Introduction
The cherry-red spot refers to the reddish area at the center of the macula surrounded by retinal opacification in certain disorders. The retinal opacification may be due to various causes, including accumulation of different materials in the ganglion cells in storage disorders, and retinal ischemia/infarction, as in central retinal arterial occlusion (CRAO). Ruling out serious life-threatening or sight-threatening diseases is paramount in a patient with a cherry-red spot at the macula. The management varies according to the cause.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The causes of the cherry-red spot include:
- Central retinal artery occlusion (CRAO)- The characteristic presentation of this disease is sudden onset unilateral visual loss in an elderly male or female. The usual cause is an embolism, which blocks the central retinal artery. The embolus may be composed of cholesterol, fibrin-platelet, or calcium. The embolus usually originates from the carotid plaque and, less commonly, from the heart or the aorta. Inflammatory conditions, like giant cell arteritis (GCA), may also cause CRAO, though branch retinal arterial occlusion (BRAO) is not a common feature in GCA. Branch retinal arterioles do not have a continuous muscular coat or internal elastic lamina.[1] GCA involves only medium and large size arteries, not arterioles (like branch retinal arteriole). However, GCA might affect the cilioretinal artery, a branch of the posterior ciliary artery. On cursory examination, an examiner might mistake cilioretinal arterial occlusion in GCA for a BRAO. Other causes of central retinal artery occlusion include myxoma or vegetation of the cardiac valves, thrombophilic disorders, and retinal migraine. Other associations of CRAO include dyslipidemia, diabetes, hypertension, smoking, and pyoderma gangrenosum.[2] In an extensive series of 248 eyes of 240 patients with central retinal artery occlusion, the cherry-red spot was present on the initial examination in 90% of permanent CRAO cases.[3] Retinal whitening and a cherry-red spot are important manifestations of acute CRAO. Other related situations causing a cherry-red spot include:
- Intraorbital hemorrhage or mass may compromise the choroidal and retinal circulation and lead to a cherry-red spot at the macula
- Retrobulbar injections (usually anesthetics) with or without retrobulbar hemorrhage may cause CRAO and a cherry red spot at the macula[4][5]
- Leukemic infiltrates of the optic nerve head may cause CRAO and cherry red spots[6]
- Optic nerve avulsion may cause a cherry-red spot at the macula[7]
- Macular infarction is another cause of cherry red spots at the macula. Causes of macular infarction include trauma,[8] retinal vascular occlusion, diabetic retinopathy, intravitreal gentamicin or amikacin, systemic lupus erythematosus, and sickle cell anemia.
- Tay-Sachs disease (GM2 gangliosidosis type 1, infantile amaurotic familial idiocy, B variant GM2 gangliosidosis)- This is the most common type of gangliosidosis due to deficiency of the alpha subunit of hexosaminidase A on chromosome 15q23. The disease has an onset of symptoms in infancy. There is an abnormal accumulation of gangliosides in the brain and ganglion cell layer of the retina. The disease is fatal, and most affected children die within the first 2 to 5 years of age. This neurodegenerative disorder is inherited in an autosomal recessive fashion. The child usually has a developmental delay. Other features include a cherry-red spot at the macula in both eyes, increased startle response, poor head control, seizures, dementia, apathy, hypotonia, deafness, blindness, and late hypertonia.[9] Incidence is very high in Ashkenazi Jews, at around 1 in 3500 to 4000 births. A cherry-red spot is present in approximately 90% of the patients affected by the disease.
- GM2 gangliosidosis type 2 or Sandhoff disease is due to mutation of the beta subunit of hexosaminidase at chromosome 5q13. Reports describe infantile, juvenile, and adult forms. It is inherited autosomal recessively. The cherry-red spot is present in a majority of cases. Clinical features are similar to Tay-Sachs disease.
- Commotio retinae - After blunt trauma, acutely retinal opacification may occur.[10] When this retina whitening involves the macula, it is called Berlin's edema. Although actual retinal thickening is not present, this macular whitening can cause a pseudo-cherry red spot.
- Niemann-Pick disease - Type A and B result from the deficiency of acid sphingomyelinase enzyme, a product of the SMPD1 gene (sphingomyelin phosphodiesterase-1) on chromosome 11p15.4. There is an accumulation of sphingomyelin in the tissues. The cause of Niemann-Pick disease type C1 and type D is a mutation in the NPC1 gene on chromosome 18q11. Niemann-Pick disease C2 is due to a mutation in the NPC2 gene at chromosome 14q24.3. Both NPC1 and NPC2 genes are involved in the transport of lipids (LDL cholesterol or low-density lipoprotein cholesterol) in endosomes and lysosomes.
