Introduction
Chromoblastomycosis is a chronic granulomatous infection of the skin and subcutaneous tissue caused by several different dematiaceous fungi (ie, fungi containing pigment in the cell wall, whether melanin or melanin-like).[1][2] The indolent infection begins with the cutaneous inoculation of fungi, usually through some portion of the fungal cell capable of reproduction or propagation (eg, conidia, mycelia), into exposed skin, typically due to trauma.[3][4] The infection has wide morphologic variability but typically begins as a pink-to-red macule or papule and evolves in later stages to a verrucous, hyperkeratotic plaque, tumor, or nodule; ultimately, the slow-growing, warty (eg, cauliflower-like) lesions may ulcerate.[2][5]
The disease is most common in tropical and subtropical areas, where it is endemic and a neglected tropical disease; as such, it should be most strongly considered in patients who have recently traveled to or originated from these areas.[6] Diagnosis of chromoblastomycosis requires the identification of multicellular clusters of pigmented fungal cells, which produce a characteristic finding called sclerotic bodies (ie, muriform bodies, copper pennies, medlar bodies) on a potassium hydroxide stain or with histopathologic analysis of a skin biopsy.[4] Although other differential diagnoses may include infectious and noninfectious verrucous lesions, the treatment of chromoblastomycosis includes surgical removal for mild disease and antifungal therapy for moderate-to-severe disease.[7] If left untreated, chromoblastomycosis complications can include debilitating tissue fibrosis, squamous cell carcinoma in the site of prior infection, lymphedema, or secondary bacterial infection.[8][9]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Many melanized fungi can cause chromoblastomycosis, but the main causes are usually from 2 genera: Fonsecaea spp (especially F pedrosoi) and Cladophialophora spp (especially C carrionii).[10][11] Demiataceous fungi (ie, phaeoid, black fungi, pigmented) pathogenic to humans include those nonopportunistic organisms that cause chromoblastomycosis and the opportunistic pathogens that cause pheohyphomycosis, the latter of which is more likely associated with internal organ involvement.[12] Important species for chromoblastomycosis include:
- Fonsecaea spp: F pedrosoi, F monophora, F nubica, F pugnacious, F brasiliensis, F erecta)
- Cladophialophora spp: C carrionii, C yegresii
- Exophiala spp: E dermatitidis (formerly Wangiella dermatitidis), E spinifera, E jeanselmei, E alcalophila
- Phialophora spp: P verrucosa, P americana, P chinensis, P macrospora
- Rhinocladiella spp: R similis, R mackenziei, R aquaspersa [10][13][14][15][16]
These etiologic organisms exist in cutaneous tissue in various yeast-like forms and reproduce through asexual reproduction, though at least 1 species has been shown to reproduce through a cleistothecium-like structure.[13][17] The saprophytic fungi have been isolated from wood, soil, and other plant material in many rural communities, thus implicating their method of entry into the host through trauma and after exposure to plant material.[7][18][7]
Epidemiology
Chromoblastomycosis is a disease in the humid, semiarid climates of the tropical and subtropical world.[19] In 1 review of over 2081 patients from South America and the Caribbean, 80.3% were male, and the mean age was 56.1 years.[20] Disease duration was 10.8 years, with lesions mostly on the lower limbs (60%), and the most common presenting symptoms were itching and pain; the most common forms of the lesion at presentation were verrucous (46.4%) or tumorous (21.7%).[20] The most frequent comorbidity in patients with chromoblastomycosis was post-kidney transplantation, though leprosy was a common concomitant infection.[20] Chromoblastomycosis leads to significant disability in agricultural workers in rural areas, particularly as the disease is spread via direct inoculation into the skin from soil or other plant material. However, adult male agricultural workers are most likely to be diagnosed with chromoblastomycosis; the condition has also been reported in children.[20][21]
The incidence of chromoblastomycosis is difficult to determine since few studies describe its distribution; however, it is a neglected tropical disease more likely to be found in tropical climates and less likely in areas like the United States.[22] Even in India, chromoblastomycosis represents 1 of the rare fungal infections seen in 1 study in the population.[22] Although underestimated because it is not classified as a notifiable disease to public authorities in many nations, it is reported to be most common in 3 different continents (South America, Africa, and Asia), with higher reported rates in Costa Rica, Dominican Republic, Venezuela, French Guyana, Comoros, Madagascar, and Gabon, among others.[23]
Pathophysiology
Chromoblastomycosis follows a similar pattern explaining its pathophysiology; namely, after traumatic inoculation from the environment leading to a chronic cutaneous granulomatous and fibrotic reaction, multiple microabscesses with tissue proliferation develop, leading to a T-helper cell type 2 (Th2) immune reaction and the embedding of sclerotic bodies in infected tissue and a polymorphous clinical appearance.[24] After cutaneous inoculation, purportedly following trauma, a dematiaceous fungal species enters the skin and rarely disseminates further.[4] Several days after traumatic implantation, the pigmented fungi differentiate into muriform cells (ie, sclerotic bodies) within phagocytic cells, which explain the fungal globe-shaped, multiseptate morphology.[25][26] The sclerotic bodies—brown septate cells that resemble copper pennies—are extruded transepidermally, ultimately appearing as black dots clinically, a characteristic clinicopathologic correlate on physical examination.[27] During phagocytosis, toll-like receptor dysfunction has been implicated in the host cell's ability to mount an appropriate eradication response, resulting in a granulomatous reaction.[28]
Few virulence factors are known in the pathogenesis of chromoblastomycosis, but many elements of organisms that cause chromoblastomycosis have been implicated.
