Introduction
Chest wall deformities are a set of congenital diseases that span a broad spectrum of disorders. These can manifest as isolated conditions or can correlate with extra-thoracic anomalies and genetic syndromes. Deleterious effects can occur from birth to adolescence, ranging from life-threatening conditions to psychosocial cosmetic concerns. The cornerstone of treatment lies with medical management, surgical repair, and genetic counseling. From a surgical standpoint, repair options are extensive and complex; therefore, a patient’s age and gender, as well as surgical timing and choice of prosthetic materials and grafts, are some factors that play a role in the decision-making process. This activity aims to review the most common chest wall deformities from a general thoracic surgery standpoint. The disorders described below are pectus excavatum, pectus carinatum, sternal clefts, ectopia cordis, and the syndromes of Poland, Jeune, and Jarcho-Levin.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
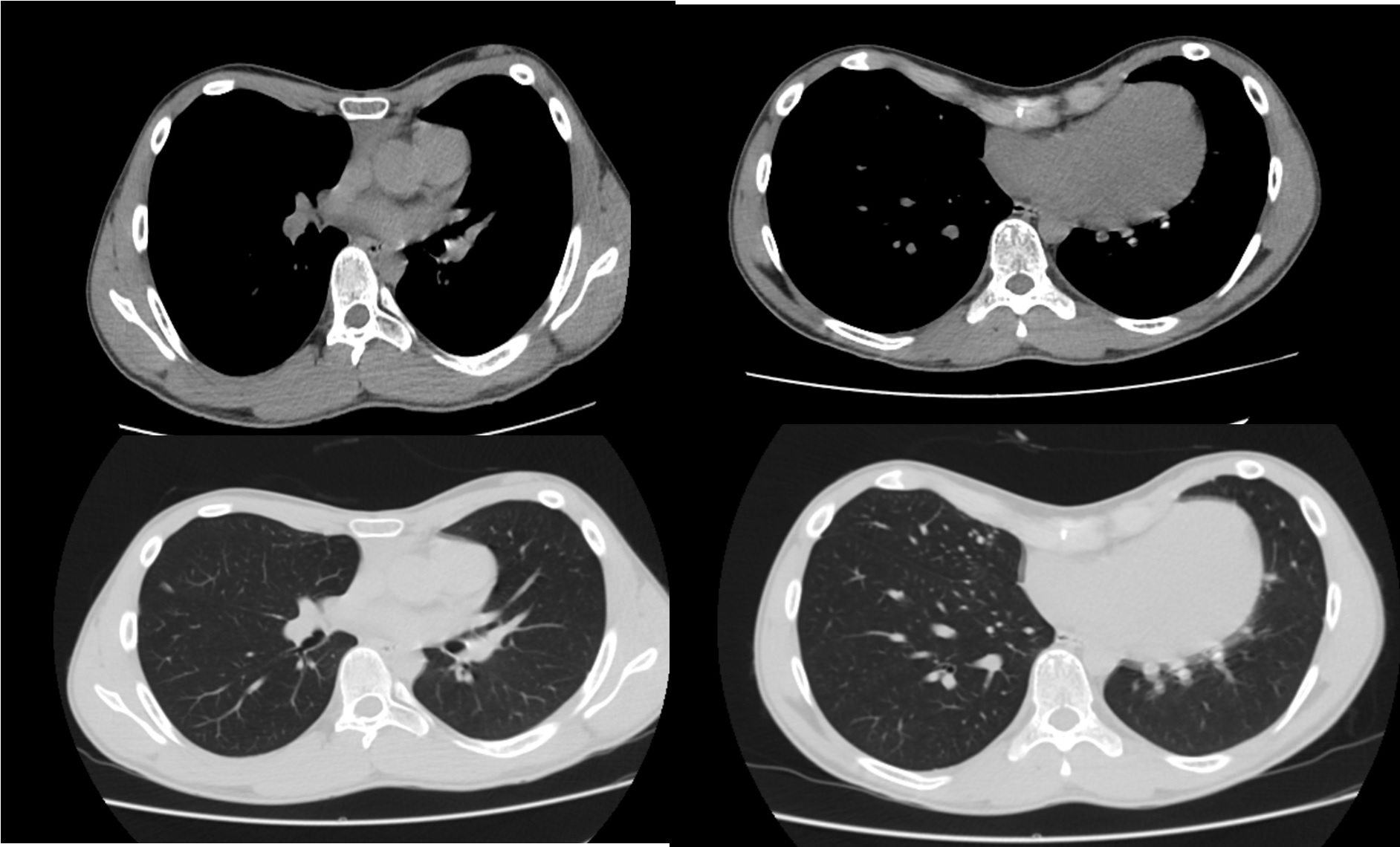
Pectus Excavatum
Exact etiology is unknown. The current hypothesis focuses on collagen metabolism impairment; this causes a sternal cartilage overgrowth that results in the sternocostal joint deformity (sternum protrusion). Pectus deformities are mostly isolated diseases; however, research has linked genetic mutation involvement. In inheritance scenarios, syndromic association highlights are; autosomal dominant (Marfan and Noonan syndromes), autosomal recessive (osteogenesis imperfecta I, III, IV types), and X-chromosomal inheritances with an underlying connective tissue strong association. Non-syndromic familiar inheritance highlight is autosomal dominant inheritance.[1][2] MASS phenotype (mitral valve prolapse, non-progressive aortic root enlargement, and skeletal and skin alterations) is related to FBNI gene mutation.[3]
Pectus Carinatum
The same pectus excavatum etiology hypothesis has been proposed. However, a mutation in the COL2A1 gene (collagen type II encoding impairment) links to pectus carinatum deformity. The familiar genetic trigger association is the same as in pectus excavatum.[4][5]
Poland Syndrome
Etiology is thought more to result from an in utero event than an inheritance situation, but familial cases should not be dismissed. The exact etiology remains undefined. The hypothesis with greater acceptance favors an intrauterine vascular insult that consists of embryonic blood supply interruption in subclavian or vertebral arteries, leading to different malformations (limb and chest wall). Another important proposed hypothesis is a lateral plate mesoderm development disruption. Lately, some research has related teratogenicity with drugs, misoprostol, and tobacco use during pregnancy.[6][4][7][8][9] In genetic scenarios association, highlights are bilateral phenotype related to autosomal dominant inheritance with genetic risk of 50%, and Mobius syndrome linked to autosomal recessive and X-chromosome inheritance with low genetic risk. The unilateral phenotype is considered non-syndromic with low genetic risk.[2]
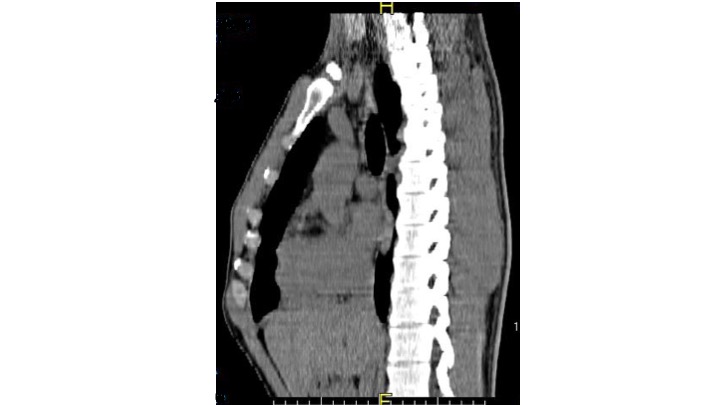
Sternal Clefts
Sternal cleft etiology consists of ventral cell migration and sternal bands fusion failure in the sixth to the ninth gestational week. This embryonic developmental disorder carries an association with Hoxb gene mutations with dominance possibility.[10][11] Syndromic genetic trigger association highlights are; Cantrell pentalogy related to X-chromosome inheritance with low genetic risk and PHACE syndrome with low genetic risk. Non-syndromic sternal clefts have a low genetic risk.[12]
Ectopia Cordis
Etiology is unknown. The deformity core is an embryonic VFM (ventral folding morphogenesis) impairment. Early chorionic and/or yolk sac rupture at third or four gestational weeks have been the most accepted hypothesis to explain this event. Lately, in genetic etiology pathways terms (gene mutation), BMP2 (bone morphogenetic protein 2) expression impairment has been linked to VFM impairment. Genetic trigger association is unknown; however, it has links with major chromosomal anomalies (e.g., Turner syndrome, 18 trisomies). The genetic risk depends on the underlying syndrome association.[13]
Jeunes Syndrome
The etiology is an autosomal recessive mutation on chromosome 7 genes, which encode proteins that participle in ITF (intraflagellar transport), specifically IFT80. However, lately, DYNC2H1 gene mutation, which encodes the dynein-2 protein complex (cilia structure component), has been reported as the main etiology core. Genetic risk is 25% (1 in 4), 50% 1 in 2), and 25% do not have the deformity.[14]
Jarcho-Levin Syndrome
Etiology depends on the Jarcho Levin syndrome phenotypes. Spondylocostal dysostosis links to DLL3 and MESP2 gene mutations, which can be a dominant or recessive autosomal inheritance. Spondylothoracic dysostosis is an autosomal recessive inheritance without DLL3 gene mutation. PAX1 and/or PAX9 (family PAX genes) mutation also has been linked to Jarcho Levin syndrome. The genetic risk depends on positive genetic carriers.[14][15]
Epidemiology
Pectus Excavatum
This anomaly represents 90% of chest wall deformities; it is more common in the White race with an incidence of 1 per 400 live births and 5 to 1 male predominance. Pectus excavatum could present as isolated non-familiar deformity; however, familiar non-syndromic positive history is present in up to 40%, highlighting an autosomal dominant inheritance patron. Pectus excavatum deformity can be part of genetic syndromes. Noonan and Marfan are the most important associated syndromes identified by POSSUM (Pictures Of Standard Syndromes and Undiagnosed Malformations version 5.7) and WBDD (Winter-Baraitser Dysmorphology Database version 1.0.14). It is of paramount importance that Poland syndrome rib hypoplasia causes secondary pectus excavatum, which could need modified Ravitch procedure repair. Less than 1% of pectus excavatum cases have an underlying connective tissue disorder. Data shows that 80% of cases get diagnosed at two years of life. Mitral valve prolapse (17%), arrhythmias (15%), and congenital heart disease (2%) are the most important cardiac anomalies related.[4][5][16] MASS phenotype is present in two-thirds of pectus excavatum patients.[3]
Pectus Carinatum
Is the second most common chest-wall deformity (5 to 6 times less common than pectus excavatum), has an incidence of 0.06% of live births with a 4 to 1 male predominance. Pectus carinatum could present as an isolated disease with a 25% to 30% positive family history, or can be part of genetic syndromes. As pectus excavatum, Marfan and Noonan are the most prevalent associated syndromes. Cardiac anomalies association could exist. Thoracolumbar scoliosis is linked to an underlying connective disorder in 12% to 14% of cases. Chondrogladiolar type is more common (95%). Pectus carinatum may present at any age; however, patients generally request medical attention at puberty.[5][17]
Poland Syndrome
