Introduction
Traumatic brain injury (TBI) is considered the most disabling of traumatic phenomenons, almost always encompassing lifelong emotional, behavioral, and permanent physical impairment.[1][2] Nearly half of hospitalized survivors of TBI experience long-term disabilities. TBI includes several types of insults to the brain. One of the most severe damage mechanisms is the hemorrhagic cerebral contusion. TBI associated with cerebral contusions increases the risks for disability and death in TBI patients.
Cerebral contusions cause permanent damage to tissues of the cerebrum. The severity of the damage is related to the primary injury that is started by the kinetic energy absorbed by the collision and the cascade of secondary injury responses that exacerbate the primary damage. The hemorrhagic lesion is produced in the immediate moments after the head impact.[3]
Contusions can progress and expand, and in many cases, other hemorrhagic contusions are present. Hemorrhagic contusions overlie brain parenchyma with loss of function. It is known that blood is very toxic to healthy brain tissue; hence brain contusions are among the most devastating secondary injury forms seen in TBI's. Brain contusions have been attributed to bleeding from the continuous flow of injured microvessels during the initial traumatic episode. This concept has suggested that the formation of a contusion might be due to an underlying or overt coagulopathy.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of cerebral contusion is trauma to the head. Most of the time, it is a closed head injury, but open injuries can also produce them. Several mechanisms can generate the traumatic event, including:
- Road traffic accidents (motor vehicle, motorcycle, pedestrian)
- Falls
- Aggression
- Recreation-related head injuries
- Sports injury
- Cyclists
- Domestic violence
- Child abuse
- Blast injury
Epidemiology
TBI is a significant cause of disability, death, and economic cost in the world. Every year, there are approximately 2.8 million TBIs in the United States, 1/3 occurring in children.[1][2] Over 250,000 patients are hospitalized for nonfatal TBI, including 10% hospitalizations in children. Over 2.5 million patients are treated in the emergency department, with 25% of these evaluations related to children. More than 50,000 patients die from TBI, with 4.5% of deaths related to children.[1] Fatalities in children are more common with falls and road traffic accidents.[4] The mortality rate has steadily declined over the past decades due to improvements in patient management.[5]
Superficial injuries and cerebral contusions are the most common sports-related injuries seeking emergency department visits.[6] One year after TBI hospitalization, 43% of Americans have a residual disability (physical, cognitive, behavioral, and psychosocial); the prevalence of residents living with disabilities is 3.2 million.[1][2] Self-reported head injury among adults 40 years old or older has a 15.7% prevalence in the United States.[7] It is higher among men (20.0%) than among women (12.0%), with non-Hispanic White respondents showing the highest (18.0%) compared to non-Hispanic Black respondents (8.9%).[7]
TBI leads to one million hospitalizations per year in the European Union, accounting for the most deaths (50,000) following road traffic accidents.[8][9] Unfortunately, three-quarters of these victims are young people. A Chinese head trauma data bank has shown important information regarding cerebral contusions. The mortality rates of patients with no cerebral contusions, single cerebral contusions, and multiple cerebral contusions are 3.9%, 7.8%, and 14.8%, respectively. The mortality rate of patients with and without traumatic subarachnoid hemorrhage was 9.5% and 5.4%. The mortality rate of patients with no intracranial hematomas, single intracranial hematoma, and multiple intracranial hematomas was 5.8%, 8.4%, and 20.6%.[10] Approximately 51.6% of the patients had a cerebral contusion, and 49.9% had a traumatic subarachnoid hemorrhage.[10]
TBI related to recreation most commonly involved cerebral contusion in 29% of the cases followed by a traumatic subarachnoid hemorrhage in 26%, subdural hematoma in 25%, and epidural hematoma in 23%.[11] In the Netherlands, road traffic accidents had a contusion incidence of 46.6% in cyclists and 74.2% in motorcyclists.[12] A cerebral contusion occurs in over 50% of patients with severe TBI.[12] In adults over 60 with TBI, cerebral contusions occur in approximately 18% of them.[13] Pediatric TBI frequently shows brain edema (72.9%), skull fracture (69.5%), and brain contusion (55.9%).[14]
The risk of a male patient having a TBI is double than for a female patient. However, after an injury is sustained, there is no statistical difference in the mortality rate between male (7.5%) and female (7.2%) patients with TBI.[10]