- Melanin: The presence of melanin has been implicated as a protective mechanism in chromoblastomycosis. Melanin is thought to have unpaired electrons to protect fungal elements from reactive oxygen species produced by macrophages and neutrophils.[29][30]
- Cell adhesion, invasion, and survival antigens: Some species that cause chromoblastomycosis have been found to contain specific elements to aid in cell adhesion.[31] One study showed that peptide-breaking enzymes of Fonsecaea spp aid in disseminating and invading fungal elements throughout the cutaneous tissue.[32] Other species have been shown to have active phospholipase, allowing easy invasion by lipolysis of the cell membrane and the conversion of arachidonate to prostaglandins (eg, prostaglandin E2), which helps downregulate macrophages, allowing microbial survival.[33][34]
- Muriform cell architecture: Muriform cells have thicker polyhedral cell walls than the thinner ones of hyphae, which may improve the organism's ability to diminish host response, resulting in fibrous tissue formation; this may be implicated in the chronicity of infection since the fungi can be dormant and proliferate with immune evasion.[35][36]
The immune response also plays an important role in propagating chromoblastomycosis infection. Complement proteins involved in opsonization can be activated by some Fonsacaea spp, resulting in chemoattract proteins (eg, C5a) increasing the local inflammatory response.[37] For unclear reasons, neutrophils have specificity in their toll-like receptor types to eliminate conidia or hyphae forms of fungi, suggesting specificity among fungal elements and the immune system for fungal elimination.[38] However, neutrophils and macrophages have limited ability to kill intracellular fungi, even with an oxidative response, likely related to melanin produced by the fungi.[39][30]
Organisms causing chromoblastomycosis have been shown to have resistance to microbicidal molecules, impacting the effects of major histocompatibility complex class II, CD80, interleukin-12, and T-helper cell type 1 (Th1) responses.[40][41] Other studies have shown the effect of chromoblastomycosis organisms on interleukin-17, interleukin-10, and Langerhans cells, promoting chronic infection.[42][43][44] Meanwhile, the humoral immune response is poorly understood, and immunoglobulins are not yet known to be involved to a great extent. However, some immunodeficiency syndromes (eg, CARD9 mutations) and genetic associations (eg, HLA-A29 or HLA-B*4802 deletion) have been associated with developing chromoblastomycosis.[45][46][45][47]
Histopathology
Once a skin biopsy is performed for both histopathologic analysis and culture, a microscopic examination reveals the findings below:
- Pseudoepitheliomatous hyperplasia of the epidermis
- Irregular acanthosis and hyperkeratosis alternating with atrophy
- Neutrophilic microabscesses with muriform cells (ie, polyhedral, chestnut-like cells measuring 5-12 μm with longitudinal septa resembling a brick wall)
- Dermal granulomas with epithelioid cells
- Increased number of dermal capillaries
- Variable dermal fibrosis associated with mononuclear cells (eg, histiocytes, plasma cells, lymphocytes)[24]
However, the most pertinent finding is the presence of sclerotic bodies (ie, medlar bodies, muriform cells, copper pennies) in the dermis (see Image. Chromomycosis of Skin).[48][49] The tissue between the bodies may show variable levels of fibrosis, depending on the extent and duration of the disease. When ulceration has occurred, there may be a secondary bacterial infection.[27]
History and Physical
Following trauma to the skin, usually in an outdoor environment with exposure to soil or plant material, fungi proliferate in the cutaneous tissue.[50] The evolution of chromoblastomycosis usually starts with a small, pink-to-red macule or papule and can progress to myriad variable morphologic varieties, which depend on the virulence, location, and advancement of the disease; some common morphologies are listed below.[5]
- Verrucous papule
- Nodules
- Scaly plaque
- Exophytic or ulcerative tumor (ie, cauliflower-like mass)
- Cicatricial plaques
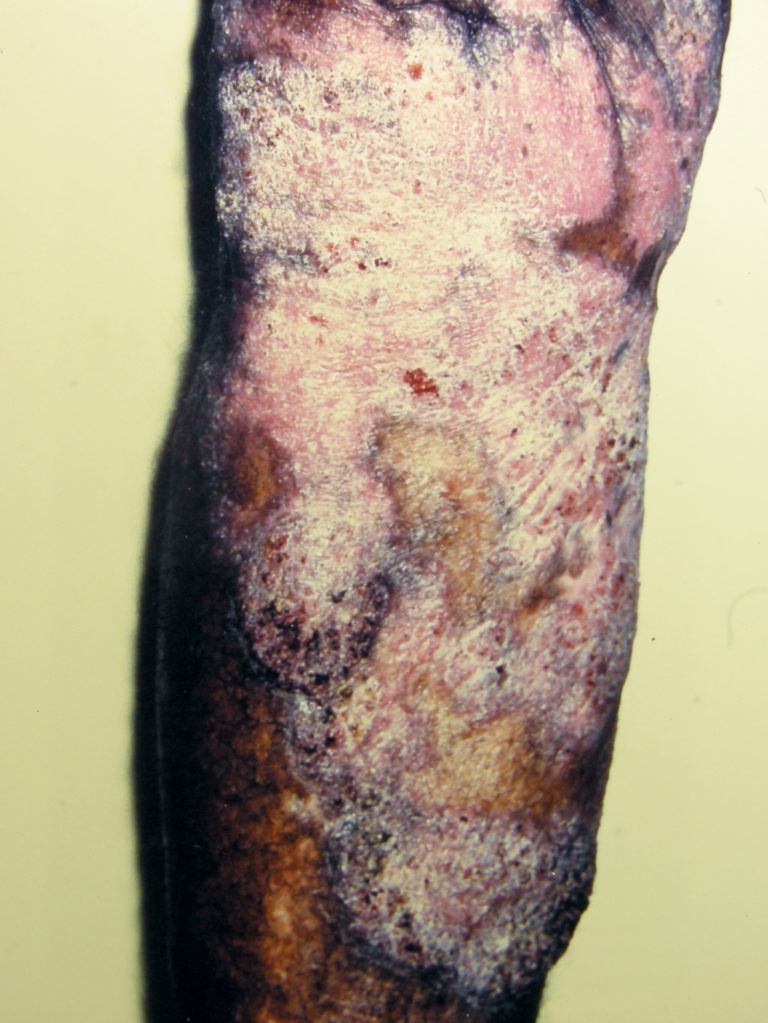
Typically, most lesions are on the lower extremities (see Image. Chromoblastomycosis), and nearly all patients with the disease have had exposure to hot, tropical environments, with the disease progressing in an indolent course, even lasting many weeks before presentation.[5] For this reason, after appreciation of the physical findings, the skin examination should focus on the extent of the disease, the presence of satellite lesions, or the involvement of deeper organs, which is rare and may represent a diagnosis of pheohyphomycosis.[51][52] Lymphatic spread should be evaluated near the existing lesion or lesions.[53]
Once the physical examination shows characteristic findings that could indicate chromoblastomycosis, history-taking should emphasize questions intended to rule out other differential diagnoses. Pruritus is a dominant complaint among patients with chromoblastomycosis, with pain at a later time; however, these findings may present late in the clinical course of the disease, particularly in trauma-prone locations.[54] Patients should be asked about:
- Recent travel to endemic tropical or subtropical areas
- History of prior surgeries or skin changes in the location (eg, squamous cell carcinoma, keloid)
- Exposure of skin to trauma or injury previously (eg, gardening)
- Outdoor recreation (eg, hiking, camping)
- Occupation (eg, farmer, agricultural worker, outdoor occupations)
- Exposure of skin to the environment (eg, walking barefoot, exposure to volcanic soil)
- History of malignancy (eg, mycosis fungoides)
- History of other infections (eg, bacterial, fungal, protozoan, viral)
- History of inflammatory diseases that can present with disfiguring skin lesions (eg, lupus, sarcoidosis)
- Family history of fungal infections
- Other issues relevant to the history or course of chromoblastomycosis [55][56][57]
The absence of these historical findings should not exclude chromoblastomycosis since patients may not remember the lesions.
Evaluation
The diagnosis of chromoblastomycosis should be suspected in patients who have indolent courses of verrucous papules, nodules, or plaques who are from or have recently traveled to endemic tropical or subtropical areas; however, it has also been found in nonendemic areas.[50][58][50] Dermoscopy may be used to identify black dots on the lesion, resembling "cayenne pepper" spots, which are associated with the transdermal extrusion of sclerotic bodies.[59] Scraping the lesion and applying a potassium hydroxide stain on the glass slide for viewing under a microscope can reveal the presence of copper pennies, the clusters of fungal cells.[2].
