Introduction
A cochlear implant is a medical device designed to restore sensorineural hearing loss by bypassing damaged components of the ear and directly stimulating the auditory nerve. Cochlear implants consist of external and internal components that work together to convert sound into electrical signals, which are transmitted to the cochlea and stimulate the auditory nerve, effectively bypassing the damaged ear components. This technology is a significant advancement, offering improved hearing and quality of life for individuals with severe to profound hearing loss.
Cochlear implants offer remarkable benefits across age groups. In children, they enhance speech development, facilitate integration into mainstream education, and reduce reliance on alternative communication methods. In adults, cochlear implants improve communication, enable safe navigation, and mitigate the social isolation and cognitive decline associated with untreated hearing loss. For elderly patients, they may even reduce the risk of dementia and improve overall quality of life.
A key challenge with cochlear implants is selecting the appropriate patients who will benefit from the technology, as this is a relatively new medical advancement that continues to evolve rapidly. Cochlear implantation involves selecting suitable candidates through a comprehensive evaluation, including medical history, audiometric testing, and radiologic assessments. Criteria for eligibility often depend on the severity of hearing loss, age, and the presence of specific anatomical or physiological conditions. The Food and Drug Administration (FDA) regulates the production of these devices in the United States; however, these FDA criteria are limited, with more significant barriers to pediatric implantation.[1] Collaboration among otolaryngologists, audiologists, and other specialists ensures that patients receive optimal care. Surgical approaches, eg, cochleostomy or round window insertion, are employed to place the electrode array, with nerve monitoring used to safeguard critical structures like the facial nerve during the procedure.
Despite their benefits, cochlear implants require ongoing postoperative care, device programming, and rehabilitation. Interprofessional teams, including audiologists, speech-language pathologists, and support personnel, work collaboratively to maximize outcomes. Advances in technology continue to expand the indications for cochlear implants, offering hope to patients with complex hearing impairments and pushing the boundaries of what is possible in auditory restoration.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Cochlear Implant Function
The fundamental goal of a cochlear implant is to transmit sound from the external world to the cochlear nerve by transducing acoustic energy into an electrical signal, which is used to stimulate surviving spiral ganglion cells of the auditory nerve.[2] A cochlear implant bypasses the external ear, middle ear, and part of the inner ear, allowing it to directly transmit sound to the cochlea instead of simply amplifying the sound like a hearing aid (see Images. Internal Ear and Cochlear Division of the Acoustic Nerve). The implant's purpose is to restore hearing for patients without other options, eg, reconstructive surgery or traditional hearing aids.
Inner Ear Physiology
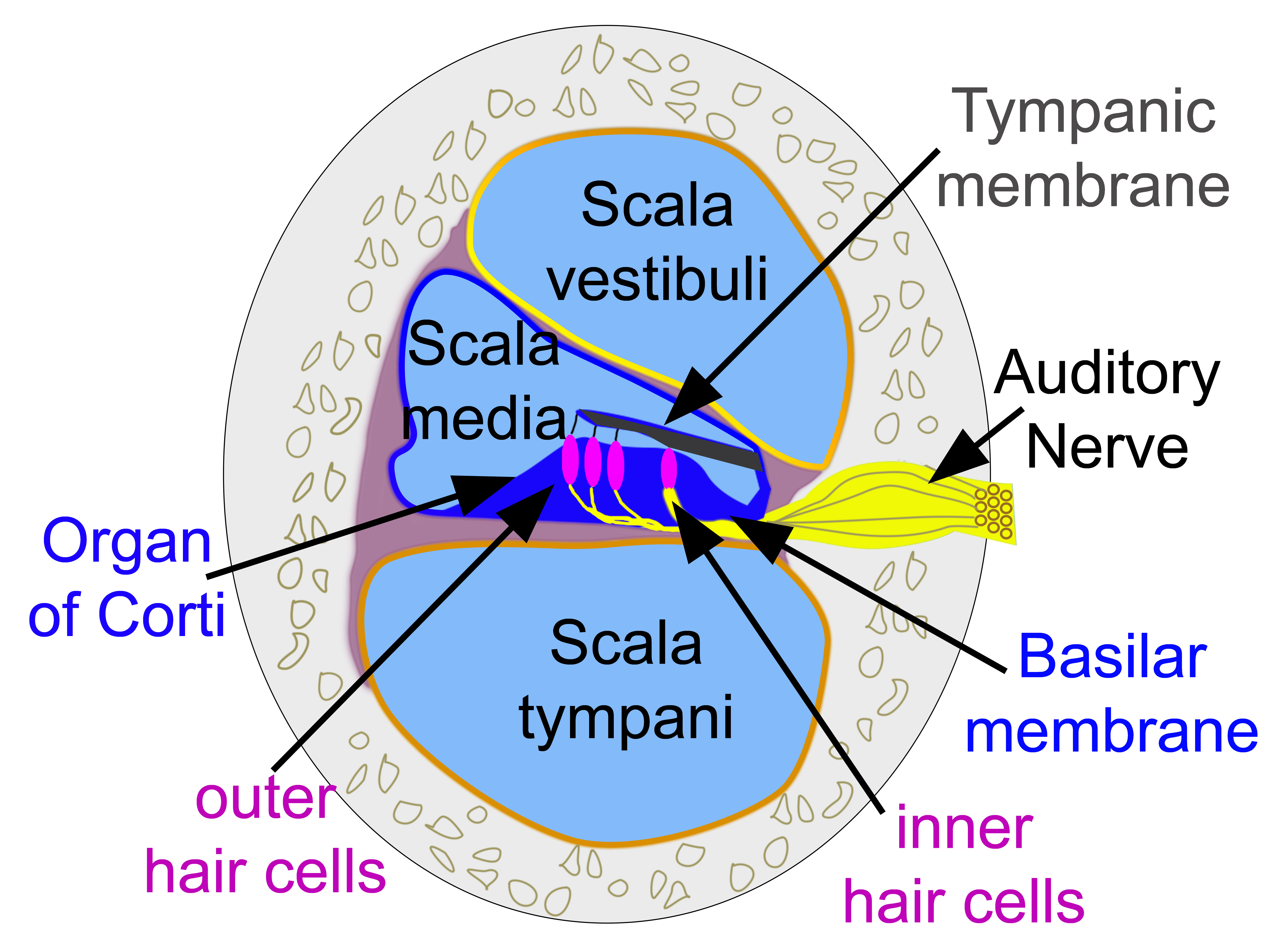
The cochlea is a spiral-shaped structure housed in the temporal bone with approximately 2.5 turns in the average individual. A normal cochlea has 3 layers: the scala tympani, scala media, and scala vestibuli.[3] The scala media houses the organ of Corti, which serves as the hearing organ that directly communicates with the cochlear nerve. The typical direction for sound to travel is from the external world, through the external ear canal, contact the tympanic membrane, transmit along the malleus, incus, and stapes, and be directed through the oval window of the cochlea where the vibrational energy of sound travels along the scala vestibuli.[4] Sound reaches its peak at the helicotrema or the apex of the cochlea and then crosses over to the scala tympani, and this energy then gets emitted from the round window (see Image. Cochlea Cross Section). The vibrational energy displaces the organ of Corti, causing cochlear nerve stimulation. The cochlea has a tonotopical organization so that high-pitched sounds are located along the base of the cochlea, and low-frequency sounds are organized around the apex.