- Type A disease (classic infantile) has an early onset and severe neurodegeneration. Hepatosplenomegaly may be present in early infancy, and there may be a failure to thrive and a suboptimal weight gain. The developmental milestones may be normal until the first birthday, after which there may be psychomotor regression or progressive loss of mental abilities and motor performance. Interstitial lung disease may be noted, and death usually occurs in early childhood. Most of the affected children have a cherry-red spot at the macula.
- Type B, usually (visceral), has a normal function of the central nervous system (CNS). Disease onset is usually in the mid-childhood, and presentation is similar to but less severe than type A. Features include short stature, hepatosplenomegaly, recurrent pulmonary infections, and thrombocytopenia. Some patients may have mixed features of type A and B. Cherry-red spot at the macula is noted in around 1/3rd of cases.
- Type C (subacute/juvenile) has a slowly progressive disease and moderate CNS damage. Features include ataxia, speech and swallowing difficulty, vertical supranuclear gaze palsy, dystonia, interstitial lung disease, intellectual decline, and seizures.
- Type D (Nova Scotia form noted in individuals from Nova Scotia of Canada who have an Acadian ancestor) is associated with late-onset disease but may lead to severe CNS involvement with time. Currently, it classifies as the same condition as type C.
- GM1 gangliosidosis type 1 (Generalized gangliosidosis or Landing disease) results from the deficiency of beta-galactosidase at chromosome 3p22.3. A cherry-red spot in the macula occurs in around 50% of cases.[11] There are three variants:
- Type I, or infantile form, is characterized by a severe disease with onset within the first year of life. Facial features are coarse with gingival hypertrophy. Other findings include developmental regression, increased startle response, skeletal anomalies, hepatosplenomegaly, intellectual disability, seizures, a cherry-red spot at the macula, corneal clouding, and death within early childhood.
- Type II or the late infantile or juvenile form presents within 18 months or five years, respectively. The severity is less than type I, and the cherry-red spot at the macula, visceromegaly, or facial features like type I are usually not seen.
- Type III or adult or chronic form is the mildest form. Features include dystonia and vertebral anomaly. The age of onset and life expectancy may be variable.
- Sialidosis or mucolipidosis type 1 or alpha-neuraminidase deficiency or sialidase deficiency, or lipomucopolysaccharidosis presents with ataxia, movement disorders, nystagmus, and myoclonic seizures. There is a deficiency of neuraminidase 1 (NEU1) or sialidase (SIAL1) at chromosome 6p21.33. Intellect is usually normal, and somatic features are generally not seen. Two types have been described according to the age of onset and severity of the disease:
- Sialidosis type I, or cherry-red spot myoclonus syndrome, is characterized by onset in the 2nd or 3rd decade, gait disturbances, myoclonus, ataxia, tremor, a cherry-red spot at the macula, and seizures. The intellect and life expectancy are usually not affected, though the patient eventually needs wheelchair assistance with time. Almost all the patients have a cherry-red spot at the macula.[12]
- Sialidosis type II causes early and more severe disease. The variants include:
- Congenital - There is either stillbirth or death soon after birth. Features include ascites, hydrops fetalis, coarse facial characteristics, dysostosis multiplex, and hepatosplenomegaly.
- Infantile - Features are similar but milder than the congenital form. There may be short stature, gingival hyperplasia, widely spaced teeth, myoclonus, a cherry-red spot at the macula, deafness, and intellectual decline. The patients may survive into adolescence.
- Juvenile form - This is the mildest variety, and life expectancy varies among the patients. Along with other features, angiokeratoma of the skin is present.
- Farber lipogranulomatosis - Ceramidase or acid ceramidase (chromosome 8p22) is deficient in this autosomal recessive (AR) disorder. The typical triad consists of painful swollen joints, subcutaneous nodules (lipogranulomas), and a weak cry or hoarse voice due to laryngeal nodules. Other features are developmental delay, visceromegaly, respiratory involvement, and neurological features, including quadriplegia, seizures, and myoclonus.
- Metachromatic leukodystrophy - There is a deficiency of arylsulfatase A, cerebroside sulfatase, or sulfatide sulfatase on chromosome 22q13 in this AR disease. An abnormal collection of sulfatides in cells producing myelin results in the progressive destruction of white matter (leukodystrophy). There is a progressive decline in intellectual and motor functions, peripheral neuropathy resulting in diminished sensations, hearing loss, incontinence, loss of speech and mobility, blindness, unresponsiveness, and eventual death. The name comes from the color of the deposited sulfatide granules on histopathological examination. A cherry-red spot at the macula is occasionally a finding.[12]
- Galactosialidosis (Goldberg Cotlier syndrome or neuraminidase deficiency with beta-galactosidase deficiency or cathepsin A deficiency) - This is an AR disorder due to a mutation in the CTSA gene (chromosome 20q13.12) coding for cathepsin A. The disease characteristically presents with seizures, mental retardation, growth retardation, dysostosis multiplex, coarse facies, short stature, skin hemangiomas, mitral and atrial valvular disease, and deafness. Ocular features include a cherry-red spot at the macula, corneal clouding, and conjunctival telangiectasia.