The average incidence is 1 in 20000 to 1 in 30000 live births with male predominance between 2 to 1 and 3 to 1.[18] Unilateral presentation (over 90%) is considered a non-genetic disease. The deformity is most commonly right-sided in men (60% to 75%), females have no side tendency. Familial cases span less than 1% of cases (rare), highlighting bilateral or non-right sided with an equal male-to-female ratio as specific features. The left side anomaly is associated with dextrocardia. Poland syndrome has correlated with; Moebius anomaly, Klippel-Feil syndrome, renal anomalies, leukemia, non-Hodgkin lymphoma, breast cancer, cervical cancer, leiosarcoma, and lung cancer.[4]
Sternal Clefts
Sternal clefts are the most common type of sternal defects and represent 0.15% of chest wall deformities.[18] Of these, 67% are superior clefts,19.5% complete clefts,11% inferior clefts, and 2.5% sternal foramen. Sternal clefts incidence is difficult to estimate; however, there are reports of a female predominance of 2 to 1. Sternal clefts can be part of PHACES syndrome (90% female), midline fusion defects, and cavernous hemangiomata (female predominance), chromosome 22 syndromes, and 1 chromosome arrangement problems have been linked to sternal clefts by the POSSUM system. The inferior sternal cleft could be considered a pathognomonic sign of Cantrell pentalogy. The superior sternal cleft is commonly an isolated deformity.[4][11]
Ectopia Cordis
It is an extremely rare chest-wall deformity with an estimated prevalence of 1 of 5.5 to 7.9 per million live births. Ectopia cordis types are thoracic (65% to 90%), thoracoabdominal (7% to 20%), and cervical (2.8 %). Ectopia cordis may occur as an isolated condition; however, it has a strong association with congenital heart diseases or other midline defects. Fallot tetralogy is the most common congenital cardiac defect. Thoracoabdominal ectopia cordis highlights include high indices of left ventricle diverticulum and Cantrell pentalogy association. Omphalocele is the most common abdominal defect associated with ectopia cordis.[13]
Jeunes Syndrome
The incidence is 1 per 120000 live births. Most deaths occur in half to three-fourths of cases in the first two years of life. Skeletal chest wall growth arrest is the most serious complication core. Clavicle, limbs, pelvis, renal, hepatic, pancreatic are the main malformations that correlate with Jeune syndrome.[14][19]
Jarcho-Levin syndrome
Epidemiological data are scarce. Spondylothoracic dysostosis incidence is unknown. It has an autosomal recessive inheritance patron linked to chromosome 2q32.1, without DLL3 gene mutation, it does not demonstrate gender predilection. Puerto Rican ancestry history is commonly positive. Most deaths (80 to 100%) occur in infancy; however, reports exist of mild phenotypes cases with a good prognosis.[20] Spondylocostal dysostosis has a reported prevalence of 0.25 per 10000 births and demonstrates a better prognosis. This phenotype has both dominant and recessive autosomal inheritance, with the DLL3 (chromosome 19) and MESP2 (chromosome 15) gene mutation. Also, MESP2 mutation has links to the ethnic Puerto Rican group. Other anomalies associated with Jarcho Levin syndrome are a short trunk, dwarfism, craniofacial anomalies, short and rigid neck, inguinal and abdominal hernias, urinary tract anomalies, and talipes equinovarus.[20][21]
Pathophysiology
Pectus Excavatum
The sternocostal joint deformity causes an abnormal sternum depression that results in a decreased chest anteroposterior diameter. Compression severity is associated with cardiopulmonary impairment and paradoxical chest wall movement that causes dyspnea and pain, especially with physical activity. Other physiologic alterations may be present with a coexisting malformation (congenital heart diseases). Psychosocial issues are of paramount importance.[22]
Pectus Carinatum
Impaired sternocostal cartilage collagen arrangement causes an abnormal sternum protrusion. Cardiopulmonary physiology impairment is not associated with this condition; however, respiratory symptoms are attributable to a fixed chest wall with augmented residual lung volumes.[4]
The pathophysiological impact will depend on the expressed phenotype. Poland syndrome usually represents an aesthetic deformity. Organic impairment and associated anomalies are rare conditions; however, some chest wall anomalies and hand deformities could cause functionality impairment (superior limb motion, lung herniation, and flail chest). Therefore, in these scenarios, regardless of age, surgical repair is mandatory. Psychosocial cosmetic issues are of paramount importance, highlighting that these are more prevalent in adolescents females with asymmetric hypoplastic breasts.[4][8]
Sternal Clefts
Sternal clefts cause mediastinum structures exposition with somatic covering. The physiological impairment will depend on the cleft type and anomalies associated, highlighting paradoxical respiration. However, the most physiopathologic impact can occur in the post-surgical stage due to cardiac compression.[4][11]
Ectopia Cordis
VFM (Ventral folding morphogenesis) impairment causes ventral body wall closure failure; the common denominator effect is an ectopic heart outside the thoracic cavity through a sternal cleft. Thoracic ectopia cordis highlights are; rotated ectopic hearth without somatic covering. Thoracoabdominal ectopia cordis highlights are a non-rotated ectopic heart with a thin membranous or cutaneous layer covering and a coexisting abdominal wall defect. Cervical ectopia is an extremely rare and lethal condition, where the heart is cranially located. In general terms, the lack of cardiac protection at birth causes severe cardiopulmonary impairment. Congenital anomalies association has a strong negative impact on survival.[4][11]
Jeunes Syndrome
The genetic mutation causes a costochondral dysplasia (short rib size). Ribs cannot extend anteriorly, resulting in anteroposterior and lateral thoracic diameters reduction (narrow chest). The highlights include alveolar hypoventilation and lung growth restriction. Respiratory impairment is progressive.[14]
Jarcho-Levin syndrome
Mesodermal somite cells signaling induction for axial skeleton development is damaged (somite segmentation failure). Spondylothoracic dysostosis highlights are; All ribs symmetrical fusion at costovertebral union across the entire spine (classic crab aspect). This condition causes a restrictive thorax with progressive respiratory failure. Spondylocostal dysostosis highlights include symmetrical intrinsic ribs anomalies. These can be; bifid, wide, or fused ribs, resulting in progressive scoliosis that cause a restrictive thorax. Both phenotypes converging points include vertebral segmentation and restrictive thorax with progressive lung development arrest.[20]
History and Physical
Pectus Excavatum
This condition is diagnosable at birth but commonly remains unrecognized until early childhood or adolescence. The clinical picture is usually a tall, thin, healthy patient with only a visible pectus defect. Highlighting; type of morphology defect, spine alteration coexistence, pain along the sternal border, and lower ribs margin detection. Psychological distress from body image issues is of paramount importance.[5]
Pectus Carinatum