However, performing a skin biopsy is most prudent to observe the granulomatous reaction with medlar bodies, the thick-walled pigmented copper bodies in the dermis; either 1 biopsy should be divided for histopathology and culture each, or 2 punch biopsies should be performed.[60] A calcifluor white stain may be used if the fungal elements are challenging to identify since the fluorescent stain binds fungal cell walls when exposed to ultraviolet light.[24][61][62][24] Fungal cultures may be used to identify the genus, in which case an antibiotic may be needed to inhibit bacterial growth, but further molecular testing is needed to identify the species causing chromoblastomycosis.[63] Serologic by immunosorbent assay (eg, enzyme-linked immunosorbent assay [ELISA]) is not commercially available but has been used in research studies.[64][65][66]
Treatment / Management
Chromoblastomycosis treatment depends on the severity and extent of the disease, including the size of lesions, number of lesions, presence of complications, and prior response to treatment.[26] There are few randomized clinical trials to evaluate treatment success. Generally, mild disease is more amenable to surgical excision, whereas moderate-to-severe disease is best addressed with antifungal therapy.[67][58] Disease severity is classified as follows:
- Mild: Scales or nodules up to 5 cm in diameter
- Moderate: Single or multiple lesions in verrucous, tumoral, or plaque forms on 1 or 2 adjacent body areas up to 15 cm in diameter
- Severe: Single or multiple lesions in extensive areas beyond moderate [26]
Mild Disease
Mild disease is most appropriately treated with procedural intervention, usually surgical excision with 5-mm margins of healthy tissue, to prevent fungal spread through lymphatic channels.[26] Mohs micrographic surgery or skin grafting can be considered on a case-by-case basis.[68] Other procedural interventions, which may be used with or without oral medications, have included:
- Photodynamic therapy
- Long-pulsed 1064-nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser
- Carbon dioxide fractional laser
- Heat therapy (beyond 46 °C for fungicidal activity)
- Cryosurgery.[69][70][71][72][73] (B3)
Moderate-to-Severe Disease
Antifungal drugs should be initiated for moderate-to-severe disease. The primary recommended treatments are terbinafine and itraconazole, administered on dual oral therapy at 200 to 400 mg/day and 250 to 500 mg/day, respectively.[26]
- Itraconazole: An azole drug that inhibits fungal 14-α demethylase, treatment with 100 to 200 mg has been used as monotherapy for 12 to 24 months with varying success.[74] Another study showed that 200 to 400 mg of itraconazole needed to be used for up to 30.5 months for the clinical cure of F pedrosoi.[75] However, monotherapy is sometimes avoided for improved efficacy, and instead, terbinafine or 5-fluorocytosine (50-150 mg/kg/day) has been used in conjunction with itraconazole at 100 to 400 mg/kg/day for chromoblastomycosis.[76][77] Pulsed itraconazole at 200 mg daily for 7 days monthly has also been used successfully in patients with chromoblastomycosis.[78]
- Amphotericin B: Amphotericin B was shown to be effective when administered locally in chromoblastomycosis lesions; however, the dose may be too high and cause significant adverse effects, including pain, hemolysis, fibrosis, and gangrene.[79][80]
- Terbinafine: Terbinafine is an allylamine that inhibits squalene epoxidase; it was previously used at 500 mg/kg/day for 6 to 12 months for monotherapy of chromoblastomycosis.[81] Terbinafine is not successful as monotherapy in other studies, but dual therapy with terbinafine and an azole antifungal (eg, itraconazole, voriconazole) is thought to be synergistic.[82][83][84]
- Posaconazole and voriconazole: Both posaconazole and voriconazole have been used for chromoblastomycosis at 800 mg (as 2 or 4 doses) or 400 mg (as 2 doses), respectively, but these are usually limited to refractory cases or cases in which cost or other medications are an issue.[85][86]
- Imiquimod: Imiquimod is a toll-like receptor 7 agonist found to significantly improve the lesions of chromoblastomycosis when used with itraconazole with or without terbinafine.[87][88]
- Glucan: Glucan is a pathogen-associated molecular pattern that was found to improve the treatment of chromoblastomycosis when used at 5 mg/week with itraconazole at 400 mg/day.[89][90]
- Retinoids: Synthetic retinoids like acitretin have been combined with other medications (eg, imiquimod, itraconazole) for chromoblastomycosis.[91]
- Melanin Inhibitors: Molecules like tricyclazole, which inhibits melanin, have been thought to help with fungicidal activity in chromoblastomycosis.[92] The use of melanin inhibitors in conjunction with antifungal therapy might be useful to reduce fungal defenses for better antifungal penetration.[93] (A1)
Other medications of interest for fungicidal properties include HIV peptidase inhibitors and ajoene (garlic extract that inhibits phosphatidylcholine biosynthesis).[94] The presence of adverse effects (eg, hepatotoxicity in terbinafine) may change drug choice, and complications of the disease (eg, fibrosis) can affect the availability of the medication in cutaneous tissue. For example, although liver function monitoring may be required for terbinafine, it has fewer drug-drug interactions compared with itraconazole, which may warrant its selection. Because fungi can spread to adjacent healthy skin, monitoring after complete clinical resolution for several months without symptoms is necessary and can include regular skin biopsies with culture and fungal assessments. Regular follow-up should be performed to ensure complete treatment, barring expected atrophic scarring.
Differential Diagnosis
The differential diagnosis of chromoblastomycosis should include infectious and noninfectious causes that can appear clinically similar. Infectious causes that appear similar and should be considered include:
- Fungal: Lobomycosis, Pheohyphomycosis, Eumycetoma, Blastomycosis, Coccidioidomycosis, Sporotrichosis, Majocchi granuloma, Paracoccidiomycosis)
- Bacterial: Tuberculosis, Actinomycetoma, Botryomycosis, Syphilis, Yaws, Nontuberculous mycobacterial infection
- Viral: Verruca vulgaris, Papilloma
- Protozoan: Leishmaniasis, Rhinosporidiosis
- Helminth: Filariasis
- Alga: Protothecosis [26]
Noninfectious causes should also be considered due to similar clinical appearance or due to complications of chromoblastomycosis, including:
- Melanoma
- Squamous cell carcinoma (eg, keratoacanthoma)
- Keloid
- Sarcoidosis
- Podoconiosis
- Systemic lupus erythematosus [26]
Prognosis
Although treatable, there are many cases refractory to treatment; therefore, early intervention with regular follow-up is essential to reduce morbidity and mortality. Because there is rare internal organ involvement and the disease remains mostly cutaneous, there is less likely to be mortality due to the disease, except in certain cases, such as those with disseminated disease.[26] Without treatment, complications are likely to occur, and with treatment, monitoring must be performed to observe patients for adverse effects of treatment (eg, hepatotoxicity).
Complications
The most common complications of chromoblastomycosis include scarring (especially atrophic scarring), disabling tissue fibrosis (which could lead to ectropion or joint immobility), ulceration, secondary bacterial infection, lymphatic spread in a sporotrichoid pattern, and lymphedema resulting in elephantiasis.[9][26] There have been rare, documented cases of malignant transformation of chromoblastomycosis to squamous cell carcinoma and other rare cases of disseminated diseases, though this is unlikely as compared with other dematiaceous fungi.[95][96][97]
Deterrence and Patient Education
Patients should receive significant education on the prevention of chromoblastomycosis. Avoidance of cutaneous trauma in endemic areas is essential, and wearing protective equipment is important if individuals work outdoors or are engaged in outdoor recreation.[26] However, many patients susceptible to chromoblastomycosis live in low-resource settings with high temperatures, so reasonable planning should be discussed with manageable patients. Vaccination studies are limited, and no commercial vaccine exists for chromoblastomycosis.[26] During treatment, patients should be educated on the expected treatment course ( which may be lengthy with multiple treatments) to ensure patient compliance with treatment. Discussion on the myriad adverse effects of treatment medications should be discussed thoroughly, and any drug-drug interactions should be discussed.
