Cerebral Cavernous Malformations
Cerebral Cavernous Malformations
Introduction
Cerebral cavernous malformations (CCMs) are abnormally large collections of "low flow" vascular channels without brain parenchyma intervening between the sinusoidal vessels (see Images. Pons Cavernoma, Head CT, Cavernous Malformation).[1][2] McCormick (1966) recognized CCMs as a class of cerebral vascular malformations, which include arteriovenous malformations, developmental venous anomalies (DVA), and capillary telangiectasia. Due to recurrent microhemorrhages and thrombosis, they are typically surrounded by hemosiderin deposits and gliosis. These lesions have slow flow and low pressure, causing the average rupture risk to be much lower than some other vascular malformations, eg, arteriovenous malformations. Cavernomas are often found incidentally but can also present during the evaluation of headaches, seizures, focal neurologic deficits, or symptomatic hemorrhage.[3]
Clinically, CCMs are highly variable in both symptomatic presentation and natural history. Adding to the confusion, CCM is referred to by various terms in the medical literature, including cavernomas, cavernous angiomas, and cavernous hemangiomas, although CCM is the preferred nomenclature (see Image. Cerebral Cavernous Hemangioma).[2] CCMs range in size from punctate to several centimeters in diameter and may occur anywhere in the central nervous system, with up to 20% of them located in the brainstem.[4] CCM may be diagnosed in both young children and adults and may develop de novo or even regress spontaneously during a patient's lifetime. A thorough understanding of this entity's natural history is paramount to avoid unnecessary and potentially morbid interventions. Given the heterogeneity of this condition, the ontogenesis, diagnosis, and management strategies for CCMs are subjects of ongoing debate among neuroscientists, and treatment paradigms continue to evolve.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Experts do not fully understand the pathogenesis of CCMs, but the genetic underpinnings have been clarified in recent years. CCMs may be sporadic or have a familial cause. Some studies report that up to 20% of cases follow a familial, autosomal dominant inheritance pattern, while others estimate that between 40% and 60% of cases are familial.[3][5][6] Sporadic cases tend to present with a single CCM, while familial cases are characterized by multiple CCMs in a single patient.
Evolving understanding of genetic associations with CCMs has led to identifying 3 homologically distinct genes responsible for CCM development: CCM1/KRIT1, CCM2/Malcavernin, and CCM3/PDCD10 genes located on the 7q, 7p, and 3p chromosomes, respectively.[7][8][9][8] Those expressed genes encoded by the CCM genes interact in neural tissue with capillary endothelial tight junctions and cytoskeletal proteins during angiogenesis.[3] A common deletion in CCM2 was found to be responsible for clustering among Ashkenazi Jews.[10] Many authors have proposed a "2-hit" hypothesis of familial CCM wherein epigenetic or environmental exposure (the second hit) results in CCM gene loss-of-function and may account for the proclivity of these lesions to accumulate over time and with radiation exposure.[11] Studies of sporadic CCM support a common pathway involving de novo mutations of CCM genes.[9]
Interactions occur between various CCM protein products as well as between these products and other cellular machinery responsible for a range of functions, including cell-cell communication and angiogenesis. The most critical dysfunction identified in CCM mutations is endothelial junction permeability, an effect mediated by Notch1 and Rho kinase activity.[12] This correlates with the characteristic histopathological appearance of CCM, which lacks mature vessel wall architecture and a mature blood-brain barrier.[13] CCMs are distinguished from other cerebral vascular malformations by the absence of direct arteriovenous communication and lack of intervening brain parenchyma.
Recently, genetic studies on the surgically resected lesions from sporadic cases lacking inherited germline mutations have shown somatic mutations of the same 3 CCM genes. This could point towards identical molecular mechanisms in both familial and sporadic CCMs.[14]
Cerebral Cavernous Malformation Risk Factors
The average annual hemorrhage rate is estimated at 0.7% to 1.1% per lesion in patients without a history of prior hemorrhage.[7] However, this risk rises to approximately 4.5% in patients who have sustained previous intracerebral hemorrhage.[7] Approximately 2 to 3 years after a hemorrhagic event, the risk of hemorrhage is thought to decrease. Furthermore, rupture risk varies based on the CCM's location, associated developmental venous anomalies, and gender.[15] Infratentorial location, deep location, young age, and female gender are associated with increased risk.[7][16] Asymptomatic familial cases are also thought to have a higher annual hemorrhage rate than asymptomatic sporadic cases.[16] CCMs may also arise de novo and grow, shrink, or remain stable over time.[17][18] Brainstem lesions and CCM3 familial cases with PDCD10/CCM3 mutations are associated with a greater risk of bleeding. Cavernous angiomas with symptomatic hemorrhage (CASH) include lesions that impact a patient’s life and merit clinical intervention.
Epidemiology
The incidence of CCMs is approximately 0.4% to 0.8% in the general population.[7] Though uncommon, CCMs are the most common cerebral vascular abnormality, accounting for 10% to 25% of all vascular malformations.[7] Moreover, CCMs are the second most common incidental vascular finding, after aneurysms, on brain magnetic resonance imaging (MRI), with a prevalence of 1 in 625 neurologically asymptomatic people.[19][20][21][22]
Clinical presentation is bimodal, with a significant number of cases detected in both adolescents and middle-aged adults. No discernible sex difference in prevalence has been noted; however, conflicting research has been reported on whether prognosis differs among men and women.[23] Familial CCM is notably prevalent among persons of northern Mexican ancestry, compared with other populations, with rates as high as 50%, an effect traced to a common founder mutation.[8][1] The incidence of incidentally detected CCM has increased substantially due to the widespread use of MRI.[24] The majority (approximately 75%) of CCMs are found in the supratentorial compartment in predictable proportion to the volume of neural tissue present.[25]
Pathophysiology
The chief mechanism underlying the clinical manifestations of CCM is the propensity for intralesional and extralesional hemorrhage. Sluggish blood flow through dysplastic channels results in recurrent thrombosis, calcification, and hemosiderin deposition along the lesion's margins. Hemorrhage into adjacent brain parenchyma can produce focal neurologic deficits (FND), seizure, or headache, prompting the patient to present for evaluation.