Cochlear Implant Surgical Approaches
The 2 main surgical approaches used for cochlear implant placement include:
- Cochleostomy: The electrode is placed into the scala tympani by creating an opening anterior and inferior to the round window.[5][6]
- Round window: Direct insertion of the electrode into the round window, with or without drilling the edges of the window, thereby creating an "extended round window."[7]
Cranial nerve VIII (vestibulocochlear nerve) travels from the brainstem (pons) through the temporal bone and directly contacts the cochlea via fibers traversing the modiolus. It runs in association with the facial nerve (cranial nerve VII). The cochlear nerve travels in the anterior inferior division, relative to Bill's bar and vertical crest of the internal auditory canal.[8][9]
Indications
All patients seeking cochlear implantation must undergo a thorough evaluation, including a detailed otologic medical history, audiometric testing, and radiographic assessments. This evaluation is essential for determining the cause of the hearing loss and ensuring that the FDA's candidacy criteria and insurance eligibility requirements are met.
Cochlear Implant Indications
The FDA criteria for cochlear implantation vary. However, general indications for implants include:
- Sensorineural hearing loss (prelingual or postlingual deafness) diagnosis has been confirmed.
- Longstanding severe or profound hearing loss predicts a poorer outcome.
- Bilateral or single-sided deafness is present.
- Most pediatric cochlear implant candidates are prelingually deafened children with genetic mutations (eg, connexin 26), perinatal environmental exposures, or idiopathic causes, and they have the best results after cochlear implantation within the second year of life.[10]
- Prelingually deafened adolescents generally have poor outcomes and limited benefits from cochlear implantation.[11]
- An age older than 6 months is preferable.
- Radiologic findings
- No contraindications to cochlear implantation are noted on imaging, including:
- Complete labyrinthine aplasia
- Cochlear aplasia
- Cochlear nerve aplasia
- Complete cochlear ossification
- Magnetic resonance imaging (MRI) is best to confirm a fluid-filled cochlear duct to receive the electrode and the presence of a cochlear nerve to carry the signal to the brainstem and auditory cortex.[12]
- No contraindications to cochlear implantation are noted on imaging, including:
- Auditory neuropathy [13]
- The patient is reliable to follow up with the audiology and otolaryngology team.
- The patient can undergo general anesthesia and surgical procedures.[2][14]
Insurance qualifications generally include:
- Bilateral moderate to profound SNHL (>40 dB) over 18 years of age (failed benefit from hearing aids)
- Bilateral severe to profound SNHL (>70 dB) in patients from 2 to 18 years of age (failed benefit from alternative hearing amplification)
- Bilateral profound SNHL (>90 dB) in children under 2 years of age (failed benefit from alternative hearing amplification)
- Minimum age of 12 months (younger if there is a concern for cochlear ossification, which is a complication after meningitis)
- Adult open sentence scores with speech recognition of <50% in the ear to be implanted and below 60% in the contralateral ear (with amplification used)
- Pediatric Multisyllabic Lexical Neighborhood Test <30% with a lack of auditory progress
- HINT Audiometric battery <50% in the implanted ear, <60% in the best-aided ear (scores depending on the manufacturer)
- MLNT audiometric battery <20% to 30% (scores depending on the manufacturer)
- For the hybrid system device that aids patients with high-frequency sensorineural hearing loss
- Patients with profound high-frequency hearing loss and preserved low-frequency hearing thresholds range from 0 to 60 dB at 125 to 500 Hz
- A severe to profound middle- to high-frequency hearing loss as defined by a threshold average of >75 dB at 2000, 3000, and 4000 Hz
- The consonant-nucleus-consonant word recognition score criteria are 10% to 60% in the ear to be implanted and up to 80% in the contralateral ear.