Enhancing Healthcare Team Outcomes
Chromoblastomycosis is a rare fungal infection and is best managed by an interprofessional team that includes an infectious disease consultant, surgeon, emergency department physician, wound care nurse, dermatologist, and an internist. Chromoblastomycosis is a chronic granulomatous infection of the skin and subcutaneous tissue caused by several different dematiaceous fungi (brown pigment-producing), resulting in the formation of slow-growing, warty plaques, cauliflower-like lesions that may ulcerate.[98][99][100] The prognosis depends on lesion size and extent. The clinician can excise single small lesions followed by antifungal therapy. Oral antifungal agents alone are an alternative approach if surgery is not feasible or the disease is advanced.[27][101]
Media
(Click Image to Enlarge)
References
Hartsough EM, Foreman RK, Martinez-Lage M, Branda J, Sohani AR, Zukerberg L. Dematiaceous fungal infections: clinical and pathologic conundrums. Journal of clinical pathology. 2024 Apr 18:77(5):352-357. doi: 10.1136/jcp-2023-209239. Epub 2024 Apr 18 [PubMed PMID: 38272660]
Shenoy MM, Girisha BS, Krishna S. Chromoblastomycosis: A Case Series and Literature Review. Indian dermatology online journal. 2023 Sep-Oct:14(5):665-669. doi: 10.4103/idoj.idoj_292_23. Epub 2023 Aug 10 [PubMed PMID: 37727562]
Level 2 (mid-level) evidenceBorman AM, Fraser M, Patterson Z, Linton CJ, Palmer M, Johnson EM. Fungal Infections of Implantation: More Than Five Years of Cases of Subcutaneous Fungal Infections Seen at the UK Mycology Reference Laboratory. Journal of fungi (Basel, Switzerland). 2022 Mar 25:8(4):. doi: 10.3390/jof8040343. Epub 2022 Mar 25 [PubMed PMID: 35448574]
Level 3 (low-level) evidenceKhan S, Khan M, Khan F, Ahmad Z, Zia-Ur-Rehman A. A Rare Case of Chromoblastomycosis in a 12-year-old boy. JPMA. The Journal of the Pakistan Medical Association. 2019 Sep:69(9):1390-1393 [PubMed PMID: 31511733]
Level 3 (low-level) evidenceMugleston BJ, Usatine RP, Rosen T. Wide Morphologic Variability of Chromoblastomycosis in the Western Hemisphere. Skinmed. 2016:14(6):423-427 [PubMed PMID: 28031127]
Enbiale W, Bekele A, Manaye N, Seife F, Kebede Z, Gebremeskel F, van Griensven J. Subcutaneous mycoses: Endemic but neglected among the Neglected Tropical Diseases in Ethiopia. PLoS neglected tropical diseases. 2023 Sep:17(9):e0011363. doi: 10.1371/journal.pntd.0011363. Epub 2023 Sep 27 [PubMed PMID: 37756346]
Garzon LM, Rueda LJ, Celis AM, Cardenas M, Guevara-Suarez M. Exophiala psychrophila: A new agent of chromoblastomycosis. Medical mycology case reports. 2019 Mar:23():31-33. doi: 10.1016/j.mmcr.2018.10.001. Epub 2018 Oct 25 [PubMed PMID: 30533349]
Level 3 (low-level) evidenceTorres E, Beristain JG, Lievanos Z, Arenas R. Chromoblastomycosis associated with a lethal squamous cell carcinoma. Anais brasileiros de dermatologia. 2010 Mar-Apr:85(2):267-70 [PubMed PMID: 20520950]
Rios JE, Paiva CB, Paula GM, Figueiredo WR, Arantes JC, Almeida FM, Limongi RM. Chromomycosis, an unusual cause of cicatricial ectropion: a case report. Arquivos brasileiros de oftalmologia. 2017 Jan-Feb:80(1):46-48. doi: 10.5935/0004-2749.20170012. Epub [PubMed PMID: 28380102]
Level 3 (low-level) evidencePotenciano da Silva KL, Moraes D, Lechner B, Lindner H, Haas H, Almeida Soares CM, Silva-Bailão MG, Bailão AM. Fonsecaea pedrosoi produces ferricrocin and can utilize different host iron sources. Fungal biology. 2023 Dec:127(12):1512-1523. doi: 10.1016/j.funbio.2023.07.002. Epub 2023 Jul 22 [PubMed PMID: 38097325]
Abadías-Granado I, Gómez-Mateo MC, Stchigel AM, López C. Chromoblastomycosis due to Cladophialophora immunda: An emerging pathogen in immunocompromised patients? Enfermedades infecciosas y microbiologia clinica (English ed.). 2023 Jan:41(1):51-53. doi: 10.1016/j.eimce.2022.11.006. Epub 2022 Nov 25 [PubMed PMID: 36443189]
Abliz P, Fukushima K, Takizawa K, Nishimura K. Identification of pathogenic dematiaceous fungi and related taxa based on large subunit ribosomal DNA D1/D2 domain sequence analysis. FEMS immunology and medical microbiology. 2004 Jan 15:40(1):41-9 [PubMed PMID: 14734185]
Voidaleski MF, Gomes RR, Azevedo CMPES, Lima BJFS, Costa FF, Bombassaro A, Fornari G, Cristina Lopes da Silva I, Andrade LV, Lustosa BPR, Najafzadeh MJ, de Hoog GS, Vicente VA. Environmental Screening of Fonsecaea Agents of Chromoblastomycosis Using Rolling Circle Amplification. Journal of fungi (Basel, Switzerland). 2020 Nov 17:6(4):. doi: 10.3390/jof6040290. Epub 2020 Nov 17 [PubMed PMID: 33212756]
Sudhadham M, de Hoog GS, Menken SB, Gerrits van den Ende AH, Sihanonth P. Rapid screening for genotypes as possible markers of virulence in the neurotropic black yeast Exophiala dermatitidis using PCR-RFLP. Journal of microbiological methods. 2010 Feb:80(2):138-42. doi: 10.1016/j.mimet.2009.11.007. Epub 2009 Dec 2 [PubMed PMID: 19961882]
Ahmed SA, Bonifaz A, González GM, Moreno LF, Menezes da Silva N, Vicente VA, Li R, de Hoog S. Chromoblastomycosis Caused by Phialophora-Proven Cases from Mexico. Journal of fungi (Basel, Switzerland). 2021 Jan 29:7(2):. doi: 10.3390/jof7020095. Epub 2021 Jan 29 [PubMed PMID: 33572699]
Level 3 (low-level) evidenceGonzález GM, Rojas OC, González JG, Kang Y, de Hoog GS. Chromoblastomycosis caused by Rhinocladiella aquaspersa. Medical mycology case reports. 2013 Aug 31:2():148-51. doi: 10.1016/j.mmcr.2013.08.001. Epub 2013 Aug 31 [PubMed PMID: 24432242]
Level 3 (low-level) evidenceIbrahim-Granet O, de Bièvre C. Study of the conidial development and cleistothecium-like structure of some strains of Fonsecaea pedrosoi. Comparison with other close Dematiaeae. Mycopathologia. 1984 Feb 15:84(2-3):181-6 [PubMed PMID: 6538933]