- Hyperalimentation causing hyperlipidemia
- Retinitis involving the central retina, including progressive outer retinal necrosis, subacute sclerosing panencephalitis[13][14][15]
- The toxicity of various drugs may also cause a cherry-red spot. These drugs include:
- Quinine
- Carbon monoxide
- Dapsone poisoning associated with hemolytic anemia and peripheral neuropathy
- Methanol
- Intravitreal gentamicin and amikacin[16]
The association between Gaucher disease and the presence of a cherry-red spot in the macula is questionable, and the cherry-red spot at the macula is not considered a feature of Gaucher disease.[17][18]
Epidemiology
A cherry-red spot at the macula is a rare finding, and the epidemiology depends on the cause. Tay-Sachs disease is estimated to affect 1 in 320000 newborns.[19] The true epidemiology of central retinal arterial occlusion is unknown. A study estimated that acute central retinal arterial occlusion (duration less than 48 hours) affects approximately 0.85 per 100000 per year or 1.13 per 10000 outpatient visits.[20]
Pathophysiology
The macula is characterized histologically by an area with more than one layer of ganglion cell layers, and the ganglion cell layer is thick at the macula. In diseases causing loss of transparency (whitening or opacification) of the inner retina, the reddish color of the vascular choroid and pigmentation of retinal pigment epithelium (RPE) is not seen through the opacified retina. However, the foveola is devoid of the inner retinal layer. The retinal layers present at the foveola are (from inside outwards)- the internal limiting membrane, outer nuclear layer, external limiting membrane, photoreceptor layer, and RPE. Inner retinal layers are absent in mature foveola. The vascular supply of the fovea is from the choroid, and in the occlusion of retinal vessels, it does not get hampered. Thus foveola, the thinnest part of the central retina, does not lose its transparency in inner retinal ischemia.
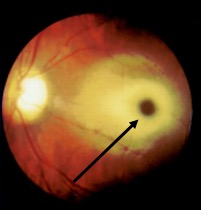
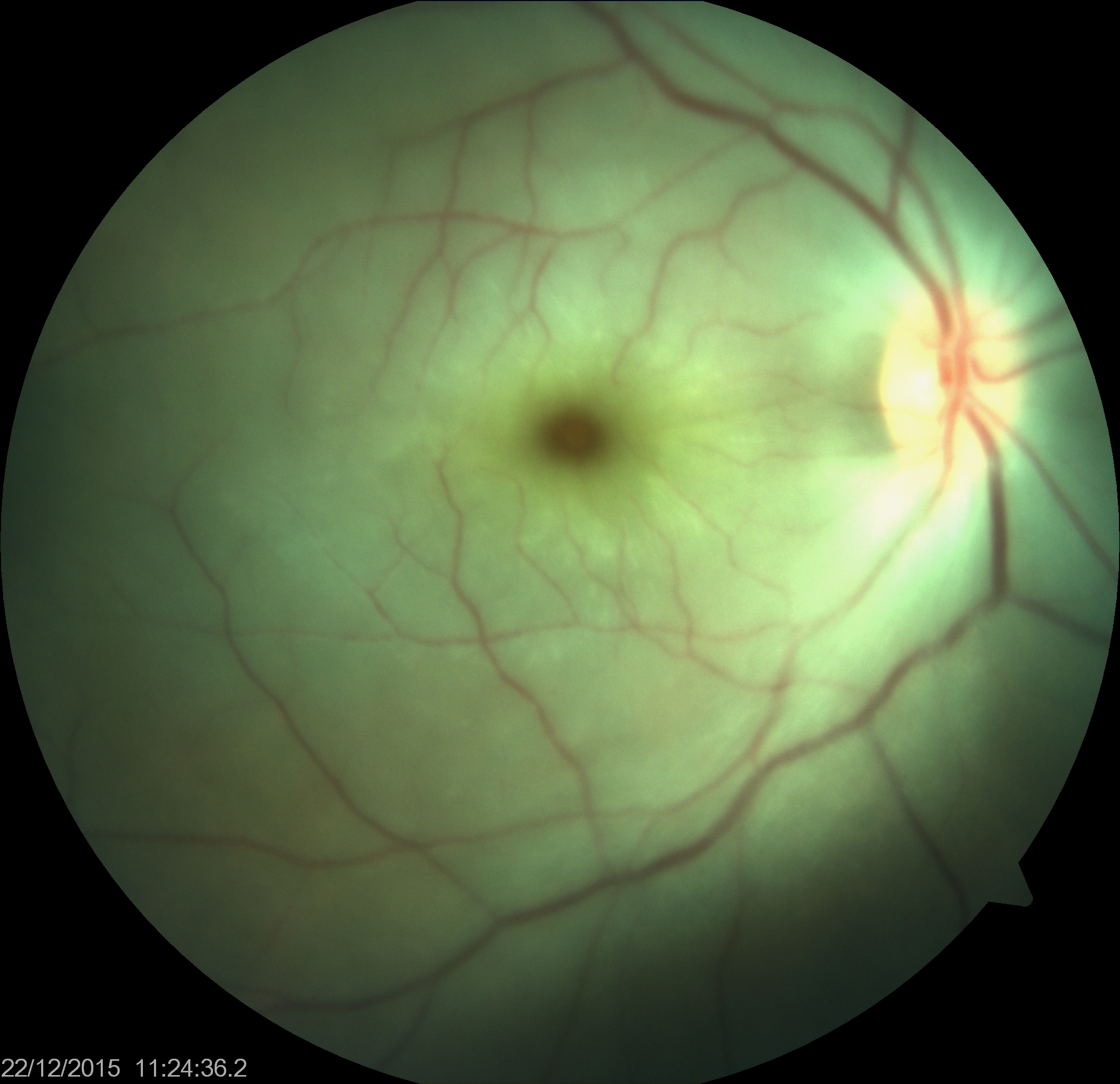
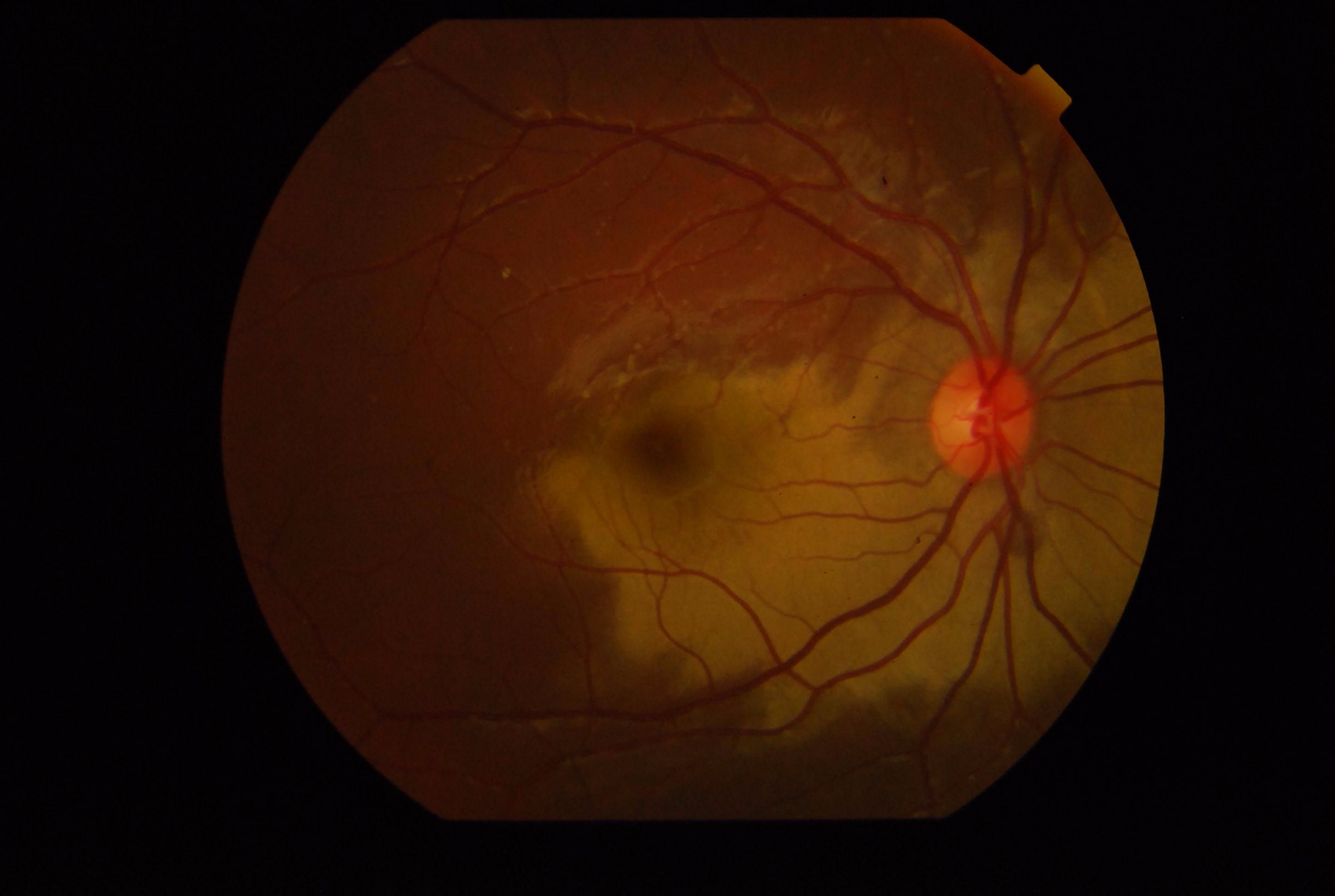
Thus, when there is inner retinal opacification, the reddish color of the vascular choroid and the RPE is still seen through the foveola, which is surrounded by an area of the white/opacified retina; this gives rise to the typical cherry-red spot. The size of the cherry-red spot depends on the size of the foveola.