- Reliability to follow up with otolaryngology and audiology to perform auditory rehabilitation
The following are expanded indications for implants that should be considered:
- Children with auditory neuropathy spectrum disorder (ANSD) have been shown to achieve reliable open-set speech recognition, and most patients with cochlear malformations (eg, Mondini deformity) who were previously not implant candidates are now being implanted safely.[15]
- Although implantation for single-sided deafness is not currently FDA-approved, increasing evidence suggests that it may be a viable option in the future.[16]
- In select cases, implanting patients with a vestibular schwannoma and neurofibromatosis type 2 may be considered.[17][18]
- Other instances in which implantation may be considered include:
- Superficial siderosis
- Pachymeningitis
- Sarcoidosis
- History of cerebral nervous system radiation
- Other brainstem lesions [19]
Contraindications
Cochlear implants are not recommended for certain patient populations. Notably, individuals who meet the eligibility criteria but choose not to undergo surgery are not considered candidates for the procedure. Furthermore, alternative forms of communication, eg, sign language, require a more detailed explanation. Significant therapy is necessary after cochlear implantation, and not every patient achieves the same level of success. Patients must also learn to interpret sounds and should work closely with audiologists and speech-language therapists during this process.
Patients born without a cochlea (cochlear aplasia) or with an absent cranial nerve VIII are not candidates for cochlear implants. However, cochlear hypoplasia, a type of altered cochlear anatomy, is not a contraindication for cochlear implantation. This includes conditions like Mondini malformation, characterized by incomplete turns or partitions in the cochlea.
Patients who cannot tolerate general anesthesia are also not suitable candidates. Additionally, those with conductive hearing loss, unilateral sensorineural hearing loss, or hearing loss that can be effectively managed with hearing aids would benefit more from alternative treatment options, which an otolaryngologist can recommend. Clinicians should understand that cochlear implants do not resolve all types of hearing loss. Therefore, a comprehensive evaluation by an otolaryngologist and an audiologist is essential to determine the type and severity of hearing loss, as well as identify the most appropriate measures to address hearing loss.
Equipment
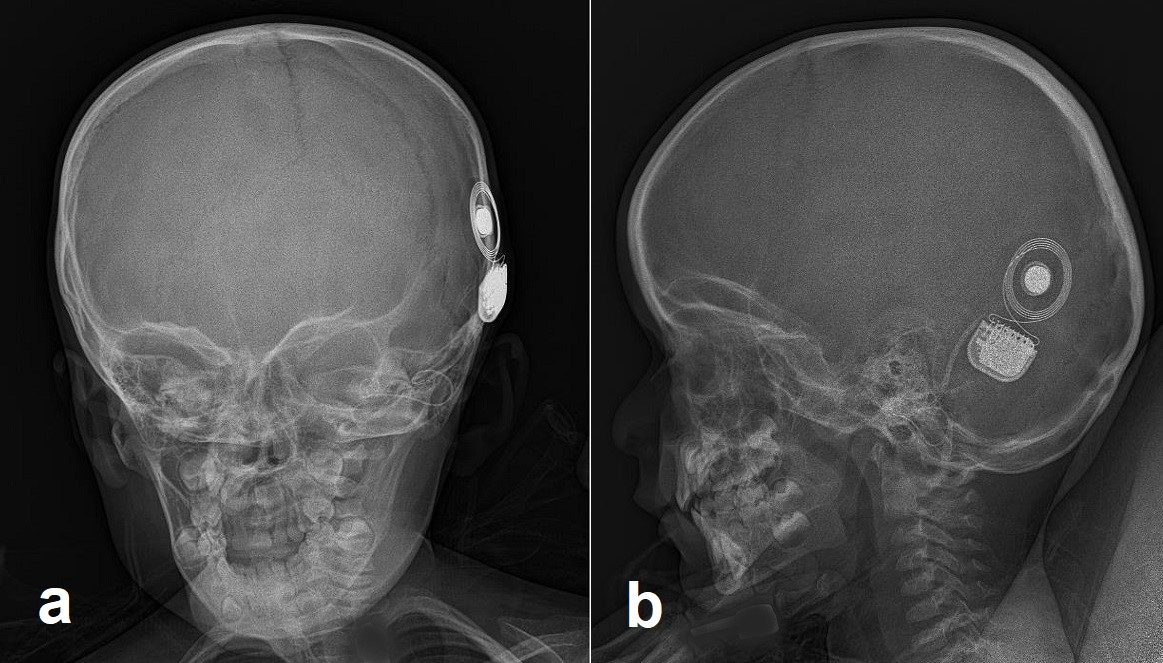
Cochlear implant systems consist of both external and internal components (see Image. Cochlear Implant Components). The external equipment includes a microphone, a sound processor, and a transmission system, while the internal device comprises a receiver/stimulator and an electrode array. The external microphone captures sounds and speech from the environment and sends this information to the sound processor. The sound processor converts these mechanical vibrations (sounds) into electrical signals. This electrical signal is then transmitted through the skin via radio frequency to the internal receiver/stimulator. For successful transmission, the external magnet on the transmitter must align with the internal magnet on the receiver/stimulator.
The receiver/stimulator accepts the electrical signal and transmits it to the electrode array positioned within the cochlea. The electrodes then stimulate the auditory nerve, allowing the signal to travel along the auditory pathway to the auditory cortex in the brain. Multiple manufacturers produce cochlear implants in the United States, each offering various specifications to customize the devices according to individual patient needs. Each manufacturer has specific device specifications and criteria for patient selection; please refer to these companies for detailed information regarding their products.
Personnel
Providing patient-centered care for individuals undergoing cochlear implantation requires collaboration among healthcare and educational professionals. The team may include internists, pediatricians, family physicians, geneticists, otolaryngologists, otologists, neurotologists, otolaryngology nurses, otolaryngology physician assistants, audiologists, speech-language pathologists, school administrators, school counselors, and cochlear implant manufacturer support personnel. Each member plays a distinct role in patient care, which can vary based on the patient's age, the age at diagnosis, whether the deafness is prelingual or postlingual, and the types of cochlear implant devices available.
