to 0.97; P = .023) but no significant difference in overall survival was seen in the stage II population. The 10-year follow-up on overall survival corroborated the aforementioned trend results. Neuropathy was seen in 92% of patients who received FOLFOX4, with 13% being severe but mostly reversible.
United States oncologists further modified the regimen, including FOLFOX6 (a single-day regimen) and FOLFOX7 (no 5-FU bolus). In a retrospective review of unselected patients with stage II disease, the 5-year DFS benefit for FOLFOX compared with 5FU/LV alone was not statistically significant (84% compared to 80%, hazard ratio for recurrence 0.84, p = 0.26). However, survival significantly exceeded 5% (82% versus 77%) for patients with stage II tumors with high-risk clinical features such as poor histological grade, T4, perforation/obstruction, less than 10 lymph nodes sampling, perineural invasion (PNI), and lymphovascular invasion (LVI).
Other trials in 2011 confirmed the value of oxaliplatin-based regimens as a component of adjuvant chemotherapy for stages II and III colon cancer, surpassing the effectiveness of 5-FU/LV monotherapy. The NSABP C-07 phase III clinical trial evaluated the impact on DFS of adding oxaliplatin to bolus weekly 5-FU/LV as surgical adjuvant therapy for stage II (29%) and III (71%) colorectal adenocarcinoma patients. After intent of curative resection, patients were randomly assigned to 1 of 2 regimens. The first is 5-FU (500 mg/m2 IV) bolus weekly for 6 weeks plus leucovorin (500 mg/m2 IV) weekly for 6 weeks during each 8-week cycle for 3 cycles. The other has the same 5-FU/LV regimen with oxaliplatin (85 mg/m2 IV) administered on weeks 1, 3, and 5 of each 8-week cycle for 3 cycles (the fluorouracil/leucovorin/oxaliplatin or “FLOX” regimen).
In the NSABP C-07 trial, the 8-year overall survival was similar between treatment groups (hazard ratio, 0.88; 95% confidence interval, 0.75 to 1.02; p = 0.08). FLOX remained superior for DFS (hazard ratio, 0.82; 95% confidence interval, 0.72 to 0.93; p = 0.002). The effect of oxaliplatin on overall survival did not differ by stage of disease (stage II compared to stage III = 0.38 for overall survival; 0.37 for DFS) but did vary by age (younger than 70 compared to older than 70; HR = 0.39) with a similar trend for DFS (p = 0.073). Oxaliplatin significantly improved overall survival in patients younger than 70 (hazard ratio, 0.80; 95% confidence interval, 0.68 to 0.95; p = 0.013). However, no positive effect was seen in older patients. FLOX regimen was associated with higher hospitalization rates for diarrhea (5.5% compared to 3%), and a total of 1.2% of patients died of any cause within 60 days of receiving chemotherapy, with no significant difference between regimens.
The XELOXA trial was a multicenter, randomized trial comparing capecitabine plus oxaliplatin (XELOX) with bolus 5-FU/LV as adjuvant therapy for individuals with stage III colorectal cancer. Patients were randomly assigned to XELOX (Oxaliplatin 130 mg/m2 on day 1 plus Capecitabine 1000 mg/m2 twice daily on days 1 to 14 every 3 weeks for 24 weeks) or the standard bolus 5-FU/LV adjuvant regimen. XELOX improved DFS and overall survival compared with bolus 5-FU/LV in patients with resected stage III colorectal cancer after a median follow-up of almost 7 years (DFS 63% compared to 56%, hazard ratio, 0.80; 95% confidence interval; 0.69; 0.93; p = 0.0045 and OS 73% compared to 67%, hazard ratio, 0.83; 95% confidence interval, 0.70 to 0.99; P = .04).
XELOX was associated with more neurotoxicity, hand-foot syndrome, and thrombocytopenia. Patients on capecitabine are advised not to take a proton pump inhibitor to avoid increasing gastric pH and lowering absorption. Another trial established that 6 months of oral capecitabine monotherapy is a safe and equally effective alternative to conventional 5-FU/LV for stage III colon cancer.
Adjuvant chemotherapy may be helpful for selected patients with stage II high-risk disease. The largest study of Stage II colorectal cancer, the UK QUick And Simple And Reliable (QUASAR) trial, randomly assigned nonstratified patients (eg, high-risk and low-risk) to receive 5-FU/LV with or without levamisole (later removed) as adjuvant chemotherapy following surgical resection. The relative risk of death from any cause with chemotherapy versus observation alone was 0.82 (95% confidence interval 0.70 to 0.95; p=0.008) and for recurrence relative risk 0.78 (0.67-0.91; p=0.001) after a 10-year follow-up. The absolute survival improvement with 5-FU/LV was 3.6%. However, the trial’s quality has been questioned due to a poor median of 6 lymph node dissection (> 60% with < 12 lymph nodes), a group of patients received radiation therapy (14%) or portal infusion chemotherapy (6%), which does not meet current standards.
The ACCENT retrospective analysis has demonstrated a trend toward an improved 10-year DFS and overall survival for stage II disease with chemotherapy compared to observation, regardless of the risk feature with high-risk disease (51% compared to 35%). The identification of high-risk prognostic factors may help identify patients at higher relapse risk who will more likely benefit from adjuvant therapy. Datasets confirmed that patients with stage II colorectal cancer without high-risk features and MSI-H/dMMR have an excellent prognosis and do not need adjuvant chemotherapy. However, the need for adjuvant chemotherapy in patients with high-risk disease remains uncertain.
Irinotecan-based regimens are effective in palliative therapy for advanced colorectal cancer. However, for unknown reasons, none of the 3 major phase III trials (Cancer and Leukemia Group B 89803 or CALGB89803, Pan-European Trials in Alimentary Tract Cancers 3 or PETACC-3, and Adjuvant Colorectal Cancer Endpoints or ACCORD) using irinotecan combination regimens with 5-FU/LV has demonstrated that the regimen confers significantly superior 3-year DFS when compared with 5-FU/LV alone.
The roles of novel targeted agents, bevacizumab (an antibody against vascular endothelial growth factor) and cetuximab (an antibody against epidermal growth factor receptor) have been investigated in the adjuvant setting in large phase III trials. Three major trials have failed to demonstrate any benefit of adjuvant bevacizumab with FOLFOX (NSABP C-08), XELOX (Avastin in Adjuvant Treatment of Colon Cancer or AVANT trial) or Capecitabine (QUASAR2). Cetuximab was also tested as part of adjuvant therapy for stage III colon cancer alongside the FOLFOX regimen. However, this agent did not enhance outcomes, even in cases of preselected KRAS wild-type tumors, and proved harmful to patients with KRAS mutations. Cetuximab's lack of efficacy was later confirmed in the PETACC-8 trial on patients with RAS and BRAF wild-type stage III colon cancer.
Based on prior clinical trial results, the standard adjuvant chemotherapy for stage III colon cancer is an oxaliplatin-containing regimen (FOLFOX, XELOX, or FLOX) administered for 6 months. Capecitabine or 5-FU/LV monotherapy should be reserved for patients not considered optimal candidates for oxaliplatin (eg, those with pre-existing neuropathy). Stage III colon cancer patients with MSI-high/dMMR tumors are intrinsically resistant to adjuvant 5-FU monotherapy, requiring the addition of oxaliplatin to overcome the resistance and impact outcomes. Patients with Stage II colon cancer and any high-risk combination should discuss the risks and benefits of adjuvant therapy. Retrospective analysis from trials did not document a significant improvement with the addition of oxaliplatin to 5-FU/LV for patients older than 70, and only younger patients had a statistically significant DFS, overall survival, and time to tumor recurrence.
