Introduction
Pediatric brain tumors are the most common type of solid childhood cancer and only second to leukemia as a cause of pediatric malignancies. They are classified into supra and infratentorial tumors. They could also be classified according to the age of diagnosis into congenital brain tumors (CBT) (diagnosed antenatally in the first 60 days of life), tumors of the infancy (younger than 1 year of age), and older children. The prognosis of pediatric brain tumors depends on the age at presentation, histological type, and extent of resection. CBT behaves in different ways than pathologically similar tumors in older children. For example, high-grade gliomas exhibit sightly better prognosis in very young children compared to their older counterparts.[1][2] With the advances in imaging techniques, molecular biology, and genetics, pediatric brain tumors are increasingly being diagnosed early in the disease course, sub-grouped, and treated with more targeted strategies.[3]
Among all childhood cancers, brain tumors are the leading cause of death.
This article highlights the congenital group with an emphasis on the most common types in this category, specifically; teratoma, choroid plexus papilloma, desmoplastic infantile tumors (DIA/DIG), glioblastoma multiforme (GBM), and medulloblastoma.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Despite advances in medical knowledge, there is no direct cause for brain tumors identified. A combination of genetic and environmental factors is blamed to come into play in the pathogenesis of brain tumors. Multiple known cancer predisposition syndromes show associations with brain tumors, for example, DICER1, Li-Fraumeni, and other neurocutaneous syndromes such as neurofibromatosis, tuberous sclerosis, and Von-Hippel Lindau.
Family history may play a role in the etiology as many studies report an association of brain tumors and siblings.
The parental age at birth may also play a role. Studies show that offspring are at high risk for brain cancer (astrocytoma and ependymoma) in women who are older than 40.
Several studies have suggested a link between infectious exposure during childhood and brain cancer, but this topic remains debatable.
High dose radiation has been linked to brain malignancies. Children who have received radiation for leukemia are known to be at risk for developing brain cancer.
Further elaboration of the pathogenesis of brain tumor is beyond this quick review.
Epidemiology
The incidence of pediatric brain tumors varies among different countries. It ranges from 1.15 to 5.14 cases per 100,000 children, with the highest rates reported in the United States.[4] The incidence of CBT ranges from approximately 0.3 to 2.9 cases per 100,000 live births in different parts of the world.[5][6] Their prognosis and survival rates depend on multiple factors including the histological subtype and location.
For the congenital group, the most common type is teratoma (26.6% to 48%). Followed by astrocytoma (7.4% to 28.8%), choroid plexus papilloma (3.7% to 13.2%), embryonal tumor (3% to 13%), craniopharyngioma (5.6% to 6.8%), and ependymoma (4.4%).[7]
Pathophysiology
Supratentorial tumors are more common than the infratentorial tumors in those younger than the age of 3 years, while posterior fossa tumors are more common between the ages of 4 to 10. After puberty, both groups occur with equal frequency.[8]
Congenital brain tumors (CBTs) are tumors that are diagnosed antenatally or within the first 2 months of life.[9][10] Teratoma, a germ cell tumor is the most common type of congenital brain tumor. The incidence of teratoma is equally distributed among both genders.[11]
Choroid plexus papillomas are the third most common mass in the pediatric population after teratomas and gliomas. They can arise from anywhere where choroid plexus is present. Typically, in infants, they arise within the lateral ventricles.[12]