Clinicians must possess the clinical skills and expertise to diagnose, evaluate, and determine a patient's candidacy for cochlear implantation. This process requires specialized surgical knowledge, an understanding of the radiographic and anatomical features of the temporal bone and facial nerve, recognition of potential complications, assessment of hearing status, and selection of the most appropriate cochlear implant for each patient. For patients who are prelingually deaf, the primary goal is to develop speech and achieve effective communication. For those who are postlingually deaf, the aim is to restore communication to a functional level. Effective communication among team members is essential for identifying, diagnosing, managing, and monitoring these individuals.[20]
An audiologist is a professional who conducts audiometry tests to assess the type and severity of hearing loss. After a cochlear implant is inserted, an audiologist works closely with patients, providing therapy to help them maximize the device's benefits. Audiologists may also adjust the device to fit the patient's specific needs. Many audiologists now hold advanced degrees in addition to their undergraduate degrees. Speech-language pathologists are communication experts who work with people of all ages. They are specially trained to help patients with speech sounds, language, social communication, voice, and fluency.
Otolaryngologists are medical doctors who diagnose sensorineural hearing loss and determine which patients are eligible for cochlear implants. They also perform the cochlear implantation surgery. Within this field, general otolaryngologists, otologists, and neurotologists may carry out this procedure. Those specializing in otology and neurotology typically undergo an additional 1 to 2 years of fellowship training after completing their otolaryngology residency.[20] After cochlear implantation, patients first follow up with an audiologist for the initial stimulation and programming of the device—essentially turning it on. The volume levels are customized based on the patient's clinical situation.
Regular follow-up appointments are essential for achieving successful outcomes after cochlear implantation. Adult cochlear implant patients should have their external devices evaluated and programmed annually. Pediatric patients, on the other hand, require evaluations at least twice a year. Many children are seen more frequently than twice a year, especially if there are concerns about their listening skills or to ensure that their devices are adjusted appropriately.
Preparation
Preparation for cochlear implantation starts with a thorough diagnosis of sensorineural hearing loss. The patient encounter begins with a detailed history and physical examination. Secondary causes of hearing loss, such as tympanic membrane perforation, middle ear effusion or infection, and canal atresia, must be ruled out. These issues must be addressed before placing a cochlear implant, as they can influence the audiological findings that determine whether patients are suitable candidates for hearing aids.
The next step involves obtaining pure tone audiometry and tympanometry. An auditory brainstem response test is necessary for children who cannot properly respond to sound. This test sends sound through the ear and assesses whether it reaches the cochlear nerve and the associated structures by measuring the electrical potential generated in response to the sound stimulation. This test is valuable for pediatric patients and in cases where hearing loss may be feigned.
Once bilateral sensorineural hearing loss is confirmed and meets the criteria for cochlear implantation, imaging becomes the subsequent step. A comprehensive history and physical examination may uncover findings that require further investigations or referrals to rule out any coexisting abnormalities or comorbidities. For pediatric patients identified as having profound sensorineural hearing loss, consideration of evaluation by geneticists should also be taken into account.[21]
Typically, a computed tomography (CT) scan of the temporal bones without contrast and an MRI of the internal auditory canals with and without contrast is obtained; however, either would suffice.[22] These images reveal the presence of the cochlear nerve and provide important anatomical details regarding the surgical anatomy for cochlear implant insertion.[9] Before considering a cochlear implant, conducting a trial of hearing amplification is essential. For newborns, hearing aids are generally recommended to be fitted by the age of 6 months, followed by a trial period of 6 months before pursuing a cochlear implant. This trial period is typically shorter in adults, ranging from 1 to 3 months. During this time, the effectiveness of hearing aids can be evaluated through repeated audiometric assessments.
If patients do not benefit from amplification and qualify for hearing aids, it is essential to discuss the risks and benefits of the cochlear implant, as well as any alternatives to surgery. Once appropriate informed consent has been obtained, the patient may schedule the surgery.[23] The CDC recommends vaccines against Streptococcus pneumoniae, specifically PCV13 and PPSV23, for individuals older than 2. PCV13 is safe for children under 2 years old and has been shown to lower the risk of meningitis related to cochlear implants.[24]
In the United States, insurance companies do not always cover bilateral cochlear implants. This creates challenges for the cochlear implant team in deciding which ear to implant. Typically, this decision takes into consideration the duration of deafness, the degree of hearing loss in each ear, and the patient's dominant hand for device manipulation. If there are no differences in hearing ability between the ears, the team usually selects the ear that is more favorable for surgery. Some studies indicate that it may not significantly matter which ear receives the implant, whether it's the better or worse-hearing ear. However, cochlear implants can provide substantial benefits to patients who meet the necessary criteria for the procedure.[25] Consider factors such as the ideal mastoid for drilling, the side with the least ossification or fibrosis in the cochlea, and the side exhibiting normal anatomy.[26][27]
Technique or Treatment
Cochlear implant surgery is performed in a hospital or surgical center's operating room. On the day of the surgery, the patient will meet with a team that includes nurses, anesthesiologists, and the otolaryngology team. The team will discuss their roles in the procedure, address any questions or concerns the patient may have, and obtain the patient's consent before proceeding to the operating room.
The surgery typically lasts 1 to 2 hours, and most patients are discharged on the same day or the following morning. Generally, patients do not experience significant pain and can resume their normal activities within 2 to 3 days. Some may experience mild dizziness for a few weeks afterward. The cochlear implant will not be activated until the incision has healed, usually taking about 4 weeks.