Clinical and lifestyle risk factors for a first symptomatic episode of CCM hemorrhage are unknown, but risk factors for repeated hemorrhage are well-studied.[2] The pathogenesis of CCM-related epilepsy has been attributed to perilesional reactive gliosis due to clinically silent microhemorrhages, which alter the conduction of adjacent white matter pathways. The observation that seizure-free outcomes are improved when the entire lesion is resected, including the surrounding hemosiderin rim, supports this.[26]
Histopathology
Histopathologically, CCMs are well-circumscribed, multilobate vascular lesions consisting of sinusoidal channels lined by a single layer of epithelium, devoid of smooth muscle and lacking intervening brain parenchyma. Gliosis and hemosiderin deposits are seen along the margins. On gross inspection, CCMs appear "mulberry-like." Light microscopy shows an absence of a smooth muscle wall layer, and electron microscopy shows abnormalities in endothelial gap junctions.[27][25]
History and Physical
Clinical Features of Cerebral Cavernous Malformations
While the clinical presentation of symptomatic CCMs varies by location, the most common clinical manifestations are seizures (50%), intracranial hemorrhage (25%), and FND without radiographic evidence of recent hemorrhage (25%).[2][28] A review by Ene and colleagues found that the most common presentation of CCMs was seizures at an average of 40.6% of CCM cases.[16] Supratentorial lesions are most commonly present with seizures, whereas FND or ataxia is the most common presentation in patients with infratentorial lesions.[29] Between 6% and 65% of patients are asymptomatic and diagnosed incidentally on brain MRI.[2][20][24][3]
When CCM is diagnosed, clinicians should perform a thorough history for evidence of prior symptomatic hemorrhage and a comprehensive neurologic exam to assess for the deficit, which may otherwise have been unrecognized. Headaches are common in patients with CCM, and determining a causal relationship may be difficult. Similarly, CCM-related epilepsy (CRE) can present a diagnostic challenge as seizure focus may be challenging to localize. The criteria for CRE have been defined by expert consensus and can be broadly categorized as "definite," "probable," or "unrelated to CCM" based on the proximity of localized seizure focus to the CCM.[30]
Given the high prevalence of familial CCM, the Angioma Alliance advocates obtaining a detailed 3-generation family history when MRI diagnoses new CCM. When multiple CCMs are present or the family history is positive, genetic screening for CCM1, CCM2, and CCM3 should be considered. Given the autosomal dominant nature of CCM inheritance, appropriate counseling regarding familial risk is warranted, and risk-benefit discussion regarding testing asymptomatic relatives should be offered.[2]
Evaluation
Imaging Modalities and Diagnostic Features
The American College of Radiology (ACR) Appropriateness Criteria provide expert consensus recommendations for acute neurologic symptoms, including a headache, FND, and altered consciousness.[31] Guidelines for imaging follow-up of known CCM are not well-established, but imaging is generally recommended that new symptoms warrant repeat imaging to assess for acute or subacute hemorrhage.[2][6]
CCMs are low-flow vascular lesions that present diagnostic challenges due to their small vessel size and angiographically occult nature. Recognition of these lesions is vital for appropriate management. A detailed understanding of imaging and diagnostic criteria, supported by advanced techniques, enhances the clinician's capability to manage CCMs effectively while minimizing unnecessary interventions.
The Angioma Alliance has established standardized definitions for CCM-related hemorrhage, emphasizing symptom-imaging concordance and biomarker identification to improve diagnostic consistency among neuroimagers and clinicians.[6]
Magnetic resonance imaging
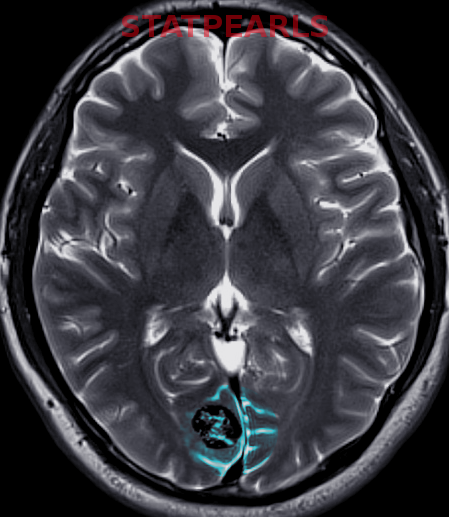
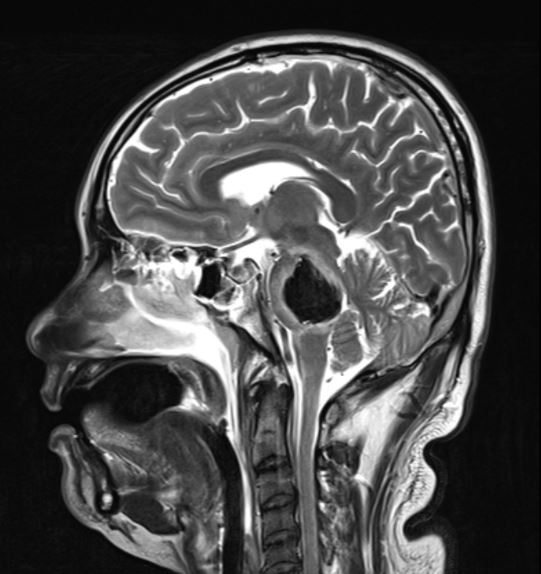
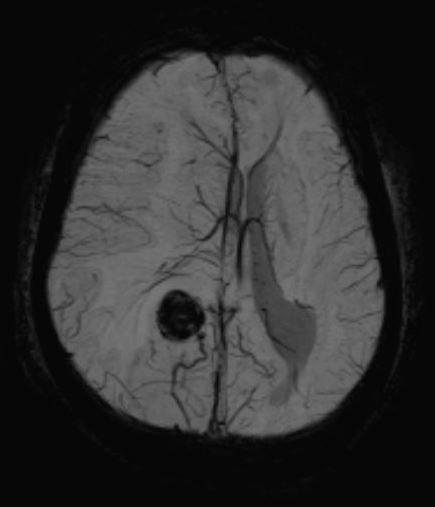
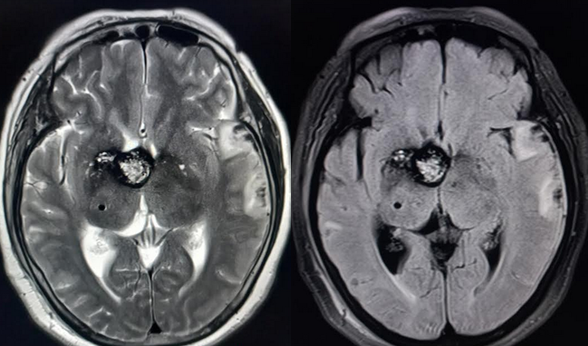