Currently, irinotecan-based and targeted therapies have no indication in colon cancer adjuvant therapy outside of investigational trials. No conclusive evidence exists to recommend standard radiation therapy alone or combined chemoradiotherapy in the adjuvant setting. Two web-based validated tools are available to calculate the relative risk of disease recurrence and mortality based on clinicopathological features and potential adjuvant benefits (an ACCENT tool by the Mayo Clinic and Adjuvant! Online). Furthermore, gene profiling by Oncotype DX Colon, Colorectal Cancer Assay (ColDX) or ColoPrint are currently used in clinical practice to assist in risk assessment. However, these tools have no valid predictive value and are thus not recommended by clinical practice guidelines.
Neoadjuvant Therapy
The role of preoperative chemotherapy for patients with colorectal cancer is currently being investigated in a phase III Fluoropyrimidine Oxaliplatin and Targeted Receptor Preoperative Therapy (FOxTROT) trial (National Clinical Trial 00647530 or NCT00647530) after the pilot study showed promising results. The FOxTROT collaborative group randomized candidates with locally advanced resectable lesions (T3 with > 5 mm invasion beyond the muscularis propia and T4) to 2 regimens. The first is 3 preoperative FOLFOX cycles (Oxaliplatin; 85 mg/m2, Leucovorin; 175 mg, Fluorouracil; 400 mg/m2 bolus, then 2400 mg/m2 via 46-hour infusion), surgery, and 9 additional cycles. The second is surgery and 12 adjuvant cycles. Findings favored neoadjuvant therapy, showing significant improvements in tumor regression (31% compared to 2%), node-positive (1% compared to 20%), and negative margins (4% compared to 20%), resulting in significant downstaging (p=0.04).
Systemic Therapy
More than half of all patients with colorectal cancer develop metastasis, with most tumors metastasizing to the liver (80% to 90%). The prognosis for advanced nonresectable and metastatic colon cancer with best supportive care is poor, with a median overall survival (mOS) of 5 to 6 months. However, a subset of patients with oligometastatic hepatic or pulmonary involvement are potentially curable with perioperative chemotherapy. Systemic therapy’s goals for advanced nonresectable and metastatic colon cancer are symptom palliation, quality of life enhancement, and survival prolongation.
Systemic therapy for colon and rectal cancers is addressed jointly by clinical research and practice. In the 1990s, 5-FU/LV monotherapy was the standard first-line therapy, with an approximate mOS of 12 months. Adding oxaliplatin and irinotecan to the 5-FU/LV backbone in the early 2000s resulted in a mOS improvement to nearly 24 months. The introduction of biologic agents, including monoclonal antibodies (MAbs) targeting vascular endothelial growth factors (VGFRs) and epidermal growth factor receptor (EGFR), further enhanced the efficacy of systemic medical therapy, with mOS reaching 36 months and an impressive 20% 5-year overall survival even at a metastatic stage after exposure to multiple lines of therapy.
The current availability of 9 antineoplastic classes and over a dozen drugs for metastatic colorectal cancer offers numerous therapeutic possibilities, with varied combinations and sequences not yet established. These agents include fluoropyrimidines, capecitabine, S-1, tegafur plus uracil (UFT), irinotecan, oxaliplatin, anti-EGFRs (ie, cetuximab or panitumumab), anti-VEGFRs (ie, bevacizumab and ramucirumab), the recombinant fusion protein aflibercept, the tyrosine kinase inhibitors regorafenib, antimetabolites like TAS-102, and immunotherapeutic agents (ie, nivolumab and pembrolizumab).
The most appropriate treatment regimen conceivably generates the highest overall response rate (ORR), greatest impact on metastases for surgical conversion, longest progression-free (PFS) and overall survival, and a favorable toxicity profile. Treatment decisions for specific patient subpopulations may be guided by predictive biomarkers (RAS/BRAF type and MMR/MSI status), primary location (tumor and metastasis), patient factors (performance status and comorbidities), and therapy goals (palliation or conversion).
For otherwise healthy candidates, the standard first-line treatment approach is to start with a FOLFOX, CAPOX, or Folinic Acid/Fluorouracil/Irinotecan (FOLFIRI) regimen plus an anti-EGFR agent when the tumor is RAS/BRAF wild-type and left-sided or anti-VGFR when the tumor is RAS/BRAF mutated or right-sided. Disease progression or unacceptable toxicity warrants switching to a second-line regimen with either continuation of prior anti-VGFR or using a new biological agent regardless of the tumor location. ASCO, ESMO, and NCCN recommend comprehensive testing of KRAS, NRAS exons 2 (codons 12 and 13), 3 (codons 59 and 61), 4 (codons 117 and 146) and BRAF V600E for anti-EGFR candidates based on the results of the Panitumumab Randomized Trial in Combination with Chemotherapy for Metastatic Colorectal Cancer to Determine Efficacy (PRIME) trial and other major meta-analyses.
Incorporating biological agents into chemotherapy regimens has markedly enhanced ORR, DFS, and overall survival in the first- and second-line settings. Cetuximab and panitumumab exhibit comparable effectiveness in first- and second-line treatments and single-agent salvage therapy. However, cross-resistance may develop from using these agents, limiting their use after anti-EGFR treatment failure. Bevacizumab may be continued into second-line therapy after first-line progression, particularly for RAS/BRAF-mutated cases, but not in combination with new anti-EGFR agents for RAS/BRAF wild-type and left-sided tumors. Patients who progress despite anti-VEGF therapy may consider adding aflibercept or ramucirumab to their chemotherapy regimen.
Sequential single agents remain a valid option for patients with metastatic colorectal cancer compared to combination treatments demonstrated in the United Kingdom Medical Research Council's Fluorouracil, Oxaliplatin, CPT-11: Use and Sequencing (FOCUS) and Dutch Colorectal Group’s capecitabine/irinotecan/oxaliplatin (CAIRO) trials. Biological agents were not used in either trial, and most patients did not receive all 3 drugs. Multiple phase III trials have confirmed similar ORR, PFS, and overall survival rates between FOLFOX and FOLFIRI regimens, even with added anti-VEGFs or anti-EGFRs. In clinical practice, the choice between FOLFOX and FOLFIRI is based on the treatment toxicity profile. The capecitabine-oxaliplatin (CAPOX) combination is a statistically noninferior substitute to the FOLFOX regimen in PFS and OS with a tolerable toxicity profile. Conversely, the capecitabine-irinotecan (XELIRI) combination has shown higher intolerable rates of gastrointestinal toxicities. Maintenance therapy with low-dose capecitabine-bevacizumab after the first-line FOLFOX-bevacizumab good response is an accepted treatment option based on CAIRO3 trial results. Complete chemotherapy discontinuation may be detrimental and remains controversial. Meanwhile, intermittent treatment has not significantly reduced overall survival but, instead, improved quality of life.
FOLFOX with irinotecan (FOLFOXIRI) with or without bevacizumab is reserved for conversion approach in potentially resectable liver/lung metastasis on selected patients with excellent performance status. Consensus-based guidelines for individuals unfit for triple or double therapies recommend 5-FU/LV IV or capecitabine PO monotherapy. Patients eligible for further chemotherapy after progression on FOLFOX, CAPOX, or FOLFIRI with anti-VEGFs and anti-EGFRs may explore regorafenib or TAS-102 as salvage therapy. Alternatively, immunotherapy is approved for MSI-H/dMMR tumors. Clinical trials may offer access to novel targeted agents following failure on various therapeutic lines.
The recommended treatment monitoring involves close observation for signs and symptoms of adverse reactions before each treatment cycle and as needed. Blood work parameters should be checked frequently, also before each treatment cycle and as indicated. Serial CEA assays are recommended every 1 to 3 months, and radiographic evaluation using Response Evaluation Criteria In Solid Tumors (RECIST) should be performed every 2 to 3 months by an interprofessional team. Patients should also be regularly screened for somatic symptoms and psychosocial distress.