The surgery is performed under sterile conditions, and a timeout confirms the patient's identity and the correct surgical site. General anesthesia with an endotracheal tube is administered. Nerve monitoring is often employed to monitor the facial nerve during the procedure. The patient also undergoes sterile prepping. A standard mastoidectomy involves exposing the facial recess, which is a triangular space in the petrous temporal bone, using a mastoid drill based on the surgeon's preference. It is crucial to achieve complete exposure of the facial recess when placing the electrode for cochlear implantation. The facial recess is bordered superiorly by the fossa incudis, medially by the mastoid segment of the facial nerve, and laterally by the chorda tympani nerve.[28][29]
This procedure should ensure clear visibility of the cochlea's round window. Round window insertion may prevent inadvertent scala vestibuli insertion and may be preferred. If necessary, the surgeon may perform a cochleostomy. The processor is then positioned beneath the temporalis fascia, or a small bony well may be created to secure the device in the mastoid bone. The electrode is inserted through the opening of the round window or the cochleostomy, following the manufacturer's recommendations. Most modern cochlear implant devices have between 12 and 22 electrodes that stimulate the auditory nerve. During the implantation, an audiologist or a device representative should be present to verify the proper alignment of the device within the cochlea. An x-ray is taken to confirm the placement before closing the skin. The surgical closure is performed in layers, ensuring a cosmetically pleasing result.[30] A temporary mastoid pressure dressing is used for the first 24 to 48 hours.
Complications
When performed by a well-trained, experienced otolaryngologist, cochlear implantation is a safe and reliable procedure with few severe complications. Intraoperative and postoperative complications include:
- Bleeding, including life-threatening bleeding
- Seroma or hematoma
- Stroke
- Infection
- Increased risk for meningitis
- Pain
- Skin breakdown overlying the area of the magnet
- Device failure, including broken portions of the device, failed device, or improper placement in the cochlea
- Skull base damage
- Trauma to the brain
- Cerebrospinal fluid leaks
- Facial nerve paralysis/paresis
- Loss of taste on the ipsilateral side of the tongue
- Dizziness/vertigo (vestibular concerns)
- Loss of residual hearing in select populations
- Complete deafness
- Death [31][32]
Long-term complications are less well-defined and may include: [33]
- Skin infections
- Mastoiditis
- Electrode issues
- Device rejection
- Recurrent otitis
- Device migration
- Cholesteatoma
- Tympanic membrane perforation
- Chronic headaches
- Cerebral spinal fluid otorrhea [33]
Clinical Significance
Ethical considerations are important when determining treatment options and respecting patient autonomy in the decision-making process. Responsibilities within the interprofessional team should be clearly defined, with each member contributing their specialized knowledge and skills to optimize patient outcomes. Effective interprofessional communication fosters a collaborative environment where information is shared, questions are encouraged, and concerns are addressed promptly. Care coordination is crucial to ensure seamless and efficient patient care. Otologic surgeons, nurses, audiologists, speech pathologists, and other healthcare professionals must work together to streamline the patient's journey from diagnosis to cochlear implantation surgery and ongoing training and follow-up. This coordination minimizes errors, reduces delays, and enhances hearing outcomes, ultimately leading to improved patient-centered care that prioritizes the well-being and satisfaction of patients, their families, and their social interactions.
The goal of achieving effective patient outcomes is to identify hearing loss as early as possible through evaluations by an otolaryngologist and an audiologist. Currently, 43 states in the United States have established newborn hearing screening programs known as Early Hearing Detection and Intervention (EHDI). These programs facilitate the earlier diagnosis and management of congenital hearing loss, allowing for a timely assessment of the condition's severity and appropriate treatment initiation. Early identification of hearing impairment in infants and children may lead to an evaluation by a geneticist. The geneticist can determine the underlying causes of the impairment and identify any associated medical issues that require attention. This comprehensive evaluation can help prevent further hearing loss and allow for preventive measures in specific cases, eg, an enlarged vestibular aqueduct.
This device has allowed individuals once considered deaf to hear again, marking a significant medical achievement. People of all ages can benefit from this technology, which impacts patients differently at various life stages. In the elderly population, individuals with severe to profound sensorineural hearing loss face a higher risk of developing dementia earlier than their peers. Social isolation is often associated with hearing loss in older adults, and combined with a significant decline in quality of life and increased emotional challenges, it contributes to cognitive decline. Hearing aids can prevent dementia progression and may reverse mild cognitive impairment.[34] Studies indicate that appropriately selected patients can experience improvements in these areas through cochlear implants when hearing aids are not an option.[35] Cochlear implants may prolong a patient's life, as dementia can lead to an earlier death compared to those without the condition.
Early recognition of hearing loss is crucial in adults, as it helps identify the cause and enables appropriate management. This proactive approach can assist individuals in returning to work and regaining their quality of life. Hearing loss without adequate treatment is a risk factor for patient injury. It can decrease the quality of life and hinder patients from performing their duties at work and earning an income.[36]
Prelingually deaf children's ability to develop speech is significantly enhanced, which may reduce their need to learn alternative forms of communication, such as sign language. It is important to note that there is nothing wrong with individuals who choose or require alternative communication methods; these choices should be respected, as they can help facilitate integration into regular classroom environments. While educational expenses for all implanted patients tend to be higher compared to children without hearing impairment, the ultimate goal of achieving educational independence for most implanted children leads to overall savings. These savings typically range from $30,000 to $100,000 per child, taking into account the costs associated with the initial cochlear implantation and postoperative rehabilitation.[37] The primary objective is to perform bilateral cochlear implant surgeries for children, as this approach has been shown to enhance learning outcomes and offer a more natural listening experience for patients.[38]
Cochlear implants significantly enhance speech development, facilitate speech restoration for those who become deaf after language acquisition, and restore hearing. This has various benefits, eg, enabling safe navigation in traffic and improving the overall quality of life, including the enjoyment of music. They also support integration into regular classroom environments, which may lead to increased earning potential. Hearing aids can be tailored to meet individual needs. After a successful cochlear implant procedure, the audiology team collaborates with the patient to customize the implant settings to suit their daily requirements best. Research and technology surrounding cochlear implants are continually advancing, resulting in more user advantages and assisting cochlear implant teams in patient selection and management.
