Introduction
A thoracostomy or chest tube is a flexible catheter introduced into the pleural space via a minimally invasive approach to facilitate drainage of air, blood, fluid, chyle, or infectious material. These tubes, typically composed of polyvinyl chloride or silicone, range from 6 to 40 French in diameter and often feature fenestrations near the insertion end to optimize drainage. A radiopaque stripe allows for imaging confirmation of proper placement.
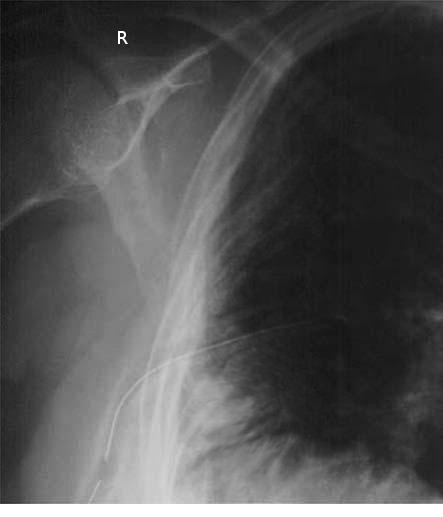
Upon insertion, the distal end of the chest tube is connected to a drainage system, which consists of 3 chambers: the suction chamber, which applies negative pressure to assist lung reexpansion; the water seal chamber, acting as a one-way valve to prevent the backflow of air into the thoracic cavity; and the collection chamber, which accumulates drained fluids. These mechanisms collectively reestablish pulmonary mechanics and improve respiratory function (see Image. Surgically-Placed Chest Tube).[1][2][3]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Successful chest tube placement necessitates a comprehensive understanding of thoracic anatomy to optimize procedural efficacy while mitigating risks of iatrogenic injury. The preferred site for placement is the fourth or fifth intercostal space at the anterior to midaxillary line, corresponding to the nipple line in males and the inframammary fold in females.[4][5] Avoiding injury to the intercostal neurovascular bundle, which courses along the inferior margin of each rib, is critical. The tube should be inserted superior to the lower rib margin to minimize trauma within the designated intercostal space.
The safe triangle is a well-defined anatomical region that minimizes complications during tube insertion. This triangle is bordered anteriorly by the lateral border of the pectoralis major, posteriorly by the anterior border of the latissimus dorsi, and inferiorly by a horizontal line at the nipple level (fifth intercostal space), with its apex just below the axilla. Utilizing this safety zone helps to avoid injury to critical structures, including the long thoracic nerve, diaphragm, heart, liver, and spleen.[4] The pleural cavity, which is bordered by the visceral and parietal pleura, serves as the target space for drainage. Misplacement may result in parenchymal injury or subcutaneous emphysema. Special attention must be given to left-sided insertions to avoid the heart and stomach, while right-sided placements should consider the proximity of the liver.[5]
Indications
Chest tube placement is required for pleural decompression or drainage in various conditions, including pneumothorax (tension, spontaneous, or traumatic), hemothorax, malignant or parapneumonic pleural effusion, empyema, and chylothorax. For tension pneumothorax, needle decompression is the first-line intervention, followed by definitive chest tube placement to prevent recurrence.[6] The decision to place a chest tube depends on the pneumothorax size and the patient’s clinical stability. Tran et al emphasized that small, stable pneumothoraces can often be managed conservatively, whereas larger or symptomatic pneumothoraces necessitate thoracostomy tube placement or small-bore catheter drainage.[7] A systematic review and meta-analysis by Beeton et al demonstrated that pigtail catheters provide comparable efficacy to traditional large-bore thoracostomy tubes while reducing procedural pain and complications in select trauma patients.[8] However, larger tubes remain preferable in cases of retained hemothorax or persistent air leaks.