Pectus carinatum assessment is similar to patients with pectus excavatum.
Poland Syndrome
Poland syndrome has a wide clinical findings constellation and can be detected during pregnancy or along from birth to adolescence. Physical examination highlights include limbs and torso symmetry and functionality evaluation, and sternal notch to the acromion, olecranon to ulnar styloid, and phalanges lengths measurements. The breast disease spectrum is of paramount importance.[23]
Sternal Clefts
Sternal clefts detection can be made with prenatal sonography or at birth. Physical findings would vary depending on the type of cleft. Highlights are; the presence of a bulging skin in a partial cleft, and direct precordial pulse in a total cleft. Identify cardiopulmonary physiologic impairment and other associated anomalies are of paramount importance.[23][10]
Ectopia Cordis
Prenatal evaluation is the recommended approach of ectopia cordis detection. Physical findings at pregnancy and birth highlights are an ectopic heart and associated anomalies coexistence.[24]
Jeunes Syndrome
Detection is possible with a prenatal ultrasound examination. Lung area and CT / AC ratio (thoracic circumference / abdominal circumference) measurements are the most specific prenatal evaluation findings. Classic physical findings at birth are narrow chest with short limbs, brachydactyly, micromelia, elevated and fixed clavicles, and small pelvis with hypoplastic iliac bones.[14][19][25]
Jarcho-Levin syndrome
Detection is possible with a prenatal ultrasound examination. Vertebral anomalies and spine Bifida are the suspicious prenatal evaluation findings (anecdotical). Physical findings highlights are short trunk dwarfism with vertebral and ribs deformities.[26]
Evaluation
Pectus Excavatum
Cardiopulmonary impairment correlates with the severity of defect or coexisting cardiac anomalies coexistence; therefore, pulmonary function tests, echocardiography, and radiological assessment are mandatory with special attention in exercise tolerance.[5] Other kinds of pulmonary and cardiac evaluation tests must follow if indicated. A computerized tomography scan is considered the gold standard tool for thoracic morphology evaluation. Bedside caliper measurement, magnetic resonance, and color-coded video raster stereography have also been useful tools for this purpose. Haller index is the most accepted score to asses sternal depression severity. To select the best treatment option, the clinician must determine the morphology type and depression severity. Pectus excavatum types morphology has lately classified as an asymmetrical or symmetrical deformity with the presence of normal or narrow thorax (platythorax) and mixed excavatum-carinatum deformity. Haller index greater than 3.25 is considered a severe defect.[27][28]
Pectus Carinatum
The same pectus excavatum workup must take place for pectus carinatum. Haller index in pectus carinatum doesn’t have a cutoff value, but in many series, the principal value of their patients is 1.9 and is associated with respiratory distress during exercise. Pectus morphology frequently classifies as symmetrical or asymmetrical chondrogladiolar or chondromanubrial defects, with the presence of normal or narrow thorax.[29][30] Chondromanubrial defect is also referred to as Currarino-Silverman syndrome.[31]
Poland Syndrome
Poland’s syndrome evaluation focuses on soft-tissue, skeletal, and upper extremity malformations, and always thinking in associated anomalies related to a specific phenotype (Right and left-sided, bilateral). TC (Computed tomography), MRI (magnetic resonance imaging), and angiography with three-dimension reconstruction, cross-sectional, and sagittal views are the most useful imaging tools.[18] Further interprofessional investigations are necessary due to other malformations and cancer associations.[1] In general terms, Poland syndrome categorizes as simple (thoracic soft tissue) or complex (thoracic skeletal, thoracic soft tissue, hand deformities). When the hand deformity is absent, Poland syndrome has been called Poland sequence.[23] Highlights anatomic defects are costochondral hypoplasia or dysplasia that affects the second through fourth or third through fifth ribs with an associated pectus deformity, sternocostal head of the pectoralis major muscle and/or pectoralis minor muscle absence, breast and nipple hypoplasia, cutaneous anterior axillary fold loss, axillary and mammary region alopecia, and unilateral brachy-syndactyly or ectrodactyly.[4][8][32][23]
Sternal Clefts
The initial evaluation focuses on associated anomalies and life-threating conditions. If indicated, preoperative evaluation tools are; chest TC scan with intravenous contrast with 3-D reconstruction and chest magnetic resonance angiogram. Ophthalmological and cardiological evaluation, lung functional tests, neuro-radiologic imaging, and laryngotracheal-bronchoscopy is often required.[1][11]
Ectopia cordis
Evaluation principles are similar to those of sternal clefts. In this specific condition, evaluation is focused on severity determination and grant neonatal vital support and protection to exposed structures.[24] Elective termination of pregnancy can be offered (first trimester of pregnancy) due to the poor prognosis and family psychological impact. If the parents decide pregnancy continuation, the follow-up is with ultrasound and magnetic resonance imaging.[4][13]
Jeunes Syndrome
The initial evaluation is focused on respiratory distress support and carry out an extended investigation to detect organic anomalies coexistence. Evaluation tools highlights are; ophthalmoscopy, chest, and extremities radiology imaging (computerized tomography, magnetic resonance, X-rays), abdominal ultrasound, laryngoscopy, and bronchoscopy. If indicated, a preoperative workup is necessary.[14][19]
Jarcho-Levin syndrome
The initial evaluation focuses on respiratory distress support and costovertebral anomalies (vertebrae segmentation and the ribs deformities morphology) identification with imaging test (computerized tomography, magnetic resonance, X-rays). Surgical treatment options are designated on a case-by-case basis. A genetic test is mandatory to inheritance patron determination.[26]
Treatment / Management
Pectus Excavatum