A strategic approach that involves evidence-based guidelines and individualized care plans tailored to each patient's unique circumstances is essential. Once patients are identified as candidates for cochlear implants, the otolaryngologist and audiologist collaborate to manage their care. The otolaryngologist performs the surgery while the audiologist assists in planning and implementing the device. An interprofessional team, including audiologists, speech-language pathologists, specially trained otolaryngology nurses, and other professionals, will be involved in postoperative care to ensure optimal device function. This team works collaboratively to maximize the benefits of the device and address any concerns that may arise during the recovery process.
Enhancing Healthcare Team Outcomes
Providing patient-centered care for individuals undergoing cochlear implantation requires a cohesive, interprofessional approach involving diverse healthcare and educational professionals. These include internists, pediatricians, family physicians, geneticists, otolaryngologists, neurotologists, audiologists, speech-language pathologists, nurses, physician assistants, school personnel, and cochlear implant manufacturer support teams. Each member contributes uniquely based on the patient’s age, the timing and type of hearing loss, and the available implant technology. Clinicians must demonstrate expertise in diagnosing and evaluating candidacy for implantation, surgical techniques, and postoperative care, tailoring strategies to meet the specific needs of prelingual or postlingual patients.
Effective interprofessional communication is critical to achieving optimal outcomes. Teams must collaborate to share information, address concerns, and develop individualized care plans grounded in evidence-based guidelines. For instance, otolaryngologists perform surgery, audiologists manage device programming, and speech-language pathologists support rehabilitation. Nurses and physician assistants are pivotal in preoperative and postoperative care, ensuring patient safety and addressing complications. School professionals and cochlear implant manufacturer representatives also provide essential support to integrate the technology into patients’ daily lives.
Clear delineation of roles and responsibilities fosters accountability and enhances team performance. Ethical considerations, including respecting patient autonomy and ensuring informed decision-making, are integral to the process. Care coordination ensures seamless transitions from diagnosis to treatment and ongoing follow-up, minimizing delays and errors while maximizing patient satisfaction and outcomes.
Nursing, Allied Health, and Interprofessional Team Monitoring
The nursing staff plays a crucial role in the success of cochlear implant surgery. Nurses are integral healthcare team members involved in all stages of cochlear implantation surgery.
- Preoperatively, nurses are responsible for preparing the patient for surgery. This includes
- Obtaining a thorough review of the patient’s medical history and checking vital signs.
- Identify any red flags that may affect the surgery, such as recent ear infections, concerns about the patient’s stability (e.g., heart attack or stroke), changes in medical history, or abnormal vital signs. If any issues arise, the surgeon should be promptly informed. For cochlear implant surgery to succeed, patients must be healthy enough to undergo it.
- Intraoperatively, nurses assist in setting up the patient and preparing the operating room with the required equipment.
- Each surgeon may have different preferences for conducting the surgery, so effective communication between the surgeon and nursing staff is vital.
- A general understanding of otologic instruments can significantly enhance surgical efficiency, which is especially important when performing surgery on young children under anesthesia.
- Nurses must be familiar with the procedure's general steps and be ready with the necessary tools for the surgeon.
- Postoperatively, nurses continue to monitor patients closely.
- Attending to the patient’s needs and accurately gathering vital signs are imperative.
- Monitoring the patient’s alertness, changing the mastoid dressing as instructed, and watching for any bleeding or purulent drainage from the incision site. The surgical team should be updated about any concerns.
- After surgery, patients typically experience mild to moderate pain, dizziness, drowsiness from anesthesia (which may be accompanied by nausea), and possible constipation due to pain medications. It is vital to recognize abnormal signs, such as facial weakness, sudden changes in mental status, bleeding that soaks through the mastoid dressing, severe pain that is not relieved with opioids, or abnormal vital signs. These symptoms require immediate communication with the surgical team for evaluation.
Communication with the healthcare team about the patient's progress after the immediate surgical period will begin with the audiologist. Audiologists will be the first to "turn on" the cochlear implant device and start the rehabilitation journey.