Determining the appropriate pneumothorax size threshold for tube placement has been extensively studied. Over the past 2 decades, smaller chest tubes have become available, are easier to insert, and are associated with significantly less pain than older chest tubes.[9][10] Ginsberg et al proposed the 35 mm rule, suggesting that pneumothoraces <35 mm on computed tomography (CT) imaging may be observed, whereas those ≥35 mm warrant chest tube placement.[11] Additionally, ultrasound is increasingly recognized as a preferred diagnostic modality, offering higher sensitivity than chest radiography and facilitating real-time bedside assessment in trauma patients.[7]
Contraindications
Relative contraindications to thoracostomy tube placement include pulmonary adhesions due to prior surgery or inflammatory disease, coagulopathy, and diaphragmatic hernia. While coagulopathy has historically been considered a contraindication, a systematic review by Fong et al found that thoracostomy tube placement does not significantly increase major bleeding risk in patients with uncorrected coagulopathy, challenging traditional concerns regarding preprocedural coagulation correction.[12] However, careful patient selection and monitoring remain critical.
Equipment
Chest tube placement requires a standardized set of equipment to ensure procedural success and minimize complications. Essential tools include a sterile chest tube of appropriate size (typically 28–36 Fr for trauma-related hemothorax and 24–28 Fr for pneumothorax), a sterile tray with surgical instruments (scalpel, Kelly clamp, needle driver, scissors), antiseptic solution, sterile gloves, gown, and drapes, and a local anesthetic (eg, lidocaine). A thoracostomy tube drainage system (water-seal or suction-based), suture material (usually 0 or 2-0 silk), and occlusive dressings are required. Ultrasound may guide insertion, particularly in patients with loculated effusions or challenging anatomy. Evidence supports that using a structured insertion kit and adherence to sterile technique significantly reduce the risk of infection and tube malposition.[13] Current trauma guidelines recommend large-bore tubes for hemothorax to optimize drainage and reduce retained blood products.
Personnel
Proper chest tube placement requires a coordinated interprofessional team to optimize safety, efficiency, and outcomes. The core personnel typically include a proceduralist (surgeon, emergency physician, or intensivist) trained in thoracostomy techniques, alongside a registered nurse and respiratory therapist. The nurse assists with patient preparation, monitoring, and postprocedural care, while the respiratory therapist manages ventilatory support and evaluates chest tube function. Studies highlight that team-based approaches—especially those using standardized protocols and simulation-based training—significantly reduce procedural complications and improve confidence and performance among team members. The presence of trained support staff also facilitates rapid recognition of complications such as tube dislodgement or tension physiology, which can be life-threatening if not addressed promptly.
Preparation
Prior to chest tube insertion, imaging guidance such as ultrasound or CT scanning is recommended for complex cases. Prophylactic antibiotic administration, typically with first-generation cephalosporins, has been shown to reduce infectious complications, particularly empyema, in trauma patients. A study by MacDonald and Long supports targeted antibiotic use in high-risk patients undergoing tube thoracostomy for hemothorax or pneumothorax.[14]
Technique or Treatment
Placement Technique
Thoracostomy tubes are typically placed at the mid-to-anterior axillary line in the fourth or fifth intercostal space. The insertion approach should ensure tracking superior to the rib margin to prevent neurovascular injury. For pneumothorax drainage, the tube should be directed upward toward the apex, whereas for hemothorax or pleural effusion, a posterior-oriented placement is preferred. Postplacement chest radiography confirms correct positioning and evaluates for complications such as malposition or residual pneumothorax.
Discontinuation and Complications
Chest tube removal should be performed during expiration to reduce the likelihood of air reentry.[15] The Valsalva maneuver can further mitigate the risk of residual pneumothorax by increasing intrathoracic pressure during removal.[16][17] Complications associated with thoracostomy tube placement include bleeding, empyema, tube dislodgement, occlusion, and reexpansion pulmonary edema. Early recognition and appropriate management of these complications are critical for optimizing patient outcomes.
Complications
The complications that can manifest with chest tubes are as follows:
- Bleeding
- Superficial site infection
- Deep organ space infection (empyema)
- Dislodgement of the tube
- Clogging of the tube
- Reexpansion pulmonary edema
- Injury to intraabdominal organs such as the spleen or liver
- Injury to the diaphragm
- Injury to intrathoracic organs, such as the heart or thoracic aorta
Clinical Significance
A chest tube provides life-saving removal of air, blood, and infectious fluids.
Management of Chest Tube
The standard management of chest tubes has yet to be scientifically determined or agreed upon by experts and is often physician-specific based on their training and anecdotal experience, not an exact science. Depending on the indication for the thoracostomy tube placement, the overall concept of managing 1 is based on the favorable opposition of the visceral and parietal pleura. The 3 options for managing a chest tube are suction, water seal, and clamping.