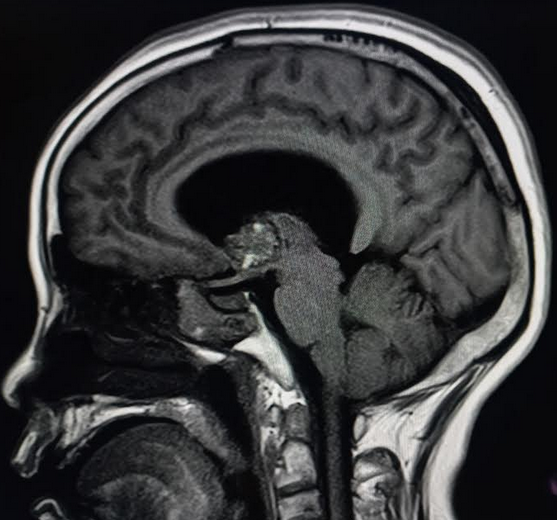
When parenchymal hemorrhage is diagnosed, follow-up imaging with contrast-enhanced MRI is indicated to assess for an underlying vascular lesion. Whether symptomatic or incidentally detected, the majority of CCMs are diagnosed by MRI (see Images. Cortical Cavernoma, Third Ventricular Cavernoma, and Third Ventricular Cavernoma, Sagittal View).[6][24][1][32] MRI is nearly 100% sensitive for CCM detection, making it the diagnostic modality of choice. MRI is particularly effective in familial CCM, where multiple lesions are often present. T2-weighted imaging reveals the characteristic "popcorn" core with a hypointense hemosiderin rim.[25] Gradient-echo (GRE) and susceptibility-weighted imaging (SWI) sequences highlight the "blooming" artifact caused by hemosiderin deposition, though this may exaggerate lesion size.[2][6]
Advanced MRI techniques, such as diffusion tensor imaging (DTI) and functional MRI, aid preoperative planning by mapping critical white matter tracts and eloquent brain regions.[7][3][33] Functional MRI techniques, eg, blood oxygen level-dependent task-activation mapping of language function, are highly accurate and noninvasive tools that have proven useful in the preoperative workup of CCM.[34] New techniques like high-field SWI and advanced MR imaging are improving lesion characterization and risk stratification, particularly for surgically complex cases. Emerging techniques like quantitative susceptibility mapping (QSM) and dynamic contrast-enhanced quantitative perfusion (DCEQP) are under investigation as biomarkers for disease activity.[35][36]
Computed tomography imaging
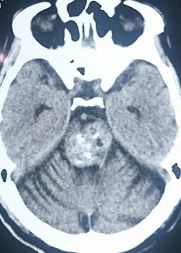
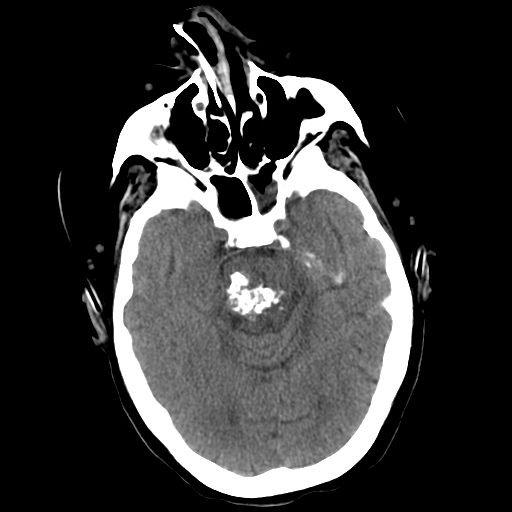
Computed tomography (CT) is less sensitive and specific for CCM but can identify hemorrhage or amorphous calcifications in symptomatic patients. Contrast enhancement may highlight the lesions, but significant edema or mass effect is rare unless a recent hemorrhage has occurred (see Image. Pons Cavernoma, Head CT). CT is primarily used to rule out acute hemorrhage, with MRI needed for definitive diagnosis unless contraindicated.[37]
Angiography
CCMs are characteristically angiographically occult lesions due to the slow transit of blood via the dysplastic channels. However, associated DVAs may be visible on CT angiography or digital subtraction angiography, often enhancing briskly and serving as indirect evidence of CCM (see Image. Cavernous Malformation).[38] The presence of DVAs can impact surgical planning and hemorrhage risk assessment. Multiple CCMs associated with a single DVA suggest a sporadic origin rather than familial.
Zabramski Classification and Hemorrhage Risk
The Zabramski classification categorizes CCMs according to the following types based on their MRI appearance (see Image. Cavernoma, Zabramski Classification):
- Type I: Hyperintense on T1 and T2 due to subacute hemorrhage
- Type II: The classic "popcorn" lesion with mixed signal intensities reflecting hemorrhages at various stages
- Type III: Chronic resolved hemorrhage presenting with an isointense core
- Type IV: Small capillary telangiectasias only visible on GRE sequences [39][3]
Type I and II lesions may carry a higher risk of hemorrhage, sometimes prompting consideration of surgical intervention.[39][3]
Treatment / Management
Management of Cerebral Cavernous Malformations
The clinical course of CCMs is highly variable and primarily determined by lesion location.[25] This variability necessitates a thorough evaluation of patient-specific factors, including risk tolerance and lesion characteristics, to guide management and therapeutic decisions. Interprofessional discussions are essential for determining the optimal approach for each patient. CCM management requires a tailored approach balancing surgical risks, natural disease progression, and patient-specific factors. Conservative management, surgical intervention, and advanced techniques form the foundation of current treatment paradigms, with ongoing research expanding therapeutic options.