Media
(Click Image to Enlarge)
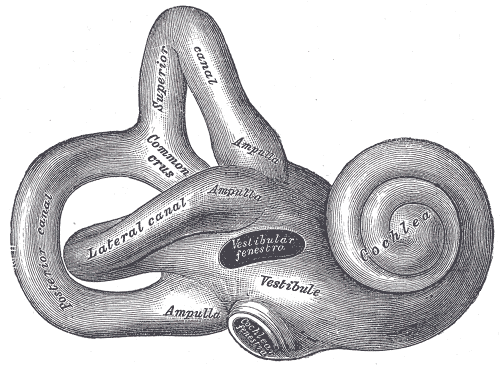
Internal Ear. Lateral view of the right osseous labyrinth with labeled components, including the lateral canal, common crus, ampulla, vestibule, and cochlea.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
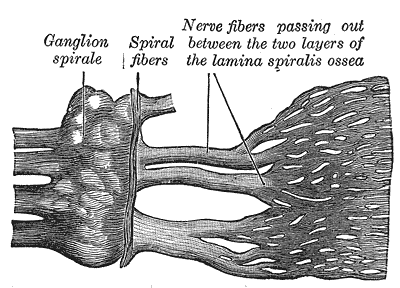
Cochlear Division of the Acoustic Nerve. Cochlear division of the acoustic nerve with labeled components, including the ganglion spirale and spiral fibers.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Park LR, Gagnon EB, Brown KD. The Limitations of FDA Criteria: Inconsistencies with Clinical Practice, Findings, and Adult Criteria as a Barrier to Pediatric Implantation. Seminars in hearing. 2021 Nov:42(4):373-380. doi: 10.1055/s-0041-1739370. Epub 2021 Dec 9 [PubMed PMID: 34912165]
Deep NL, Dowling EM, Jethanamest D, Carlson ML. Cochlear Implantation: An Overview. Journal of neurological surgery. Part B, Skull base. 2019 Apr:80(2):169-177. doi: 10.1055/s-0038-1669411. Epub 2018 Sep 6 [PubMed PMID: 30931225]
Level 3 (low-level) evidenceLim DJ. Functional structure of the organ of Corti: a review. Hearing research. 1986:22():117-46 [PubMed PMID: 3525482]
Level 3 (low-level) evidenceSzymanski A, Geiger Z. Anatomy, Head and Neck, Ear. StatPearls. 2025 Jan:(): [PubMed PMID: 29262017]
Jiam NT, Jiradejvong P, Pearl MS, Limb CJ. The Effect of Round Window vs Cochleostomy Surgical Approaches on Cochlear Implant Electrode Position: A Flat-Panel Computed Tomography Study. JAMA otolaryngology-- head & neck surgery. 2016 Sep 1:142(9):873-80. doi: 10.1001/jamaoto.2016.1512. Epub [PubMed PMID: 27355198]
Richard C, Fayad JN, Doherty J, Linthicum FH Jr. Round window versus cochleostomy technique in cochlear implantation: histologic findings. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2012 Sep:33(7):1181-7. doi: 10.1097/MAO.0b013e318263d56d. Epub [PubMed PMID: 22892806]
Roland PS, Wright CG, Isaacson B. Cochlear implant electrode insertion: the round window revisited. The Laryngoscope. 2007 Aug:117(8):1397-402 [PubMed PMID: 17585282]
Nadol JB Jr. Comparative anatomy of the cochlea and auditory nerve in mammals. Hearing research. 1988 Aug:34(3):253-66 [PubMed PMID: 3049492]
Level 3 (low-level) evidenceErixon E, Högstorp H, Wadin K, Rask-Andersen H. Variational anatomy of the human cochlea: implications for cochlear implantation. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2009 Jan:30(1):14-22. doi: 10.1097/MAO.0b013e31818a08e8. Epub [PubMed PMID: 18833017]
Tajudeen BA, Waltzman SB, Jethanamest D, Svirsky MA. Speech perception in congenitally deaf children receiving cochlear implants in the first year of life. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2010 Oct:31(8):1254-60. doi: 10.1097/MAO.0b013e3181f2f475. Epub [PubMed PMID: 20814343]
Sharma A, Gilley PM, Dorman MF, Baldwin R. Deprivation-induced cortical reorganization in children with cochlear implants. International journal of audiology. 2007 Sep:46(9):494-9 [PubMed PMID: 17828665]
Parry DA, Booth T, Roland PS. Advantages of magnetic resonance imaging over computed tomography in preoperative evaluation of pediatric cochlear implant candidates. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2005 Sep:26(5):976-82 [PubMed PMID: 16151346]
Gibson WP, Graham JM. Editorial: 'auditory neuropathy' and cochlear implantation - myths and facts. Cochlear implants international. 2008 Mar:9(1):1-7. doi: 10.1179/cim.2008.9.1.1. Epub [PubMed PMID: 18246533]
Level 3 (low-level) evidenceManrique M, Zubicaray J, Ruiz de Erenchun I, Huarte A, Manrique-Huarte R. [Guidelines for cochlear implant indication in Navarre]. Anales del sistema sanitario de Navarra. 2015 May-Aug:38(2):289-96 [PubMed PMID: 26486535]
Tucci DL, Telian SA, Zimmerman-Phillips S, Zwolan TA, Kileny PR. Cochlear implantation in patients with cochlear malformations. Archives of otolaryngology--head & neck surgery. 1995 Aug:121(8):833-8 [PubMed PMID: 7619406]
Schleich P, Nopp P, D'Haese P. Head shadow, squelch, and summation effects in bilateral users of the MED-EL COMBI 40/40+ cochlear implant. Ear and hearing. 2004 Jun:25(3):197-204 [PubMed PMID: 15179111]
Carlson ML, Breen JT, Driscoll CL, Link MJ, Neff BA, Gifford RH, Beatty CW. Cochlear implantation in patients with neurofibromatosis type 2: variables affecting auditory performance. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2012 Jul:33(5):853-62. doi: 10.1097/MAO.0b013e318254fba5. Epub [PubMed PMID: 22664900]
Ahsan S, Telischi F, Hodges A, Balkany T. Cochlear implantation concurrent with translabyrinthine acoustic neuroma resection. The Laryngoscope. 2003 Mar:113(3):472-4 [PubMed PMID: 12616199]
Modest MC, Carlson ML, Wanna GB, Driscoll CL. Cochlear Implantation in Patients With Superficial Siderosis: Seven Cases and Systematic Review of the Literature. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2015 Aug:36(7):1191-6. doi: 10.1097/MAO.0000000000000792. Epub [PubMed PMID: 26065403]
