Introduction
Clonorchis sinensis is a trematode known as the Chinese or Oriental liver fluke. This parasitic infection is most commonly found in Eastern Asia, including Korea, China, and Vietnam, but it can be endemic in far eastern regions of Russia. These liver flukes are common parasites of fish-eating mammals. Cats and dogs of endemic areas are the most common hosts, but C sinensis can be transmitted to humans who eat infected fish. When infected, C sinensis can live for years within the biliary system of humans and result in various symptoms, including cholecystitis, cholangitis, and cholangiocarcinoma. This can be extremely burdensome for Asian immigrants who emigrate from an area of endemic infection and develop ongoing symptoms years after the initial infection. In parts of Asia, liver flukes are a public health problem, and more than 200 million people are at risk of infection in these regions.[1][2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Causes of clonorchiasis are mostly limited to the ingestion of fish encysted with C sinensis in endemic regions of the world, including East Asia.
The Lifecycle of C sinensis
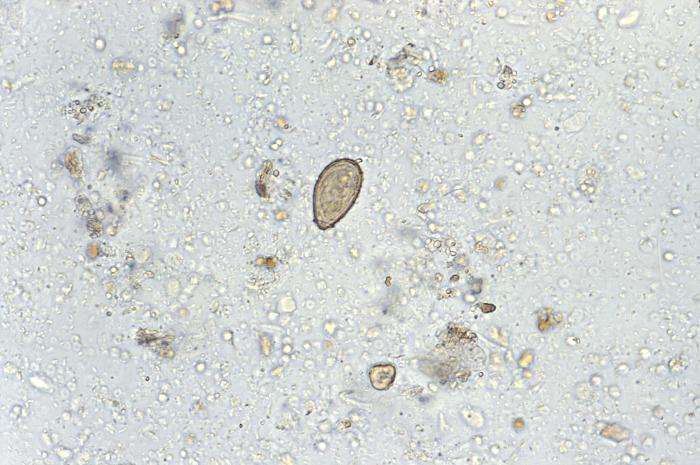
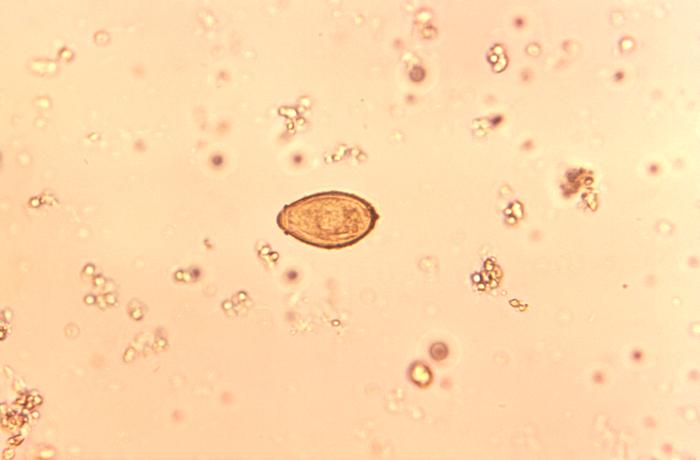
Eggs of C sinensis enter the water environment from feces (see Image. C Sinensis eggs). The first intermediate host then consumes these eggs, the freshwater snails, in which the eggs release miracidia, which undergo a series of developmental stages, such as sporocyst, rediae, and cercariae. The cercariae are released from the snail and are free-swimming in the water until they penetrate a freshwater fish. These cercariae encyst into the flesh of freshwater fish and mature to metacercariae. The infected fish is then ingested by a higher mammal, such as humans, where the metacercariae encyst as juvenile flukes in the duodenum. Hermaphroditic adult C sinensis ascends through the ampulla of Vater into the bile system and can often be found in the intrahepatic bile ducts. Complete maturation can take up to a month. [3] The life cycle can take up to 3 months before eggs show up in the sputum or feces of an infected adult.