Conservative management
For asymptomatic patients with solitary lesions, observation is the preferred strategy.[2] This approach is supported for cases with supratentorial lesions in noneloquent areas and for patients whose risk of surgery outweighs the potential benefits. Conservative management typically includes clinical and radiographic surveillance.(B3)
Surgical management
Microsurgical resection remains the only definitive treatment for CCMs but is associated with significant challenges and potential postoperative morbidity, which can sometimes exceed the risks posed by the untreated lesion.[40] The following considerations are involved with surgical management:(A1)
- Indications for surgery
- Symptomatic lesions: Surgery is often considered for symptomatic lesions, especially in noneloquent, supratentorial locations where resection is associated with high success rates and low complication rates.[41]
- Medically refractory epilepsy: Early surgery is favored for patients whose epilepsy is linked to a solitary CCM confirmed as the epileptogenic focus. This approach may prevent a "kindling" effect, improving seizure outcomes.[2][26][30]
- Brainstem lesions: CCMs located in the deep gray nuclei and brainstem pose a much more significant challenge. Studies using DTI and diffusion tensor tractography have shown that up to 82% of patients with brainstem lesions have involvement of the corticospinal tract and other major fiber tracts[42], highlighting the extreme difficulty neurosurgeons face with patient and approach selection (see Images. Brainstem Cavernoma, Pontine Cavernoma, and Pontine Cavernoma, Sagittal). While good outcomes may be achieved in surgically resected brainstem lesions at high-volume, specialized centers, complication rates are high, and new postoperative neurologic deficits are expected (53% of cases).[43] While surgery is not typically recommended for brainstem CCMs due to a significant risk of complications, many recommend operating after a second symptomatic bleed due to a potentially aggressive natural course. Indications for resection of brainstem CCMs after a single disabling hemorrhage or spinal CCMs are more controversial.[2] Favorable outcomes are more likely for pial-presenting brainstem lesions. With image guidance, appropriate patient and approach selection, and detailed knowledge of the intrinsic brainstem anatomy, these lesions can be safely resected with good outcomes.[44]
-
Surgical techniques
- Complete lesionectomy is the primary goal, with resection of hemosiderin-stained gliotic brain when seizures are the indication.[26] Techniques include frameless stereotactic guidance, electrocorticography, and intraoperative MRI for precision.[45]
- DVAs, which often coexist with CCMs, must be preserved to avoid venous infarction.
- Surgical strategies depend on lesion size; smaller lesions may be excised en bloc, while larger ones often require piecemeal excision.[25]
- Postoperative MRI within 72 hours is recommended to confirm complete resection, as incomplete removal is associated with a high rebleeding risk (approximately 40%).
(B2)
Stereotactic radiosurgery
Stereotactic radiosurgery (SRS) is a noninvasive alternative for treating anatomically inaccessible or high-risk symptomatic lesions. SRS is highly accurate and allows targeted delivery of high-dose radiation (typically 11 to 15 Gy) with sparing of adjacent, healthy brain parenchyma. The mechanism of therapeutic SRS is uncertain; lesion size may decrease, remain stable, or even increase, and no reliable imaging biomarker for successful CCM obliteration has been established, as with metastasis and high-flow vascular lesions.[46] SRS is most effective after a latency period of about 2 years, during which hemorrhage risk reduction is observed. While it may reduce annualized hemorrhage rates (eg, from 33% to 12.3% within 2 years and <1% after).[47] A recent meta-analysis found a modest reduction in hemorrhage rates with a substantial incidence of radiation-related complications (11%), including new focal neurologic deficits, hydrocephalus, and painful paresthesia.[46] SRS is also strongly linked to the development of de novo CCM, although such cases rarely become symptomatic.[48] Because radiation-induced damage in the brainstem can be devastating, SRS is not recommended as a treatment option for brainstem CCMs.[44](A1)
Complications include radiation-induced FND, hydrocephalus, and paresthesia (approximately 11% incidence). Brainstem CCMs are generally not treated with SRS due to the potential for devastating complications. SRS is still seen with an eye of suspicion as its benefit is not conferred for at least 2 to 3 years after treatment, concurrent with the period of temporal hemorrhage clustering. New techniques, such as MRI-guided laser interstitial thermal therapy (LIIT), show promise as minimally invasive alternatives for select cases.[49]
Medical management and experimental therapies
Medical treatment for CCMs remains investigational. Preclinical studies suggest that Rho-kinase inhibitors, statins, and vitamin D may reduce symptoms and lesion progression.[50][51] Anticoagulant and antiplatelet therapies have not been associated with increased hemorrhage risk in patients with CCMs treated for other conditions.[52](B2)
Genetic considerations
Genetic testing is advised for patients with multiple CCMs or a family history of the condition. Genetic counseling should be offered to the patient and their family if mutations are identified.[2] Screening MRIs are recommended for first-degree relatives of patients with familial CCMs.(B3)
Special considerations and guidelines
The following recommendations should be kept in mind in the management of CCMs:
- According to the 2017 Angioma Alliance Care Guidelines, surgical resection is not recommended for asymptomatic lesions, particularly those in eloquent or deep brain regions.
- Surgery may be considered for solitary, asymptomatic CCMs in accessible, noneloquent locations to prevent future hemorrhages and alleviate psychological burden or lifestyle restrictions.
- Brainstem CCMs may warrant surgery after a second symptomatic bleed due to their potentially aggressive natural history.