When a new air leak is noted, the chest tube, connecting tubing, evacuation system, and a patient's wound should be examined for any loose connections or dislodgement of the tube. The fenestrated holes should not be outside of the body. Factors that put a patient at high risk for persistent air leaks include steroid use, emphysematous lungs, reoperation with extensive scar tissue, or significant trauma to the lungs.
Suction is usually the initial chest tube management for most indications, not including specialized thoracic surgeries. The tube can then be placed on a water seal if there is no air leak or pneumothorax on a chest x-ray (see Image. Chest Tube on Plain Radiograph). Chest tubes placed for pleurodesis and decortication usually need to be on suction longer to aid in opposition of the pleura before discontinuing the tube. The chest tube can be discontinued once no air leak is visualized, output is serosanguinous with no signs of bleeding, output is less than 150 to 400 cc over 24 hours (this range is wide because it is debatable among researchers), there is nonexistent or stable mild pneumothorax on chest x-ray, and the patient is minimized on positive pressure from the ventilator. A thoracic surgical specialist must be consulted if a patient has a persistent leak after the previous management mentioned above. Suppose the thoracostomy tube is placed for traumatic hemothorax. In that case, the indications for a thoracotomy include an initial sanguineous output of 1500 cc or an average of 200 cc/hr over 4 consecutive hours.
The Eastern Association for the Surgery of Trauma (EAST) 2021 practice management guideline provides evidence-based recommendations for managing simple and retained hemothorax. Patel et al suggest that for hemodynamically stable patients with traumatic hemothorax, pigtail catheters can be an effective alternative to traditional large-bore thoracostomy tubes, with similar efficacy and reduced procedural pain.[18]
For posttraumatic retained hemothorax, the literature is shifting toward early video-assisted thoracic surgery (VATS) if thoracostomy tube management fails.[19] A 2024 multicenter study by Carver et al examined the role of thoracic irrigation in reducing the need for secondary interventions after chest tube placement for hemothorax. Their findings suggest that irrigation with saline or heparinized solution may improve clearance of retained hemothorax and reduce the incidence of late surgical intervention.[20] This approach aligns with the evolving trend in trauma surgery, where optimizing initial chest tube drainage can potentially decrease the need for more invasive procedures. Anderson et al highlight the importance of individualized management strategies for patients, emphasizing that the decision to remove or replace a tube should be guided by clinical context rather than fixed numerical thresholds.[21]
Considerations
Small thoracostomy tubes (such as Wayne catheters) are meant to treat pneumothorax over hemothorax or effusion, secondary to the risk of clogging. Larger chest tubes, usually 28 Fr or larger, are needed for drainage of blood or pus in adults.
Enhancing Healthcare Team Outcomes
Effective chest tube management requires a multidisciplinary approach to optimize patient-centered care, improve outcomes, and ensure patient safety. Chest tubes are now routinely used in most hospitals for various indications, necessitating physicians, nurses, and respiratory therapists to be proficient in their management. Physicians and advanced practitioners must be skilled in proper chest tube placement, troubleshooting complications, and determining when removal is appropriate. Nurses are crucial in monitoring drainage output, ensuring connections are secure, and performing regular dressing changes to prevent infection. They must also know how to manage dislodgement or disconnections and assess the patient for signs of complications. Listening to breath sounds and obtaining regular chest x-rays are essential components of ongoing assessment. Respiratory therapists contribute by optimizing pulmonary function, managing suction settings, and assisting with patient breathing exercises. Pharmacists play a role in pain control and antibiotic stewardship when necessary. If there is any doubt about the function or necessity of the chest tube, consulting the surgeon is imperative.
Interprofessional teamwork is critical in preventing complications such as tube dislodgement, occlusion, or retained hemothorax. Physicians, nurses, and respiratory therapists must collaborate closely to monitor lung re-expansion, assess respiratory status, and ensure proper drainage kit function (see Image. Chest Tube Drainage System). Clear documentation and communication regarding indications for continued chest tube placement or removal streamline decision-making and reduce hospital length of stay. Engaging physical therapists to encourage ambulation and deep breathing exercises further enhances recovery. A well-coordinated, team-based approach ensures that patients receive comprehensive, evidence-based care, ultimately improving outcomes and reducing procedural risks.