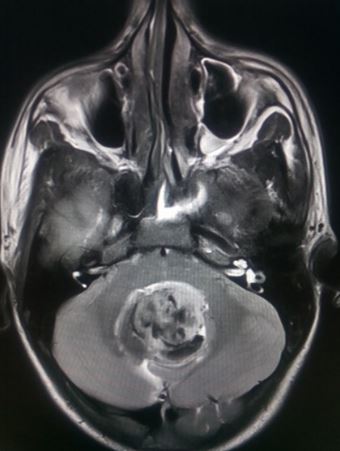
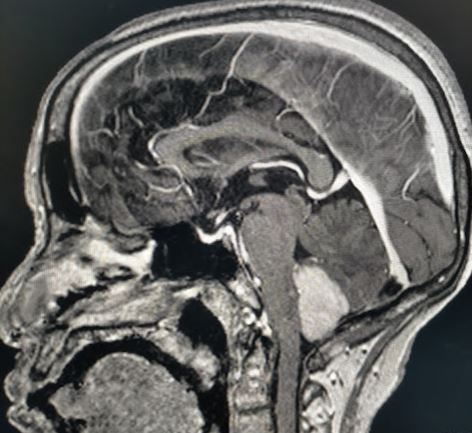
Medulloblastoma is one of the common perinatal tumors and is the most common posterior fossa tumor in the pediatric age group.[13] Medulloblastomas arise from the vermis of the cerebellum. It can cause obstructive hydrocephalus and leptomeningeal seeding along the spinal cord.[14]
Histopathology
The histopathology and molecular basis of brain tumors vary tremendously among the subtypes. For example:
Teratomas
Teratomas are derived from all 3 germ layers.[11] They can be mature, immature, or malignant. The malignant form is the least common among congenital brain tumors.[15]
Gliomas
Gliomas arise from glial precursor cells of the brain and spinal cord. Astrocytic tumors make a large group of neoplasms that have different clinicopathologic and histological subtypes and grades from (WHO grade I to IV).[16] Gliomas are classified as low grade (WHO grade I/II) and high grade (WHO grade III/IV). For example, pilocytic astrocytoma (WHO grade I) and glioblastoma multiforme (WHO grade IV). Desmoplastic infantile astrocytoma and ganglioglioma (DIA/DIG) are WHO grade I tumors.[16] Congenital (GBM) is a very rare brain tumor (WHO grade IV). This lesion shows clinical and molecular characteristics that are different from their pediatric or adult counterparts and has a better prognosis than the pediatric and adult GBM.[17]
Medulloblastoma
Medulloblastoma is one of the embryonal brain tumors. Molecular studies have shown that medulloblastomas are not a single entity but rather a group of different neoplasms that have a different cell of origin and thus behave differently from each other. In the revised 2016 WHO classification of brain tumor, there are currently four identified subgroups of medulloblastoma wingless(WNT), sonic hedgehog(SHH), group 3, and group 4.
History and Physical
CBTs are occasionally discovered during the second or third-trimester ultrasound. Antenatal ultrasound may show intracranial mass, hydrocephalus, polyhydramnios, among other signs. Fetal MRI is increasingly used to confirm findings visualized on ultrasound. Postnatally, common presentations are hydrocephalus, macrocrania, and localizing neurological signs.[8]
In neonates and older children, the clinical presentation depends on the site of tumor involvement. Supratentorial tumors may present with limb weakness, convulsions, and altered level of consciousness. Infratentorial tumors commonly present with signs of raised intracranial pressure (HCP) and imbalance.[18]
Evaluation
Teratoma typically visualized as a mixed echogenic and cystic intracranial mass on antenatal ultrasound during the second and third trimester.[19] MRI shows heterogeneous signal intensity on T1/T2. The presence of fat signal intensity on T1 is almost pathognomonic. It appears as a mixed density lesion in CT with frequent coarse calcification. again, the presence of fat is a good clue to the diagnosis. Supratentorial location is more common than infratentorial.
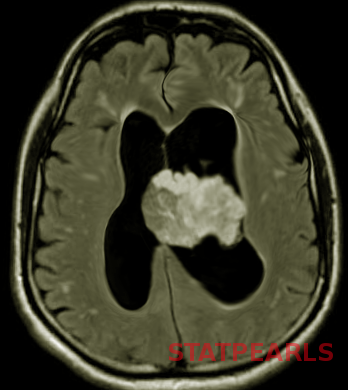
Glioblastoma multiforme is a high-grade astrocytoma, typically presents as a large, ill-defined, intracranial mass occupying most of one hemisphere or spread through the corpus callosum into the other hemisphere. It appears as an echogenic heterogeneous mass on ultrasonography and commonly shows intralesional hemorrhage and necrosis.[20] Diffusion restriction in MRI is a key feature that reflects the high-grade nature of the lesion. GBM usually presents with features of raised ICP.[20]
Choroid plexus papilloma typically presents as enhancing intraventricular mass on CT, and iso-hypo intense on T1 and iso-hyperintense on T2 MRI.[21] It commonly causes significant hydrocephalus.