Selected Landmark Clinical Trials
Pivotal studies have shaped the standard of care for colorectal cancer treatment. Introducing new colorectal cancer therapies has significantly improved both efficacy and safety profiles, offering patients better outcomes and quality of life. These interventions are described below.
Cytotoxic chemotherapy
Cytotoxic agents target rapidly dividing cancer cells, thereby inhibiting tumor proliferation and promoting cancer cell death. These medications include 5-FU/LV, capecitabine, irinotecan, and oxaliplatin.
An updated meta-analysis compared 5-FU/LV to 5-FU alone. The combination regimen produced a higher ORR (21% compared to 11%) and 1-year overall survival (47% compared to 37%). The 2 most commonly used regimens in the United States include the Mayo regimen (bolus 5-FU 425 mg/m2 and leucovorin 20 mg/m2 on days 1 to 5 every 4 to 5 weeks) and the Roswell Park regimen (bolus 5-FU 500 mg/m2 and leucovorin 500 mg/m2 administered weekly for 6 out of 8 weeks).
The outcomes further improve by using the de Gramont regimen, with leucovorin 200 mg/m2 given as a 2-hour infusion followed by bolus 5-FU 400 mg/m2 and 22-hour infusion 5-FU 600 mg/m2 for 2 consecutive days every 2 weeks). Outcome measures include better ORR (33% compared to 14%), longer PFS (7 compared to 5 months) and overall survival (15.5 versus 14.2 months), and significantly less grade 3/4 toxicities (23.9% compared to 11.1%) than the Mayo regimen.
For capecitabine, Hoff PM et al’s phase III clinical trial prospectively randomly assigned patients to either oral capecitabine (1250 mg/m2 twice daily for 14 days every 21 days) or 5-FU/LV Mayo regimen, resulting in noninferior ORR (24.8% compared to 15.5%), time to progression (TTP 4.3 compared to 4.7 months) and mOS (12.5 compared to 13.3 months). Capecitabine’s common side effects included diarrhea (overlapping toxicities with irinotecan), hyperbilirubinemia, and hand-foot syndrome. Capecitabine has never been directly compared with infusional 5-FU/LV using the de Gramont regimen. However, CAPOX and infusional FOLFOX have been shown to have similar efficacy in advanced colon cancer treatment by the Three Regimens of Eloxatin Evaluation-1 (TREE-1) and Arbeitsgemeinschaft Internistische Onkologie (AIO) trials.
In clinical practice in the United States, reducing the dose of capecitabine by approximately 20% (to 1000 mg/m2) either alone or in combination with other regimens does not seem to diminish treatment effectiveness. However, this strategy significantly enhances the treatment's safety profile. Additionally, alternative oral fluoropyrimidines unapproved in the United States include different agents derived from S1: tegafur-uracil, gimeracil, and oteracil, as well as raltitrexed.
For irinotecan, a landmark phase II clinical trial was conducted where patients with 5-FU-refractory metastatic colon cancer were randomly assigned to either single-agent irinotecan (300 mg/m2 every 3 weeks) or best supportive care (BSC). The drug regimen demonstrated a significant 1-year overall survival (36% compared to 14%) with improved quality of life (better performance status, weight, and pain levels). Following this outcome, 3 key trials were conducted to test irinotecan’s role versus 5-FU/LV in the front-line setting. A 3-arm trial compared 3 treatment regimens: the Roswell Park regimen, irinotecan with 5-FU/LV weekly bolus regimen (the IFL or Saltz regimen), and irinotecan alone. The trial results significantly favored IFL with an ORR (39% compared to 21%) and mOS (14.8 compared to 12.6 months).
In Europe, 3 pivotal phase III trials compared the 5-FU/LV and FOLFIRI regimens. The Douillard FOLFIRI regimen included irinotecan (180 mg/m2 on day 1), 5-FU (400 mg/m2 bolus followed by 600 mg/m2 over 22 hours, both on days 1 and 2), and leucovorin (200 mg/m2 on days 1 and 2). The Douillard FOLFIRI regimen trial demonstrated a significant ORR (49% compared to 31%), TTP (6.7 compared to 4.4 months), and mOS (17.4 compared to 14.1 months). However, higher incidences of diarrhea, myelosuppression, and alopecia were observed in the irinotecan group. Irinotecan combinations require adequate biliary function for the drug’s active glucuronide metabolite, SN-38, to be excreted. Approximately 10% of United States patients are homozygous for the UGT1A1*28 allele polymorphism, which increases SN-38 bioavailability and, therefore, requires starting with a lower irinotecan dose.
Oxaliplatin has very limited activity in colorectal cancer as a single agent thus not recommended unless synergist with fluoropyrimidines. In the phase III trial, FU/LV (de Gramont regimen) was compared with or without OXA (OXA: 85 mg/m2 on day 1 over 2 hours FOLFOX4 regimen) as first-line therapy for patients with mCRC. FOLFOX4 regimen had significantly higher ORR (51% versus 22%) and PFS (9 compared to 6 months) but comparable mOS (16.2 versus 14.7 months) with more grade 3/4 neutropenia, diarrhea, and neurotoxicity. Because no overall survival benefit was achieved in these first-line trials, the FDA did not approve oxaliplatin for colorectal cancer until years later for second-line therapy that showed prolonged PFS and increased ORR compared with FU/LV for patients who experienced disease progression while receiving first-line irinotecan regimens. The most important side effect and dose-limiting toxicity of oxaliplatin is neurotoxicity. It may present as an acute and reversible, cold-triggered sensory neuropathy, or a chronic dose-limiting cumulative sensory neurotoxicity.
Oxaliplatin has very limited activity in colorectal cancer as a single agent and is thus not recommended unless it is used to synergize with fluoropyrimidines. In a phase III trial, 5-FU/LV using the de Gramont regimen was compared with or without oxaliplatin (oxaliplatin: 85 mg/m2 on day 1 over 2 hours, FOLFOX4 regimen) as first-line therapy for patients with metastatic colorectal cancer. The FOLFOX4 regimen showed a significantly higher ORR (51% compared to 22%) and PFS (9 compared to 6 months) but comparable mOS (16.2 compared to 14.7 months) and with more grade 3/4 neutropenia, diarrhea, and neurotoxicity.
Since no overall survival benefit was achieved in these first-line trials, the FDA did not approve oxaliplatin for colorectal cancer until years later for second-line therapy, which showed prolonged PFS and increased ORR compared with 5-FU/LV for patients who experienced disease progression while receiving first-line irinotecan-based regimens. Oxaliplatin’s most important, dose-limiting adverse effect is neurotoxicity, which may present as acute and reversible cold-triggered sensory neuropathy or chronic dose-limiting cumulative sensory neurotoxicity.
With oxaliplatin and irinotecan’s encouraging trial results in the United States and Europe, the North Central Cancer Treatment Group (NCCTG)/Intergroup trial N9741 performed a pivotal and practice-changing trial comparing FOLFOX4, the standard combination IFL, and the new irinotecan oxaliplatin (IROX) combination (oxaliplatin 85 mg/m2 with irinotecan 200 mg/m2, both on day 1 every 3 weeks). The N9741 results demonstrated FOLFOX’s superiority over IFL and IROX as first-line therapy for metastatic colon cancer with ORR (45% compared to 31% and 36%), PFS (8.7 compared to 6.9 and 6.7 months) and mOS (19.5 compared to 15 and 17.3 months). The toxicity profile likewise favored FOLFOX, except for neurotoxicity. FOLFOX emerged as the new standard first-line therapy with rapid and widespread adaptation in the United States.
VEGF inhibitors
VEGF inhibitors work by inhibiting VEGF, a key signaling protein involved in angiogenesis. In colon cancer, this mechanism helps suppress the formation of new blood vessels within tumors, thus reducing their blood supply and growth. The widely used agents in this class are bevacizumab, aflibercept, and ramucirumab.