As the color of choroid and RPE varies with racial variation, the color of the foveola or the central dot may change according to race. A true cherry-red spot presents in whites. The color of foveola was brown, causing a cherry brown spot in a Canadian aboriginal child with Sandhoff disease, and black in a patient of east Indian race creating a cherry black spot in Sandhoff disease.[21] Thus an alternate name of 'perifoveal white patch' has been suggested.[21]
Therefore for a cherry-red spot to be seen, the vascularity of the choroid needs to be intact. In cases of ophthalmic artery occlusion, there is a compromise to the vascular supply to the eye, and even the choroid is not perfused. In such cases, a cherry red spot at the macula may not be seen.
The opacification of inner retinal may be due to the following:
- Retinal ischemia due to occlusion of the central retinal artery- The retinal whitening is typically most prominent in the posterior pole, and the opaque retina obscures the details of the choroidal vessels. The whitening of the retina becomes less prominent a little peripheral to the arcades, which may be related to the reduced inner retinal thickness at the peripheral retina. There is a cessation of axoplasmic transport and the opacification of both the ganglion cell and the nerve fiber layer in a CRAO.
- Deposition or accumulation of material in the ganglion cell layer- In Tay-Sachs disease and other lipid storage disorders (sphingolipidoses), there is a progressive abnormal excessive intracellular accumulation of various glycolipids/phospholipids in the ganglion cell layer leading to opacification of the inner retina. The foveola is devoid of the ganglion cell layer and hence remains transparent and allows transmission of the color of the choroid and the RPE. However, the damaged ganglion cells are lost with increasing age, and the cherry-red spot becomes less prominent. In such cases, the optic disc has consecutive optic atrophy and pallor due to the degeneration of the ganglion cell layer and nerve fiber layer.
Other causes of retinal whitening include retinitis and trauma (commotion retinae). Sphingolipidoses are disorders of lysosomal metabolism, which involve conjugated products of ceramide and sugar or phospholipids. Sphingolipidoses include gangliosidosis, Niemann-Pick disease, Farber disease, and metachromatic leukodystrophy.
Histopathology
Tay-Sachs disease demonstrates an accumulation of GM2 gangliosides in the brain, spinal cord, and ganglion cells of the retina.[22] Typically, a ballooned appearance of the neurons is a feature that has vacuoles in the cytoplasm composed of distended lysosomes filled with gangliosides. Stain with oil red O or Sudan black B is positive.[23]
Electron microscopy reveals whorled deposits within the lysosome, which have a multilayered appearance like a cut section of an onion.[22] In CRAO, the early changes are ischemic changes and swelling of the inner retina, which is followed by thinning of inner retinal layers with time.[24]
History and Physical
Central retinal artery occlusion usually presents with sudden onset vision loss in the elderly. Usually, there is a relative afferent pupillary defect in the affected eye.[25] The fundus examination reveals a whitened opaque retina at the posterior pole blocking the visibility of the choroidal vessels. The foveola remains transparent, giving rise to the cherry-red spot. Other features include fragmentation of blood column in retinal vessels ("boxcarring"), which may be noted to move sluggishly on prolonged observation. An embolus within the central retinal artery or its branches may be present. In the late phase, retinal thinning, pigmentary changes at the fovea, and optic atrophy may occur.