Surgery is indicated in diverse clinic scenarios. These scenarios include protrusion cardiopulmonary impairment, psycho-social stress issues, and Haller index over3.25 with or without cardiopulmonary symptomatology.[1][5] As a general rule, surgical correction should be performed in adolescence (12 to 14 years); however, it is also an option in pediatric and adult patients.[33]Surgical technique options are MARPE (minimal repair of pectus excavatum) and open surgery; Nuss and modified Ravitch, respectively, are the specific hallmarks procedures. The Nuss procedure is a recommendation in pediatric patients, and symmetrical deformities and the modified Ravitch procedure are recommended options in asymmetrical or complex deformities. Special Nuss procedure highlights are; use rib stabilators to fix the retrosternal bar, and avoid cardiac injury. Special modified Ravitch procedure highlights are the meticulous preservation of the perichondrium and its non-recommendation to perform in pediatrics, especially in children under four years (Acquired Jeunes syndrome risk).[16] The most common open surgical techniques modifications are Leonard modification, Welch procedure, Willtal-Hegemann procedure, Robiscsek procedure, non-prosthetic repair with muscle flap, and PLIER (pectus less invasive extrapleural repair); these procedures merit consideration in very severe deformations.[33][34][35][36][37][38] The retrosternal bar highlights are; duration time (6 to 8 months in the open procedure and 2 to 3 years in MARPE), the possibility of two bars if necessary, and a mandatory second procedure for bar remotion. The use of a retrosternal rib graft in the modified Ravitch procedure has been used in some centers, the highlight of this alternative is that the bone graft biodegrades or remodeled in 2 years, so a second intervention is no longer necessary. Non-operative management focuses on physical rehabilitation programs that improve posture and muscular strength. Some centers have used suction devices; however, long-term results are lacking.[39](B2)
Pectus Carinatum
Surgical repair is indicated in thoracic pain, respiratory symptoms, psycho-social issues, and non-operative techniques failure.[5] As a general rule, a surgical correction occurs after puberty; however, there are significative reports of surgery in 3 to 65 years old patients. Modified Ravitch procedure is the most used technique for pectus carinatum repair. Technical details highlights are; wedge osteotomies, less costal cartilage resection and imbrication, detachment, and reattachment of lower perichondria cartilage, and placement of a metal bar in the anterior lower sternum if necessary. In chondromanubrial deformity, a wedge osteotomy is performed at the point of the maximal sternal protrusion. Minimally invasive techniques are also available; however, their use is yet to be adopted.[2][40] The non-operative orthotic bracing device has been used in some centers as the first treatment option in 10 to 15 years patients with chondrogladiolar deformity.[41](B2)
Poland Syndrome
Surgical repair is indicated in psycho-social, and chest wall and/or functionality impairment. Timing and surgical options depend on age, gender, and functionality impairment severity, As a general principle, surgery is recommended after puberty. Surgical correction goals are; chest wall symmetry improvement, anterior axillary fold creation, and hand reconstruction if indicated.[23] Children's surgical correction is indicated in cardiopulmonary impairment scenarios, so the surgery is made in two stages. Stage one surgery focuses on skeletal reconstruction, as general rule Poland syndrome skeletal defect consist in a unilateral hypoplasia or aplasia of the second through fourth or third through fifth ribs with an associated pectus deformity, when ribs hypoplasia is present, modified Ravitch procedure should be performed, Nuss procedure may be a consideration too, and when rib aplasia is present, a rib graft is used for defect bridging. Stage two surgery is performed after puberty and focuses on thoracic soft tissue reconstruction.[42] Post-puberty chest wall surgery repair takes place in one stage. Skeletal bony defects are repairable with metal struts and synthetic meshes, and soft tissue reconstruction is performed with flap +/- implant (females). Flaps options can include local or distant (pedicle or free), simple (muscular), and multiple (myocutaneous) flaps. Autologous fat grafting has been used to improve the anterior axillary fold. Hand defects undergo repair within the first 18 to 24 months of life.[43][44]
Sternal Clefts
The surgical repair depends on the cleft severity and other anomalies association. Follow-up with observation has been recommended in asymptomatic patients with minor defects. Partial and complete clefts surgical goals are; mediastinum structures protection, improve respiratory dynamics, and provide a normal growth thorax development. As a general rule, surgical correction must take place in the neonatal period. The surgical technique is to perform sternal bars dissection with careful perichondrium and pericardium separation to avoid phrenic nerve injury. In partial clefts, perform a complete cleft with a wedge osteotomy in the intact sternum has been recommended to grant symmetrical sternum sides re-approximation. At over three months, the patient's thoracic skeletal structures are more rigid; therefore, a surgical approximation may cause thoracic compartment syndrome to approach this scenario, costal cartilage sliding-plasty, autologous tissue bridges, and prosthetic material protection has been utilized.[10]
Ectopia Cordis
Provide vital support to the newborn is global management. There is no better surgical approach for ectopia cordis if it is possible; however, a multi-stage procedure is the recommendation. The first stage is focussed on immediate omphalocele closure if indicated and temporally heart soft tissue covering. Second stage surgery focuses on heart reduction into the thoracic cavity, cardiac defects repair, and chest wall reconstruction.[13][24](B3)
Jeunes Syndrome
Treatment directs towards aggressive respiratory support. Surgery is indicated in severe respiratory distress (thoracic insufficiency syndrome) instauration, and this commonly occurs between 8 and 18 months of age. Providing chest wall diameter augment is the surgical goal.[19] Sternal split interposition, VEPTR (vertical expandable prosthetic titanium rib), LTE (lateral thoracic expansion technique), and Nuss technique are the most popular surgical repair options. Major surgical chest wall reconstruction is controversial due to the high frequency of progressive renal failure in these patients.[45][46]
Jarcho-Levin syndrome
Treatment focuses on aggressive respiratory support. In spondylothoracic dysostosis, surgical repair has no benefit due to the deformity being limited to the spine's posterior aspect. In spondylocostal dysostosis phenotype, VEPTR surgical repair has been performed to grant thoracic spine augment, thus permitting expansion of the thoracic volume to allow lung development. Surgery is the recommendation when scoliosis and respiratory distress begins to progress (6 months of age).[47][48](B2)
Differential Diagnosis
Pectus deformities
Marfan syndrome is the hallmark entity of differential diagnosis; however, all related syndromes to pectus deformities are part of differential diagnosis constellation.[5][16]
Poland Syndrome
Differential diagnoses in Poland syndrome highlights include normal breast asymmetry in females, and Swayer-James syndrome and giant bulla in hyper-lucent hemithorax RX setting.[4]
Sternal Clefts
The differential diagnosis depends on syndromic association constellation. Highlights are limb body wall complex, amniotic band syndrome, and Cantrell pentalogy.[11]
Ectopia Cordis
Differential diagnosis highlights are; trisomy 18 and Turner syndrome.[49]
Jeunes Syndrome
Differential diagnosis highlights are; Ellis-Van Creveld syndrome, short rib polydactyly syndrome (I-IV types), Barnes syndrome, and Shwachman-Diamond syndrome.[45][46]
Jarcho-Levin syndrome