Pathophysiology
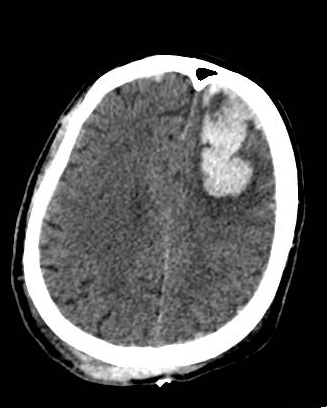
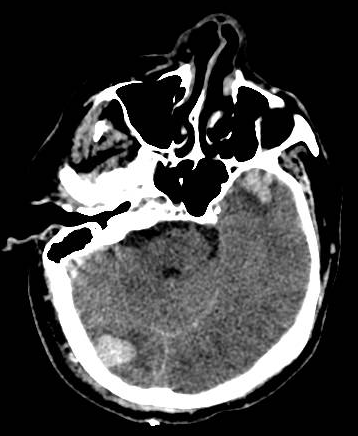
Cerebral contusions are usually seen in the temporal and frontal lobes, even though other sites can be implicated through a coup (beneath the impact) and contrecoup (opposite to impact). The brain contusions are lesions with a hemorrhagic character that begins in the brain cortical area and, more commonly, at crests of the gyri of the cerebrum. They can progress to the subcortical white matter in the more severe forms of injury. Despite the primary cortical area's preference, they can also develop at the white/gray border with expansion into overlying grey matter. The occurrence of hemorrhage within the contusion can cause ischemia and edema in the local area, which progresses to destruction of tissues, necrosis of neuronal structures, and cavitation with overlying reactive gliosis.
There are many instances where the progression and expansion of the hemorrhagic components in the areas surrounding the initial contusion have been observed. Contusion progression occurred with a frequency of 63%-70%.[15][16] Progression usually occurs in the first 12 hours, but may develop even in the 3–4 days after TBI.[17] Historically, it was postulated that the hemorrhagic brain contusion progression occurred secondary to a coagulopathic event associated with TBI.[17][18][19]
In TBI, the contused brain is one of the major sources of the release of the tissue factor. Recent studies have proposed that the cerebrum's vasculature is mechanosensitive, activating endothelial cells in the penumbra that did not suffer the contusion core's initial damaging effect. In this situation, the contusion penumbra experiences activation of two transcription factors (endothelial mechanosensitive mechanisms), which are the specificity protein 1 (transcription factor1) and nuclear factor kappaB.[20] The importance of these factors in the control of sulfonylurea receptor 1-transient receptor potential melastatin 4, which forms the regulatory subunit of the NCCa-ATP channel, is of interest in the damage cascade.[21][22][23][24]
The nuclear factor kappaB leads to apoptosis, whereas the specificity factor 1 causes fragmentation of the vessels. It has also been related to dysfunction or damage of vascular entities, causing microvascular failure and endothelial necrosis.[17][25] Contusion progression risk factors independently associated with the hematoma growth included: an initial volume, cisternal compression, decompressive craniectomy, age, falls as the mechanism of trauma, multiple hematomas, and hypoxia.[15]
The initial trauma from the brain injury can lead to immediate cell death through necrosis. The cell lyses and releases harmful substances such as inflammatory chemokines and cytokines, reactive oxygen species, and proteases. There is a release of inflammatory mediators, pro-inflammatory cytokines, blood-brain barrier breakdown, and subsequent cerebral edema development. Secondary injury from excitatory amino acids, including glutamate and aspartate, increased intercellular cytosolic calcium concentration, acidosis, and free radical production. Glutamate excitotoxicity can cause persistent membrane depolarization, which activates ion channels, particularly the N-methyl-D-aspartate channel. Secondary to the permanent opening of N-methyl-D-aspartate channels, sodium and calcium penetrate cells while potassium is extruded to the extracellular space.[26] A shift of potassium into the extracellular space will result in rapid swelling of astrocytes, which absorb potassium to preserve ionic homeostasis.[27]
Free radicals cause genetic material injury, with downregulation of genes and mitochondrial disruption. This results in ion dyshomeostasis and consequent neuronal and astrocytic swelling, terminating with cell death. Once the autoregulatory mechanisms have been abolished, cerebral blood flow (CBF) passively follows changes in arterial blood pressure (ABP) and impaired cerebral pressure. In the central core area, the cerebral blood flow is 4.7 ml/100g/min, and in the peripheral zone is 16-18 ml/100g/min.[27] Irreversible neuronal death occurs with cerebral blood flow below 8 cc/100g/min, while neurons can recover if they have a 9 to 18 cc/100g/min cerebral blood flow.[28]
Brain edema formation is started with vasogenic edema resulting from the breakdown of the blood-brain barrier and extravasations of fluid into the extracellular space, which sets in only after 12-24 hours. Then a secondary injury caused by a cascade of mechanisms initiated at the moment of injury sets in after 24 to 72 hours and progresses for 7-10 days. A third phase occurs with the lysis of red blood cells in the intracerebral clot.