Snails are important intermediate hosts, including Bithynia fuchsiana, Alocinma longicornis, and Parafossarulus striatulus. These snails are often found in the local waterways, ponds, lakes, and paddy fields. Eating raw or uncooked freshwater fish (mostly of the Cyprinidae family) can lead to parasitic infection. Still, contamination of utensils and food with Metacercariae is also a potential infection route, especially in children and women.
Epidemiology
C sinensis is predominantly endemic to the Eastern hemispheres of the world, mainly East Asia and parts of Russia. Previously, it was also prevalent in Japan, but it virtually disappeared after the Second World War following the modernization of agriculture.[4][5]
Additionally, emigrants or travelers from endemic areas increase the risk of disease transmission to other countries.[6] It is estimated that about 15 to 20 million people are currently infected with C sinensis, and 13 million are alone in China, which indicates a dramatic increase compared to an estimated 7 million infections in the 1990s.[7][8] More than 200 million people are at risk of infection in these regions due to the frequent ingestion of improperly cooked fish; the World Health Organization (WHO) therefore listed C sinensis among the most neglected tropical diseases globally.[9] Large fish are commonly less contaminated with metacercariae, but repetitive exposure through frequent ingestion can result in an overall accumulation of adults in the human bile ducts. Additionally, over 31 types of fish and shrimp are hosts of C sinensis, many of which are common fish consumed by humans and are served in restaurants in endemic regions.[10][11]
Qian et al. indicated in a recent study the presence of C sinensis eggs in almost 20% of the feces of 500 school children aged 10 to 17 years in the highly affected Hunan province of southeastern China.[12] Other affected areas in China include the Guangdong province, which borders Hong Kong and Macau, and the Guangxi province.[2][11]
Men often have a better understanding of the existence of clonorchiasis, but because of still existing misconceptions (eg, "alcohol can prevent infection") and deeply embedded cultural beliefs that eating raw fish is healthy.[13][6] Infection rates show a preference for males over females, with an infection rate increasing with age, climaxing in the 50s.[14][13] Other potential ways of infection include contamination of utensils and food with metacercariae, as cooked and uncooked food is rarely separated. This method of transmission is commonly responsible for infections in children and women.
Pathophysiology
C sinensis is 1 of the most common parasitic infections in the world (see Image. Pathology, Trematode, Parasite, Clonorchis Sinensis eggs, Infection). In 2009, C sinensis was classified as a carcinogen by the International Agency for Cancer Research.[15] Approximately 25 to 35 per 100.000 cholangiocarcinomas are attributable to clonorchiasis in endemic areas.[8] The exact events leading to cholangiocarcinoma are unknown; several mechanisms are believed to play a role. One mechanism includes mechanical injury related to damage to the bile duct mucosa related to the feeding activities of the parasite. Secondly, local accumulation of worms results in bile stasis, favoring bacterial growth, inflammation, and, subsequently, recurrent cholangitis. Thirdly, the toxic effects of excretory-secretory products (ESPs) released by the parasite also result in inflammation.[16]
History and Physical
The clinical consequences of infection are directly related to the intensity and duration of infection. The worm burden can be estimated by the number of eggs in feces, which become detectable in stool after 3 to 4 weeks.[17] The more eggs are present, the more symptoms can be expected.[18][19] Symptoms occur 10 to 30 days after ingesting an infected fish and are present for at least 2 to 4 weeks. Chronic symptoms are often observed in patients with a heavy burden of liver flukes. These chronic symptoms are generally a result of chronic mechanical and physical injuries of the bile ducts.
