Introduction
The blood supply to the brain divides into an anterior and posterior circulation. The anterior circulation derives blood from the bilateral internal carotid arteries (ICA) and supplies blood to the majority of the cerebral hemispheres, including the frontal lobes, parietal lobes, lateral temporal lobes and anterior part of deep cerebral hemispheres. The posterior circulation derives blood from the bilateral vertebral arteries (VA). It supplies the brainstem, cerebellum, occipital lobes, medial temporal lobes and posterior part of the deep hemisphere, mainly the thalamus. The circle of Willis (CoW) is an anatomical structure that provides an anastomotic connection between the anterior and posterior circulations, providing collateral flow to affected brain regions in the event of arterial incompetency.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Structure
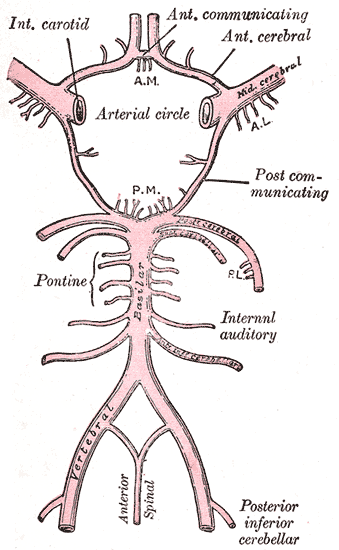
The circle of Willis is a ring of vessels connecting the anterior and posterior circulations of the brain. The ring is bounded anteriorly by a single anterior communicating artery (ACom), which connects the bilateral anterior cerebral arteries (ACA). The ACAs course posterolaterally until reaching their lateral-most connection to the ICA, which runs cephalically through the neck and into the brain. As each ICA runs its course, they individually give off an ophthalmic artery. At the point of connection between the ACA and the ICA, the lateral continuation of the ICA becomes the middle cerebral artery (MCA). Coursing posteromedially from each ACA-ICA junction is the posterior communicating artery (PCom). The PCom connects the MCA with the posterior cerebral arteries (PCA), which form the posterior-most aspect of the CoW. The bilateral PCAs fuse to become the basilar artery (BA). The BA courses caudally along the anterior pons, giving off many branches, including the superior cerebellar arteries, and pontine arteries, and the anterior inferior cerebellar artery. The BA then divides into the bilateral VAs, which each gives off a posterior inferior cerebellar artery (PICA) and contributes to the formation of a single anterior spinal artery.[1][2][3][4]
Function
The circle of Willis acts to provide collateral blood flow between the anterior and posterior circulations of the brain, protecting against ischemia in the event of vessel disease or damage in one or more areas.
Embryology
The formation of the cerebral circulation begins with the development of six pairs of branchial arch arteries. The third branchial arch arteries contribute to the formation of the ICAs early in embryonic life. The second branchial arch arteries help form the ventral pharyngeal arteries, which fuse proximally with the ICAs to form the common carotid arteries (CCA). Around 28 days of development, the ICA branches into anterior and posterior divisions. The anterior ICA gives rise to the ACA, MCA, and anterior choroidal artery; the posterior division gives rise to the PCA and the posterior choroidal artery.[2]
The growth of the occipital lobe and brainstem trigger the development of the posterior circulation. Early in development, the hindbrain receives vascular supply from the carotid-vertebrobasilar connections via the trigeminal artery (TA), the otic artery (OA), the hypoglossal artery (HA), and the proatlantal artery (ProA). When the PCom forms and connects to the distal BA, the TA, OA, and HA regress. The ProA remains until the VAs develop.[2]
The development of the MCA begins around 35 days from the anterior division of the ICA. Meanwhile, the ACA grows medially, leading to the development of the ACom. The formation of the ACA and ACom marks the full development of the CoW, typically occurring at 6 to 7 weeks of development.[2]
Blood Supply and Lymphatics
Blood Supply
The anterior circulation of the brain derives from the bilateral ICAs, branches of the common carotid arteries (CCA). The posterior circulation derives from the bilateral VAs, branches of the subclavian arteries.[1][2][3]
Lymphatics
There is no evidence to date supporting the existence of conventional lymphatic vessels within the central nervous system (CNS). Recent research, however, has described functional lymphatic vessels in the dural sinuses of the CNS and a perivascular network of glial cells (the glymphatic network) that acts to eliminate waste and distribute molecules throughout the brain.[5][6]
Nerves
The circle of Willis lies at the base of the brain, near several cranial nerves. The optic chiasm lies in the anterior portion of the circle, between the ICA-MCA junction and the bilateral ACAs. The oculomotor (CN3) and trochlear (CN4) nerves both flow posteriorly to the PCA.