History and Physical
An initial trauma assessment is performed. Adequate resuscitation is followed by rapid imaging studies with the identification of possible surgical injuries. Proper immobilization should be maintained until the stability of the entire spine has been confirmed. The mechanism of trauma is obtained from the patient, relatives, or paramedics.
A detailed neurologic examination is performed. If sedation was given, it should be stopped for an adequate neurological assessment. The patient is evaluated for response to stimuli with a response in eye, motor, and verbal forms. The Glasgow coma scale (GCS) was established in 1974 by Teasdale and Jennett.[29] It helps obtain the best response in a patient and presents the status to the healthcare team. It also helps in comparing future evaluations. The pupillary response is critical in the initial assessment.
Glasgow coma scale:[29]
Eye opening (Score 1-4)
- Spontaneous – 4
- To speech – 3
- To pain – 2
- No response – 1
Verbal function (Score 1-5)
- Alert and oriented – 5
- Confused/disoriented – 4
- Inappropriate words – 3
- Incomprehensible sounds – 2
- No response – 1
Motor function (Score 1-6)
- Obeys commands – 6
- Localizes pain – 5
- Withdraws from pain – 4
- Decorticate flexion – 3
- Decerebrate extension – 2
- No response – 1
Evaluation
X-rays should routinely be performed for a trauma workup. An entire spine, chest film, and pelvis should be done, and any other evident bones involved.
Laboratories should include a complete blood count, comprehensive metabolic panel, international normalized ratio, and toxicology. Arterial blood gases are required for intubated patients and those with respiratory distress.
Head computed tomographic (CT) scan should be done for patients with impaired consciousness or history of loss of consciousness, focal neurologic findings, persistent vomiting, seizures, and an abnormal GCS score.[30] Initially, the CT may show isodense or hypodense areas, sometimes mixed-density lesions commonly surrounded by perilesional hypodense areas, but later more surrounding edema with progression over time. Multiple focal contusions may have a “salt and pepper” appearance. The bone window is used for the evaluation of skull fractures.
Brain magnetic resonance imaging (MRI) is not indicated in the acute management of TBI.[30] If acutely done, there are isointense to hyperintense areas on T1-weighted images and hyperintense on T2-weighted images. Fluid-attenuated inversion recovery images demonstrate petechial hemorrhages that are isointense to the brain. The MRI can show ischemic areas and changes from diffuse axonal injury. It can be used later for cognitive problems.
Treatment / Management
The airway, ventilation, and circulation are assessed. Intravenous access is obtained, and intubation is performed if necessary. Hypoxia and hypotension have to be avoided as they are detrimental to outcomes.
After the head CT scan is obtained, management is decided. In patients with no immediate neurosurgical lesions, the priority is the management of intracranial pressure (ICP). Monitoring ICP is performed if the GCS is below 8. An external ventricular drain (EVD) has the advantage of cerebrospinal fluid (CSF) drainage. Intraparenchymal devices can also be used. In patients with cerebral contusions, seizure prophylaxis is started for seven days to prevent post-traumatic seizures. Cerebral perfusion pressure (CPP) is defined as the difference between the mean arterial pressure (MAP) and the ICP. CPP = MAP - ICP. The goals in treatment are to keep the ICP under 20 mm Hg and the CPP above 60 mm Hg. Patients with increased ICP are managed using sedation, diuresis, and hypertonic saline.
Some patients need immediate craniotomy for evacuation of the contusion; however, there is still debate about the value in removing intraparenchymal lesions. Indications for surgical removal include progressive neurological deterioration, signs of mass effect on brain CT, unresponsive increased ICP, midline deviation of more than 5 mm, cistern compression evidenced on brain CT, temporal contusions of greater than 20 cc of volume.[31] In extreme cases, unresponsive to the usual mechanisms to reduce the ICP, decompressive craniectomy can be performed and results in lower mortality but also with more patients in a vegetative state.[32]
Hemorrhage/contusion progression in up to 87% of the cases is a potential complication of decompressive craniectomy.[16][33] Corticosteroids are not recommended for the management of increased ICP. Early enteral nutritional support should be started within 72 hours. The Brain Trauma Foundation has specific guideline recommendations with the level of evidence for each one that can be used to develop treatment protocols.[34][35](B2)
The American College of Surgeons published in 2015 a three-tiered protocol for the management of increased intracranial pressure.