In the early phase of infection, most infected patients are considered to have an innocuous infection (less than 100 flukes) and rarely have symptoms. Patients with a very heavy parasitic load (>20 000) can present with symptoms compatible with acute cholangitis, including jaundice, right upper quadrant abdominal pain, nausea, vomiting, anorexia, malaise, and fevers. [6] Chronic clonorchiasis can result in several symptoms involving the liver and biliary system. Cholelithiasis is commonly observed and is likely related to C sinensis, predisposing to the formation of gallstones, especially calcium carbonate stones. C sinensis induces hyperplastic changes in the gallbladder wall, resulting in decreased gallbladder contractions and the subsequent precipitation of bilirubinate and mucin on the parasitic eggs.[20][21]
Furthermore, pancreatitis and liver abscesses appear more frequently in patients with clonorchiasis.[22] Chronic obstruction and strictures of the bile ducts can also result in bile stasis and recurrent ascending cholangitis mediated by Escherichia coli.[6] Clonorchis-associated cholangiocarcinoma is another complication of chronic inflammation and induced cellular proliferation and is often adenomatous in histology.[23] Children with a high parasitic load are at risk for potential developmental delays related to ongoing diarrhea, malnutrition, and anemia.[6]
Evaluation
The gold standard of diagnosing C sinensis is detecting eggs in the stool, providing insight into the intensity of infection.[24] Another marker of infection intensity includes bile and serum concentration of IgG4.[6] The absence of eggs in feces does not rule out clonorchiasis, as eggs are usually undetectable in stool during biliary obstruction.[25] Therefore, direct evidence of parasites can also be obtained in duodenal aspirates or biliary secretions via endoscopic retrograde cholangiopancreatography (ERCP).[7][3]
Standard diagnostic tests include a direct fecal smear via the Kato-Katz method, which is a method of preparing stool samples where stool is pressed through a mesh, and the remaining sample is a piece of cellophane soaked in glycerol which clears the stool from the eggs. The sensitivity of the test correlates with the intensity of infection, and the sensitivity of the test in low-intensity infections can be increased with repetitive assessments of stool samples. As differentiation between Clonorchis sinensis and Opisthorchis viverrine eggs is difficult (they appear morphologically similar), other factors like geographic exposure must be considered.
Alternatively, the formalin-ether centrifuge sedimentation method concentrates the eggs in the feces and has better sensitivity than the Kato-Katz method, especially in suspected low-intensity cases.[26][14] Serodiagnostic tests like enzyme-linked immunosorbent assay (ELISA) are commonly used and are equally helpful for diagnosing C sinensis. Specific antigens used in ELISA include ESPs and crude extract antigens, with the caveat that these assays can potentially cross-react with infections caused by other trematodes.[1]
Polymerase chain reaction (PCR) is also frequently used and has high sensitivity and specificity for diagnosing C sinensis, but it might not be readily available. Newer diagnostic tools include loop-mediated isothermal amplification (LAMP), which targets C sinensis DNA and is optimal for point-of-care diagnosis.[27][14] Despite these technical achievements within the last few years, the underdiagnosis of C sinensis is still a major issue due to the lack of diagnostic tools and training within the affected areas.[6]
Imaging modalities like ultrasound, CT, and MRI are useful to determine the infection's location, progression, and extent. On ultrasound, diffuse dilatation of intrahepatic bile ducts is commonly seen in patients infected with Clonorchis sinensis. Still, the finding is too unspecific for diagnostic purposes and is significantly operator-dependent.[28][29] Choi et al. indicated in a sonographic study with 457 subjects that enhanced periductal echogenicity and floating echogenic foci in the gallbladder are useful for diagnosing clonorchiasis.[30] CT imaging can help delineate the diagnosis of clonorchiasis, as it shows a regular caliber of the unaffected larger bile ducts/extrahepatic ducts compared to the more frequently affected intrahepatic bile ducts.[22] MR cholangiographic imaging is also helpful in the diagnosis of C sinensis. A classic sign of this imaging modality includes "too many intrahepatic ducts"- a sign resulting from parasitic obstruction of the smaller intrahepatic bile ducts in the periphery.[31]
Treatment / Management
The most effective treatment for clonorchiasis is praziquantel. The World Health Organization (WHO) recommends 25 mg/kg orally 3 times daily for 2 to 3 days or 40mg/kg in a single administration, resulting in cure rates above 90%.[32][33] Regional policies might vary, including higher dosing with less frequent administration, eg, 75 mg/kg of praziquantel twice daily within 48 hours.[32] In the case of heavy parasitic infections or the continuous presence of eggs in feces after treatment, re-treatment is necessary.[32] Associated side effects of praziquantel include abdominal discomfort, vomiting, and cephalgias.[34] (B3)