Differential diagnosis highlights include spondyloepiphyseal dysplasia, Morquio syndrome, chondrodysplasia, Klippel-Feil syndrome, and short rib polydactyly syndrome.[26]
Prognosis
Pectus Excavatum
Surgical correction is achieved with open and MARPE surgery with satisfactory results (86% to 98.1%), from a functional standpoint, cardio-pulmonary fitness performance improve at 6 to 12 months after surgery.[50][51][52] Mitral valve prolapse related to pectus excavatum resolution occurs in 50 % of cases after surgical repair.[53]
Pectus Carinatum
Highlighting that minimally invasive surgery still needs more evidence support and acceptance, pectus carinatum surgical repair results are based on the modified Ravitch repair principles, therefore in several series, outcomes, and prognosis (esthetic satisfaction and symptomatology improvement) homogeneity results between both pectus deformities have been demonstrated. For bracing treatment, anatomical changes can be observed within 2 to 3 months of its use, bracing treatment duration is from 2 to 2.5 years; however, there are reports that the bracing device is poorly tolerated.[51]
Poland Syndrome
Prognosis will depend on age, gender, phenotype severity, associated cancer if coexisting, and type of surgical reconstruction if indicated.[32][23]
Sternal Clefts
Prognosis and outcomes will depend on defect phenotype, age, and associated anomalies. Superior sternal clefts commonly present as an isolated feature with an orthotropic hearth without significant intra-cardiac abnormalities, so they have a good prognosis. In general, a better prognosis is achievable with an appropriate surgical repair timing (neonatal period); however, survival will depend more on the associated anomalies than sternal cleft.[1]
Ectopia Cordis
Ectopia cordis general prognosis is poor. Thoracic ectopia cordis, complex congenital cardiac defects, and Cantrell pentalogy have the poorest prognosis. Thoracoabdominal ectopia cordis has been reported with a better prognosis due to the non-malrotated ectopic heart.[13][24]
Jeunes Syndrome
Generally, Jeune's syndrome prognosis is poor, and it has a reported mortality rate of 60 to 80%; however, some centers have reported up to a 50% chance of survival with surgery in mild cases. Survival prognosis may improve with age progression.[54][55]
Jarcho-Levin syndrome
Prognosis will depend on phenotype. Spondylothoracic dysostosis generally is considered a lethal condition and surgery has no benefit; however, in some cases series, mild forms have been reported with up to 56% survival with aggressive medical treatment focused on respiratory function.[21] Spondylocostal dysostosis has a better prognosis. VEPTR surgery has a more probable better prognosis. Both phenotypes have normal intellectual development, and the overall prognosis improves if patients reach six months of age.[56]
Complications
Pectus Excavatum
Complications related to any thoracic surgery such; bleeding, surgical site infections, seroma, pneumothorax, and other pulmonary issues are equally in open and MARPE surgery. In the NSQIP database, the Nuss procedure morbidity rate complications are of 3.8%. Pectus excavatum recurrence (2 to 37%) is higher due to open surgery resulting from perichondrium surgical disruption, highlighting acquired asphyxiating thoracic dystrophy development in patients under four years old. Underlying connective tissue disease and too early bar removal have been linked to pectus recurrence too. The highlight complication associated with MARPE is bar displacement (5.7 to 12%).[50][57]
Pectus Carinatum
Complications are the same as the modified Ravitch procedure for pectus excavatum; however, it is important to highlight that the recurrence rates are reduced by making less costal cartilage resection with intact growth centers. Bracing treatment recurrence risk is 5 to 15%. Bracing complications highlight is the device's poor tolerance.[41]
Poland Syndrome
Complications will depend on phenotype, associated anomalies, and surgical repair (case-by-case basis). Breast cancer risk in a female is a paramount highlight.[32][44]
Sternal Clefts
Will depend more on the associated anomalies than sternal cleft; however, post-surgical complications are of special interest, bein post-surgical repair cardiac compression the most life-threatening complication.[10]
Ectopia Cordis
Infection, cardiac failure, and hypoxemia have been reported as the most common causes of death.[13]
Jeunes Syndrome
The main cause of death is respiratory failure. Terminal kidney disease can develop in 30% of cases. Associated anomalies highlights are; Liver, pancreatic, and retinal anomalies. The most frequently reported deaths are during the first year of life, while the older the child, the lower the risks.[54][58]
Jarcho-Levin syndrome
Respiratory complications, congestive heart failure, and pulmonary hypertension are the leading causes of death.[26]
Postoperative and Rehabilitation Care
Pectus Excavatum
General postoperative care as; pain control, wound/drainage-care, and possible complications vigilance are equal in both surgical techniques. Respiratory exercises with a spirometer is a paramount highlight. Some postoperative care differences exist between open and MARPE surgery. In open surgery, an early regular walk is encouraged, while in MARPE, short interval steps with gradually increasing are recommended, paying special attention that Nuss bar twisting does not occur. Patients get discharged at 3 to 5 postoperative days. Muscle weakness (thorax, limbs, abdomen) and poor posture are a constant feature in pectus deformities and correlate with enhanced deformity recurrence or causing a wrong esthetic post-surgical result perception; therefore physical rehabilitation program is mandatory.[29] Activity and sports restoration are similar for both techniques. Open surgery activity restoration highlights are; avoid lifting heavy weights for eight weeks, start light exercises at four weeks with gradually incrementing at three months. MARPE activity restoration highlights are; avoid lifting heavy weights for eight weeks, start light exercises gradually at 12 weeks in MARPE. For both surgical techniques, contact sports are avoided until bar removal. Follow-up control focuses on possible complications detection and satisfaction perception evaluation.[51][52]
Pectus Carinatum
Postoperative management is the same as pectus excavatum open surgery. Specific pectus carinatum post-surgical care highlights are; spirometer using, start lightweights lifting at three weeks, start a gym at five months, and if utilizing a metal strut, it is removable at four months.[51] A physical rehabilitation program focuses on biceps, deltoids, and abdominal strength improvement.[29] Bracing treatment follow-up consists of periodic visits with adjustment tightening at less frequent intervals.[41]
Jeunes Syndrome
Devries published a follow-up protocol in which the frequency of clinical vigilance and realization of para-clinic studies depends on the patient's age.[54][55]
Poland Syndrome, Sternal Clefts, Ectopia Cordis, and Jarcho-Levin syndrome
Postoperative and rehabilitation care is determined case by case basis.