In lipid storage disorders, there might be evidence of neurodegeneration and visceromegaly. In the early phase, there is a perifoveal white donut-like area around the fovea with a cherry-red spot appearance. In the late phase, there is atrophy of inner retinal layers, the disappearance of the cherry-red spot, and optic atrophy. Family history and examination of family members are of paramount importance. Commotio retinae patients have a history of blunt trauma. Signs of blunt trauma, including hyphema, anterior uveitis, and damage to the seven rings of trauma, may be visible. Retinitis may be associated with intraocular inflammation, vitritis, and retinal necrosis that is more prominently visible on the optical coherence tomography scan.[26][27][28]
Evaluation
Color fundus photo helps in documenting the fundus features, counseling the patients, and evaluating the progression of the disease and treatment response.[29]
Fundus fluorescein angiography (FFA)[30] shows delayed arterial circulation, a slowly progressing front edge of the dye, and late filling of the retinal veins in CRAO. There may be an abrupt ending to the flow of the dye at the location of an embolus in the central retinal artery or its branches. The FFA may even be normal in reperfused retinas in CRAO or transient CRAO.[3] FFA may show an area of hypofluorescence (block fluorescence) 360 degrees around the foveola in lipid storage disorders.[31]
The optical coherence tomography (OCT) of the macula in CRAO shows hyperreflectivity of inner retinal layers and retinal thickening. In lipid storage disorders, there is hyperreflectivity of the nerve fiber layer and the ganglion cell layer on OCT.[32] In commotio retinae, OCT usually does not show an increased retinal thickness. Still, there may be hyperreflectivity of the inner segment-outer segment (IS-OS) junction with or without defects in the IS-OS junction, external limiting membrane (ELM), or cone outer segment tips (COST) line.[33]
Autofluorescence may show hyper autofluorescence around the fovea in sialidosis.[34] In CRAO, there is hypo autofluorescence in the area of retinal whitening.[35]
In suspected lipid storage disorder, evaluation for enzyme deficiency and multisystem involvement is essential. In retinitis, evaluation includes tests for immune status, neurological examination for some patients, and, if needed, vitreous or aqueous tap to look for various infectious etiologies.[26]
Treatment / Management
The management of a cherry-red spot depends on the cause.
For CRAO, the options for management when the present patients early include ocular massage, anterior chamber paracentesis, reduction of intraocular pressure by systemic and topical medications, hyperbaric oxygen therapy, and intraarterial thrombolysis. These acute measures may provide benefits; however, there is no clear evidence at this point that these measures improve the patient's final visual acuity.[36] Intravenous pulse steroid forms the mainstay of treatment for GCA-related CRAO to prevent vision loss in the fellow eye.
For various storage diseases, management requires a team of various specialties. Generalized pearls for managing such patients include avoiding/reducing intake of specific molecules, enzyme replacement therapy, and symptomatic management.
Retinitis patients will need individualized therapy based on the clinical characteristics, imaging features, and results of investigations.[26][27][28][37][38] Patients with commotio retinae or Berlin's edema should be examined thoroughly to look for traumatic optic neuropathy, uveitis, hyphema, iris-sphincter tears, iridodialysis, retinal dialysis, and other evidence of trauma. The associated ocular injuries should be treated and may need topical or systemic steroids. Most cases with commotio retinae resolve spontaneously.[39][26](B2)
Differential Diagnosis
The possible differential diagnoses of cherry red spot include
- Macular hole
- Macular pseudohole
- Laser injury to the fovea
- Macular telangiectasia type 2
- Choroidal neovascular membrane
- Macular hemorrhage[40]
Prognosis
The prognosis of a cherry-red spot at the macula varies according to the cause. Most cases of commotion retinae resolve with time, and visual recovery is usually good. CRAO and macular infarction, on the other hand, has a poor visual prognosis, and these eyes may become painful blind eyes due to neovascular glaucoma. Cases with progressive outer retinal necrosis (PORN) usually lose perception of light. Tay Sachs disease is usually fatal by five years of age.
Complications
Complications of a cherry red spot at the macula will depend on the cause. In CRAO, atrophy of the retina and optic disc usually ensues in the long term. Commotio retinae may rarely be followed by pigmentary retinopathy and retinal thinning. In cases with lipid storage disorder, optic nerve pallor and retinal thinning may appear as time progresses. Retinal detachments may complicate cases with necrotizing retinitis.
Deterrence and Patient Education
The cause, prognosis, and management must be discussed with the patients with cherry red spots at the macula. Possible life-threatening or vision-threatening implications should be explained in detail so the patient can make an informed decision about their treatment. Genetic counseling is crucial for lipid storage disorders. For CRAO, control of vascular risk factors is of paramount importance.