Of note, the recovery period for pathological changes of the bile duct may be prolonged for weeks, and symptoms can persist despite adequate treatment. As symptoms tend to be unspecific and large populations are unknowingly inhabited by the parasite, pilot studies elucidated the effects of mass treatments in endemic areas with praziquantel, indicating positive effects of(semi-)annual treatment with praziquantel reducing the prevalence from 59.5% in 2001 to 7.5% in 2004.[35]
An alternative regimen includes albendazole (10mg/kg) for 5 to 7 days.[36][6] Patients’ family members and close contacts should also be tested for infection because it is more than likely that they may have eaten the same contaminated food or were exposed to metacercariae by contaminated surfaces.(B2)
According to recent studies, another alternative includes tribendimidine, a relatively new drug with promising cure rates and a favorable side effect profile.[37][32] Researchers have been trying to develop a vaccine, but these have only been tested in animals.(B3)
Differential Diagnosis
The differential diagnosis includes acute hepatitis, cholecystitis, choledocholithiasis, cholangiocarcinoma, primary sclerosing cholangitis, primary biliary cholangitis, and other parasitic infections, including schistosomiasis, fascioliasis, and ascariasis.[1]
Prognosis
An initial prognosis can be based on the number of eggs found in feces, as more eggs indicate more pronounced disease intensity.[17][18][19] Initial infections are often untreated as most patients remain asymptomatic or have non-specific symptoms. The reversibility of pathological changes induced by C sinensis is largely dependent on the timing of initiation of treatment, meaning that when praziquantel treatment is delayed, the recovery period for pathological changes of the bile duct may be up to 12 weeks, and even longer or irreversible if the infection is severe or prolonged.[38]
Initial symptoms of C sinensis are frequently unspecific so that they can result in a missed diagnosis and the inhabitation of C sinensis adult worms for many years.[19] Chronic complications like cholecystitis, cholangitis, or liver abscesses usually respond to symptomatic treatment. The prognosis of cholangiocarcinoma is sinister, with a median overall survival of fewer than 30 months.[39]
Complications
In 2009, C sinensis was classified as a carcinogen by the International Agency for Cancer Research. The liver flukes reside within the biliary tree and cause obstruction, inflammatory irritation, and fibrosis, resulting in the potential development of cholangiocarcinoma (CCA).[8] One study even reported Clonorchis sinensis as a risk factor for hepatic carcinoma, potentially synergistically occurring in a co-infected patient with HBV infection or alcohol use disorder.[40] As initial symptoms are often non-specific and missed for many years, histological changes like the epithelial proliferation of bile ducts and periductal fibrosis go unnoticed and can result in adenomatous hyperplasia, giving rise to neoplastic changes.[19][41]
Long-standing infection also increases the risk of strictures of the bile ducts and, together with the mechanical obstruction caused by the parasite, can result in recurrent pyogenic cholangitis, pancreatitis, and liver abscesses.[22] Regarding cholangiocarcinoma, C sinensis infection increases the risk of intrahepatic and extrahepatic CCA.[42][41] Children with a high parasitic load are at risk for potential developmental delays related to ongoing diarrhea, malnutrition, and anemia.[6]
Deterrence and Patient Education
Preventing C sinensis infection is heavily based on patient education and proper food preparation. Endemic areas promote education with public awareness programs, health guide booklets, and educating school children. These educational programs enforce proper food preparation and avoidance of undercooked or raw fish. Removing farm animals from nearby fish farms and recognizing symptoms is also essential. These areas must undergo cultural changes to begin the decline of infection rates of C sinensis, including replanting family farms to move farm animals from water sources and decreasing the consumption of raw fish in their diet.[43]
Enhancing Healthcare Team Outcomes
While most C sinensis infections are asymptomatic with a parasite load of approximately 100, acute infections require a more detailed and rigorous management strategy with several medical teams on board. Patients with a heavy parasite load of approximately 20,000 are at a higher risk for chronic, long-term issues. Such risks include ascending cholangitis, cholangiocarcinoma, and hepatitis. Once it has become clear that a parasitic liver infection has developed, an interprofessional healthcare team, including gastrointestinal (GI)/surgery, internal medicine, and infectious disease specialists, must have a clear line of communication to begin therapy as soon as possible.