Level 1 (high-level) evidenceAlkhamra RA. Cochlear implants in children implanted in Jordan: A parental overview. International journal of pediatric otorhinolaryngology. 2015 Jul:79(7):1049-54. doi: 10.1016/j.ijporl.2015.04.025. Epub 2015 Apr 27 [PubMed PMID: 25990943]
Level 3 (low-level) evidenceRehm HL, Morton CC. A new age in the genetics of deafness. Genetics in medicine : official journal of the American College of Medical Genetics. 1999 Sep-Oct:1(6):295-302; quiz 303 [PubMed PMID: 11258632]
Gürtler N, Gysin C, Schmid N, Pieren C, Vischer M, Schumacher S, Oppermann P, Leuba D, Veraguth D. Bilateral congenital deafness: What investigations should be performed? Swiss medical weekly. 2017:147():w14416. doi: 10.4414/smw.2017.14416. Epub 2017 Mar 3 [PubMed PMID: 28322432]
Choi KJ, Kaylie DM. What is the role of preoperative imaging for cochlear implants in adults with postlingual deafness? The Laryngoscope. 2017 Feb:127(2):287-288. doi: 10.1002/lary.26084. Epub 2016 Jun 12 [PubMed PMID: 27292097]
Kahue CN, Sweeney AD, Carlson ML, Haynes DS. Vaccination recommendations and risk of meningitis following cochlear implantation. Current opinion in otolaryngology & head and neck surgery. 2014 Oct:22(5):359-66. doi: 10.1097/MOO.0000000000000092. Epub [PubMed PMID: 25101934]
Level 3 (low-level) evidenceFriedland DR, Venick HS, Niparko JK. Choice of ear for cochlear implantation: the effect of history and residual hearing on predicted postoperative performance. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2003 Jul:24(4):582-9 [PubMed PMID: 12851549]
Level 2 (mid-level) evidenceSürmelioğlu O, Cetik F, Tarkan O, Ozdemir S, Tuncer U, Kiroğlu M, Sahin R. Choice of cochlear implant side in a paediatric population. The Journal of laryngology and otology. 2014 Jun:128(6):504-7. doi: 10.1017/S0022215114001212. Epub 2014 Jun 3 [PubMed PMID: 24892225]
Level 2 (mid-level) evidenceAmaral MSAD, Damico TA, Gonçales AS, Reis ACMB, Isaac ML, Massuda ET, Hyppolito MA. Is there a best side for cochlear implants in post-lingual patients? Brazilian journal of otorhinolaryngology. 2018 Sep-Oct:84(5):560-565. doi: 10.1016/j.bjorl.2017.06.012. Epub 2017 Jul 29 [PubMed PMID: 28890230]
Öztürk K, Göde S, Çelik S, Orhan M, Bilge O, Bilgen C, Kirazlı T, Saylam CY. Revisiting the Anatomy of the Facial Recess: The Boundaries of the Round Window Exposure. Balkan medical journal. 2016 Sep:33(5):552-555 [PubMed PMID: 27761285]
Bielamowicz SA, Coker NJ, Jenkins HA, Igarashi M. Surgical dimensions of the facial recess in adults and children. Archives of otolaryngology--head & neck surgery. 1988 May:114(5):534-7 [PubMed PMID: 3355691]
Luers JC, Hüttenbrink KB, Beutner D. Surgical anatomy of the round window-Implications for cochlear implantation. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2018 Apr:43(2):417-424. doi: 10.1111/coa.13048. Epub 2018 Jan 3 [PubMed PMID: 29240305]
Farinetti A, Ben Gharbia D, Mancini J, Roman S, Nicollas R, Triglia JM. Cochlear implant complications in 403 patients: comparative study of adults and children and review of the literature. European annals of otorhinolaryngology, head and neck diseases. 2014 Jun:131(3):177-82. doi: 10.1016/j.anorl.2013.05.005. Epub 2014 Jun 2 [PubMed PMID: 24889283]
Level 2 (mid-level) evidenceCohen NL, Hoffman RA. Complications of cochlear implant surgery in adults and children. The Annals of otology, rhinology, and laryngology. 1991 Sep:100(9 Pt 1):708-11 [PubMed PMID: 1952660]
Terry B, Kelt RE, Jeyakumar A. Delayed Complications After Cochlear Implantation. JAMA otolaryngology-- head & neck surgery. 2015 Nov:141(11):1012-7. doi: 10.1001/jamaoto.2015.2154. Epub [PubMed PMID: 26469680]
Mosnier I, Vanier A, Bonnard D, Lina-Granade G, Truy E, Bordure P, Godey B, Marx M, Lescanne E, Venail F, Poncet C, Sterkers O, Belmin J. Long-Term Cognitive Prognosis of Profoundly Deaf Older Adults After Hearing Rehabilitation Using Cochlear Implants. Journal of the American Geriatrics Society. 2018 Aug:66(8):1553-1561. doi: 10.1111/jgs.15445. Epub 2018 Aug 8 [PubMed PMID: 30091185]
Clark JH, Yeagle J, Arbaje AI, Lin FR, Niparko JK, Francis HW. Cochlear implant rehabilitation in older adults: literature review and proposal of a conceptual framework. Journal of the American Geriatrics Society. 2012 Oct:60(10):1936-45. doi: 10.1111/j.1532-5415.2012.04150.x. Epub 2012 Sep 13 [PubMed PMID: 22974240]
Albernaz PL. History of cochlear implants. Brazilian journal of otorhinolaryngology. 2015 Mar-Apr:81(2):124-5. doi: 10.1016/j.bjorl.2014.12.006. Epub 2014 Dec 27 [PubMed PMID: 25681092]
Francis HW, Koch ME, Wyatt JR, Niparko JK. Trends in educational placement and cost-benefit considerations in children with cochlear implants. Archives of otolaryngology--head & neck surgery. 1999 May:125(5):499-505 [PubMed PMID: 10326806]
Spencer LJ, Barker BA, Tomblin JB. Exploring the language and literacy outcomes of pediatric cochlear implant users. Ear and hearing. 2003 Jun:24(3):236-47 [PubMed PMID: 12799546]