Medulloblastoma typically presents as a midline posterior fossa tumor growing into the fourth ventricle. It appears hyperdense on CT-scan, has variable intensity on T1/T2 MRI with diffusion restriction and varying contrast enhancement.[22]
Scanning the whole neural axis is mandatory to look for CSF seeding and spinal drop metastasis.
Treatment / Management
The principal treatment for CBT is surgery aiming for gross total resection.[23] The extent of the CBT and the general condition of the patient are important determinants of surgical outcome.[8] Adjuvant therapies are not commonly used in neonates. Craniospinal radiotherapy results in significant developmental retardation and is avoided during the first 3 years of life.[24](B2)
Differential Diagnosis
Abscess
Neonatal brain abscess could occur as a complication of bacterial meningitis in 1.3% to 4.0% neonates.[25] Several congenital CNS infections like TORCH (toxoplasmosis, rubella, cytomegalovirus, and herpes simplex) infections may mimic metastatic disease from extracranial neoplasms in children.
Intracranial Hemorrhage
Variable stages of evolving hematoma may mimic tumors on imaging especially in the subacute and chronic stages which show central clot retraction and peripheral enhancement with/without surrounding edema on CT and MRI. MRI sequences like SWI for blood products, DWI and MRS, could aid in the differentiation as well as follow-up images. If underlying vascular malformation or vascular lesion is suspected, then a dedicated MRI angiography or CT angiogram can be performed.[26]
Ischemic Stroke
Ischemic arterial or venous stroke may mimic tumor on imaging. A diagnostic dilemma arises typically when imaging shows mass effects and/or irregular contrast enhancements, advanced MRI sequences like DWI and perfusion MRI can throw further light. As a general rule, if the edema shows restricted diffusion, then tumoral edema is the culprit. However, clinical history and knowledge of arterial and venous territories are of paramount importance for an accurate diagnosis.
Neurocutaneous Syndromes
Neurofibromatosis (NF), tuberous sclerosis(TS), Sturge-Weber syndrome, Von-Hippel Lindau (VHL), and hereditary telangiectasia are well-known syndromes with central nervous system (CNS) manifestations that are non-neoplastic. These syndromes are known to predispose to specific types of brain tumors. For example, NF-1 could be associated with low-grade gliomas. TS may be associated with SEGA (subependymal giant cell astrocytomas), and hemangioblastomas are seen in VHL. Physicians may find it challenging to differentiate between the non-neoplastic NF-related myelin vacuolation spots and low-grade glioma in settings of NF-1. Cortical and subcortical T2 hyperintense tubers in tuberous sclerosis may mimic gliomas.
Prognosis
Congenital brain tumors have a poor prognosis with overall survival of less than 30%.[8] Several factors such as malignant histological type, size, and location of the tumor, stage of fetal development, and treatment-related complications play a significant role in survival outcomes.[27]
Complications
Complications vary with type, site and size of the tumor, and treatment protocol.
Deterrence and Patient Education
Teamwork is key in the successful management of brain tumors. Full and detailed education of families and involvement in the decision-making process as appropriate is vital. The treatment protocols are usually discussed at multidisciplinary meetings to explore the best treatment options available and the expected prognosis of the patient. Support and counseling to the family during treatment makes a big difference. Genetic testing and profiling are offered on certain occasions for families to help in prognosis and future family planning.
Enhancing Healthcare Team Outcomes
Early detection of brain tumors and prompt referral to pediatric neurosurgery and pediatric neuro-oncology teams is vital. This usually starts with the pediatrician, nurse practitioner, family physician or emergency physician. The optimum care of patients with brain tumors occurs through an interprofessional collaboration including oncology, diagnostic imaging, pathology, radiotherapy, nursing, rehabilitation, and social workers.