- The risks of surgical morbidity should be weighed against the natural history of the disease. While microsurgical resection is curative for intractable cases, most patients with supratentorial CCMs are managed conservatively either with radiographic and clinical observation alone or in addition to antiepileptic drugs, as the current first-line management strategy.[25]
Differential Diagnosis
Classic CCM rarely poses a diagnostic dilemma as the radiographic differential diagnosis for isolated T2 artifact, a nonenhancing lesion on MRI, is limited. When numerous small CCMs are present, as is often the case with familial CCM, the differential diagnosis is broad and includes other etiologies of diffuse cerebral microbleeds, including cerebral amyloid angiopathy, chronic hypertension, and hemorrhagic or previously treated metastases, among others. Lesion calcification, which can be detected on routine noncontrast head CT, favors the diagnosis of CCM over other types of microbleeds. Finally, the coexistence of a DVA strongly supports the diagnosis of CCM.[38]
Prognosis
The natural history of CCM has been characterized in several large studies.[5][23][39][28] The overall annualized hemorrhage rate in untreated CCM is estimated at 2.4%, with a predicted cumulative 5-year risk of hemorrhage of 15.8% from the time of diagnosis.[32][22] For patients with incidentally detected CCM, the risk of hemorrhage is substantially lower, estimated to be 0.33% per year.[5]
Rates of epilepsy in incidental lesions are similarly low at 1% to 2%.[30] Conversely, patients who have a documented history of CCM hemorrhage are at significantly greater risk of repeat hemorrhage (23% 5-year rate), a finding which has been replicated in multiple large case series and meta-analyses to date.[5][24][32][53] CCMs display a phenomenon termed temporal clustering wherein hemorrhage tends to reoccur within the first 2 to 3 years after a prior hemorrhage. After this initial clustering of hemorrhage events, a relatively quiescent period where no overt hemorrhages occur may be seen.[25][54] Prior hemorrhage is a significant risk factor for future hemorrhagic events.[25]
Several factors have been associated with CCM rupture, including lesion location, size, multiplicity, and an associated DVA.[55][53][32] Studies have shown that supratentorial lobar CCMs have a much more benign prognosis than deep lesions in the thalamus, basal ganglia, or posterior fossa. In one study, the event rate for superficial lesions was 0% per year, while that for deep lesions was 10.6% per year (P = .0001).[25][56] Brainstem CCMs are the most dangerous and have a high relative event rate (4- to 7-times more likely to rupture than isolated supratentorial lesions).[53][43] In one meta-analysis, nonbrainstem hemorrhage rates were reported to be 0.3% per year vs. 2.8% per year for brainstem lesions.[53]
Also of note, the initial presentation of patients with intracranial hemorrhage or FND and brainstem location was independently associated with a hemorrhage over the 5 years after the initial diagnosis.[22][25] Female gender as a risk factor for hemorrhage remains a topic of debate.[23][22] In familial CCM, more aggressive CCM behavior has been observed in CCM3 mutants, contrasting with a more benign clinical course in CCM1 deletions.[57][9]
Complications
The risks and benefits of surgical or radiotherapeutic intervention must be assessed on a case-by-case basis, and the prospective risks of untreated CCM must be balanced with the anticipated intervention morbidity. The overall mortality associated with CCM hemorrhage is low, estimated at 2.2%, but progressive neurologic deficits can accumulate and reduce a patient's quality of life.[53] In the hands of experienced surgeons with appropriate patient selection, postoperative morbidity can be quite low, with a recent estimate of 1.5%.[58] Nonetheless, when feasible, conservative management may be favorable, as shown in a recent prospective study in which CCM excision worsened short-term disability and increased the risk of neurologic deficit or recurrent hemorrhage.[40]
Postoperative and Rehabilitation Care
Although there are no guidelines on the role of antiepileptics following surgical resection of CCM, patients are typically maintained on antiepileptic monotherapy following surgery.[59] Seizure-free outcomes following surgery are dependent on various factors such as preoperative seizure frequency, the extent of CCM resection, the extent of perilesional "hemosiderin-ring" resection, and timing of surgery relative to the initial presentation.[60][61] Antiepileptic drug withdrawal following surgery should be planned with appropriate dose tapering to reduce the risk of seizure recurrence.[62][63]
Consultations
Neurosurgery consultation is recommended for the management of patients with a cerebral or spinal CCM.
Deterrence and Patient Education
Patients with CCM are encouraged to explore the official website of the multidisciplinary Angioma Alliance. The Angioma Alliance is dedicated to providing up-to-date patient resources, including educational videos. Information is provided regarding genetic testing, participation in ongoing clinical research, and tissue banking. The Angioma Alliance also offers social support via online forums and social media sites, allowing patients and family members to support one another and share their experiences with CCM.
Some activity restrictions, including mountain climbing above 10,000 feet, smoking, water activities, and contact sports, may be considered for people with CCMs, though the effectiveness of these recommendations is not proven. Most studies suggest that antiplatelet medications (for other conditions, if needed) are safe in these patients.
Pearls and Other Issues
RhoA/Rho kinase pathway is seen as a potential target for the pharmacotherapeutic treatment of CCMs. Normally, CCM2 and CCM1 act together to suppress RhoA. CCM1 and CCM2 deficiency leads to constitutively active Rho-kinase (ROCK), destabilizing endothelial cell junctions and vascular permeability.[64] ROCK suppressants have been experimentally shown to enable vasculogenesis in CCM1-, CCM2-, and CCM3-deficient cells.[65] Fasudil, a ROCK inhibitor, has been shown to decrease the lesion burden in CCM1-deficient mice.[66]
While CCM1, CCM2, or CCM3 deficiencies have been shown to activate bone morphogenic protein (BMP) and transform growth factor-beta (TGF-beta), causing an endothelial-to-mesenchymal transition (EndoMT), inhibiting either BMP or TGF-beta was found to decrease the lesion burden in CCM1-deficient mice representing another avenue of research in CCM therapy.[67] Similarly, suppressing Beta-catenin was also found to reduce the number and size of CCMs in a CCM3-deficient mice model.[68] These findings demonstrate the importance of a thorough understanding of the molecular biology underpinning CCM.
Enhancing Healthcare Team Outcomes
Effective management of CCMs relies on a collaborative, patient-centered approach involving physicians, advanced practitioners, nurses, pharmacists, and other healthcare professionals. Each team member contributes specialized skills and assumes distinct responsibilities, emphasizing interprofessional communication and care coordination to enhance patient safety, clinical outcomes, and team performance. Physicians, including neurologists and neurosurgeons, play a central role in diagnosing CCMs, determining treatment plans, and performing surgical interventions when indicated. They work closely with advanced practitioners, who provide detailed patient education, conduct follow-ups, and coordinate care transitions, ensuring continuity and adherence to the chosen management strategy.
Nurses are pivotal in providing bedside care, monitoring symptoms, and offering psychosocial support, particularly for patients undergoing observation or recovering from surgery. Pharmacists contribute by reviewing medication regimens to prevent interactions, optimizing treatment for comorbid conditions, and exploring emerging pharmacological interventions, eg, Rho-kinase inhibitors and statins, currently in trial for CCM management. Genetic counselors are essential for patients with familial or multiple CCMs, offering guidance on genetic testing and implications for the patient and their family members.
Interprofessional communication ensures a seamless flow of information between team members, facilitating shared decision-making and personalized care. Neurosurgical consultations are integral to evaluating treatment options, including observation with serial imaging, surgical resection, or stereotactic radiosurgery. Care coordination across disciplines is vital for managing complex cases, such as those involving brainstem CCMs or refractory epilepsy, requiring alignment on risk-benefit analyses and tailored intervention strategies. This collaborative approach extends to patient education and empowerment, ensuring that patients and their families are informed about their condition and involved in care decisions. By leveraging the collective expertise of the healthcare team and maintaining open lines of communication, care delivery is optimized to enhance patient outcomes, improve safety, and promote a cohesive and effective care environment for individuals with CCMs.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
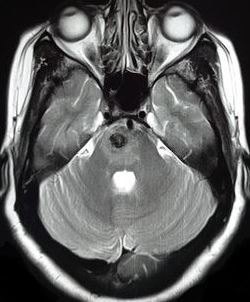
Pontine Cavernoma. Image showing pontine cerebral cavernous malformation. About 82% of patients with brainstem lesions have involvement of the corticospinal tract and other major fiber tracts, highlighting the extreme difficulty neurosurgeons face with patient and approach selection.