Enhancing Healthcare Team Outcomes
Ruling out serious life-threatening or sight-threatening diseases is of paramount importance in a patient with a cherry-red spot at the macula. The management varies according to the cause. When the primary care provider, including PAs and NPs, pediatricians, or emergency department physicians, encounters patients with vision alterations, referral to an ophthalmologist is a strong recommendation. Given the variety of potential etiologies for this condition, an interprofessional team approach involving clinicians and nursing professionals is the best way to ensure proper diagnosis and treatment for the underlying disease state. Nurse specialists in ophthalmology and genetics coordinate the care, monitoring, and education of patients and their families. This interprofessional team approach can improve patient outcomes. [Level 5]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Hayreh SS, Acute retinal arterial occlusive disorders. Progress in retinal and eye research. 2011 Sep; [PubMed PMID: 21620994]
Level 3 (low-level) evidenceTripathy K, Mazumdar S, Sarma B. Central retinal arterial occlusion in a patient with pyoderma gangrenosum. Indian journal of ophthalmology. 2018 Jul:66(7):1019-1021. doi: 10.4103/ijo.IJO_1229_17. Epub [PubMed PMID: 29941761]
Hayreh SS, Zimmerman MB. Fundus changes in central retinal artery occlusion. Retina (Philadelphia, Pa.). 2007 Mar:27(3):276-89 [PubMed PMID: 17460582]
Jung EH,Park KH,Woo SJ, Iatrogenic Central Retinal Artery Occlusion Following Retrobulbar Anesthesia for Intraocular Surgery. Korean journal of ophthalmology : KJO. 2015 Aug [PubMed PMID: 26240507]
Vasavada D,Baskaran P,Ramakrishnan S, Retinal Vascular Occlusion Secondary to Retrobulbar Injection: Case Report and Literature Review. Middle East African journal of ophthalmology. 2017 Jan-Mar [PubMed PMID: 28546695]
Level 3 (low-level) evidenceZhao C,Wei D,Shi X,Zhao M, Unilateral isolated optic nerve infiltration combined with central retinal artery occlusion in a patient with acute myeloid leukemia. American journal of ophthalmology case reports. 2022 Jun [PubMed PMID: 35321253]
Level 3 (low-level) evidenceTripathy K, Kumawat B, Chawla R, Sharma YR, Bypareddy R. Acute Vision Loss Due to Central Retinal Arterial Occlusion, Partial Optic Nerve Avulsion, and Hemorrhage "Spurting Out" from Optic Disc after Blunt Trauma. Journal of ophthalmic & vision research. 2017 Jul-Sep:12(3):351-352. doi: 10.4103/jovr.jovr_4_15. Epub [PubMed PMID: 28791073]
Goel N,Rajput M,Sawhney A,Sardana T, Macular infarction and traumatic optic neuropathy following blunt ocular trauma. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2016 Jan-Mar; [PubMed PMID: 26949360]
Ahmed NR, Tripathy K, Kumar V, Gogia V. Choroidal coloboma in a case of tay-sachs disease. Case reports in ophthalmological medicine. 2014:2014():760746. doi: 10.1155/2014/760746. Epub 2014 Sep 10 [PubMed PMID: 25295204]
Level 3 (low-level) evidenceTripathy K, Chawla R. Extensive commotio retinae involving peripheral retina. The National medical journal of India. 2017 Jul-Aug:30(4):242. doi: 10.4103/0970-258X.218686. Epub [PubMed PMID: 29162764]
Chen H,Chan AY,Stone DU,Mandal NA, Beyond the cherry-red spot: Ocular manifestations of sphingolipid-mediated neurodegenerative and inflammatory disorders. Survey of ophthalmology. 2014 Jan-Feb; [PubMed PMID: 24011710]
Level 3 (low-level) evidenceSuvarna JC,Hajela SA, Cherry-red spot. Journal of postgraduate medicine. 2008 Jan-Mar; [PubMed PMID: 18296811]
Tripathy K,Chawla R,Mittal K,Farmania R,Venkatesh P,Gulati S, Ophthalmic examination as a means to diagnose Subacute Sclerosing Panencephalitis: an optical coherence tomography and ultrawide field imaging evaluation. Eye and vision (London, England). 2017 [PubMed PMID: 28116334]
Chawla R,Tripathy K,Gogia V,Venkatesh P, Progressive outer retinal necrosis-like retinitis in immunocompetent hosts. BMJ case reports. 2016 Aug 10; [PubMed PMID: 27511757]
Level 3 (low-level) evidenceYiu G,Young LH, Progressive outer retinal necrosis presenting as cherry red spot. Ocular immunology and inflammation. 2012 Oct; [PubMed PMID: 22957726]