Tier 1
- The head of the bed elevated at 30 degrees.
- Sedation and analgesia using short-acting agents for intubated patients.
- EVD with intermittent drainage.
- Repeat head CT imaging if no improvement.
- If ICP remains ≥ 20 - 25 mmHg, proceed to Tier 2.
Tier 2
- If using a parenchymal ICP device, change to an EVD.
- Hyperosmolar therapy (mannitol or hypertonic saline), given intermittently.
- Mannitol administered in intermittent boluses (0.25 to 1 gm/ kg body weight). Check frequently the serum sodium and osmolality; additional doses held if serum osmolality exceeds 320 mOsm/L.
- Hypertonic saline 3% sodium chloride solution administered in intermittent boluses of 250 ml over 0.5 hours (2-5 mL/kg over 10-20 minutes). Serum sodium and osmolality checked every 6 hours. Additional doses held if serum sodium exceeds 160 mEq/L. A bolus of up to 23.4% sodium chloride solution can be given for refractory increased ICP.
- CPP no less than 50 mm Hg. (Goal between 60 and 70 mm Hg).
- PaCO2 goal of 30 to 35 mmHg should be maintained.
- Repeat head CT imaging if no improvement.
- Neuromuscular paralysis bolus test dose.
- If ICP remains ≥ 20 to 25 mmHg, proceed to Tier 3.
Tier 3
- Decompressive hemicraniectomy or bilateral craniectomy.
- Neuromuscular paralysis via continuous infusion (titrated to maintain at least two twitches out of a train of four). Adequate sedation must be utilized.
- Barbiturate or propofol coma. Hypotension is a common side effect. Continuous EEG is used to achieve burst suppression.
- Hypothermia below 36 degrees C is not indicated as an initial TBI treatment. Use only as salvage therapy after all previous Tier 3 treatments have failed.
Differential Diagnosis
After a traumatic event, it is difficult to confuse a cerebral contusion with other disorders. However, on rare occasions, a patient may first have a non-traumatic hemorrhage, which then provoked a traumatic event, including:
- Brain tumor with an acute hemorrhage
- Intraparenchymal hemorrhage from a ruptured aneurysm
- Hypertensive hemorrhage
- Stroke with reperfusion hemorrhage
Prognosis
Mortality increases as the GCS reduces. The patient's age and mechanism of injury are the most critical factors in the outcome.[36] A predictor of disability is a more extended period of coma. For moderate head injuries, approximately 60% of the patients will make a positive recovery, but 25% have a residual moderate degree of disability. In severely head-injured patients, only 25% to 33% have positive outcomes. Patients can remain with impaired concentration, attention, and memory. Cognitive deficits and personality changes may persist. A brain contusion is an important factor in the development of post-traumatic seizures.[37]
The number of non-reacting pupils (0-2) has recently been used for prognostic information in TBI.[38] The formula is composed of the GCS minus the number of non-reactive pupils. In patients with GCS of 3 and no reactive pupils, mortality six months after injury is 74%, and an unfavorable outcome six months after injury is 90%. If one pupil is reactive, the mortality six months after injury is 47%, and an unfavorable outcome six months after injury is 70%. If both pupils are reactive, the mortality six months after injury is 28%, and an unfavorable outcome six months after injury is 50%. Very similar results are found in patients with GCS of 4. If those patients with GCS of 5 and no reactive pupil, the mortality six months after injury is 54%, and an unfavorable outcome six months after injury is 82%.
Increasing age is a significant factor for mortality and unfavorable recovery six months after TBI.[39] The same study found that the outcomes based on the single CT findings (hematoma, cisterns, or subarachnoid hemorrhage) are very alike between patients, but when the three findings are combined, a progression for an unfavorable outcome, based on the number of abnormalities present in the study.
The Glasgow outcome scale is used for outcome assessment using a five-point scale.[40]
- Score 5- Good recovery: Minor disabilities, but able to resume normal life
- Score 4- Moderate disability: More significant disabilities, but still able to live independently. Can use public transportation, work in an assisted situation, etc.