In a randomized placebo-controlled phase III trial, IFL with bevacizumab (5 mg/kg every 2 weeks) was assessed as a first-line therapy for metastatic colorectal cancer. For the first time, the addition of an anti-VEGF inhibitor was validated as an efficacious antineoplastic treatment by significantly improving ORR, PFS, and mOS. This trial was the first phase III validation of an antiangiogenic agent as an effective treatment option in human malignancy. Subsequently, FOLFOX with bevacizumab (FOLFOX-BEV) also showed improved mOS in the first-line and second-line settings.
The Avastin in Elderly Colorectal Cancer Patients (AVEX) phase III trial selected patients age 70 or older ineligible for oxaliplatin or irinotecan-based chemotherapy first-line regimens to receive capecitabine regimen alone or with bevacizumab (7.5 mg/kg intravenously on day 1 given every 3 weeks). The study showed a significantly longer median progression-free survival (mPFS) and a remarkable improvement in mOS for capecitabine-bevacizumab (CAP-BEV) than with capecitabine alone.
Prolonged VEGF inhibition with bevacizumab beyond the first-line progression was evaluated by the European Therapy for Metastatic Colorectal Cancer (ML18147) phase III trial. Patients who experienced progression within 3 months were randomly assigned to either continue second-line therapy with or without bevacizumab. The mOS, the study’s primary endpoint, favored continuing with bevacizumab with chemotherapy over chemotherapy alone. PFS improvement confirmed this effect but not ORR. The toxicity profile observed with bevacizumab included hypertension, bleeding, gastrointestinal perforations, impaired wound healing, and arterial-venous thrombotic events.
In the placebo-controlled phase III Aflibercept Plus FOLFIRI vs. Placebo Plus FOLFIRI in Second-Line Metastatic Colorectal Cancer (VELOUR) trial, aflibercept was assessed in the second-line setting for patients who had progressed on oxaliplatin-based chemotherapy, with or without bevacizumab, in the first line. Patients randomly assigned to receive FOLFIRI with aflibercept (4 mg/kg IV every 2 weeks) demonstrated a significant improvement in ORR (19.8% compared to 11.1%), PFS (6.9 compared to 4.7 months), and mOS (13.5 compared to 12.1 months). However, the aflibercept arm exhibited an increased incidence of grade 3/4 diarrhea, mucositis, neutropenia, infection, fatigue, hypertension, proteinuria, hemorrhage, and arterial-venous thromboembolic events, typically observed in second-line FOLFIRI with bevacizumab (FOLFIRI-BEV).
In the double-blinded, placebo-controlled Ramucirumab in Colorectal Cancer: Efficacy and Safety (RAISE) trial, adding ramucirumab (8 mg intravenously every two weeks) to FOLFIRI as second-line therapy for patients who progressed with FOLFOX plus bevacizumab showed improved PFS (5.7 compared to 4.5 months) and mOS (13.3 compared to 11.7 months). However, the combination arm had worse grade 3/4 neutropenia, hypertension, diarrhea, and fatigue.
Anti-EGFR monoclonal antibodies
Anti-EGFR monoclonal antibodies block EGFR on cancer cells, inhibiting downstream signaling cascades involved in cell proliferation, survival, and angiogenesis. These agents cause tumor growth inhibition and enhanced immune response against cancer cells. Agents in this caregory include cetuximab and panitumumab.
Cetuximab monotherapy (400 mg/m2 followed by a weekly infusion of 250 mg/m2) in patients who had experienced disease progression on prior 5-FU, oxaliplatin, and irinotecan-based therapy had an improved ORR (40% compared to 11%) and mOS (6.1 compared to 4.6 months) with BSC as the comparator.
The Evaluation of Panitumumab in Colorectal Cancer to Improve Survival (EPIC) and Biomarker Oncology Research and Development (BOND) trials proved the PFS and ORR superiority of irinotecan-cetuximab (IRI-CET) over irinotecan alone on patients with prior irinotecan failure, without significantly improving survival. A large multicenter randomized phase III Cetuximab Combined With Irinotecan in First-Line Therapy for Metastatic Colorectal Cancer (CRYSTAL) trial, using the regimen FOLFIRI with or without cetuximab in treatment-naive colorectal cancer patients, confirmed that selected patients with wild-type KRAS have a significantly higher ORR, PFS, and mOS (23.5 compared to 20 months).
The benefit of FOLFOX-cetuximab (FOLFOX-CET) remains uncertain. Three trials, the Oxaliplatin and Cetuximab in First-Line Treatment of Metastatic Colorectal Cancer (OPUS), Cancer and Leukemia Group B (CALGB) 80203, and CALGB 80405, showed that the regimen only had modest significant ORR and PFS improvements but no mOS benefit. Three other trials, the Medical Research Council (MRC) COIN, NORDIC VII, and New EPOC demonstrated the treatment’s lack of benefit. The cetuximab arm had higher-grade diarrhea, magnesium-wasting syndrome, infusion reaction, and ocular-skin toxicity, with the last correlating to response rate.
Single-agent panitumumab (6 mg/kg every 2 weeks) was compared to BSC in a large international phase III trial in patients with chemotherapy-refractory metastatic colorectal cancer. Panitumumab had an ORR of 37%, similar to cetuximab, and a modestly prolonged mPFS of 8 compared to 7.3 weeks. However, mOS was not increased, likely due to patients crossing over from the BSC arm to the panitumumab arm. The A Study of Panitumumab Efficacy and Safety Compared to Cetuximab (ASPECCT) phase III trial directly compared panitumumab with cetuximab monotherapies in patients with chemotherapy-refractory disease and showed noninferior mOS (10 months) with a similar expected toxicity profile. The FOLFOX4-panitumumab (FOLFOX4-PAN) benefit in the first-line setting was seen in the phase III Panitumumab Randomized Trial in Combination with Chemotherapy for Metastatic Colorectal Cancer to Determine Efficacy (PRIME) trial with an mPFS improvement (9.6 compared to 8 months) and a trend for mOS (23.0 compared to 19.7 months), although not significant at 55 weeks follow-up.
Comparing VEGF inhibitors with anti-EGFR monoclonal antibodies
The FOLFIRI Plus Cetuximab versus FOLFIRI plus Bevacizumab as First-Line Treatment for Patients with Metastatic Colorectal Cancer (FIRE-3) trial enrolled treatment-naïve patients with metastatic colorectal cancer with KRAS exon2 wild-type status and randomly assigned them to receive FOLFIRI with cetuximab (FOLFIRI-CET) or FOLFIRI with bevacizumab (FOLFIRI-BEV). The trial’s primary endpoint, which looked at the ORR using an intention-to-treat approach, did not show a significant difference between the 2 groups (cetuximab’s 62% compared to bevacizumab’s 58%, p = 0.18). While progression-free survival (PFS) was similar between the 2 arms (cetuximab’s 10 months compared to bevacizumab’s 10.3 months), the mOS was significantly longer in the FOLFIRI-CET arm (cetuximab’s 28.7 months compared to bevacizumab’s 25.0 months; hazard ratio, 0.77; p = 0.017).
An updated analysis, considering additional mutations in KRAS exon 3 and 4 and NRAS exon 2 and 3 showed an even longer mOS of 33.1 months for FOLFIRI-CET. However, the U.S. Intergroup study, CALGB/Southwest Oncology Group (SWOG) 80405 trial, which compared FOLFOX or FOLFIRI (chosen by the physician) with cetuximab versus bevacizumab as first-line therapy, found no difference in mOS (cetuximab had 30 months compared to bevacizumab’s 29.9 months), even with expanded RAS-mutated analysis. Preliminary retrospective analysis suggests that patients with KRAS wild-type left-sided tumors treated with cetuximab had a significantly higher mOS compared to individuals with right-sided tumors (33.3 months and 19.4 months, respectively). Combination therapy with VEGF inhibitors and anti-EGFR monoclonal antibodies has shown no benefit and even harm in multiple trials (BOND-2, PACCE, CAIRO-2), and is therefore not recommended.