Guevara A, Nery AF, de Souza Carvalho Melhem M, Bonfietti L, Rodrigues AM, Hagen F, de Carvalho JA, de Camargo ZP, de Souza Lima BJF, Vicente VA, Hahn RC. Molecular epidemiology and clinical-laboratory aspects of chromoblastomycosis in Mato Grosso, Brazil. Mycoses. 2022 Dec:65(12):1146-1158. doi: 10.1111/myc.13505. Epub 2022 Sep 29 [PubMed PMID: 35869803]
Queiroz-Telles F. CHROMOBLASTOMYCOSIS: A NEGLECTED TROPICAL DISEASE. Revista do Instituto de Medicina Tropical de Sao Paulo. 2015 Sep:57 Suppl 19(Suppl 19):46-50. doi: 10.1590/S0036-46652015000700009. Epub [PubMed PMID: 26465369]
Guevara A, Siqueira NP, Nery AF, Cavalcante LRDS, Hagen F, Hahn RC. Chromoblastomycosis in Latin America and the Caribbean: Epidemiology over the past 50 years. Medical mycology. 2021 Dec 8:60(1):. pii: myab062. doi: 10.1093/mmy/myab062. Epub [PubMed PMID: 34637525]
Verma S, Thakur BK, Raphael V, Thappa DM. Epidemiology of Subcutaneous Mycoses in Northeast India: A Retrospective Study. Indian journal of dermatology. 2018 Nov-Dec:63(6):496-501. doi: 10.4103/ijd.IJD_16_18. Epub [PubMed PMID: 30504979]
Level 2 (mid-level) evidenceRay A, Aayilliath K A, Banerjee S, Chakrabarti A, Denning DW. Burden of Serious Fungal Infections in India. Open forum infectious diseases. 2022 Dec:9(12):ofac603. doi: 10.1093/ofid/ofac603. Epub 2022 Dec 26 [PubMed PMID: 36589484]
Santos DWCL, de Azevedo CMPES, Vicente VA, Queiroz-Telles F, Rodrigues AM, de Hoog GS, Denning DW, Colombo AL. The global burden of chromoblastomycosis. PLoS neglected tropical diseases. 2021 Aug:15(8):e0009611. doi: 10.1371/journal.pntd.0009611. Epub 2021 Aug 12 [PubMed PMID: 34383752]
Queiroz-Telles F, de Hoog S, Santos DW, Salgado CG, Vicente VA, Bonifaz A, Roilides E, Xi L, Azevedo CM, da Silva MB, Pana ZD, Colombo AL, Walsh TJ. Chromoblastomycosis. Clinical microbiology reviews. 2017 Jan:30(1):233-276 [PubMed PMID: 27856522]
López Martínez R, Méndez Tovar LJ. Chromoblastomycosis. Clinics in dermatology. 2007 Mar-Apr:25(2):188-94 [PubMed PMID: 17350498]
Passero LFD, Cavallone IN, Belda W Jr. Reviewing the Etiologic Agents, Microbe-Host Relationship, Immune Response, Diagnosis, and Treatment in Chromoblastomycosis. Journal of immunology research. 2021:2021():9742832. doi: 10.1155/2021/9742832. Epub 2021 Nov 1 [PubMed PMID: 34761009]
Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. Anais brasileiros de dermatologia. 2018 Jul-Aug:93(4):495-506. doi: 10.1590/abd1806-4841.20187321. Epub [PubMed PMID: 30066754]
Level 2 (mid-level) evidenceQueiroz-Telles F, Fahal AH, Falci DR, Caceres DH, Chiller T, Pasqualotto AC. Neglected endemic mycoses. The Lancet. Infectious diseases. 2017 Nov:17(11):e367-e377. doi: 10.1016/S1473-3099(17)30306-7. Epub 2017 Jul 31 [PubMed PMID: 28774696]
Peltroche-Llacsahuanga H, Schnitzler N, Jentsch S, Platz A, De Hoog S, Schweizer KG, Haase G. Analyses of phagocytosis, evoked oxidative burst, and killing of black yeasts by human neutrophils: a tool for estimating their pathogenicity? Medical mycology. 2003 Feb:41(1):7-14 [PubMed PMID: 12627799]
Rozental S, Alviano CS, de Souza W. The in vitro susceptibility of Fonsecaea pedrosoi to activated macrophages. Mycopathologia. 1994 May:126(2):85-91 [PubMed PMID: 8065435]
Kneipp LF, Magalhães AS, Abi-Chacra EA, Souza LO, Alviano CS, Santos AL, Meyer-Fernandes JR. Surface phosphatase in Rhinocladiella aquaspersa: biochemical properties and its involvement with adhesion. Medical mycology. 2012 Aug:50(6):570-8. doi: 10.3109/13693786.2011.653835. Epub 2012 Feb 9 [PubMed PMID: 22320857]
Palmeira VF, Goulart FRV, Granato MQ, Alviano DS, Alviano CS, Kneipp LF, Santos ALS. Fonsecaea pedrosoi Sclerotic Cells: Secretion of Aspartic-Type Peptidase and Susceptibility to Peptidase Inhibitors. Frontiers in microbiology. 2018:9():1383. doi: 10.3389/fmicb.2018.01383. Epub 2018 Jun 29 [PubMed PMID: 30008700]
Montoya AM, Montesino CA, Carrión-Álvarez D, González GM, Rojas OC. A comparative study of extracellular enzymes from chromoblastomycosis agents reveals the potential association of phospholipase with the severity of the lesions. Microbial pathogenesis. 2020 Oct:147():104367. doi: 10.1016/j.micpath.2020.104367. Epub 2020 Jul 8 [PubMed PMID: 32649963]
Level 2 (mid-level) evidencePalmeira VF, Kneipp LF, Alviano CS, dos Santos AL. Phospholipase and esterase production by clinical strains of Fonsecaea pedrosoi and their interactions with epithelial cells. Mycopathologia. 2010 Jul:170(1):31-7. doi: 10.1007/s11046-010-9293-6. Epub 2010 Mar 1 [PubMed PMID: 20195904]
Siqueira IM, de Castro RJA, Leonhardt LCM, Jerônimo MS, Soares AC, Raiol T, Nishibe C, Almeida N, Tavares AH, Hoffmann C, Bocca AL. Modulation of the immune response by Fonsecaea pedrosoi morphotypes in the course of experimental chromoblastomycosis and their role on inflammatory response chronicity. PLoS neglected tropical diseases. 2017 Mar:11(3):e0005461. doi: 10.1371/journal.pntd.0005461. Epub 2017 Mar 29 [PubMed PMID: 28355277]
Ricard-Blum S, Esterre P, Grimaud JA. Collagen cross-linking by pyridinoline occurs in non-reversible skin fibrosis. Cellular and molecular biology (Noisy-le-Grand, France). 1993 Nov:39(7):723-7 [PubMed PMID: 8268758]
Torinuki W, Okohchi K, Takematsu H, Tagami H. Activation of the alternative complement pathway by Fonsecaea pedrosoi. The Journal of investigative dermatology. 1984 Oct:83(4):308-10 [PubMed PMID: 6481182]
Breda LCD, Breda CNS, de Almeida JRF, Paulo LNM, Jannuzzi GP, Menezes IG, Albuquerque RC, Câmara NOS, Ferreira KS, de Almeida SR. Fonsecaeapedrosoi Conidia and Hyphae Activate Neutrophils Distinctly: Requirement of TLR-2 and TLR-4 in Neutrophil Effector Functions. Frontiers in immunology. 2020:11():540064. doi: 10.3389/fimmu.2020.540064. Epub 2020 Oct 21 [PubMed PMID: 33193308]
Bocca AL, Brito PP, Figueiredo F, Tosta CE. Inhibition of nitric oxide production by macrophages in chromoblastomycosis: a role for Fonsecaea pedrosoi melanin. Mycopathologia. 2006 Apr:161(4):195-203 [PubMed PMID: 16552481]