Contributed by S Munakomi, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
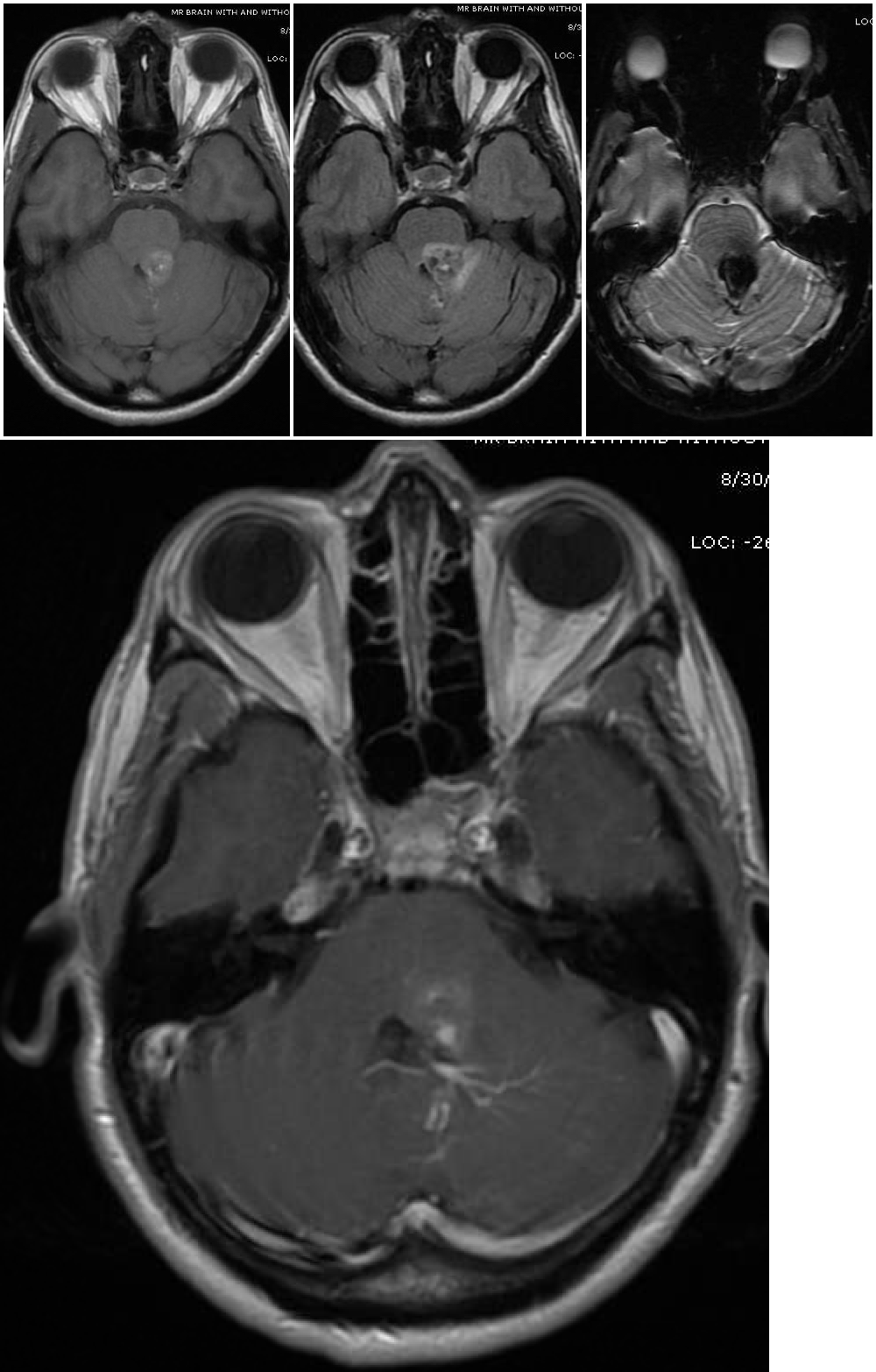
Cavernous Malformation. The top 3 images show magnetic resonance imaging depicting a cerebral cavernous malformation in the left middle cerebellar peduncle of a 42-year-old woman who presented with right-sided facial numbness and ataxia. Varying signal intensities on T1- and T2-weighted sequences and blood detection on susceptibility-weighted sequences (left, middle, and right images, respectively) characteristic of a cavernoma are seen. The bottom image — a weighted image with contrast enhancement — shows a large developmental venous anomaly (DVA) associated with the adjacent cavernous malformation.
Contributed by G Gould, MD, and S Zyck, MD
(Click Image to Enlarge)
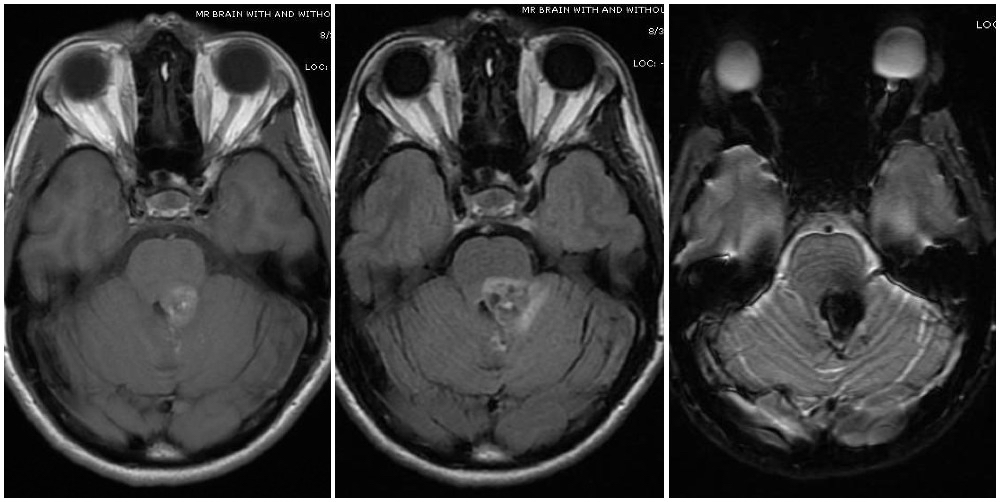
Cavernoma, Zabramski Classification. Magnetic resonance imaging depicts a cavernous malformation in the left middle cerebellar peduncle of a patient who presented with right-sided facial numbness and ataxia. Varying signal intensities on T1—and T2-weighted sequences and blood detection on susceptibility-weighted sequences (left, middle, and right images, respectively) characteristic of a cavernoma are seen. Imaging findings are consistent with a Zabramski type 2 cavernoma with mixed signal intensities on both type 1 and type 2, giving it a "popcorn-like" appearance.