Specialty-trained infection control and gastroenterology nurses should educate patients and their families, monitor patients, and provide status reports to the interprofessional team. Pharmacists evaluate the appropriateness of medications prescribed, assess drug-drug interactions, and participate in education. It is also important to accurately diagnose which parasite is the culprit of the current disease, as there are several types of different parasitic liver infections. Clear communication between all involved specialties ensures that the patient and any close contacts are treated quickly and appropriately to avoid severe consequences.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Hong ST, Fang Y. Clonorchis sinensis and clonorchiasis, an update. Parasitology international. 2012 Mar:61(1):17-24. doi: 10.1016/j.parint.2011.06.007. Epub 2011 Jun 30 [PubMed PMID: 21741496]
Level 3 (low-level) evidenceQian MB, Chen YD, Yan F. Time to tackle clonorchiasis in China. Infectious diseases of poverty. 2013 Feb 19:2(1):4. doi: 10.1186/2049-9957-2-4. Epub 2013 Feb 19 [PubMed PMID: 23849773]
Level 2 (mid-level) evidenceKim TS, Pak JH, Kim JB, Bahk YY. Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: a brief review. BMB reports. 2016 Nov:49(11):590-597 [PubMed PMID: 27418285]
Yoshida Y. Clonorchiasis--a historical review of contributions of Japanese parasitologists. Parasitology international. 2012 Mar:61(1):5-9. doi: 10.1016/j.parint.2011.06.003. Epub 2011 Jul 3 [PubMed PMID: 21749930]
Level 3 (low-level) evidence. Control of foodborne trematode infections. Report of a WHO Study Group. World Health Organization technical report series. 1995:849():1-157 [PubMed PMID: 7740791]
Level 3 (low-level) evidenceQian MB, Utzinger J, Keiser J, Zhou XN. Clonorchiasis. Lancet (London, England). 2016 Feb 20:387(10020):800-10. doi: 10.1016/S0140-6736(15)60313-0. Epub 2015 Aug 21 [PubMed PMID: 26299184]
Level 2 (mid-level) evidenceFürst T, Keiser J, Utzinger J. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. The Lancet. Infectious diseases. 2012 Mar:12(3):210-21. doi: 10.1016/S1473-3099(11)70294-8. Epub 2011 Nov 20 [PubMed PMID: 22108757]
Level 1 (high-level) evidenceQian MB, Chen YD, Liang S, Yang GJ, Zhou XN. The global epidemiology of clonorchiasis and its relation with cholangiocarcinoma. Infectious diseases of poverty. 2012 Oct 25:1(1):4. doi: 10.1186/2049-9957-1-4. Epub 2012 Oct 25 [PubMed PMID: 23849183]
Level 2 (mid-level) evidenceSripa B. Concerted action is needed to tackle liver fluke infections in Asia. PLoS neglected tropical diseases. 2008 May 28:2(5):e232. doi: 10.1371/journal.pntd.0000232. Epub 2008 May 28 [PubMed PMID: 18509525]
Level 3 (low-level) evidenceFang YY, Chen YD, Li XM, Wu J, Zhang QM, Ruan CW. [Current prevalence of Clonorchis sinensis infection in endemic areas of China]. Zhongguo ji sheng chong xue yu ji sheng chong bing za zhi = Chinese journal of parasitology & parasitic diseases. 2008 Apr:26(2):99-103, 109 [PubMed PMID: 24812810]