Deterrence and Patient Education
Chest wall deformities are unpopular diseases for the general population due to their low incidence. Patients' education global approach should focus on genetic conditions due to the syndromic environment of these diseases; therefore, genetic counseling is of paramount importance for all patients and families so that the genetic risks of chest wall deformities can be made clear. From a specific disease standpoint, patient education is determined on a case-by-case basis with special attention on disease prognosis and post-operative rehabilitation if indicated.
Enhancing Healthcare Team Outcomes
Chest wall deformities are a broad set of congenital diseases that can present with different phenotypes and clinical manifestations, ranging from life-threatening conditions to psychosocial cosmetic concerns, with medical assistance searching interval from prenatal to adulthood for these reasons chest wall deformities workup and management is a difficult task that needs an interprofessional team. Medical genetics, maternal-fetal medicine, neonatologist, pediatric, and neonatal intensive care, pediatric surgery, cardiothoracic surgery, plastic surgery, orthopedic surgery, and psychology are the specialties involved in the management of chest wall deformities. Due to the low incidence, level 1 evidence is lacking for these diseases. Meta-analysis for pectus deformities are available, they are focused on differences between Nuss procedure and modified Ravitch procedure with level III evidence.[59] Management recommendations for Poland syndrome, sternal defects, Jeunes, and Jarcho Levyn syndrome have their basis in case-report series due to the low incidence. There is no global accepted general classification for chest wall deformities. Also, treatment options are customized, and a general approach does not exist. Thus, the importance of chest wall deformities knowledge is essential to have coordinated interprofessional care in these patients.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Blanco FC, Elliott ST, Sandler AD. Management of congenital chest wall deformities. Seminars in plastic surgery. 2011 Feb:25(1):107-16. doi: 10.1055/s-0031-1275177. Epub [PubMed PMID: 22294949]
Fonkalsrud EW. Surgical correction of pectus carinatum: lessons learned from 260 patients. Journal of pediatric surgery. 2008 Jul:43(7):1235-43. doi: 10.1016/j.jpedsurg.2008.02.007. Epub [PubMed PMID: 18639675]
Tocchioni F, Ghionzoli M, Pepe G, Messineo A. Pectus excavatum and MASS phenotype: an unknown association. Journal of laparoendoscopic & advanced surgical techniques. Part A. 2012 Jun:22(5):508-13. doi: 10.1089/lap.2012.0009. Epub 2012 May 8 [PubMed PMID: 22568544]
Level 2 (mid-level) evidenceKotzot D, Schwabegger AH. Etiology of chest wall deformities--a genetic review for the treating physician. Journal of pediatric surgery. 2009 Oct:44(10):2004-11. doi: 10.1016/j.jpedsurg.2009.07.029. Epub [PubMed PMID: 19853763]
Colombani PM. Preoperative assessment of chest wall deformities. Seminars in thoracic and cardiovascular surgery. 2009 Spring:21(1):58-63. doi: 10.1053/j.semtcvs.2009.04.003. Epub [PubMed PMID: 19632564]
Koumbourlis AC. Chest wall abnormalities and their clinical significance in childhood. Paediatric respiratory reviews. 2014 Sep:15(3):246-54; quiz 254-5. doi: 10.1016/j.prrv.2013.12.003. Epub 2013 Dec 18 [PubMed PMID: 24462760]
Sharma CM, Kumar S, Meghwani MK, Agrawal RP. Poland syndrome. Indian journal of human genetics. 2014 Jan:20(1):82-4. doi: 10.4103/0971-6866.132764. Epub [PubMed PMID: 24959021]
Level 3 (low-level) evidenceMoir CR, Johnson CH. Poland's syndrome. Seminars in pediatric surgery. 2008 Aug:17(3):161-6. doi: 10.1053/j.sempedsurg.2008.03.005. Epub [PubMed PMID: 18582821]
Rosa RF, Travi GM, Valiatti F, Zen PR, Pinto LL, Kiss A, Graziadio C, Paskulin GA. Poland syndrome associated with an aberrant subclavian artery and vascular abnormalities of the retina in a child exposed to misoprostol during pregnancy. Birth defects research. Part A, Clinical and molecular teratology. 2007 Jun:79(6):507-11 [PubMed PMID: 17393483]
Level 3 (low-level) evidenceAlshomer F, Aldaghri F, Alohaideb N, Aljehani R, Murad MA, Hashem F. Reconstruction of Congenital Sternal Clefts: Surgical Experience and Literature Review. Plastic and reconstructive surgery. Global open. 2017 Nov:5(11):e1567. doi: 10.1097/GOX.0000000000001567. Epub 2017 Nov 20 [PubMed PMID: 29263968]
Torre M, Rapuzzi G, Carlucci M, Pio L, Jasonni V. Phenotypic spectrum and management of sternal cleft: literature review and presentation of a new series. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2012 Jan:41(1):4-9. doi: 10.1016/j.ejcts.2011.05.049. Epub [PubMed PMID: 21737294]
Level 3 (low-level) evidencevan der Merwe AE, Weston DA, Oostra RJ, Maat GJ. A review of the embryological development and associated developmental abnormalities of the sternum in the light of a rare palaeopathological case of sternal clefting. Homo : internationale Zeitschrift fur die vergleichende Forschung am Menschen. 2013 Apr:64(2):129-41. doi: 10.1016/j.jchb.2013.01.003. Epub 2013 Mar 7 [PubMed PMID: 23473075]
Level 3 (low-level) evidenceGabriel A, Donnelly J, Kuc A, Good D, Doros G, Matusz P, Loukas M. Ectopia cordis: a rare congenital anomaly. Clinical anatomy (New York, N.Y.). 2014 Nov:27(8):1193-9. doi: 10.1002/ca.22402. Epub 2014 Apr 19 [PubMed PMID: 24753313]
Schmidts M, Arts HH, Bongers EM, Yap Z, Oud MM, Antony D, Duijkers L, Emes RD, Stalker J, Yntema JB, Plagnol V, Hoischen A, Gilissen C, Forsythe E, Lausch E, Veltman JA, Roeleveld N, Superti-Furga A, Kutkowska-Kazmierczak A, Kamsteeg EJ, Elçioğlu N, van Maarle MC, Graul-Neumann LM, Devriendt K, Smithson SF, Wellesley D, Verbeek NE, Hennekam RC, Kayserili H, Scambler PJ, Beales PL, UK10K, Knoers NV, Roepman R, Mitchison HM. Exome sequencing identifies DYNC2H1 mutations as a common cause of asphyxiating thoracic dystrophy (Jeune syndrome) without major polydactyly, renal or retinal involvement. Journal of medical genetics. 2013 May:50(5):309-23. doi: 10.1136/jmedgenet-2012-101284. Epub 2013 Mar 1 [PubMed PMID: 23456818]