Muscles
The ophthalmic artery, a branch of the ICA, typically courses inferiorly to the optic nerve (CN2) as it passes into the orbit via the optic canal. It then gives off several branches that go on to supply the eyeball and ocular muscles.[7]
Physiologic Variants
A complete Circle of Willis is present in a minority of the population, with many physiologic variants containing duplicated, fenestrated, hypoplastic, or absent vessels in certain regions of the ring.[1][2][3][4] Fenestrations occur when a single vessel’s lumen divides into two channels that later fuse back together. Duplication occurs when two arteries with distinct origins fuse into a single, downstream segment. Fenestrations and duplications are more common in the anterior circulation; the most commonly involved artery is the ACom. Hypoplastic arteries are the most common anomalies seen in the CoW, most frequently affecting the PCom or ACom.
The most common variation in the CoW involves changes in the ACom. Examples include ACom duplication (up to 18% prevalence), fenestration (up to 21% prevalence), and an azygous ACA. An azygous ACA occurs when the two ACA vessels fuse to form a single, midline vessel, accompanied by the absence of the ACom; this occurs in up to 2% of the population.[2]
Surgical Considerations
Surgical intervention at the base of the brain carries the risk of damaging not only the circle of Willis but other critical structures that lie near it. Such is the case with intervention for cerebral aneurysms that often occur within the CoW, or intervention to the pituitary gland.[8] There is a risk for potential damage to the optic chiasm with interventions affecting the anterior or middle CoW or the pituitary gland, and risk for damage to the oculomotor and trochlear nerves with procedures near the posterior CoW. Damage to the CoW itself may result in brain ischemia or infarction, depending on the severity of the injury.
Clinical Significance
The circle of Willis is of great clinical significance due to its structure, function, and location. As the connection between the anterior and posterior cerebral circulations, the CoW perfuses the brain and protects against ischemia (at least in those with a complete or mostly-complete ring of vessels). It is, however, one of the most common locations for intracranial aneurysms. An estimated 85% of intracranial aneurysms occur in the anterior circulation, either at the ICA-PCom junction, within the ACom, or the MCA.[4][8] Aneurysms in the posterior circulation are common at the bifurcation of the basilar artery or the VA-PICA junction.[8] Aneurysms large enough may cause symptomatic mass effects such as headaches or third nerve palsy. Intracranial aneurysms also pose a risk for subsequent development of intra-aneurysmal thrombi, which may embolize in a downstream vessel and cause distal ischemia.
A phenomenon called subclavian steal occurs when there is significant stenosis or occlusion of the subclavian artery proximal to the origin of the VA. In such cases, blood is carried from the contralateral VA to the BA and then retrogradely through the ipsilateral VA to the subclavian artery, distal to the blockage. This arrangement provides collateral flow to the affected arm.[8] Subclavian steal syndrome describes symptoms of posterior circulation ischemia such as vertigo or ataxia precipitated by the exercise of the upper extremity supplied by the stenotic subclavian artery.