Contributed by G Gould, MD
(Click Image to Enlarge)
References
Rigamonti D, Hadley MN, Drayer BP, Johnson PC, Hoenig-Rigamonti K, Knight JT, Spetzler RF. Cerebral cavernous malformations. Incidence and familial occurrence. The New England journal of medicine. 1988 Aug 11:319(6):343-7 [PubMed PMID: 3393196]
Akers A, Al-Shahi Salman R, A Awad I, Dahlem K, Flemming K, Hart B, Kim H, Jusue-Torres I, Kondziolka D, Lee C, Morrison L, Rigamonti D, Rebeiz T, Tournier-Lasserve E, Waggoner D, Whitehead K. Synopsis of Guidelines for the Clinical Management of Cerebral Cavernous Malformations: Consensus Recommendations Based on Systematic Literature Review by the Angioma Alliance Scientific Advisory Board Clinical Experts Panel. Neurosurgery. 2017 May 1:80(5):665-680. doi: 10.1093/neuros/nyx091. Epub [PubMed PMID: 28387823]
Level 3 (low-level) evidenceDalyai RT, Ghobrial G, Awad I, Tjoumakaris S, Gonzalez LF, Dumont AS, Chalouhi N, Randazzo C, Rosenwasser R, Jabbour P. Management of incidental cavernous malformations: a review. Neurosurgical focus. 2011 Dec:31(6):E5. doi: 10.3171/2011.9.FOCUS11211. Epub [PubMed PMID: 22133177]
Level 3 (low-level) evidenceOtten P, Pizzolato GP, Rilliet B, Berney J. [131 cases of cavernous angioma (cavernomas) of the CNS, discovered by retrospective analysis of 24,535 autopsies]. Neuro-Chirurgie. 1989:35(2):82-3, 128-31 [PubMed PMID: 2674753]
Level 2 (mid-level) evidenceFlemming KD, Link MJ, Christianson TJ, Brown RD Jr. Prospective hemorrhage risk of intracerebral cavernous malformations. Neurology. 2012 Feb 28:78(9):632-6. doi: 10.1212/WNL.0b013e318248de9b. Epub 2012 Feb 1 [PubMed PMID: 22302553]
Level 2 (mid-level) evidenceAl-Shahi Salman R, Berg MJ, Morrison L, Awad IA, Angioma Alliance Scientific Advisory Board. Hemorrhage from cavernous malformations of the brain: definition and reporting standards. Angioma Alliance Scientific Advisory Board. Stroke. 2008 Dec:39(12):3222-30. doi: 10.1161/STROKEAHA.108.515544. Epub 2008 Oct 30 [PubMed PMID: 18974380]
Level 1 (high-level) evidenceMouchtouris N, Chalouhi N, Chitale A, Starke RM, Tjoumakaris SI, Rosenwasser RH, Jabbour PM. Management of cerebral cavernous malformations: from diagnosis to treatment. TheScientificWorldJournal. 2015:2015():808314. doi: 10.1155/2015/808314. Epub 2015 Jan 5 [PubMed PMID: 25629087]
Kim J. Introduction to cerebral cavernous malformation: a brief review. BMB reports. 2016 May:49(5):255-62 [PubMed PMID: 26923303]
Labauge P, Denier C, Bergametti F, Tournier-Lasserve E. Genetics of cavernous angiomas. The Lancet. Neurology. 2007 Mar:6(3):237-44 [PubMed PMID: 17303530]
Gallione CJ, Solatycki A, Awad IA, Weber JL, Marchuk DA. A founder mutation in the Ashkenazi Jewish population affecting messenger RNA splicing of the CCM2 gene causes cerebral cavernous malformations. Genetics in medicine : official journal of the American College of Medical Genetics. 2011 Jul:13(7):662-6. doi: 10.1097/GIM.0b013e318211ff8b. Epub [PubMed PMID: 21543988]
Jain R, Robertson PL, Gandhi D, Gujar SK, Muraszko KM, Gebarski S. Radiation-induced cavernomas of the brain. AJNR. American journal of neuroradiology. 2005 May:26(5):1158-62 [PubMed PMID: 15891176]
Level 2 (mid-level) evidenceFischer A, Zalvide J, Faurobert E, Albiges-Rizo C, Tournier-Lasserve E. Cerebral cavernous malformations: from CCM genes to endothelial cell homeostasis. Trends in molecular medicine. 2013 May:19(5):302-8. doi: 10.1016/j.molmed.2013.02.004. Epub 2013 Mar 15 [PubMed PMID: 23506982]
Level 3 (low-level) evidenceZawistowski JS, Stalheim L, Uhlik MT, Abell AN, Ancrile BB, Johnson GL, Marchuk DA. CCM1 and CCM2 protein interactions in cell signaling: implications for cerebral cavernous malformations pathogenesis. Human molecular genetics. 2005 Sep 1:14(17):2521-31 [PubMed PMID: 16037064]
Level 3 (low-level) evidenceMcDonald DA, Shi C, Shenkar R, Gallione CJ, Akers AL, Li S, De Castro N, Berg MJ, Corcoran DL, Awad IA, Marchuk DA. Lesions from patients with sporadic cerebral cavernous malformations harbor somatic mutations in the CCM genes: evidence for a common biochemical pathway for CCM pathogenesis. Human molecular genetics. 2014 Aug 15:23(16):4357-70. doi: 10.1093/hmg/ddu153. Epub 2014 Apr 3 [PubMed PMID: 24698976]
Wurm G, Schnizer M, Fellner FA. Cerebral cavernous malformations associated with venous anomalies: surgical considerations. Neurosurgery. 2005 Jul:57(1 Suppl):42-58; discussion 42-58 [PubMed PMID: 15987569]
Ene C, Kaul A, Kim L. Natural history of cerebral cavernous malformations. Handbook of clinical neurology. 2017:143():227-232. doi: 10.1016/B978-0-444-63640-9.00021-7. Epub [PubMed PMID: 28552144]
Massa-Micon B, Luparello V, Bergui M, Pagni CA. De novo cavernoma case report and review of literature. Surgical neurology. 2000 May:53(5):484-7 [PubMed PMID: 10874148]