Media
(Click Image to Enlarge)
References
Larouche V, Huang A, Bartels U, Bouffet E. Tumors of the central nervous system in the first year of life. Pediatric blood & cancer. 2007 Dec:49(7 Suppl):1074-82 [PubMed PMID: 17943961]
El-Ayadi M, Ansari M, Sturm D, Gielen GH, Warmuth-Metz M, Kramm CM, von Bueren AO. High-grade glioma in very young children: a rare and particular patient population. Oncotarget. 2017 Sep 8:8(38):64564-64578. doi: 10.18632/oncotarget.18478. Epub 2017 Jun 14 [PubMed PMID: 28969094]
Ostrom QT, de Blank PM, Kruchko C, Petersen CM, Liao P, Finlay JL, Stearns DS, Wolff JE, Wolinsky Y, Letterio JJ, Barnholtz-Sloan JS. Alex's Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007-2011. Neuro-oncology. 2015 Jan:16 Suppl 10(Suppl 10):x1-x36. doi: 10.1093/neuonc/nou327. Epub [PubMed PMID: 25542864]
Level 3 (low-level) evidenceJohnson KJ, Cullen J, Barnholtz-Sloan JS, Ostrom QT, Langer CE, Turner MC, McKean-Cowdin R, Fisher JL, Lupo PJ, Partap S, Schwartzbaum JA, Scheurer ME. Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014 Dec:23(12):2716-36. doi: 10.1158/1055-9965.EPI-14-0207. Epub 2014 Sep 5 [PubMed PMID: 25192704]
Oi S, Kokunai T, Matsumoto S. Congenital brain tumors in Japan (ISPN Cooperative Study): specific clinical features in neonates. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1990 Mar:6(2):86-91 [PubMed PMID: 2340534]
Carstensen H, Juhler M, Bøgeskov L, Laursen H. A report of nine newborns with congenital brain tumours. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2006 Nov:22(11):1427-31 [PubMed PMID: 16804715]
Level 2 (mid-level) evidenceAlamo L, Beck-Popovic M, Gudinchet F, Meuli R. Congenital tumors: imaging when life just begins. Insights into imaging. 2011 Jun:2(3):297-308 [PubMed PMID: 22347954]
Isaacs H Jr. II. Perinatal brain tumors: a review of 250 cases. Pediatric neurology. 2002 Nov:27(5):333-42 [PubMed PMID: 12504200]
Level 3 (low-level) evidenceParkes SE, Muir KR, Southern L, Cameron AH, Darbyshire PJ, Stevens MC. Neonatal tumours: a thirty-year population-based study. Medical and pediatric oncology. 1994:22(5):309-17 [PubMed PMID: 8127254]
Level 2 (mid-level) evidenceIsaacs H Jr. Perinatal (congenital and neonatal) neoplasms: a report of 110 cases. Pediatric pathology. 1985:3(2-4):165-216 [PubMed PMID: 3879355]
Level 3 (low-level) evidenceGoyal N, Kakkar A, Singh PK, Sharma MC, Chandra PS, Mahapatra AK, Sharma BS. Intracranial teratomas in children: a clinicopathological study. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2013 Nov:29(11):2035-42. doi: 10.1007/s00381-013-2091-y. Epub 2013 Apr 9 [PubMed PMID: 23568500]
Level 2 (mid-level) evidenceSeverino M, Schwartz ES, Thurnher MM, Rydland J, Nikas I, Rossi A. Congenital tumors of the central nervous system. Neuroradiology. 2010 Jun:52(6):531-48. doi: 10.1007/s00234-010-0699-0. Epub 2010 Apr 29 [PubMed PMID: 20428859]
Packer RJ, Cogen P, Vezina G, Rorke LB. Medulloblastoma: clinical and biologic aspects. Neuro-oncology. 1999 Jul:1(3):232-50. doi: 10.1093/neuonc/1.3.232. Epub [PubMed PMID: 11550316]
Level 3 (low-level) evidenceMcComb JG, Davis RL, Isaacs H Jr. Extraneural metastatic medulloblastoma during childhood. Neurosurgery. 1981 Nov:9(5):548-51 [PubMed PMID: 7322317]