Moyamoya disease is a chronic vascular disease characterized by bilateral stenosis of the terminal portion of the ICA. As a consequence, there are extensive fine collaterals developed with time trying to compensate for the distal ICA territory ischemia and hypoperfusion. The fine collaterals appear in angiography like a "puff of smoke," which gives rise to the Japanese meaning of moyamoya. It is most prevalent in Japan and is more common in females compared to males. The etiology of the disease is unknown; however, research suggests a possible genetic association.[9][10] The presentation is bimodal with a peak around the age of 10 and another peak around the 30s. The presentation is predominantly ischemic in children and both ischemic and hemorrhagic in adults.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
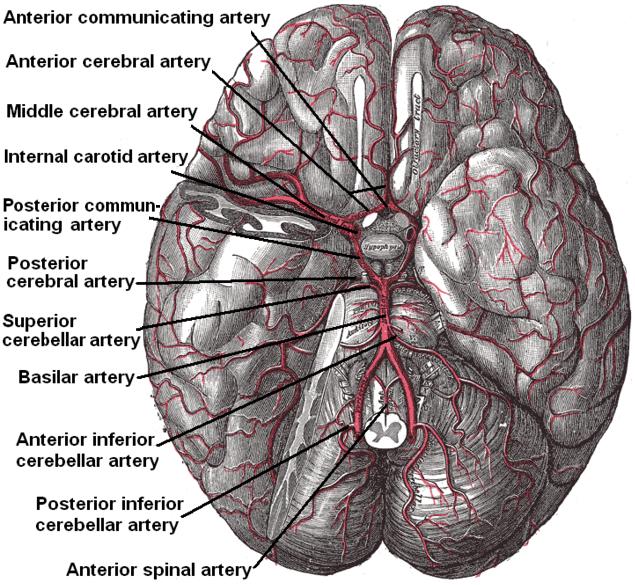
Arterial Circulation of the Brain. This inferior view shows the circle of Willis at the base of the brain formed by the anterior communicating, anterior cerebral, middle cerebral, internal carotid, posterior communicating, posterior cerebral, and basilar arteries. The temporal pole of the cerebrum and a portion of the cerebellar hemisphere have been removed on the right side. Other arteries in this illustration include the superior cerebellar, anterior inferior cerebellar, posterior inferior cerebellar, and anterior spinal arteries.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
References
Prince EA, Ahn SH. Basic vascular neuroanatomy of the brain and spine: what the general interventional radiologist needs to know. Seminars in interventional radiology. 2013 Sep:30(3):234-9. doi: 10.1055/s-0033-1353475. Epub [PubMed PMID: 24436544]
Menshawi K, Mohr JP, Gutierrez J. A Functional Perspective on the Embryology and Anatomy of the Cerebral Blood Supply. Journal of stroke. 2015 May:17(2):144-58. doi: 10.5853/jos.2015.17.2.144. Epub 2015 May 29 [PubMed PMID: 26060802]
Level 3 (low-level) evidenceKrishnaswamy A, Klein JP, Kapadia SR. Clinical cerebrovascular anatomy. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2010 Mar 1:75(4):530-9. doi: 10.1002/ccd.22299. Epub [PubMed PMID: 20049963]
Robben D, Türetken E, Sunaert S, Thijs V, Wilms G, Fua P, Maes F, Suetens P. Simultaneous segmentation and anatomical labeling of the cerebral vasculature. Medical image analysis. 2016 Aug:32():201-15. doi: 10.1016/j.media.2016.03.006. Epub 2016 Apr 1 [PubMed PMID: 27131026]
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015 Jul 16:523(7560):337-41. doi: 10.1038/nature14432. Epub 2015 Jun 1 [PubMed PMID: 26030524]
Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner's Guide. Neurochemical research. 2015 Dec:40(12):2583-99. doi: 10.1007/s11064-015-1581-6. Epub 2015 May 7 [PubMed PMID: 25947369]
Hayreh SS. Orbital vascular anatomy. Eye (London, England). 2006 Oct:20(10):1130-44 [PubMed PMID: 17019411]
Schievink WI. Intracranial aneurysms. The New England journal of medicine. 1997 Jan 2:336(1):28-40 [PubMed PMID: 8970938]
Scott RM, Smith ER. Introduction: moyamoya disease. Neurosurgical focus. 2009 Apr:26(4):E1. doi: 10.3171/2009.2.FOCUS.APR09.INTRO. Epub [PubMed PMID: 19335125]
Kim JS. Moyamoya Disease: Epidemiology, Clinical Features, and Diagnosis. Journal of stroke. 2016 Jan:18(1):2-11. doi: 10.5853/jos.2015.01627. Epub 2016 Jan 29 [PubMed PMID: 26846755]