Level 3 (low-level) evidenceWang KX, Zhang RB, Cui YB, Tian Y, Cai R, Li CP. Clinical and epidemiological features of patients with clonorchiasis. World journal of gastroenterology. 2004 Feb 1:10(3):446-8 [PubMed PMID: 14760777]
Level 3 (low-level) evidenceQian MB, Zhuang SF, Zhu SQ, Deng XM, Li ZX, Zhou XN. Epidemiology and determinants of clonorchiasis in school children in southeastern China. Acta tropica. 2021 Apr:216():105752. doi: 10.1016/j.actatropica.2020.105752. Epub 2020 Nov 12 [PubMed PMID: 33188749]
Level 2 (mid-level) evidenceQian MB, Chen YD, Fang YY, Tan T, Zhu TJ, Zhou CH, Wang GF, Xu LQ, Zhou XN. Epidemiological profile of Clonorchis sinensis infection in one community, Guangdong, People's Republic of China. Parasites & vectors. 2013 Jul 1:6():194. doi: 10.1186/1756-3305-6-194. Epub 2013 Jul 1 [PubMed PMID: 23816055]
Level 3 (low-level) evidenceNa BK, Pak JH, Hong SJ. Clonorchis sinensis and clonorchiasis. Acta tropica. 2020 Mar:203():105309. doi: 10.1016/j.actatropica.2019.105309. Epub 2019 Dec 17 [PubMed PMID: 31862466]
Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V, WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens--Part B: biological agents. The Lancet. Oncology. 2009 Apr:10(4):321-2 [PubMed PMID: 19350698]
Zheng M, Hu K, Liu W, Hu X, Hu F, Huang L, Wang P, Hu Y, Huang Y, Li W, Liang C, Yin X, He Q, Yu X. Proteomic analysis of excretory secretory products from Clonorchis sinensis adult worms: molecular characterization and serological reactivity of a excretory-secretory antigen-fructose-1,6-bisphosphatase. Parasitology research. 2011 Sep:109(3):737-44. doi: 10.1007/s00436-011-2316-5. Epub 2011 Mar 22 [PubMed PMID: 21424807]
Level 3 (low-level) evidenceQian MB, Zhou XN. Clonorchis sinensis. Trends in parasitology. 2021 Nov:37(11):1014-1015. doi: 10.1016/j.pt.2021.05.011. Epub 2021 Jul 3 [PubMed PMID: 34229953]
Level 2 (mid-level) evidenceKim JH, Choi MH, Bae YM, Oh JK, Lim MK, Hong ST. Correlation between discharged worms and fecal egg counts in human clonorchiasis. PLoS neglected tropical diseases. 2011 Oct:5(10):e1339. doi: 10.1371/journal.pntd.0001339. Epub 2011 Oct 4 [PubMed PMID: 21991401]
Level 3 (low-level) evidenceLun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, Fang YY. Clonorchiasis: a key foodborne zoonosis in China. The Lancet. Infectious diseases. 2005 Jan:5(1):31-41 [PubMed PMID: 15620559]
Level 3 (low-level) evidenceQiao T, Ma RH, Luo ZL, Yang LQ, Luo XB, Zheng PM. Clonorcis sinensis eggs are associated with calcium carbonate gallbladder stones. Acta tropica. 2014 Oct:138():28-37. doi: 10.1016/j.actatropica.2014.06.004. Epub 2014 Jun 16 [PubMed PMID: 24945791]
Level 3 (low-level) evidenceChoi D, Lim JH, Lee KT, Lee JK, Choi SH, Heo JS, Choi DW, Jang KT, Lee NY, Kim S, Hong ST. Gallstones and Clonorchis sinensis infection: a hospital-based case-control study in Korea. Journal of gastroenterology and hepatology. 2008 Aug:23(8 Pt 2):e399-404 [PubMed PMID: 18070015]