Zhang J, Wang L, Xi L, Huang H, Hu Y, Li X, Huang X, Lu S, Sun J. Melanin in a meristematic mutant of Fonsecaea monophora inhibits the production of nitric oxide and Th1 cytokines of murine macrophages. Mycopathologia. 2013 Jun:175(5-6):515-22. doi: 10.1007/s11046-012-9588-x. Epub 2012 Oct 3 [PubMed PMID: 23054330]
Hayakawa M, Ghosn EE, da Gloria Teixeria de Sousa M, Ferreira KS, Almeida SR. Phagocytosis, production of nitric oxide and pro-inflammatory cytokines by macrophages in the presence of dematiaceous [correction of dematiaceus] fungi that cause chromoblastomycosis. Scandinavian journal of immunology. 2006 Oct:64(4):382-7 [PubMed PMID: 16970678]
Teixeira de Sousa Mda G, Ghosn EE, Almeida SR. Absence of CD4+ T cells impairs host defence of mice infected with Fonsecaea pedrosoi. Scandinavian journal of immunology. 2006 Dec:64(6):595-600 [PubMed PMID: 17083615]
d'Avila SC, Pagliari C, Duarte MI. The cell-mediated immune reaction in the cutaneous lesion of chromoblastomycosis and their correlation with different clinical forms of the disease. Mycopathologia. 2003:156(2):51-60 [PubMed PMID: 12733624]
Alves de Lima Silva A, Criado PR, Nunes RS, Kanashiro-Galo L, Seixas Duarte MI, Sotto MN, Pagliari C. Langerhans Cells Express IL-17A in the Epidermis of Chromoblastomycosis Lesions. Biomedicine hub. 2017 May-Aug:2(2):1-8. doi: 10.1159/000477954. Epub 2017 Jul 25 [PubMed PMID: 31988913]
Tsuneto LT, Arce-Gomez B, Petzl-Erler ML, Queiroz-Telles F. HLA-A29 and genetic susceptibility to chromoblastomycosis. Journal of medical and veterinary mycology : bi-monthly publication of the International Society for Human and Animal Mycology. 1989:27(3):181-5 [PubMed PMID: 2778577]
Level 3 (low-level) evidenceNaranjo F, Márquez I, Gendzekhadze K, Zhang S, Fernández-Mestre M, Yegres F, Richard-Yegres N, Navas T, Montagnani S, Ogando V, Layrisse Z. Human leukocyte antigen class I and MICA haplotypes in a multicase family with Cladophialophora carrionii chromoblastomycosis. Tissue antigens. 2006 Oct:68(4):287-92 [PubMed PMID: 17026462]
Level 3 (low-level) evidenceHuang C, Deng W, Zhang Y, Zhang K, Ma Y, Song Y, Wan Z, Wang X, Li R. CARD9 deficiency predisposing chromoblastomycosis: A case report and comparative transcriptome study. Frontiers in immunology. 2022:13():984093. doi: 10.3389/fimmu.2022.984093. Epub 2022 Sep 9 [PubMed PMID: 36159827]
Level 2 (mid-level) evidenceSali AP, Sahay A. Chromoblastomycosis of the leg. Polish journal of pathology : official journal of the Polish Society of Pathologists. 2017:68(2):182-184. doi: 10.5114/pjp.2017.69695. Epub [PubMed PMID: 29025254]
Gajurel K, Ahrens WA. Medlar bodies of chromoblastomycosis. Transplant infectious disease : an official journal of the Transplantation Society. 2023 Jun:25(3):e14047. doi: 10.1111/tid.14047. Epub 2023 Feb 28 [PubMed PMID: 36852755]
Dokic Y, Verstovsek G, Rosen T. Chromoblastomycosis Presenting as a Solitary Lesion in a Non-endemic Region. Cureus. 2023 Dec:15(12):e49791. doi: 10.7759/cureus.49791. Epub 2023 Dec 1 [PubMed PMID: 38164315]
Dupont C, Duong TA, Mallet S, Mamzer-Bruneel MF, Thervet E, Bougnoux ME, Dupont B. Unusual presentation of chromoblastomycosis due to Cladophialophora carrionii in a renal and pancreas transplant recipient patient successfully treated with posaconazole and surgical excision. Transplant infectious disease : an official journal of the Transplantation Society. 2010 Apr:12(2):180-3. doi: 10.1111/j.1399-3062.2009.00477.x. Epub 2009 Dec 7 [PubMed PMID: 20002358]
Schieffelin JS, Garcia-Diaz JB, Loss GE Jr, Beckman EN, Keller RA, Staffeld-Coit C, Garces JC, Pankey GA. Phaeohyphomycosis fungal infections in solid organ transplant recipients: clinical presentation, pathology, and treatment. Transplant infectious disease : an official journal of the Transplantation Society. 2014 Apr:16(2):270-8. doi: 10.1111/tid.12197. Epub 2014 Mar 17 [PubMed PMID: 24628809]
Kimura TFE, Romera LMD, de Almeida SR. Fonsecaea pedrosoi Conidia Induces Activation of Dendritic Cells and Increases CD11c(+) Cells in Regional Lymph Nodes During Experimental Chromoblastomycosis. Mycopathologia. 2020 Apr:185(2):245-256. doi: 10.1007/s11046-020-00429-w. Epub 2020 Feb 1 [PubMed PMID: 32008205]
Shen Y, Jiang B, Zhang H, Feng J, Hua H. Combination therapy for an elderly patient with chromoblastomycosis caused by Fonsecaea monophora: a case report. Annals of translational medicine. 2022 Jan:10(2):114. doi: 10.21037/atm-21-6119. Epub [PubMed PMID: 35282094]
Level 3 (low-level) evidenceGuevara A, Vicente VA, de Souza Lima BJF, Nery AF, Hagen F, Hahn RC. Chromoblastomycosis-Leprosy Co-Infection in Central West Brazil. Presentation of Three Cases and Literature Review. Mycopathologia. 2022 Aug:187(4):363-374. doi: 10.1007/s11046-022-00646-5. Epub 2022 Jun 28 [PubMed PMID: 35764905]
Level 3 (low-level) evidenceWang J, Zhu M, Wang P. Chromoblastomycosis by Exophiala jeanselmei associated with squamous cell carcinoma. Journal de mycologie medicale. 2021 Mar:31(1):101105. doi: 10.1016/j.mycmed.2020.101105. Epub 2021 Jan 7 [PubMed PMID: 33422739]
Mutalik VS, Bissonnette C, Kalmar JR, McNamara KK. Unique Oral Presentations of Deep Fungal Infections: A Report of Four Cases. Head and neck pathology. 2021 Jun:15(2):682-690. doi: 10.1007/s12105-020-01217-0. Epub 2020 Sep 5 [PubMed PMID: 32889592]
Level 3 (low-level) evidenceLanzoni A, Rapparini L, Pagliara A, Misciali C, Starace M, Piraccini BM. Chromoblastomycosis, A Neglected Fungal Infection. Mycopathologia. 2023 Dec:188(6):1103-1105. doi: 10.1007/s11046-023-00803-4. Epub 2023 Oct 19 [PubMed PMID: 37856009]
Kim JSTW, Santos FGD, Enokihara MMSES, Hirata SH, Tomimori J, Ogawa MM. Cutaneous chromoblastomycosis mimicking melanoma in a renal transplant recipient. Medical mycology case reports. 2022 Dec:38():41-43. doi: 10.1016/j.mmcr.2022.10.003. Epub 2022 Nov 2 [PubMed PMID: 36393996]