Level 3 (low-level) evidenceAbhayambika K,Chacko A,Mahadevan K,Najeeb OM, Peripheral neuropathy and haemolytic anaemia with cherry red spot on macula in dapsone poisoning. The Journal of the Association of Physicians of India. 1990 Aug; [PubMed PMID: 2174032]
Level 3 (low-level) evidenceGoker-Alpan O,Schiffmann R,Park JK,Stubblefield BK,Tayebi N,Sidransky E, Phenotypic continuum in neuronopathic Gaucher disease: an intermediate phenotype between type 2 and type 3. The Journal of pediatrics. 2003 Aug [PubMed PMID: 12970647]
Winter AW,Salimi A,Ospina LH,Roos JCP, Ophthalmic manifestations of Gaucher disease: the most common lysosomal storage disorder. The British journal of ophthalmology. 2019 Mar [PubMed PMID: 30612093]
Lew RM, Burnett L, Proos AL, Delatycki MB. Tay-Sachs disease: current perspectives from Australia. The application of clinical genetics. 2015:8():19-25. doi: 10.2147/TACG.S49628. Epub 2015 Jan 21 [PubMed PMID: 25653550]
Level 3 (low-level) evidenceRumelt S, Dorenboim Y, Rehany U. Aggressive systematic treatment for central retinal artery occlusion. American journal of ophthalmology. 1999 Dec:128(6):733-8 [PubMed PMID: 10612510]
Level 1 (high-level) evidenceOspina LH,Lyons CJ,McCormick AQ, [PubMed PMID: 16391625]
Ramani PK,Parayil Sankaran B, Tay-Sachs Disease. StatPearls. 2022 Jan [PubMed PMID: 33232090]
Ferreira CR, Gahl WA. Lysosomal storage diseases. Translational science of rare diseases. 2017 May 25:2(1-2):1-71. doi: 10.3233/TRD-160005. Epub 2017 May 25 [PubMed PMID: 29152458]
Hayreh SS, Zimmerman MB, Kimura A, Sanon A. Central retinal artery occlusion. Retinal survival time. Experimental eye research. 2004 Mar:78(3):723-36 [PubMed PMID: 15106952]
Level 3 (low-level) evidenceSimakurthy S, Tripathy K. Marcus Gunn Pupil. StatPearls. 2024 Jan:(): [PubMed PMID: 32491607]
Level 3 (low-level) evidenceGupta N, Tripathy K. Retinitis. StatPearls. 2024 Jan:(): [PubMed PMID: 32809355]
Geetha R, Tripathy K. Chorioretinitis. StatPearls. 2024 Jan:(): [PubMed PMID: 31869169]
Bergstrom R,Tripathy K, Acute Retinal Necrosis. StatPearls. 2022 Jan [PubMed PMID: 29262034]
Mishra C,Tripathy K, Fundus Camera StatPearls. 2022 Jan; [PubMed PMID: 36256758]
Ruia S, Tripathy K. Fluorescein Angiography. StatPearls. 2024 Jan:(): [PubMed PMID: 35015403]
Heroman JW,Rychwalski P,Barr CC, Cherry red spot in sialidosis (mucolipidosis type I). Archives of ophthalmology (Chicago, Ill. : 1960). 2008 Feb; [PubMed PMID: 18268224]
Level 3 (low-level) evidenceWang IH,Lin TY,Kao ST, Optical coherence tomography features in a case of Type I sialidosis. Taiwan journal of ophthalmology. 2017 Apr-Jun [PubMed PMID: 29018767]
Level 3 (low-level) evidenceAhn SJ,Woo SJ,Kim KE,Jo DH,Ahn J,Park KH, Optical coherence tomography morphologic grading of macular commotio retinae and its association with anatomic and visual outcomes. American journal of ophthalmology. 2013 Nov; [PubMed PMID: 23972302]
Level 2 (mid-level) evidenceZou W,Wang X,Tian G, Fundus autofluorescence and optical coherence tomography of a macular cherry-red spot in a case report of sialidosis. BMC ophthalmology. 2016 Mar 22; [PubMed PMID: 27004518]
Level 3 (low-level) evidenceMathew R,Papavasileiou E,Sivaprasad S, Autofluorescence and high-definition optical coherence tomography of retinal artery occlusions. Clinical ophthalmology (Auckland, N.Z.). 2010 Oct 21; [PubMed PMID: 21060665]
Level 3 (low-level) evidenceVarma DD,Cugati S,Lee AW,Chen CS, A review of central retinal artery occlusion: clinical presentation and management. Eye (London, England). 2013 Jun [PubMed PMID: 23470793]
Tripathy K,Venkatesh P,Chawla R, Cytomegaloviral retinitis. The National medical journal of India. 2016 Jan-Feb [PubMed PMID: 27492038]
Chawla R,Tripathy K,Temkar S,Venkatesh P,Kumar A, An imaging-based treatment algorithm for posterior focal retinitis. Therapeutic advances in ophthalmology. 2018 Jan-Dec [PubMed PMID: 29998221]
Level 3 (low-level) evidencePark JY,Nam WH,Kim SH,Jang SY,Ohn YH,Park TK, Evaluation of the central macula in commotio retinae not associated with other types of traumatic retinopathy. Korean journal of ophthalmology : KJO. 2011 Aug [PubMed PMID: 21860574]
Level 2 (mid-level) evidenceMajumdar S, Tripathy K. Macular Hole. StatPearls. 2023 Jan:(): [PubMed PMID: 32644626]