Level 3 (low-level) evidenceLim JH. Radiologic findings of clonorchiasis. AJR. American journal of roentgenology. 1990 Nov:155(5):1001-8 [PubMed PMID: 2120925]
Papachristou GI, Schoedel KE, Ramanathan R, Rabinovitz M. Clonorchis sinensis-associated cholangiocarcinoma: a case report and review of the literature. Digestive diseases and sciences. 2005 Nov:50(11):2159-62 [PubMed PMID: 16240232]
Level 3 (low-level) evidenceHong ST, Choi MH, Kim CH, Chung BS, Ji Z. The Kato-Katz method is reliable for diagnosis of Clonorchis sinensis infection. Diagnostic microbiology and infectious disease. 2003 Sep:47(1):345-7 [PubMed PMID: 12967748]
Level 3 (low-level) evidenceJoo KR, Bang SJ. A bile based study of Clonorchis sinensis infections in patients with biliary tract diseases in Ulsan, Korea. Yonsei medical journal. 2005 Dec 31:46(6):794-8 [PubMed PMID: 16385655]
Level 3 (low-level) evidenceQian MB, Yap P, Yang YC, Liang H, Jiang ZH, Li W, Utzinger J, Zhou XN, Keiser J. Accuracy of the Kato-Katz method and formalin-ether concentration technique for the diagnosis of Clonorchis sinensis, and implication for assessing drug efficacy. Parasites & vectors. 2013 Oct 29:6(1):314. doi: 10.1186/1756-3305-6-314. Epub 2013 Oct 29 [PubMed PMID: 24499644]
Level 3 (low-level) evidenceKim EM, Verweij JJ, Jalili A, van Lieshout L, Choi MH, Bae YM, Lim MK, Hong ST. Detection of Clonorchis sinensis in stool samples using real-time PCR. Annals of tropical medicine and parasitology. 2009 Sep:103(6):513-8. doi: 10.1179/136485909X451834. Epub [PubMed PMID: 19695156]
Level 3 (low-level) evidenceHong ST, Yoon K, Lee M, Seo M, Choi MH, Sim JS, Choi BI, Yun CK, Lee SH. Control of clonorchiasis by repeated praziquantel treatment and low diagnostic efficacy of sonography. The Korean journal of parasitology. 1998 Dec:36(4):249-54 [PubMed PMID: 9868890]
Level 3 (low-level) evidenceChoi D, Hong ST. Imaging diagnosis of clonorchiasis. The Korean journal of parasitology. 2007 Jun:45(2):77-85 [PubMed PMID: 17570969]
Choi D, Hong ST, Lim JH, Cho SY, Rim HJ, Ji Z, Yuan R, Wang S. Sonographic findings of active Clonorchis sinensis infection. Journal of clinical ultrasound : JCU. 2004 Jan:32(1):17-23 [PubMed PMID: 14705173]
Level 3 (low-level) evidenceJeong YY, Kang HK, Kim JW, Yoon W, Chung TW, Ko SW. MR imaging findings of clonorchiasis. Korean journal of radiology. 2004 Jan-Mar:5(1):25-30 [PubMed PMID: 15064556]
Xu LL, Jiang B, Duan JH, Zhuang SF, Liu YC, Zhu SQ, Zhang LP, Zhang HB, Xiao SH, Zhou XN. Efficacy and safety of praziquantel, tribendimidine and mebendazole in patients with co-infection of Clonorchis sinensis and other helminths. PLoS neglected tropical diseases. 2014 Aug:8(8):e3046. doi: 10.1371/journal.pntd.0003046. Epub 2014 Aug 14 [PubMed PMID: 25122121]