- Score 3- Severe disability: Conscious, but dependent upon others for daily care, often institutionalized
- Score 2- Persistent vegetative state: Not conscious, though eyes may be open and may "track" movement
- Score 1- Death
Classifications to predict outcomes on TBI have been developed and published. The Marshall CT classification evaluates midline shift, cistern patency, hematoma size, and if surgery was performed.[41] The Rotterdam CT score evaluates midline shift, cistern patency, epidural mass presence, and intraventricular or subarachnoid blood.[42][43] The Rotterdam CT score has been validated recently in the literature.[44]
Complications
- Seizures
- Hydrocephalus
- Posttraumatic anosmia
- Severe disability
- Coma
- Death
- Loss of school time
- Loss of workdays
- Employment disability
- Impaired concentration, attention, and memory
- Cognitive deficits
- Personality changes
- Posttraumatic stress disorder
- Surgical infections
Consultations
- Emergency clinician
- Trauma surgeon
- Neurosurgeon
- Intensivist
- Neuroradiologist
- Respiratory therapy
- Physical and rehabilitation medicine
Deterrence and Patient Education
Brain contusion is a significant cause of disability to the patient. It can be devastating, causing coma and death to the patient. It brings a substantial burden to the patient's relatives and creates a high economic cost to the health system. Most of these costs are related to prolonged hospitalizations and rehabilitation to the patient.
Specific recommendations to patients or parents for prevention include:
- Use of helmets by cyclists.
- Use of automobile seatbelts.
- Use of child restraints.
- Supervise younger children at all times.
- Avoid uneven or unpaved surfaces when cycling or skateboarding.
- For some sports, use helmets.
Enhancing Healthcare Team Outcomes
While the emergency clinician and trauma surgeon are almost always involved in the initial care of patients with cerebral contusions associated with TBI, it is essential to consult with an interprofessional team of specialists that include a neurosurgeon and intensivist. Prompt consultation with an interprofessional group of specialists is recommended to improve outcomes. The nurses are also vital members of the interprofessional group, as they will monitor the patient's vital signs. The blood bank and the clinical laboratory will provide key elements in the treatment and management decisions. If the patient is intubated, respiratory therapy is essential for managing ventilators with the coordination by the neurointensivist. The radiologist also plays a vital role in the radiological monitoring of imaging changes.
A significant complication in these patients that is associated with high morbidity is deep vein thrombosis. Many patients can be placed in medical prophylaxis if there is no evidence of the contusion's progression. Physical therapists must be consulted for early ambulation. An integrated care pathway using an evidence-based approach and following management guidelines are crucial elements for improving outcomes.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Corrigan JD, Selassie AW, Orman JA. The epidemiology of traumatic brain injury. The Journal of head trauma rehabilitation. 2010 Mar-Apr:25(2):72-80. doi: 10.1097/HTR.0b013e3181ccc8b4. Epub [PubMed PMID: 20234226]
Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, Servadei F, Walters BC, Wilberger JE, Surgical Management of Traumatic Brain Injury Author Group. Surgical management of acute subdural hematomas. Neurosurgery. 2006 Mar:58(3 Suppl):S16-24; discussion Si-iv [PubMed PMID: 16710968]
McGinn MJ, Povlishock JT. Pathophysiology of Traumatic Brain Injury. Neurosurgery clinics of North America. 2016 Oct:27(4):397-407. doi: 10.1016/j.nec.2016.06.002. Epub 2016 Aug 10 [PubMed PMID: 27637392]