Level 3 (low-level) evidenceAssefa W, Sinatayehu R, Sendeku MA, Dires M. Chromoblastomycosis: delayed diagnosis with extensive cutaneous lesions. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2023 Jun:131():50-52. doi: 10.1016/j.ijid.2023.03.040. Epub 2023 Mar 25 [PubMed PMID: 36972782]
Hamza SH, Mercado PJ, Skelton HG, Smith KJ. An unusual dematiaceous fungal infection of the skin caused by Fonsecaea pedrosoi: a case report and review of the literature. Journal of cutaneous pathology. 2003 May:30(5):340-3 [PubMed PMID: 12753177]
Level 3 (low-level) evidenceSousa IS, Mello TP, Pereira EP, Granato MQ, Alviano CS, Santos ALS, Kneipp LF. Biofilm Formation by Chromoblastomycosis Fungi Fonsecaea pedrosoi and Phialophora verrucosa: Involvement with Antifungal Resistance. Journal of fungi (Basel, Switzerland). 2022 Sep 15:8(9):. doi: 10.3390/jof8090963. Epub 2022 Sep 15 [PubMed PMID: 36135688]
Maubon D, Garnaud C, Ramarozatovo LS, Fahafahantsoa RR, Cornet M, Rasamoelina T. Molecular Diagnosis of Two Major Implantation Mycoses: Chromoblastomycosis and Sporotrichosis. Journal of fungi (Basel, Switzerland). 2022 Apr 9:8(4):. doi: 10.3390/jof8040382. Epub 2022 Apr 9 [PubMed PMID: 35448613]
Borges JR, Ximenes BÁS, Miranda FTG, Peres GBM, Hayasaki IT, Ferro LCC, Ianhez M, Garcia-Zapata MTA. Accuracy of direct examination and culture as compared to the anatomopathological examination for the diagnosis of chromoblastomycosis: a systematic review. Anais brasileiros de dermatologia. 2022 Jul-Aug:97(4):424-434. doi: 10.1016/j.abd.2021.09.007. Epub 2022 May 25 [PubMed PMID: 35643736]
Level 1 (high-level) evidenceOberto-Perdigón L, Romero H, Pérez-Blanco M, Apitz-Castro R. [An ELISA test for the study of the therapeutic evolution of chromoblastomycosis by Cladophialophora carrionii in the endemic area of Falcon State, Venezuela]. Revista iberoamericana de micologia. 2005 Mar:22(1):39-43 [PubMed PMID: 15813682]
Vidal MS, de Castro LG, Cavalecate SC, Lacaz Cda S. Immunoprecipitation techniques and Elisa in the detection of anti-Fonsecaea pedrosoi antibodies in chromoblastomycosis. Revista do Instituto de Medicina Tropical de Sao Paulo. 2003 Nov-Dec:45(6):315-8 [PubMed PMID: 14762630]
Rolon AM, Tolaymat LM, Sokumbi O, Bodiford K. The Role of Excision for Treatment of Chromoblastomycosis: A Cutaneous Fungal Infection Frequently Mistaken for Squamous Cell Carcinoma. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2023 Jul 1:49(7):649-653. doi: 10.1097/DSS.0000000000003800. Epub 2023 Apr 14 [PubMed PMID: 37093678]
Pavlidakey GP, Snow SN, Mohs FE. Chromoblastomycosis treated by Mohs micrographic surgery. The Journal of dermatologic surgery and oncology. 1986 Oct:12(10):1073-5 [PubMed PMID: 3760315]
Luo J, Feng P, Hu Y, Yang Y, Zhou S, Huang S, Jadad A, Zhong Z, Zheng Y, Liu K, Lu Y, Hu Y, Zhou X. [Long-pulsed 1064 nm Nd: YAG laser combined with terbinafine against chromoblastomycosis caused by Fonsecaea nubica and the effect of laser therapy in a Wistar rat model]. Nan fang yi ke da xue xue bao = Journal of Southern Medical University. 2019 Jun 30:39(6):712-717. doi: 10.12122/j.issn.1673-4254.2019.06.13. Epub [PubMed PMID: 31270051]
Lan Y, Lu S, Zhang J. Retinoid combined with photodynamic therapy against hyperkeratotic chromoblastomycosis: A case report and literature review. Mycoses. 2021 Jan:64(1):18-23. doi: 10.1111/myc.13190. Epub 2020 Oct 14 [PubMed PMID: 32989774]
Level 3 (low-level) evidenceTsianakas A, Pappai D, Basoglu Y, Metze D, Tietz HJ, Luger TA, Bonsmann G. Chromomycosis--successful CO2 laser vaporization. Journal of the European Academy of Dermatology and Venereology : JEADV. 2008 Nov:22(11):1385-6. doi: 10.1111/j.1468-3083.2008.02649.x. Epub 2008 Mar 7 [PubMed PMID: 18331317]
Bonifaz A, Martínez-Soto E, Carrasco-Gerard E, Peniche J. Treatment of chromoblastomycosis with itraconazole, cryosurgery, and a combination of both. International journal of dermatology. 1997 Jul:36(7):542-7 [PubMed PMID: 9268758]
Huang TH, Lan CE. Cutaneous chromoblastomycosis effectively treated with local heat monotherapy. Clinical and experimental dermatology. 2019 Jun:44(4):461-462. doi: 10.1111/ced.13780. Epub 2018 Oct 2 [PubMed PMID: 30280420]
Restrepo A, Gonzalez A, Gomez I, Arango M, de Bedout C. Treatment of chromoblastomycosis with itraconazole. Annals of the New York Academy of Sciences. 1988:544():504-16 [PubMed PMID: 2850755]
Queiroz-Telles F, Purim KS, Fillus JN, Bordignon GF, Lameira RP, Van Cutsem J, Cauwenbergh G. Itraconazole in the treatment of chromoblastomycosis due to Fonsecaea pedrosoi. International journal of dermatology. 1992 Nov:31(11):805-12 [PubMed PMID: 1330949]
Antonello VS, Appel da Silva MC, Cambruzzi E, Kliemann DA, Santos BR, Queiroz-Telles F. Treatment of severe chromoblastomycosis with itraconazole and 5-flucytosine association. Revista do Instituto de Medicina Tropical de Sao Paulo. 2010 Nov-Dec:52(6):329-31 [PubMed PMID: 21225217]
Level 3 (low-level) evidencePolak A. Combination therapy with antifungal drugs. Mykosen. Supplement. 1988:2():45-53 [PubMed PMID: 3078023]
Ranawaka RR, Amarasinghe N, Hewage D. Chromoblastomycosis: combined treatment with pulsed itraconazole therapy and liquid nitrogen cryotherapy. International journal of dermatology. 2009 Apr:48(4):397-400. doi: 10.1111/j.1365-4632.2009.03744.x. Epub [PubMed PMID: 19335426]
DERBES VJ, FRIEDMAN L, KRAFCHUK JD. Chromoblastomycosis treated by vibrapuncture injection of amphotericin B. Archives of dermatology. 1959 Sep:80():286-7 [PubMed PMID: 13816006]
Whiting DA. Treatment of chromoblastomycosis with high local concentrations of amphotericin B. The British journal of dermatology. 1967 Jun:79(6):345-51 [PubMed PMID: 6027196]