Cornier AS, Staehling-Hampton K, Delventhal KM, Saga Y, Caubet JF, Sasaki N, Ellard S, Young E, Ramirez N, Carlo SE, Torres J, Emans JB, Turnpenny PD, Pourquié O. Mutations in the MESP2 gene cause spondylothoracic dysostosis/Jarcho-Levin syndrome. American journal of human genetics. 2008 Jun:82(6):1334-41. doi: 10.1016/j.ajhg.2008.04.014. Epub 2008 May 15 [PubMed PMID: 18485326]
Level 3 (low-level) evidenceCobben JM, Oostra RJ, van Dijk FS. Pectus excavatum and carinatum. European journal of medical genetics. 2014 Aug:57(8):414-7. doi: 10.1016/j.ejmg.2014.04.017. Epub 2014 May 10 [PubMed PMID: 24821303]
Robicsek F, Watts LT, Fokin AA. Surgical repair of pectus excavatum and carinatum. Seminars in thoracic and cardiovascular surgery. 2009 Spring:21(1):64-75. doi: 10.1053/j.semtcvs.2009.03.002. Epub [PubMed PMID: 19632565]
Fokin AA. Thoracic defects: cleft sternum and Poland syndrome. Thoracic surgery clinics. 2010 Nov:20(4):575-82. doi: 10.1016/j.thorsurg.2010.06.001. Epub [PubMed PMID: 20974442]
Drebov RS, Katsarov A, Gagov E, Atanasova N, Penev Z, Iliev A. Is Asphyxiating Thoracic Dystrophy (Jeune's Syndrome) Deadly and Should We Insist on Treating It? Reconstructive Surgery "On Demand". Surgery journal (New York, N.Y.). 2017 Jan:3(1):e17-e22. doi: 10.1055/s-0037-1598043. Epub 2017 Feb 17 [PubMed PMID: 28825014]
Campbell RM Jr. Spine deformities in rare congenital syndromes: clinical issues. Spine. 2009 Aug 1:34(17):1815-27. doi: 10.1097/BRS.0b013e3181ab64e9. Epub [PubMed PMID: 19644333]
Kulkarni ML, Navaz SR, Vani HN, Manjunath KS, Matani D. Jarcho-Levin syndrome. Indian journal of pediatrics. 2006 Mar:73(3):245-7 [PubMed PMID: 16567923]
Level 3 (low-level) evidenceDavid VL, Izvernariu DA, Popoiu CM, Puiu M, Boia ES. Morphologic, morphometrical and histochemical proprieties of the costal cartilage in children with pectus excavatum. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie. 2011:52(2):625-9 [PubMed PMID: 21655653]
Seyfer AE, Fox JP, Hamilton CG. Poland syndrome: evaluation and treatment of the chest wall in 63 patients. Plastic and reconstructive surgery. 2010 Sep:126(3):902-911. doi: 10.1097/PRS.0b013e3181e60435. Epub [PubMed PMID: 20811224]
Shad J, Budhwani K, Biswas R. Thoracic ectopia cordis. BMJ case reports. 2012 Sep 30:2012():. doi: 10.1136/bcr.11.2011.5241. Epub 2012 Sep 30 [PubMed PMID: 23035158]
Level 3 (low-level) evidenceMistry KA, Suthar PP, Bhesania SR, Patel A. Antenatal Diagnosis of Jeune Syndrome (Asphyxiating Thoracic Dysplasia) with Micromelia and Facial Dysmorphism on Second-Trimester Ultrasound. Polish journal of radiology. 2015:80():296-9. doi: 10.12659/PJR.894188. Epub 2015 Jun 7 [PubMed PMID: 26124900]
Kurup PM, Tanigasalam V, Bhat BV. Spondylocostal Dysostosis (Jarcho Levin Syndrome). Indian journal of pediatrics. 2018 Jun:85(6):486. doi: 10.1007/s12098-017-2549-0. Epub 2017 Dec 22 [PubMed PMID: 29270795]
Kragten HA, Siebenga J, Höppener PF, Verburg R, Visker N. Symptomatic pectus excavatum in seniors (SPES): a cardiovascular problem? : A prospective cardiological study of 42 senior patients with a symptomatic pectus excavatum. Netherlands heart journal : monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation. 2011 Feb:19(2):73-8. doi: 10.1007/s12471-010-0067-z. Epub [PubMed PMID: 22020944]
Choi JH, Park IK, Kim YT, Kim WS, Kang CH. Classification of Pectus Excavatum According to Objective Parameters From Chest Computed Tomography. The Annals of thoracic surgery. 2016 Dec:102(6):1886-1891. doi: 10.1016/j.athoracsur.2016.05.079. Epub 2016 Aug 12 [PubMed PMID: 27526652]
Willital GH, Saxena AK, Schütze U, Richter W. Chest-deformities: a proposal for a classification. World journal of pediatrics : WJP. 2011 May:7(2):118-23. doi: 10.1007/s12519-011-0263-y. Epub 2011 May 15 [PubMed PMID: 21574027]
Coelho Mde S, Guimarães Pde S. Pectus carinatum. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2007 Jul-Aug:33(4):463-74 [PubMed PMID: 17982540]
Kuzmichev V, Ershova K, Adamyan R. Surgical correction of pectus arcuatum. Journal of visualized surgery. 2016:2():55. doi: 10.21037/jovs.2016.02.28. Epub 2016 Mar 16 [PubMed PMID: 29078483]
Baldelli I, Gallo F, Crimi M, Fregatti P, Mellini L, Santi P, Ciliberti R. Experiences of patients with Poland syndrome of diagnosis and care in Italy: a pilot survey. Orphanet journal of rare diseases. 2019 Nov 21:14(1):269. doi: 10.1186/s13023-019-1253-8. Epub 2019 Nov 21 [PubMed PMID: 31753026]
Level 3 (low-level) evidenceHamaji M, Hiraoka K, Jaroszewski DE, Deschamps C. Modified Robicsek procedure for pectus excavatum in adult patients. Interactive cardiovascular and thoracic surgery. 2014 May:18(5):611-4. doi: 10.1093/icvts/ivt555. Epub 2014 Jan 23 [PubMed PMID: 24457817]
Level 2 (mid-level) evidenceAntonoff MB,Erickson AE,Hess DJ,Acton RD,Saltzman DA, When patients choose: comparison of Nuss, Ravitch, and Leonard procedures for primary repair of pectus excavatum. Journal of pediatric surgery. 2009 Jun; [PubMed PMID: 19524726]
Lorenzo GR, Gutiérrez Dueñas JM, Ardela E, Martín Pinto F. [Preliminary results in the correction of the pectus excavatum with the Acastello modified Welch technique]. Cirugia pediatrica : organo oficial de la Sociedad Espanola de Cirugia Pediatrica. 2011 Oct:24(4):201-7 [PubMed PMID: 23155632]
Schulz-Drost S, Syed J, Luber AM, Carbon RT, Besendörfer M. From pullout-techniques to modular elastic stable chest repair: the evolution of an open technique in the correction of pectus excavatum. Journal of thoracic disease. 2019 Jul:11(7):2846-2860. doi: 10.21037/jtd.2019.07.01. Epub [PubMed PMID: 31463114]
Makarawo TP, Steyn RS, Naidu BV. Prosthesis-free repair of pectus chest deformity. The British journal of surgery. 2011 Nov:98(11):1660-5. doi: 10.1002/bjs.7596. Epub 2011 Jul 12 [PubMed PMID: 21751180]
Saxena AK. Pectus less invasive extrapleural repair (PLIER). Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2009 May:62(5):663-8. doi: 10.1016/j.bjps.2007.12.027. Epub 2008 Jan 28 [PubMed PMID: 18222741]