Nursing, Allied Health, and Interprofessional Team Monitoring
Effective monitoring of patients with chest tubes requires active engagement from nursing, allied health, and the broader interprofessional team to ensure early detection of complications and optimize patient outcomes. Nursing staff play a critical role in frequent assessment of tube patency, drainage output, dressing integrity, and signs of infection or subcutaneous emphysema. Allied health professionals such as respiratory therapists monitor pulmonary function, support ventilatory needs, and help assess air leak resolution. Evidence supports that structured interprofessional protocols and checklists improve adherence to best practices and reduce complications such as tube dislodgement, retained hemothorax, or tension physiology. Interdisciplinary rounding, shared documentation, and coordinated decision-making further enhance patient safety and continuity of care.
Media
(Click Image to Enlarge)
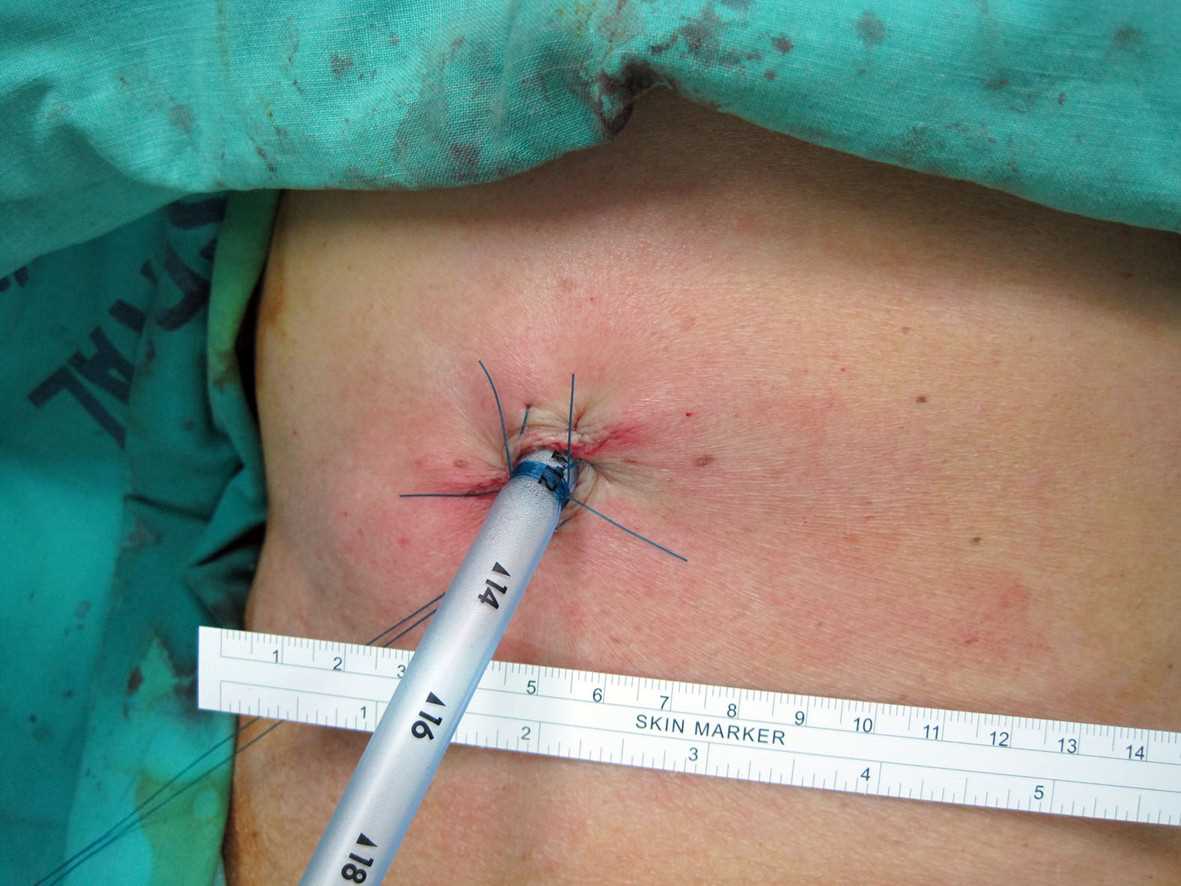
Surgically-Placed Chest Tube. The chest tube is inserted along the chest wall to a prespecified depth, ensuring that the sentinel port (the last hole on the tube that divides the radioopaque line) is completely within the chest wall.