Lalwani S, Hasan F, Khurana S, Mathur P. Epidemiological trends of fatal pediatric trauma: A single-center study. Medicine. 2018 Sep:97(39):e12280. doi: 10.1097/MD.0000000000012280. Epub [PubMed PMID: 30278499]
Level 2 (mid-level) evidenceLu J, Marmarou A, Choi S, Maas A, Murray G, Steyerberg EW, Impact and Abic Study Group. Mortality from traumatic brain injury. Acta neurochirurgica. Supplement. 2005:95():281-5 [PubMed PMID: 16463866]
Level 2 (mid-level) evidenceNalliah RP, Anderson IM, Lee MK, Rampa S, Allareddy V, Allareddy V. Epidemiology of hospital-based emergency department visits due to sports injuries. Pediatric emergency care. 2014 Aug:30(8):511-5. doi: 10.1097/PEC.0000000000000180. Epub [PubMed PMID: 25062295]
Level 2 (mid-level) evidenceSchneider ALC, Wang D, Ling G, Gottesman RF, Selvin E. Prevalence of Self-Reported Head Injury in the United States. The New England journal of medicine. 2018 Sep 20:379(12):1176-1178. doi: 10.1056/NEJMc1808550. Epub [PubMed PMID: 30231228]
Bullock R, Chesnut RM, Clifton G, Ghajar J, Marion DW, Narayan RK, Newell DW, Pitts LH, Rosner MJ, Wilberger JW. Guidelines for the management of severe head injury. Brain Trauma Foundation. European journal of emergency medicine : official journal of the European Society for Emergency Medicine. 1996 Jun:3(2):109-27 [PubMed PMID: 9028756]
Level 1 (high-level) evidenceMaas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. The Lancet. Neurology. 2008 Aug:7(8):728-41. doi: 10.1016/S1474-4422(08)70164-9. Epub [PubMed PMID: 18635021]
Jiang JY, Chinese Head Trauma Study Collaborators. Head trauma in China. Injury. 2013 Nov:44(11):1453-7. doi: 10.1016/j.injury.2012.08.045. Epub 2012 Oct 13 [PubMed PMID: 23068139]
Parchani A, El-Menyar A, Al-Thani H, Tuma M, Zarour A, Abdulrahman H, Peralta R, Asim M, Latifi R. Recreational-related head injuries in Qatar. Brain injury. 2013:27(12):1450-3. doi: 10.3109/02699052.2013.823664. Epub 2013 Aug 7 [PubMed PMID: 23924056]
Level 2 (mid-level) evidenceLeijdesdorff HA, van Dijck JT, Krijnen P, Vleggeert-Lankamp CL, Schipper IB, Regional Trauma Center West-Netherlands’ Research Group. Injury pattern, hospital triage, and mortality of 1250 patients with severe traumatic brain injury caused by road traffic accidents. Journal of neurotrauma. 2014 Mar 1:31(5):459-65. doi: 10.1089/neu.2013.3111. Epub 2013 Dec 21 [PubMed PMID: 24093437]
Fernández-Abinader JA, González-Colón K, Feliciano CE, Mosquera-Soler AM. Traumatic Brain Injury Profile of an Elderly Population in Puerto Rico. Puerto Rico health sciences journal. 2017 Dec:36(4):237-239 [PubMed PMID: 29220069]
Egbohou P, Mouzou T, Tchetike P, Sama HD, Assenouwe S, Akala-Yoba G, Randolph L, Tomta K. Epidemiology of Pediatric Traumatic Brain Injury at Sylvanus Olympio University Hospital of Lomé in Togo. Anesthesiology research and practice. 2019:2019():4038319. doi: 10.1155/2019/4038319. Epub 2019 Aug 1 [PubMed PMID: 31467523]
Cepeda S, Gómez PA, Castaño-Leon AM, Martínez-Pérez R, Munarriz PM, Lagares A. Traumatic Intracerebral Hemorrhage: Risk Factors Associated with Progression. Journal of neurotrauma. 2015 Aug 15:32(16):1246-53. doi: 10.1089/neu.2014.3808. Epub 2015 Apr 15 [PubMed PMID: 25752340]
Level 2 (mid-level) evidenceCepeda S, Castaño-León AM, Munarriz PM, Paredes I, Panero I, Eiriz C, Gómez PA, Lagares A. Effect of decompressive craniectomy in the postoperative expansion of traumatic intracerebral hemorrhage: a propensity score-based analysis. Journal of neurosurgery. 2019 Apr 26:132(5):1623-1635. doi: 10.3171/2019.2.JNS182025. Epub 2019 Apr 26 [PubMed PMID: 31026834]
Kurland D, Hong C, Aarabi B, Gerzanich V, Simard JM. Hemorrhagic progression of a contusion after traumatic brain injury: a review. Journal of neurotrauma. 2012 Jan 1:29(1):19-31. doi: 10.1089/neu.2011.2122. Epub 2011 Dec 5 [PubMed PMID: 21988198]