Funk JF, Gross C, Placzek R. Patient satisfaction and clinical results 10 years after modified open thoracoplasty for pectus deformities. Langenbeck's archives of surgery. 2011 Dec:396(8):1213-20. doi: 10.1007/s00423-011-0827-2. Epub 2011 Jul 16 [PubMed PMID: 21779828]
Level 2 (mid-level) evidenceSuh JW, Joo S, Lee GD, Haam SJ, Lee S. Minimally Invasive Repair of Pectus Carinatum in Patients Unsuited to Bracing Therapy. The Korean journal of thoracic and cardiovascular surgery. 2016 Apr:49(2):92-8. doi: 10.5090/kjtcs.2016.49.2.92. Epub 2016 Apr 5 [PubMed PMID: 27066432]
Frey AS, Garcia VF, Brown RL, Inge TH, Ryckman FC, Cohen AP, Durrett G, Azizkhan RG. Nonoperative management of pectus carinatum. Journal of pediatric surgery. 2006 Jan:41(1):40-5; discussion 40-5 [PubMed PMID: 16410105]
Level 2 (mid-level) evidenceRodriguez IE, Heare T, Bruny J, Deleyiannis FW. Customized Titanium Implant for Chest Wall Reconstruction in Complex Poland Syndrome. Plastic and reconstructive surgery. Global open. 2014 Feb:2(2):e112 [PubMed PMID: 25243105]
Sanna S, Brandolini J, Pardolesi A, Argnani D, Mengozzi M, Dell'Amore A, Solli P. Materials and techniques in chest wall reconstruction: a review. Journal of visualized surgery. 2017:3():95. doi: 10.21037/jovs.2017.06.10. Epub 2017 Jul 26 [PubMed PMID: 29078657]
Buckwalter V JA, Shah AS. Presentation and Treatment of Poland Anomaly. Hand (New York, N.Y.). 2016 Dec:11(4):389-395. doi: 10.1177/1558944716647355. Epub 2016 Oct 10 [PubMed PMID: 28149203]
Zivkovic V, Büchler P, Ovadia D, Riise R, Stuecker R, Hasler C. Extraspinal ossifications after implantation of vertical expandable prosthetic titanium ribs (VEPTRs). Journal of children's orthopaedics. 2014 May:8(3):237-44. doi: 10.1007/s11832-014-0585-0. Epub 2014 Apr 22 [PubMed PMID: 24752718]
Kikuchi N, Kashiwa H, Ogino T, Kato M, Hayasaka K. The Nuss technique for Jeune asphyxiating thoracic dystrophy repair in siblings. Annals of plastic surgery. 2010 Aug:65(2):214-8. doi: 10.1097/SAP.0b013e3181c9c375. Epub [PubMed PMID: 20606585]
Karlin JG, Roth MK, Patil V, Cordell D, Trevino H, Simmons J, Campbell RM, Joshi AP. Management of thoracic insufficiency syndrome in patients with Jarcho-Levin syndrome using VEPTRs (vertical expandable prosthetic titanium ribs). The Journal of bone and joint surgery. American volume. 2014 Nov 5:96(21):e181. doi: 10.2106/JBJS.M.00185. Epub [PubMed PMID: 25378514]
Level 2 (mid-level) evidenceJoshi AP, Roth MK, Simmons JW, Shardonofsky F, Campbell RM Jr. Expansion Thoracoplasty for Thoracic Insufficiency Syndrome Associated with Jarcho-Levin Syndrome. JBJS essential surgical techniques. 2015 May 27:5(2):e12. doi: 10.2106/JBJS.ST.N.00017. Epub 2015 Jun 24 [PubMed PMID: 30473920]
Kylat RI. Complete and Incomplete Pentalogy of Cantrell. Children (Basel, Switzerland). 2019 Oct 6:6(10):. doi: 10.3390/children6100109. Epub 2019 Oct 6 [PubMed PMID: 31590448]
Hebra A. Minimally invasive repair of pectus excavatum. Seminars in thoracic and cardiovascular surgery. 2009 Spring:21(1):76-84. doi: 10.1053/j.semtcvs.2009.04.005. Epub [PubMed PMID: 19632566]
Fonkalsrud EW, Mendoza J. Open repair of pectus excavatum and carinatum deformities with minimal cartilage resection. American journal of surgery. 2006 Jun:191(6):779-84 [PubMed PMID: 16720148]
Level 2 (mid-level) evidenceNeviere R, Montaigne D, Benhamed L, Catto M, Edme JL, Matran R, Wurtz A. Cardiopulmonary response following surgical repair of pectus excavatum in adult patients. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2011 Aug:40(2):e77-82. doi: 10.1016/j.ejcts.2011.03.045. Epub 2011 May 12 [PubMed PMID: 21570313]
Dimitrakakis G, von Oppell UO, Miller C, Kornaszewska M. Simultaneous mitral valve and pectus excavatum repair with a Nuss bar. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2012 Oct:42(4):e86-8. doi: 10.1093/ejcts/ezs392. Epub 2012 Aug 22 [PubMed PMID: 22918181]
Level 3 (low-level) evidencede Vries J, Yntema JL, van Die CE, Crama N, Cornelissen EA, Hamel BC. Jeune syndrome: description of 13 cases and a proposal for follow-up protocol. European journal of pediatrics. 2010 Jan:169(1):77-88. doi: 10.1007/s00431-009-0991-3. Epub 2009 May 10 [PubMed PMID: 19430947]
Level 3 (low-level) evidenceSacco Casamassima MG, Goldstein SD, Salazar JH, Papandria D, McIltrot KH, O'Neill DE, Abdullah F, Colombani PM. Operative management of acquired Jeune's syndrome. Journal of pediatric surgery. 2014 Jan:49(1):55-60; discussion 60. doi: 10.1016/j.jpedsurg.2013.09.027. Epub 2013 Oct 5 [PubMed PMID: 24439581]
Level 2 (mid-level) evidenceErdoğan S, Polat B, Atıcı Y, Özyalvaç ON, Öztürk Ç. Comparison of the Effects of Magnetically Controlled Growing Rod and Tradiotinal Growing Rod Techniques on the Sagittal Plane in the Treatment of Early-Onset Scoliosis. Journal of Korean Neurosurgical Society. 2019 Sep:62(5):577-585. doi: 10.3340/jkns.2019.0094. Epub 2019 Aug 30 [PubMed PMID: 31484232]
Sacco-Casamassima MG, Goldstein SD, Gause CD, Karim O, Michailidou M, Stewart D, Colombani PM, Abdullah F. Minimally invasive repair of pectus excavatum: analyzing contemporary practice in 50 ACS NSQIP-pediatric institutions. Pediatric surgery international. 2015 May:31(5):493-9. doi: 10.1007/s00383-015-3694-z. Epub 2015 Mar 27 [PubMed PMID: 25814003]
Level 2 (mid-level) evidenceDagoneau N, Goulet M, Geneviève D, Sznajer Y, Martinovic J, Smithson S, Huber C, Baujat G, Flori E, Tecco L, Cavalcanti D, Delezoide AL, Serre V, Le Merrer M, Munnich A, Cormier-Daire V. DYNC2H1 mutations cause asphyxiating thoracic dystrophy and short rib-polydactyly syndrome, type III. American journal of human genetics. 2009 May:84(5):706-11. doi: 10.1016/j.ajhg.2009.04.016. Epub [PubMed PMID: 19442771]
Mao YZ, Tang S, Li S. Comparison of the Nuss versus Ravitch procedure for pectus excavatum repair: an updated meta-analysis. Journal of pediatric surgery. 2017 Oct:52(10):1545-1552. doi: 10.1016/j.jpedsurg.2017.05.028. Epub 2017 Jun 3 [PubMed PMID: 28606386]
Level 1 (high-level) evidence