Doc James, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Goncalves Mendes Neto A, Jabuonski TA. Pigtail Catheter vs Chest Tube as the Initial Treatment for Pneumothorax. Chest. 2018 Sep:154(3):725. doi: 10.1016/j.chest.2018.05.040. Epub [PubMed PMID: 30195359]
Özturan İU, Doğan NÖ, Alyeşil C, Pekdemir M, Yılmaz S, Sezer HF. Factors predicting the need for tube thoracostomy in patients with iatrogenic pneumothorax associated with computed tomography-guided transthoracic needle biopsy. Turkish journal of emergency medicine. 2018 Sep:18(3):105-110. doi: 10.1016/j.tjem.2018.05.002. Epub 2018 May 24 [PubMed PMID: 30191189]
Taylor AC, Bates KE, Kipps AK. Variability in paediatric cardiac postoperative chest tube management. Cardiology in the young. 2018 Dec:28(12):1471-1474. doi: 10.1017/S104795111800152X. Epub 2018 Sep 10 [PubMed PMID: 30198449]
Wraight WM, Tweedie DJ, Parkin IG. Neurovascular anatomy and variation in the fourth, fifth, and sixth intercostal spaces in the mid-axillary line: a cadaveric study in respect of chest drain insertion. Clinical anatomy (New York, N.Y.). 2005 Jul:18(5):346-9 [PubMed PMID: 15971216]
Bowness JS, Nicholls K, Kilgour PM, Ferris J, Whiten S, Parkin I, Mooney J, Driscoll P. Finding the fifth intercostal space for chest drain insertion: guidelines and ultrasound. Emergency medicine journal : EMJ. 2015 Dec:32(12):951-4. doi: 10.1136/emermed-2015-205222. Epub 2015 Oct 5 [PubMed PMID: 26438727]
DeMaio A, Semaan R. Management of Pneumothorax. Clinics in chest medicine. 2021 Dec:42(4):729-738. doi: 10.1016/j.ccm.2021.08.008. Epub [PubMed PMID: 34774178]
Tran J, Haussner W, Shah K. Traumatic Pneumothorax: A Review of Current Diagnostic Practices And Evolving Management. The Journal of emergency medicine. 2021 Nov:61(5):517-528. doi: 10.1016/j.jemermed.2021.07.006. Epub 2021 Aug 29 [PubMed PMID: 34470716]
Beeton G, Ngatuvai M, Breeding T, Andrade R, Zagales R, Khan A, Santos R, Elkbuli A. Outcomes of Pigtail Catheter Placement versus Chest Tube Placement in Adult Thoracic Trauma Patients: A Systematic Review and Meta-Analysis. The American surgeon. 2023 Jun:89(6):2743-2754. doi: 10.1177/00031348231157809. Epub 2023 Feb 20 [PubMed PMID: 36802811]
Level 1 (high-level) evidenceSantos C, Gupta S, Baraket M, Collett PJ, Xuan W, Williamson JP. Outcomes of an initiative to improve inpatient safety of small bore thoracostomy tube insertion. Internal medicine journal. 2019 May:49(5):644-649. doi: 10.1111/imj.14110. Epub [PubMed PMID: 30230151]
Lu C, Jin YH, Gao W, Shi YX, Xia X, Sun WX, Tang Q, Wang Y, Li G, Si J. Variation in nurse self-reported practice of managing chest tubes: A cross-sectional study. Journal of clinical nursing. 2018 Mar:27(5-6):e1013-e1021. doi: 10.1111/jocn.14127. Epub 2018 Feb 21 [PubMed PMID: 29076204]
Level 2 (mid-level) evidenceBou Zein Eddine S, Boyle KA, Dodgion CM, Davis CS, Webb TP, Juern JS, Milia DJ, Carver TW, Beckman MA, Codner PA, Trevino C, de Moya MA. Observing pneumothoraces: The 35-millimeter rule is safe for both blunt and penetrating chest trauma. The journal of trauma and acute care surgery. 2019 Apr:86(4):557-564. doi: 10.1097/TA.0000000000002192. Epub [PubMed PMID: 30629009]