An updated analysis, which accounted for additional mutations in KRAS exons 3 and 4, as well as NRAS mutations exons 2 and 3, demonstrated a longer mOS survival of 33.1 months for FOLFIRI-CET. In contrast, the U.S. Intergroup study, CALGB/South-west Oncology Group (SWOG) 80405 trial that compared FOLFOX or FOLFIRI (dealer’s choice) with CET compared with BEV as first-line therapy had no difference in mOS (30 versus 29.9 months, respectively), not even whit expanded RAS-mutated analysis. The preliminary retrospective analysis suggests that patients treated with CET for KRAS wild-type left-sided tumors mOS are significantly higher than right-sided counterparts by 33.3 versus 19.4 months, respectively. Regimens combining VEGF inhibitors and anti-EGFRs monoclonal antibodies have failed, even proved detrimental, in multiple trials (BOND-2, PACCE, and CAIRO-2) and are thus not recommended.
Immune checkpoint inhibitors
Immune checkpoint inhibitors work by targeting specific proteins that act as brakes on the immune system's ability to attack cancer cells. In a phase II study, pembrolizumab 10 mg/kg every 14 days was given to 11 heavily pretreated patients with dMMR metastatic colorectal cancer with a remarkable ORR of 71% and PFS rate of 67%. In an expanded cohort of 54 patients, pembrolizumab showed an ORR of 50% and an 89% disease control rate with a durable response for more than a year, not seen in pMMR colon cancer. In CheckMate-142, nivolumab (3mg/kg every 2 weeks) was given to 74 patients with dMMR colon cancer, resulting in 31% objective response, 69% disease control, and median duration response not reached at 12 months.
Salvage therapy for refractory disease
The efficacy of regorafenib 160 mg PO daily for 3 weeks in a 4-week cycle was investigated in a placebo-controlled, multicenter international, randomized, phase III CORRECT trial after the progression of multiple therapies. Regorafenib had a higher ORR (41% compared to 15%) and PFS (1.9 compared to 1.7 months) than placebo, with modest but significant mOS benefit (6.4 compared to 5.0 months, p = 0.0052). The observed toxicity profile with regorafenib included hand-foot skin reaction, fatigue, hypertension, diarrhea, and rash, with a 1.6% fatal hepatic failure rate. The CONCUR trial later confirmed regorafenib’s benefit.
The international, double-blind, placebo-controlled, randomized-controlled, phase III RECOURSE study of patients with refractory disease showed that trifluridine-tipiracil (TAS-102) 35 mg/m2 orally twice daily on days 1 through 5 and 8 to 12 of each 28-day cycle improved ORR (44% versus 16%), DFS and mOS (7.1 versus 5.3 months). The most serious adverse events were neutropenia (38%), febrile neutropenia (4%), and a treatment-related death.
| Pause and Reflect |
How does early detection impact the treatment options for colon cancer? What factors influence the choice of chemotherapy or targeted therapy in colon cancer treatment? How might genetic testing affect treatment decisions for a patient with colon cancer? What are the potential adverse effects of colon cancer treatments, and how can they be managed? How can healthcare providers ensure that patients with advanced colon cancer receive optimal palliative care? |
Staging
The tumor, node, metastasis (TNM) staging system of the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) 2017 is currently the preferred staging system for colorectal cancer.[37] The staging system is explained below.
T: Primary Tumor
- TX: Primary tumor cannot be assessed
- T0: No evidence of primary tumor
- Tis: Carcinoma in situ: intramucosal carcinoma (lamina propria involvement without extending through muscularis mucosae)
- T1: Tumor invades the submucosa (through the muscularis mucosa but not into the muscularis propria)
- T2: Tumor invades the muscularis propria
- T3: Tumor invades through the muscularis propria into pericolorectal tissues (see Image. Colorectal Cancer with Pathological T3 Staging)
- T4: Tumor invades the visceral peritoneum or invades or adheres to adjacent organ or structure (see Image. Colorectal Cancer with Pathological T4 Staging)
- T4a: Tumor invades through the visceral peritoneum (including gross perforation of the bowel by the tumor and continuous invasion of tumor cells through inflamed areas to the visceral peritoneum surface)
- T4b: Tumor directly invades or adheres to adjacent organs or structures
N: Regional Lymph Nodes
- NX: Regional lymph nodes cannot be assessed
- N0: No regional lymph node metastasis
- N1: 1 to 3 regional lymph nodes are positive (tumor in lymph nodes measuring ≥0.2 mm), or any number of tumor deposits are present, and all identifiable lymph nodes are negative
- N1a: 1 regional lymph node is positive
- N1b: 2 or 3 regional lymph nodes are positive
- N1c: No regional lymph nodes are positive, but tumor deposits are present in the subserosa, mesentery, nonperitonealized pericolic, or perirectal or mesorectal tissues
- N2: 4 or more regional lymph nodes are positive
- N2a: 4 to 6 regional lymph nodes are positive
- N2b: 7 or more regional lymph nodes are positive
M: Distant Metastasis
- M0: No distant metastasis by imaging; no evidence of tumor in distant sites or organs
- M1: Metastasis to 1 or more distant sites or organs or peritoneal metastasis is identified (see Image. Liver Metastasis in Colorectal Cancer)
- M1a: Metastasis to 1 site or organ is identified without peritoneal metastasis
- M1b: Metastasis to 2 or more sites or organs is identified without peritoneal metastasis
- M1c: Metastasis to the peritoneal surface is identified alone or with metastases to other sites or organs
Prognostic Groups based on TNM
- Stage 0: Tis N0 M0
- Stage I: T1, T2 N0 M0
- Stage IIA: T3 N0 M0
- Stage IIB: T4a N0 M0
- Stage IIC: T4b N0 M0
- Stage IIIA: T1-T2 N1/N1c M0 T1 N2a M0
- Stage IIIB: T3-T4a N1/N1c M0 T2-T3 N2a M0 T1-T2 N2b M0
- Stage IIIC: T4a N2a M0 T3-T4a N2b M0 T4b N1-N2 M0
- Stage IVA: Any T Any N M1a
- Stage IVB: Any T Any N M1b
- Stage IVC: Any T Any N M1c
Prognosis
The prognosis for colon cancer depends on the stage. The most important prognostic indicator is the pathological stage, supported by SEER data.[38] The 5-year overall survival rate for colon cancer stage I is 74%, for stage IIA is 66%, for stage IIB is 58%, for stage IIC is 37%, for stage IIIA is 73%, for stage IIIB is 46%, for stage IIIC is 28%, and stage IV is 5%. These figures correspond to 5-year survival rates of 90% for localized disease, 73% for regional disease, 13% for distant disease, and 63% for all cases. Patient factors such as age, comorbidities, and tumor-related factors also influence prognosis.
Complications
Colon cancer's complications are diverse. Complications related to the primary tumor include bleeding, obstruction, perforation, and fistulization to surrounding organs. Metastatic disease can present with different symptoms based on the site of metastasis. Complications of surgery include bleeding, surgical site and organ space infections, anastomotic leaks, injury to surrounding structures like the ureters and duodenum, and longer-term complications like incisional hernias. Chemotherapy's sequelae are described in a separate section and are beyond this activity's scope.