Esterre P, Inzan CK, Ramarcel ER, Andriantsimahavandy A, Ratsioharana M, Pecarrere JL, Roig P. Treatment of chromomycosis with terbinafine: preliminary results of an open pilot study. The British journal of dermatology. 1996 Jun:134 Suppl 46():33-6; discussion 40 [PubMed PMID: 8763467]
Level 3 (low-level) evidenceBonifaz A, Saúl A, Paredes-Solis V, Araiza J, Fierro-Arias L. Treatment of chromoblastomycosis with terbinafine: experience with four cases. The Journal of dermatological treatment. 2005 Feb:16(1):47-51 [PubMed PMID: 15897168]
Level 3 (low-level) evidenceCriado PR, Careta MF, Valente NY, Martins JE, Rivitti EA, Spina R, Belda W Jr. Extensive long-standing chromomycosis due to Fonsecaea pedrosoi: three cases with relevant improvement under voriconazole therapy. The Journal of dermatological treatment. 2011 Jun:22(3):167-74. doi: 10.3109/09546630903585074. Epub 2010 Jul 28 [PubMed PMID: 20666671]
Level 3 (low-level) evidenceYu J, Li R, Zhang M, Liu L, Wan Z. In vitro interaction of terbinafine with itraconazole and amphotericin B against fungi causing chromoblastomycosis in China. Medical mycology. 2008 Nov:46(7):745-7. doi: 10.1080/13693780802163438. Epub [PubMed PMID: 18608889]
da Silva Hellwig AH, Heidrich D, Zanette RA, Scroferneker ML. In vitro susceptibility of chromoblastomycosis agents to antifungal drugs: A systematic review. Journal of global antimicrobial resistance. 2019 Mar:16():108-114. doi: 10.1016/j.jgar.2018.09.010. Epub 2018 Sep 25 [PubMed PMID: 30266638]
Level 1 (high-level) evidenceLima AM, Sacht GL, Paula LZ, Aseka GK, Goetz HS, Gheller MF, Torraca PF. Response of chromoblastomycosis to voriconazole. Anais brasileiros de dermatologia. 2016 Sep-Oct:91(5):679-681. doi: 10.1590/abd1806-4841.20165142. Epub [PubMed PMID: 27828652]
de Sousa Mda G, Belda W Jr, Spina R, Lota PR, Valente NS, Brown GD, Criado PR, Benard G. Topical application of imiquimod as a treatment for chromoblastomycosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014 Jun:58(12):1734-7. doi: 10.1093/cid/ciu168. Epub 2014 Mar 14 [PubMed PMID: 24633683]
Belda W Jr, Criado PR, Passero LFD. Successful treatment of chromoblastomycosis caused by Fonsecaea pedrosoi using imiquimod. The Journal of dermatology. 2020 Apr:47(4):409-412. doi: 10.1111/1346-8138.15225. Epub 2020 Jan 21 [PubMed PMID: 31960479]
Batbayar S, Lee DH, Kim HW. Immunomodulation of Fungal β-Glucan in Host Defense Signaling by Dectin-1. Biomolecules & therapeutics. 2012 Sep:20(5):433-45. doi: 10.4062/biomolther.2012.20.5.433. Epub [PubMed PMID: 24009832]
Azevedo Cde M, Marques SG, Resende MA, Gonçalves AG, Santos DV, da Silva RR, de Sousa Mda G, de Almeida SR. The use of glucan as immunostimulant in the treatment of a severe case of chromoblastomycosis. Mycoses. 2008 Jul:51(4):341-4. doi: 10.1111/j.1439-0507.2007.01485.x. Epub 2008 Apr 28 [PubMed PMID: 18444974]
Level 3 (low-level) evidenceBelda W, Criado PR, Domingues Passero LF. Case Report: Treatment of Chromoblastomycosis with Combinations including Acitretin: A Report of Two Cases. The American journal of tropical medicine and hygiene. 2020 Nov:103(5):1852-1854. doi: 10.4269/ajtmh.20-0471. Epub [PubMed PMID: 32815507]
Level 3 (low-level) evidenceHeidrich D, Pagani DM, Koehler A, Alves KO, Scroferneker ML. Effect of Melanin Biosynthesis Inhibition on the Antifungal Susceptibility of Chromoblastomycosis Agents. Antimicrobial agents and chemotherapy. 2021 Jul 16:65(8):e0054621. doi: 10.1128/AAC.00546-21. Epub 2021 Jul 16 [PubMed PMID: 33972246]
Tawade Y, Gaikwad A, Deodhar A, Bhide D, Romi E, Pradhan A, Satpute M. Uncommon presentation of chromoblastomycosis. Cutis. 2018 Jun:101(6):442;447;448 [PubMed PMID: 30063772]
San-Bias G, Urbina JA, Marchán E, Contreras LM, Sorais F, San-Blas F. Inhibition of Paracoccidioides brasiliensis by ajoene is associated with blockade of phosphatidylcholine biosynthesis. Microbiology (Reading, England). 1997 May:143 ( Pt 5)():1583-1586. doi: 10.1099/00221287-143-5-1583. Epub [PubMed PMID: 9168609]
Sharma N, Marfatia YS. Genital elephantiasis as a complication of chromoblastomycosis: A diagnosis overlooked. Indian journal of sexually transmitted diseases and AIDS. 2009 Jan:30(1):43-5. doi: 10.4103/0253-7184.55486. Epub [PubMed PMID: 21938115]
Level 3 (low-level) evidencede Azevedo CM, Gomes RR, Vicente VA, Santos DW, Marques SG, do Nascimento MM, Andrade CE, Silva RR, Queiroz-Telles F, de Hoog GS. Fonsecaea pugnacius, a Novel Agent of Disseminated Chromoblastomycosis. Journal of clinical microbiology. 2015 Aug:53(8):2674-85. doi: 10.1128/JCM.00637-15. Epub 2015 Jun 17 [PubMed PMID: 26085610]
Queiróz AJR, Pereira Domingos F, Antônio JR. Chromoblastomycosis: clinical experience and review of literature. International journal of dermatology. 2018 Nov:57(11):1351-1355. doi: 10.1111/ijd.14185. Epub 2018 Aug 16 [PubMed PMID: 30113072]
Bhattacharjee R, Narang T, Chatterjee D. Cutaneous Chromoblastomycosis: A Prototypal Case. Journal of cutaneous medicine and surgery. 2019 Jan/Feb:23(1):98. doi: 10.1177/1203475418789029. Epub [PubMed PMID: 30789034]
Level 3 (low-level) evidenceHuang X, Han K, Wang L, Peng X, Zeng K, Li L. Successful treatment of chromoblastomycosis using ALA-PDT in a patient with leukopenia. Photodiagnosis and photodynamic therapy. 2019 Jun:26():13-14. doi: 10.1016/j.pdpdt.2019.02.013. Epub 2019 Feb 12 [PubMed PMID: 30769166]
Rojas-García OC, García-Martínez JM, Carrión-Álvarez D. [Chromoblastomycosis in Mexico. A forgotten disease]. Salud publica de Mexico. 2019 Ene-Feb:61(1):3. doi: 10.21149/9459. Epub [PubMed PMID: 30753765]
He L, Ma J, Mei X, Lu S, Li X, Xi L. Successful treatment of chromoblastomycosis of 10-year duration due to Fonsecaea nubica. Mycoses. 2018 Apr:61(4):231-236. doi: 10.1111/myc.12732. Epub 2017 Dec 12 [PubMed PMID: 29178398]