Level 3 (low-level) evidenceKeiser J, Utzinger J. The drugs we have and the drugs we need against major helminth infections. Advances in parasitology. 2010:73():197-230. doi: 10.1016/S0065-308X(10)73008-6. Epub [PubMed PMID: 20627144]
Level 3 (low-level) evidenceLee SH. Large scale treatment of Clonorchis sinensis infections with praziquantel under field conditions. Arzneimittel-Forschung. 1984:34(9B):1227-30 [PubMed PMID: 6542401]
Choi MH, Park SK, Li Z, Ji Z, Yu G, Feng Z, Xu L, Cho SY, Rim HJ, Lee SH, Hong ST. Effect of control strategies on prevalence, incidence and re-infection of clonorchiasis in endemic areas of China. PLoS neglected tropical diseases. 2010 Feb 16:4(2):e601. doi: 10.1371/journal.pntd.0000601. Epub 2010 Feb 16 [PubMed PMID: 20169061]
Liu YH, Wang XG, Gao P, Qian MX. Experimental and clinical trial of albendazole in the treatment of Clonorchiasis sinensis. Chinese medical journal. 1991 Jan:104(1):27-31 [PubMed PMID: 1879192]
Level 3 (low-level) evidenceQian MB, Yap P, Yang YC, Liang H, Jiang ZH, Li W, Tan YG, Zhou H, Utzinger J, Zhou XN, Keiser J. Efficacy and safety of tribendimidine against Clonorchis sinensis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 Apr:56(7):e76-82. doi: 10.1093/cid/cis1011. Epub 2012 Dec 7 [PubMed PMID: 23223597]
Level 3 (low-level) evidenceLee SH, Hong ST, Kim CS, Sohn WM, Chai JY, Lee YS. Histopathological changes of the liver after praziquantel treatment in Clonorchis sinensis infected rabbits. Kisaengch'unghak chapchi. The Korean journal of parasitology. 1987 Dec:25(2):110-122 [PubMed PMID: 12886061]
Zhang GW, Lin JH, Qian JP, Zhou J. Identification of risk and prognostic factors for patients with clonorchiasis-associated intrahepatic cholangiocarcinoma. Annals of surgical oncology. 2014 Oct:21(11):3628-37. doi: 10.1245/s10434-014-3710-x. Epub 2014 Apr 30 [PubMed PMID: 24781504]
Level 3 (low-level) evidenceTan SK, Qiu XQ, Yu HP, Zeng XY, Zhao YN, Hu L. [Evaluation of the risk of clonorchiasis inducing primary hepatocellular carcinoma]. Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chinese journal of hepatology. 2008 Feb:16(2):114-6 [PubMed PMID: 18304427]
Level 3 (low-level) evidenceChoi BI, Han JK, Hong ST, Lee KH. Clonorchiasis and cholangiocarcinoma: etiologic relationship and imaging diagnosis. Clinical microbiology reviews. 2004 Jul:17(3):540-52, table of contents [PubMed PMID: 15258092]
Level 3 (low-level) evidenceChoi D, Lim JH, Lee KT, Lee JK, Choi SH, Heo JS, Jang KT, Lee NY, Kim S, Hong ST. Cholangiocarcinoma and Clonorchis sinensis infection: a case-control study in Korea. Journal of hepatology. 2006 Jun:44(6):1066-73 [PubMed PMID: 16480786]
Level 3 (low-level) evidenceTang ZL, Huang Y, Yu XB. Current status and perspectives of Clonorchis sinensis and clonorchiasis: epidemiology, pathogenesis, omics, prevention and control. Infectious diseases of poverty. 2016 Jul 6:5(1):71. doi: 10.1186/s40249-016-0166-1. Epub 2016 Jul 6 [PubMed PMID: 27384714]
Level 3 (low-level) evidence