Fong C, Tan CWC, Tan DKY, See KC. Safety of Thoracentesis and Tube Thoracostomy in Patients With Uncorrected Coagulopathy: A Systematic Review and Meta-analysis. Chest. 2021 Nov:160(5):1875-1889. doi: 10.1016/j.chest.2021.04.036. Epub 2021 Apr 24 [PubMed PMID: 33905681]
Level 1 (high-level) evidenceBailey RC. Complications of tube thoracostomy in trauma. Journal of accident & emergency medicine. 2000 Mar:17(2):111-4 [PubMed PMID: 10718232]
MacDonald AG, Long B. What Is the Utility of Antibiotic Prophylaxis in Adult Trauma Patients With Hemothorax or Pneumothorax Who Undergo Tube Thoracostomy? Annals of emergency medicine. 2023 Nov:82(5):624-626. doi: 10.1016/j.annemergmed.2023.03.012. Epub 2023 Apr 13 [PubMed PMID: 37865490]
Haider S, Kamal MT, Shoaib N, Zahid M. Thoracostomy tube withdrawal during latter phases of expiration or inspiration: a systematic review and meta-analysis. European journal of trauma and emergency surgery : official publication of the European Trauma Society. 2023 Dec:49(6):2389-2400. doi: 10.1007/s00068-023-02306-9. Epub 2023 Jun 22 [PubMed PMID: 37347296]
Level 1 (high-level) evidenceBozzay JD, Walker PF, Ronaldi AE, Elster EA, Rodriguez CJ, Bradley MJ. Tube Thoracostomy Management in the Combat Wounded. The American surgeon. 2018 Aug 1:84(8):1355-1362 [PubMed PMID: 30185316]
Tan TX, Buchanan P, Quattromani E. Teaching Residents Chest Tubes: Simulation Task Trainer or Cadaver Model? Emergency medicine international. 2018:2018():9179042. doi: 10.1155/2018/9179042. Epub 2018 Jul 24 [PubMed PMID: 30140461]
Patel NJ, Dultz L, Ladhani HA, Cullinane DC, Klein E, McNickle AG, Bugaev N, Fraser DR, Kartiko S, Dodgion C, Pappas PA, Kim D, Cantrell S, Como JJ, Kasotakis G. Management of simple and retained hemothorax: A practice management guideline from the Eastern Association for the Surgery of Trauma. American journal of surgery. 2021 May:221(5):873-884. doi: 10.1016/j.amjsurg.2020.11.032. Epub 2020 Nov 17 [PubMed PMID: 33487403]
Lai Y, Wang X, Zhou H, Kunzhou PL, Che G. Is it safe and practical to use a Foley catheter as a chest tube for lung cancer patients after lobectomy? A prospective cohort study with 441 cases. International journal of surgery (London, England). 2018 Aug:56():215-220. doi: 10.1016/j.ijsu.2018.06.028. Epub 2018 Jun 21 [PubMed PMID: 29936194]
Level 3 (low-level) evidenceCarver TW, Berndtson AE, McNickle AG, Boyle KA, Haan JM, Campion EM, Biffl WL, Carroll AN, Sise MJ, Berndt KS, Burris JM, Kopelman TR, Blank JJ, Seamon MJ, Peschman JR, Morris RS, Kugler NW, Conrardy RD, Szabo A, de Moya MA. Thoracic irrigation for prevention of secondary intervention after thoracostomy tube drainage for hemothorax: A Western Trauma Association multicenter study. The journal of trauma and acute care surgery. 2024 Nov 1:97(5):724-730. doi: 10.1097/TA.0000000000004364. Epub 2024 May 20 [PubMed PMID: 38764139]
Level 2 (mid-level) evidenceAnderson D, Chen SA, Godoy LA, Brown LM, Cooke DT. Comprehensive Review of Chest Tube Management: A Review. JAMA surgery. 2022 Mar 1:157(3):269-274. doi: 10.1001/jamasurg.2021.7050. Epub [PubMed PMID: 35080596]