Deterrence and Patient Education
Colon cancer is the third most common malignancy in both men and women. Most cancers arise from polyps and develop over several years. Colonoscopy is effective in detecting polyps and screening for colon cancer and should be started at age 45 in most normal-risk individuals. Those at a higher risk for colon cancer, including patients with a hereditary polyposis syndrome like FAP or HNPCC or strong family history of colon cancer, should begin screening earlier. Most local and regional disease is treated with surgery, with postoperative chemotherapy indicated in cases of greater tumor invasion depth or lymph node involvement. Metastatic disease is best managed with chemotherapy, with surgery reserved for symptoms or palliation. Isolated liver, peritoneum, or lung metastasis can sometimes be managed surgically and is associated with the best outcomes. Overall, the 5-year survival for all patients with colon cancer is 63% and continues to improve.
Sample questions are provided below to help guide patients into reflective thinking about colon cancer risk and prevention. Questions such as these can help strengthen dialogue between clinicians and patients and prompt deepening of conversations.
| Pause and Reflect |
How can you assess your risk for colon cancer? If you have a family history of colon cancer, what steps could you take to reduce your risk? How might lifestyle changes such as diet and exercise impact colon prevention? What questions would you ask your healthcare provider about screening options and genetic counseling? What strategies could you use to encourage a loved one to prioritize colon cancer screening? |
Enhancing Healthcare Team Outcomes
Colorectal cancer is the third leading cause of cancer-related mortality globally and presents significant diagnostic and treatment complexities due to its varied etiologies, clinical manifestations, and stages. Early detection through effective screening methods such as colonoscopy is pivotal in improving survival rates by identifying premalignant lesions and early-stage tumors. Treatment strategies depend on cancer staging, with surgical resection as the cornerstone for localized disease, supplemented by adjuvant chemotherapy for advanced cases and systemic therapies for metastatic disease. Emerging therapies, including biologics and immune checkpoint inhibitors, offer hope for improved outcomes, particularly for high-risk and advanced-stage patients.
Patients with colon cancer require coordinated, interprofessional care, given the treatments' range and rapid advancements. Management must start with appropriate screening of at-risk individuals. Healthcare teams must continue to encourage screening colonoscopies to facilitate earlier diagnosis. Colonoscopy is a proven and effective tool but remains underutilized. Healthy behaviors, including lifestyle changes, such as following a healthy diet, obtaining and maintaining an ideal body weight, establishing an active exercise routine, minimizing alcohol consumption, and quitting smoking, should be encouraged.[39][40]
Care coordination is central to ensuring seamless and efficient patient care, especially for patients receiving multiple treatments. Physicians, advanced practitioners, nurses, pharmacists, and other healthcare professionals must work together to streamline the patient's journey, from diagnosis through treatment and follow-up. Coordination minimizes errors, reduces delays, and enhances patient safety, ultimately leading to improved outcomes and patient-centered care that prioritizes the well-being and satisfaction of patients with colon cancer.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
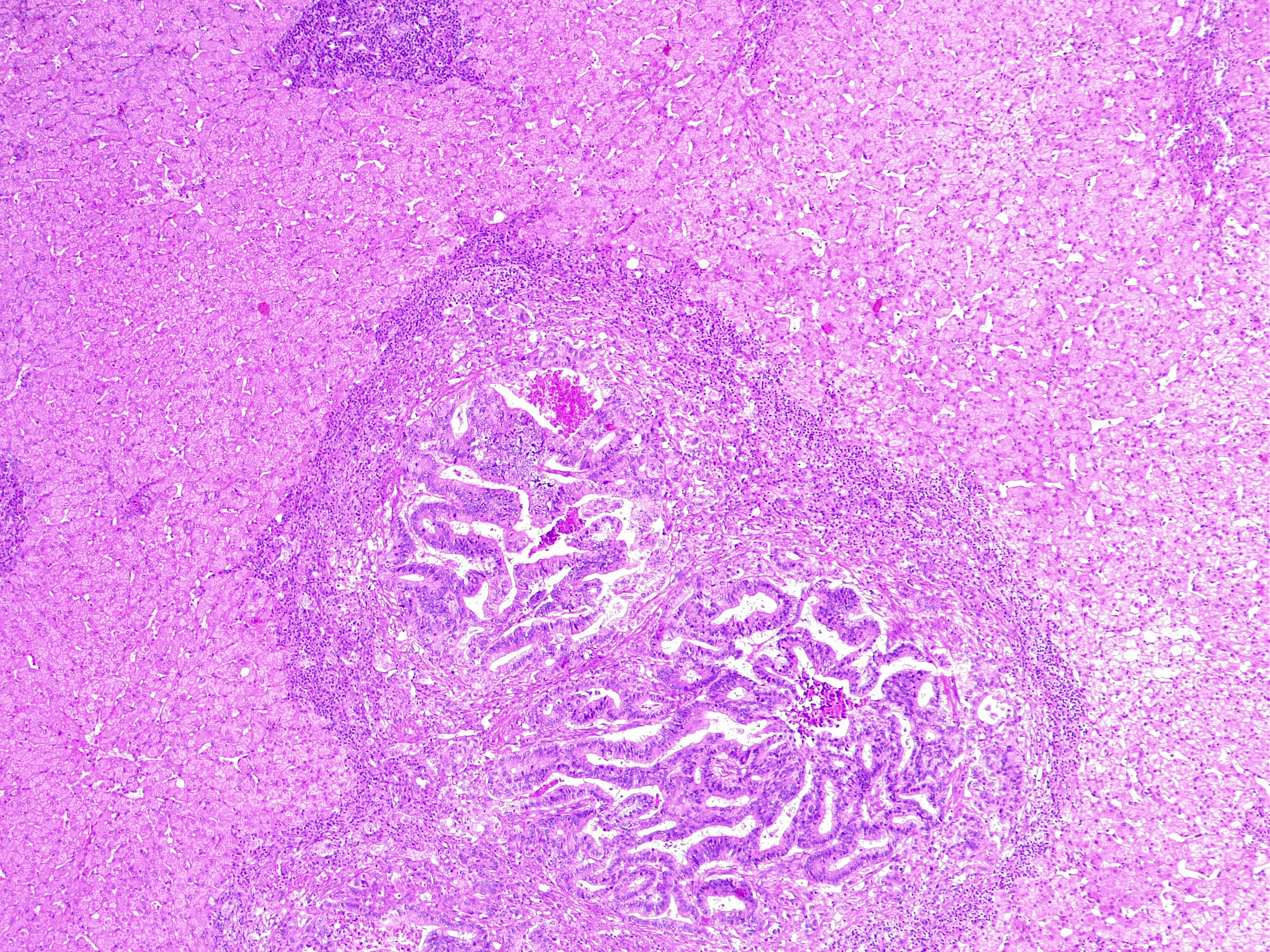
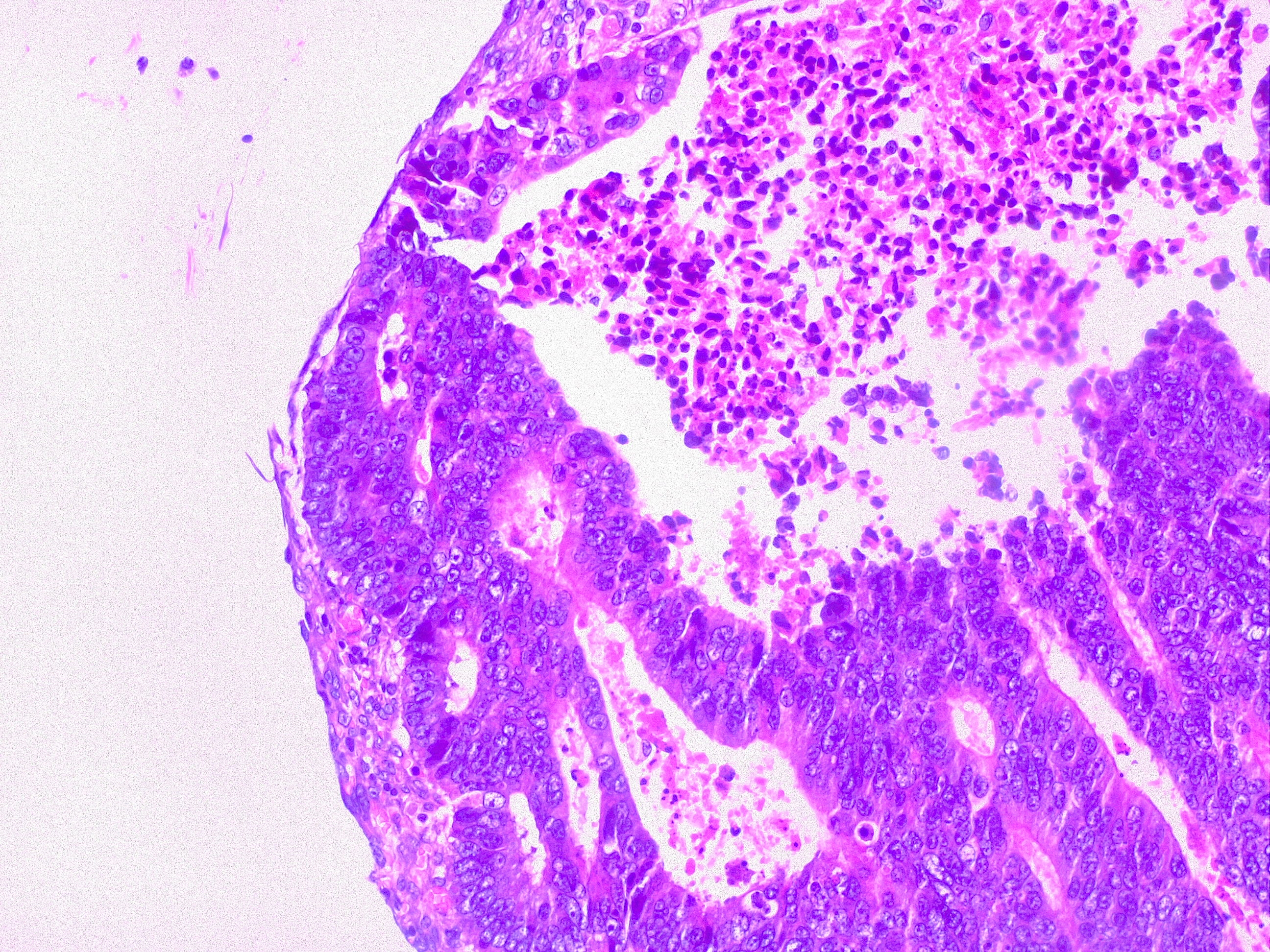
Liver Metastasis in Colorectal Cancer. This microscopic image shows a neoplastic nodule composed of glandular structures surrounded by fibrous and inflammatory tissue. This case is an example of metastatic colorectal cancer, adenocarcinoma type. This finding classifies as adenocarcinoma TNM stage pM1.
Contributed by Fabiola Farci, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: a cancer journal for clinicians. 2023 Jan:73(1):17-48. doi: 10.3322/caac.21763. Epub [PubMed PMID: 36633525]
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians. 2021 May:71(3):209-249. doi: 10.3322/caac.21660. Epub 2021 Feb 4 [PubMed PMID: 33538338]
Stoffel EM, Murphy CC. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology. 2020 Jan:158(2):341-353. doi: 10.1053/j.gastro.2019.07.055. Epub 2019 Aug 5 [PubMed PMID: 31394082]
Xiao JB, Leng AM, Zhang YQ, Wen Z, He J, Ye GN. CUEDC2: multifunctional roles in carcinogenesis. Frontiers in bioscience (Landmark edition). 2019 Mar 1:24(5):935-946 [PubMed PMID: 30844721]