Juratli TA, Zang B, Litz RJ, Sitoci KH, Aschenbrenner U, Gottschlich B, Daubner D, Schackert G, Sobottka SB. Early hemorrhagic progression of traumatic brain contusions: frequency, correlation with coagulation disorders, and patient outcome: a prospective study. Journal of neurotrauma. 2014 Sep 1:31(17):1521-7. doi: 10.1089/neu.2013.3241. Epub 2014 Jul 8 [PubMed PMID: 24738836]
Level 2 (mid-level) evidenceZhang J, Jiang R, Liu L, Watkins T, Zhang F, Dong JF. Traumatic brain injury-associated coagulopathy. Journal of neurotrauma. 2012 Nov 20:29(17):2597-605. doi: 10.1089/neu.2012.2348. Epub 2012 Oct 31 [PubMed PMID: 23020190]
Level 3 (low-level) evidenceHang CH, Chen G, Shi JX, Zhang X, Li JS. Cortical expression of nuclear factor kappaB after human brain contusion. Brain research. 2006 Sep 13:1109(1):14-21 [PubMed PMID: 16857176]
Simard JM, Kilbourne M, Tsymbalyuk O, Tosun C, Caridi J, Ivanova S, Keledjian K, Bochicchio G, Gerzanich V. Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. Journal of neurotrauma. 2009 Dec:26(12):2257-67. doi: 10.1089/neu.2009.1021. Epub [PubMed PMID: 19604096]
Level 3 (low-level) evidenceMartínez-Valverde T, Vidal-Jorge M, Martínez-Saez E, Castro L, Arikan F, Cordero E, Rădoi A, Poca MA, Simard JM, Sahuquillo J. Sulfonylurea Receptor 1 in Humans with Post-Traumatic Brain Contusions. Journal of neurotrauma. 2015 Oct 1:32(19):1478-87. doi: 10.1089/neu.2014.3706. Epub 2015 Jun 3 [PubMed PMID: 26398596]
Gerzanich V, Stokum JA, Ivanova S, Woo SK, Tsymbalyuk O, Sharma A, Akkentli F, Imran Z, Aarabi B, Sahuquillo J, Simard JM. Sulfonylurea Receptor 1, Transient Receptor Potential Cation Channel Subfamily M Member 4, and KIR6.2:Role in Hemorrhagic Progression of Contusion. Journal of neurotrauma. 2019 Apr 1:36(7):1060-1079. doi: 10.1089/neu.2018.5986. Epub 2018 Oct 4 [PubMed PMID: 30160201]
Jha RM, Bell J, Citerio G, Hemphill JC, Kimberly WT, Narayan RK, Sahuquillo J, Sheth KN, Simard JM. Role of Sulfonylurea Receptor 1 and Glibenclamide in Traumatic Brain Injury: A Review of the Evidence. International journal of molecular sciences. 2020 Jan 9:21(2):. doi: 10.3390/ijms21020409. Epub 2020 Jan 9 [PubMed PMID: 31936452]
Simard JM, Kahle KT, Gerzanich V. Molecular mechanisms of microvascular failure in central nervous system injury--synergistic roles of NKCC1 and SUR1/TRPM4. Journal of neurosurgery. 2010 Sep:113(3):622-9. doi: 10.3171/2009.11.JNS081052. Epub [PubMed PMID: 20035575]
Level 3 (low-level) evidenceMoscote-Salazar LR, M Rubiano A, Alvis-Miranda HR, Calderon-Miranda W, Alcala-Cerra G, Blancas Rivera MA, Agrawal A. Severe Cranioencephalic Trauma: Prehospital Care, Surgical Management and Multimodal Monitoring. Bulletin of emergency and trauma. 2016 Jan:4(1):8-23 [PubMed PMID: 27162922]
Ragaisis V. [Brain contusion: morphology, pathogenesis and treatment]. Medicina (Kaunas, Lithuania). 2002:38(3):243-9; quiz 354 [PubMed PMID: 12474694]
Bouma GJ, Muizelaar JP. Cerebral blood flow in severe clinical head injury. New horizons (Baltimore, Md.). 1995 Aug:3(3):384-94 [PubMed PMID: 7496746]
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet (London, England). 1974 Jul 13:2(7872):81-4 [PubMed PMID: 4136544]
Shetty VS, Reis MN, Aulino JM, Berger KL, Broder J, Choudhri AF, Kendi AT, Kessler MM, Kirsch CF, Luttrull MD, Mechtler LL, Prall JA, Raksin PB, Roth CJ, Sharma A, West OC, Wintermark M, Cornelius RS, Bykowski J. ACR Appropriateness Criteria Head Trauma. Journal of the American College of Radiology : JACR. 2016 Jun:13(6):668-79. doi: 10.1016/j.jacr.2016.02.023. Epub [PubMed PMID: 27262056]
Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, Servadei F, Walters BC, Wilberger J, Surgical Management of Traumatic Brain Injury Author Group. Surgical management of traumatic parenchymal lesions. Neurosurgery. 2006 Mar:58(3 Suppl):S25-46; discussion Si-iv [PubMed PMID: 16540746]
Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, Anderson I, Bulters DO, Belli A, Eynon CA, Wadley J, Mendelow AD, Mitchell PM, Wilson MH, Critchley G, Sahuquillo J, Unterberg A, Servadei F, Teasdale GM, Pickard JD, Menon DK, Murray GD, Kirkpatrick PJ, RESCUEicp Trial Collaborators. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. The New England journal of medicine. 2016 Sep 22:375(12):1119-30. doi: 10.1056/NEJMoa1605215. Epub 2016 Sep 7 [PubMed PMID: 27602507]
Stiver SI. Complications of decompressive craniectomy for traumatic brain injury. Neurosurgical focus. 2009 Jun:26(6):E7. doi: 10.3171/2009.4.FOCUS0965. Epub [PubMed PMID: 19485720]
Level 2 (mid-level) evidenceCarney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017 Jan 1:80(1):6-15. doi: 10.1227/NEU.0000000000001432. Epub [PubMed PMID: 27654000]
Hawryluk GWJ, Rubiano AM, Totten AM, O'Reilly C, Ullman JS, Bratton SL, Chesnut R, Harris OA, Kissoon N, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Lumba-Brown A, Ghajar J. Guidelines for the Management of Severe Traumatic Brain Injury: 2020 Update of the Decompressive Craniectomy Recommendations. Neurosurgery. 2020 Sep 1:87(3):427-434. doi: 10.1093/neuros/nyaa278. Epub [PubMed PMID: 32761068]
Nakamura N, Yamaura A, Shigemori M, Ogawa T, Tokutomi T, Ono J, Kawamata T, Sakamoto T. Final report of the Japan Neurotrauma Data Bank project 1998-2001: 1,002 cases of traumatic brain injury. Neurologia medico-chirurgica. 2006 Dec:46(12):567-74 [PubMed PMID: 17185881]
Level 3 (low-level) evidenceWang XP, Zhong J, Lei T, Wang HJ, Zhu LN, Chu S, Liu L. Epidemiology of traumatic brain injury-associated epilepsy in western China: An analysis of multicenter data. Epilepsy research. 2020 Aug:164():106354. doi: 10.1016/j.eplepsyres.2020.106354. Epub 2020 May 11 [PubMed PMID: 32438297]
Brennan PM, Murray GD, Teasdale GM. Simplifying the use of prognostic information in traumatic brain injury. Part 1: The GCS-Pupils score: an extended index of clinical severity. Journal of neurosurgery. 2018 Jun:128(6):1612-1620. doi: 10.3171/2017.12.JNS172780. Epub 2018 Apr 10 [PubMed PMID: 29631516]
Murray GD, Brennan PM, Teasdale GM. Simplifying the use of prognostic information in traumatic brain injury. Part 2: Graphical presentation of probabilities. Journal of neurosurgery. 2018 Jun:128(6):1621-1634. doi: 10.3171/2017.12.JNS172782. Epub 2018 Apr 10 [PubMed PMID: 29631517]
Jennett B,Bond M, Assessment of outcome after severe brain damage. Lancet (London, England). 1975 Mar 1; [PubMed PMID: 46957]
Marshall LF, Marshall SB, Klauber MR, Van Berkum Clark M, Eisenberg H, Jane JA, Luerssen TG, Marmarou A, Foulkes MA. The diagnosis of head injury requires a classification based on computed axial tomography. Journal of neurotrauma. 1992 Mar:9 Suppl 1():S287-92 [PubMed PMID: 1588618]
Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005 Dec:57(6):1173-82; discussion 1173-82 [PubMed PMID: 16331165]
Huang YH, Deng YH, Lee TC, Chen WF. Rotterdam computed tomography score as a prognosticator in head-injured patients undergoing decompressive craniectomy. Neurosurgery. 2012 Jul:71(1):80-5. doi: 10.1227/NEU.0b013e3182517aa1. Epub [PubMed PMID: 22382208]
Level 2 (mid-level) evidenceCharry JD, Falla JD, Ochoa JD, Pinzón MA, Tejada JH, Henriquez MJ, Solano JP, Calvache C. External Validation of the Rotterdam Computed Tomography Score in the Prediction of Mortality in Severe Traumatic Brain Injury. Journal of neurosciences in rural practice. 2017 Aug:8(Suppl 1):S23-S26. doi: 10.4103/jnrp.jnrp_434_16. Epub [PubMed PMID: 28936067]
Level 1 (high-level) evidence