Raisanen JM, Davis RL. Congenital brain tumors. Pathology (Philadelphia, Pa.). 1993:2(1):103-16 [PubMed PMID: 9420933]
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta neuropathologica. 2016 Jun:131(6):803-20. doi: 10.1007/s00401-016-1545-1. Epub 2016 May 9 [PubMed PMID: 27157931]
Arslanca SB, Söylemez F, Koç A. Congenital glioblastoma multiforme presented with intracranial bleeding: a case report. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2019 Apr:39(3):427-428. doi: 10.1080/01443615.2018.1475470. Epub 2018 Sep 18 [PubMed PMID: 30226408]
Level 3 (low-level) evidenceNdubuisi CA, Ohaegbulam SC, Ejembi GO. Paediatric brain tumours managed in Enugu, Southeast Nigeria: Review of one centre experience. The Nigerian postgraduate medical journal. 2018 Jul-Sep:25(3):186-190. doi: 10.4103/npmj.npmj_132_18. Epub [PubMed PMID: 30264771]
Sherer DM, Onyeije CI. Prenatal ultrasonographic diagnosis of fetal intracranial tumors: a review. American journal of perinatology. 1998 May:15(5):319-28 [PubMed PMID: 9643639]
Lee DY, Kim YM, Yoo SJ, Cho BK, Chi JG, Kim IO, Wang KC. Congenital glioblastoma diagnosed by fetal sonography. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1999 Apr:15(4):197-201 [PubMed PMID: 10361971]
Level 3 (low-level) evidenceWagle V, Melanson D, Ethier R, Bertrand G, Villemure JG. Choroid plexus papilloma: magnetic resonance, computed tomography, and angiographic observations. Surgical neurology. 1987 May:27(5):466-8 [PubMed PMID: 3563861]
Level 3 (low-level) evidenceDangouloff-Ros V, Varlet P, Levy R, Beccaria K, Puget S, Dufour C, Boddaert N. Imaging features of medulloblastoma: Conventional imaging, diffusion-weighted imaging, perfusion-weighted imaging, and spectroscopy: From general features to subtypes and characteristics. Neuro-Chirurgie. 2021 Feb:67(1):6-13. doi: 10.1016/j.neuchi.2017.10.003. Epub 2018 Aug 28 [PubMed PMID: 30170827]
Lang SS, Beslow LA, Gabel B, Judkins AR, Fisher MJ, Sutton LN, Storm PB, Heuer GG. Surgical treatment of brain tumors in infants younger than six months of age and review of the literature. World neurosurgery. 2012 Jul:78(1-2):137-44. doi: 10.1016/j.wneu.2011.09.012. Epub 2011 Nov 7 [PubMed PMID: 22120270]
Level 2 (mid-level) evidenceShin HJ, Kwon YJ, Park HJ, Park BK, Shin SH, Kim JY, Lee SH, Kim HS, Kim DW. An infant with prenatally diagnosed congenital anaplastic astrocytoma who remains disease-free after proton therapy. Journal of Korean medical science. 2013 Sep:28(9):1394-8. doi: 10.3346/jkms.2013.28.9.1394. Epub 2013 Aug 28 [PubMed PMID: 24015049]
Level 3 (low-level) evidenceFeferbaum R, Diniz EM, Valente M, Giolo CR, Vieira RA, Galvani AL, Ceccon ME, Araujo MC, Krebs VL, Vaz FA. Brain abscess by citrobacter diversus in infancy: case report. Arquivos de neuro-psiquiatria. 2000 Sep:58(3A):736-40 [PubMed PMID: 10973119]
Level 3 (low-level) evidenceHakimi R, Garg A. Imaging of Hemorrhagic Stroke. Continuum (Minneapolis, Minn.). 2016 Oct:22(5, Neuroimaging):1424-1450 [PubMed PMID: 27740983]
Sato J, Shimamura N, Naraoka M, Terui K, Asano K, Itou E, Ohkuma H. Long-term tumor-free survival case of congenital embryonal tumor with various pathological components. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2013 Jun:29(6):921-6. doi: 10.1007/s00381-013-2052-5. Epub 2013 Feb 21 [PubMed PMID: 23686409]
Level 3 (low-level) evidence