Gajendran M, Loganathan P, Jimenez G, Catinella AP, Ng N, Umapathy C, Ziade N, Hashash JG. A comprehensive review and update on ulcerative colitis(). Disease-a-month : DM. 2019 Dec:65(12):100851. doi: 10.1016/j.disamonth.2019.02.004. Epub 2019 Mar 2 [PubMed PMID: 30837080]
Sokic-Milutinovic A. Appropriate Management of Attenuated Familial Adenomatous Polyposis: Report of a Case and Review of the Literature. Digestive diseases (Basel, Switzerland). 2019:37(5):400-405. doi: 10.1159/000497207. Epub 2019 Mar 5 [PubMed PMID: 30836352]
Level 3 (low-level) evidenceFabregas JC, Ramnaraign B, George TJ. Clinical Updates for Colon Cancer Care in 2022. Clinical colorectal cancer. 2022 Sep:21(3):198-203. doi: 10.1016/j.clcc.2022.05.006. Epub 2022 Jun 3 [PubMed PMID: 35729033]
Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, Wender RC. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA: a cancer journal for clinicians. 2019 May:69(3):184-210. doi: 10.3322/caac.21557. Epub 2019 Mar 15 [PubMed PMID: 30875085]
Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2021 Mar 2:19(3):329-359. doi: 10.6004/jnccn.2021.0012. Epub 2021 Mar 2 [PubMed PMID: 33724754]
Level 1 (high-level) evidenceVen Fong Z, Chang DC, Lillemoe KD, Nipp RD, Tanabe KK, Qadan M. Contemporary Opportunity for Prehabilitation as Part of an Enhanced Recovery after Surgery Pathway in Colorectal Surgery. Clinics in colon and rectal surgery. 2019 Mar:32(2):95-101. doi: 10.1055/s-0038-1676473. Epub 2019 Feb 28 [PubMed PMID: 30833857]
Drago L. Probiotics and Colon Cancer. Microorganisms. 2019 Feb 28:7(3):. doi: 10.3390/microorganisms7030066. Epub 2019 Feb 28 [PubMed PMID: 30823471]
PDQ Adult Treatment Editorial Board. Colon Cancer Treatment (PDQ®): Health Professional Version. PDQ Cancer Information Summaries. 2002:(): [PubMed PMID: 26389297]
Level 3 (low-level) evidenceAllen J, Sears CL. Impact of the gut microbiome on the genome and epigenome of colon epithelial cells: contributions to colorectal cancer development. Genome medicine. 2019 Feb 25:11(1):11. doi: 10.1186/s13073-019-0621-2. Epub 2019 Feb 25 [PubMed PMID: 30803449]
Snyder C, Hampel H. Hereditary Colorectal Cancer Syndromes. Seminars in oncology nursing. 2019 Feb:35(1):58-78. doi: 10.1016/j.soncn.2018.12.011. Epub 2019 Jan 19 [PubMed PMID: 30665732]
Birch RJ, Burr N, Subramanian V, Tiernan JP, Hull MA, Finan P, Rose A, Rutter M, Valori R, Downing A, Morris EJA. Inflammatory Bowel Disease-Associated Colorectal Cancer Epidemiology and Outcomes: An English Population-Based Study. The American journal of gastroenterology. 2022 Nov 1:117(11):1858-1870. doi: 10.14309/ajg.0000000000001941. Epub 2022 Aug 12 [PubMed PMID: 36327438]
Loh NY, Wang W, Noordam R, Christodoulides C. Obesity, Fat Distribution and Risk of Cancer in Women and Men: A Mendelian Randomisation Study. Nutrients. 2022 Dec 9:14(24):. doi: 10.3390/nu14245259. Epub 2022 Dec 9 [PubMed PMID: 36558416]
Lu L, Mullins CS, Schafmayer C, Zeißig S, Linnebacher M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer communications (London, England). 2021 Nov:41(11):1137-1151. doi: 10.1002/cac2.12220. Epub 2021 Sep 25 [PubMed PMID: 34563100]
Vernia F, Longo S, Stefanelli G, Viscido A, Latella G. Dietary Factors Modulating Colorectal Carcinogenesis. Nutrients. 2021 Jan 3:13(1):. doi: 10.3390/nu13010143. Epub 2021 Jan 3 [PubMed PMID: 33401525]
Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA: a cancer journal for clinicians. 2023 May-Jun:73(3):233-254. doi: 10.3322/caac.21772. Epub 2023 Mar 1 [PubMed PMID: 36856579]
Worthley DL, Leggett BA. Colorectal cancer: molecular features and clinical opportunities. The Clinical biochemist. Reviews. 2010 May:31(2):31-8 [PubMed PMID: 20498827]
Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. International journal of molecular sciences. 2017 Jan 19:18(1):. doi: 10.3390/ijms18010197. Epub 2017 Jan 19 [PubMed PMID: 28106826]
Level 3 (low-level) evidenceHuang J, Soupir AC, Schlick BD, Teng M, Sahin IH, Permuth JB, Siegel EM, Manley BJ, Pellini B, Wang L. Cancer Detection and Classification by CpG Island Hypermethylation Signatures in Plasma Cell-Free DNA. Cancers. 2021 Nov 9:13(22):. doi: 10.3390/cancers13225611. Epub 2021 Nov 9 [PubMed PMID: 34830765]
Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, Bot BM, Morris JS, Simon IM, Gerster S, Fessler E, De Sousa E Melo F, Missiaglia E, Ramay H, Barras D, Homicsko K, Maru D, Manyam GC, Broom B, Boige V, Perez-Villamil B, Laderas T, Salazar R, Gray JW, Hanahan D, Tabernero J, Bernards R, Friend SH, Laurent-Puig P, Medema JP, Sadanandam A, Wessels L, Delorenzi M, Kopetz S, Vermeulen L, Tejpar S. The consensus molecular subtypes of colorectal cancer. Nature medicine. 2015 Nov:21(11):1350-6. doi: 10.1038/nm.3967. Epub 2015 Oct 12 [PubMed PMID: 26457759]
Level 3 (low-level) evidenceSchaad N, Berezowska S, Perren A, Hewer E. Impact of template-based synoptic reporting on completeness of surgical pathology reports. Virchows Archiv : an international journal of pathology. 2024 Jan:484(1):31-36. doi: 10.1007/s00428-023-03533-6. Epub 2023 Apr 5 [PubMed PMID: 37017774]
Kinugasa T, Mizobe T, Shiraiwa S, Akagi Y, Shirouzu K. Perineural Invasion Is a Prognostic Factor and Treatment Indicator in Patients with Rectal Cancer Undergoing Curative Surgery: 2000-2011 Data from a Single-center Study. Anticancer research. 2017 Jul:37(7):3961-3968 [PubMed PMID: 28668901]
Qu Q, Wu D, Li Z, Yin H. Tumor budding and the prognosis of patients with metastatic colorectal cancer: a meta-analysis. International journal of colorectal disease. 2023 May 24:38(1):141. doi: 10.1007/s00384-023-04423-8. Epub 2023 May 24 [PubMed PMID: 37222838]
Level 1 (high-level) evidenceDawson H, Kirsch R, Messenger D, Driman D. A Review of Current Challenges in Colorectal Cancer Reporting. Archives of pathology & laboratory medicine. 2019 Jul:143(7):869-882. doi: 10.5858/arpa.2017-0475-RA. Epub 2019 Jan 23 [PubMed PMID: 30672337]
K N M, P C S, Prabhu GK. Domain-Based Analysis of Colon Polyp in CT Colonography Using Image-Processing Techniques. Asian Pacific journal of cancer prevention : APJCP. 2019 Feb 26:20(2):629-637 [PubMed PMID: 30806070]
Grimm IS, McGill SK. Look, but don't touch: what not to do in managing large colorectal polyps. Gastrointestinal endoscopy. 2019 Mar:89(3):479-481. doi: 10.1016/j.gie.2018.10.008. Epub [PubMed PMID: 30784495]
Lowe D, Saleem S, Arif MO, Sinha S, Brooks G. Role of Endoscopic Resection Versus Surgical Resection in Management of Malignant Colon Polyps: a National Cancer Database Analysis. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2020 Jan:24(1):177-187. doi: 10.1007/s11605-019-04356-0. Epub 2019 Aug 19 [PubMed PMID: 31428961]
Dumoulin FL, Hildenbrand R. Endoscopic resection techniques for colorectal neoplasia: Current developments. World journal of gastroenterology. 2019 Jan 21:25(3):300-307. doi: 10.3748/wjg.v25.i3.300. Epub [PubMed PMID: 30686899]
Ma MX, Bourke MJ. Complications of endoscopic polypectomy, endoscopic mucosal resection and endoscopic submucosal dissection in the colon. Best practice & research. Clinical gastroenterology. 2016 Oct:30(5):749-767. doi: 10.1016/j.bpg.2016.09.009. Epub 2016 Sep 14 [PubMed PMID: 27931634]
Wells KO, Senagore A. Minimally Invasive Colon Cancer Surgery. Surgical oncology clinics of North America. 2019 Apr:28(2):285-296. doi: 10.1016/j.soc.2018.11.004. Epub 2018 Dec 26 [PubMed PMID: 30851829]
Pinto JC, Rosa I, Martins C, Marques I, da Silva JP, Fonseca R, Freire J, Pereira AD. Colon Adenocarcinoma Stage IIA-Can We Predict Relapse? Journal of gastrointestinal cancer. 2020 Mar:51(1):116-120. doi: 10.1007/s12029-019-00218-9. Epub [PubMed PMID: 30834501]
Liu Q, Huang Y, Luo D, Zhang S, Cai S, Li Q, Ma Y, Li X. Evaluating the Guiding Role of Elevated Pretreatment Serum Carcinoembryonic Antigen Levels for Adjuvant Chemotherapy in Stage IIA Colon Cancer: A Large Population-Based and Propensity Score-Matched Study. Frontiers in oncology. 2019:9():37. doi: 10.3389/fonc.2019.00037. Epub 2019 Feb 13 [PubMed PMID: 30815388]
Costas-Chavarri A, Nandakumar G, Temin S, Lopes G, Cervantes A, Cruz Correa M, Engineer R, Hamashima C, Ho GF, Huitzil FD, Malekzadeh Moghani M, Sharara AI, Stern MC, Teh C, Vázquez Manjarrez SE, Verjee A, Yantiss R, Shah MA. Treatment of Patients With Early-Stage Colorectal Cancer: ASCO Resource-Stratified Guideline. Journal of global oncology. 2019 Feb:5():1-19. doi: 10.1200/JGO.18.00214. Epub [PubMed PMID: 30802158]
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA: a cancer journal for clinicians. 2017 Mar:67(2):93-99. doi: 10.3322/caac.21388. Epub 2017 Jan 17 [PubMed PMID: 28094848]
Lin J, McGlynn KA, Shriver CD, Zhu K. Comparison of Survival among Colon Cancer Patients in the U.S. Military Health System and Patients in the Surveillance, Epidemiology, and End Results (SEER) Program. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2021 Jul:30(7):1359-1365. doi: 10.1158/1055-9965.EPI-20-1267. Epub 2021 Jun 23 [PubMed PMID: 34162655]
Thygesen MK, Baatrup G, Petersen C, Qvist N, Kroijer R, Kobaek-Larsen M. Screening individuals' experiences of colonoscopy and colon capsule endoscopy; a mixed methods study. Acta oncologica (Stockholm, Sweden). 2019:58(sup1):S71-S76. doi: 10.1080/0284186X.2019.1581372. Epub 2019 Mar 1 [PubMed PMID: 30821625]
Barnell GM, Ajayi O, Tolan-Riley A, Dixon MR. A Team-Based Approach to Anal Cancer Screening and Prevention. Diseases of the colon and rectum. 2019 Mar:62(3):e13. doi: 10.1097/DCR.0000000000001317. Epub [PubMed PMID: 30741771]