Level 3 (low-level) evidenceClatterbuck RE, Moriarity JL, Elmaci I, Lee RR, Breiter SN, Rigamonti D. Dynamic nature of cavernous malformations: a prospective magnetic resonance imaging study with volumetric analysis. Journal of neurosurgery. 2000 Dec:93(6):981-6 [PubMed PMID: 11117871]
Greving JP, Wermer MJ, Brown RD Jr, Morita A, Juvela S, Yonekura M, Ishibashi T, Torner JC, Nakayama T, Rinkel GJ, Algra A. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. The Lancet. Neurology. 2014 Jan:13(1):59-66. doi: 10.1016/S1474-4422(13)70263-1. Epub 2013 Nov 27 [PubMed PMID: 24290159]
Level 2 (mid-level) evidenceMorris Z, Whiteley WN, Longstreth WT Jr, Weber F, Lee YC, Tsushima Y, Alphs H, Ladd SC, Warlow C, Wardlaw JM, Al-Shahi Salman R. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ (Clinical research ed.). 2009 Aug 17:339():b3016. doi: 10.1136/bmj.b3016. Epub 2009 Aug 17 [PubMed PMID: 19687093]
Level 1 (high-level) evidenceVernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, Niessen WJ, Breteler MM, van der Lugt A. Incidental findings on brain MRI in the general population. The New England journal of medicine. 2007 Nov 1:357(18):1821-8 [PubMed PMID: 17978290]
Horne MA, Flemming KD, Su IC, Stapf C, Jeon JP, Li D, Maxwell SS, White P, Christianson TJ, Agid R, Cho WS, Oh CW, Wu Z, Zhang JT, Kim JE, Ter Brugge K, Willinsky R, Brown RD Jr, Murray GD, Al-Shahi Salman R, Cerebral Cavernous Malformations Individual Patient Data Meta-analysis Collaborators. Clinical course of untreated cerebral cavernous malformations: a meta-analysis of individual patient data. The Lancet. Neurology. 2016 Feb:15(2):166-173. doi: 10.1016/S1474-4422(15)00303-8. Epub 2015 Dec 2 [PubMed PMID: 26654287]
Level 1 (high-level) evidenceDel Curling O Jr, Kelly DL Jr, Elster AD, Craven TE. An analysis of the natural history of cavernous angiomas. Journal of neurosurgery. 1991 Nov:75(5):702-8 [PubMed PMID: 1919691]
Level 2 (mid-level) evidenceMoore SA, Brown RD Jr, Christianson TJ, Flemming KD. Long-term natural history of incidentally discovered cavernous malformations in a single-center cohort. Journal of neurosurgery. 2014 May:120(5):1188-92. doi: 10.3171/2014.1.JNS131619. Epub 2014 Mar 14 [PubMed PMID: 24628608]
Level 2 (mid-level) evidenceEllis JA, Barrow DL. Supratentorial cavernous malformations. Handbook of clinical neurology. 2017:143():283-289. doi: 10.1016/B978-0-444-63640-9.00027-8. Epub [PubMed PMID: 28552151]
Baumann CR, Schuknecht B, Lo Russo G, Cossu M, Citterio A, Andermann F, Siegel AM. Seizure outcome after resection of cavernous malformations is better when surrounding hemosiderin-stained brain also is removed. Epilepsia. 2006 Mar:47(3):563-6 [PubMed PMID: 16529622]
Level 2 (mid-level) evidenceWong JH, Awad IA, Kim JH. Ultrastructural pathological features of cerebrovascular malformations: a preliminary report. Neurosurgery. 2000 Jun:46(6):1454-9 [PubMed PMID: 10834648]
Al-Shahi Salman R, Hall JM, Horne MA, Moultrie F, Josephson CB, Bhattacharya JJ, Counsell CE, Murray GD, Papanastassiou V, Ritchie V, Roberts RC, Sellar RJ, Warlow CP, Scottish Audit of Intracranial Vascular Malformations (SAIVMs) collaborators. Untreated clinical course of cerebral cavernous malformations: a prospective, population-based cohort study. The Lancet. Neurology. 2012 Mar:11(3):217-24. doi: 10.1016/S1474-4422(12)70004-2. Epub 2012 Jan 31 [PubMed PMID: 22297119]
Washington CW, McCoy KE, Zipfel GJ. Update on the natural history of cavernous malformations and factors predicting aggressive clinical presentation. Neurosurgical focus. 2010 Sep:29(3):E7. doi: 10.3171/2010.5.FOCUS10149. Epub [PubMed PMID: 20809765]
Level 2 (mid-level) evidenceRosenow F, Alonso-Vanegas MA, Baumgartner C, Blümcke I, Carreño M, Gizewski ER, Hamer HM, Knake S, Kahane P, Lüders HO, Mathern GW, Menzler K, Miller J, Otsuki T, Ozkara C, Pitkänen A, Roper SN, Sakamoto AC, Sure U, Walker MC, Steinhoff BJ, Surgical Task Force, Commission on Therapeutic Strategies of the ILAE. Cavernoma-related epilepsy: review and recommendations for management--report of the Surgical Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2013 Dec:54(12):2025-35. doi: 10.1111/epi.12402. Epub 2013 Oct 17 [PubMed PMID: 24134485]
Expert Panel on Neurologic Imaging:, Salmela MB, Mortazavi S, Jagadeesan BD, Broderick DF, Burns J, Deshmukh TK, Harvey HB, Hoang J, Hunt CH, Kennedy TA, Khalessi AA, Mack W, Patel ND, Perlmutter JS, Policeni B, Schroeder JW, Setzen G, Whitehead MT, Cornelius RS, Corey AS. ACR Appropriateness Criteria(®) Cerebrovascular Disease. Journal of the American College of Radiology : JACR. 2017 May:14(5S):S34-S61. doi: 10.1016/j.jacr.2017.01.051. Epub [PubMed PMID: 28473091]
Gross BA, Lin N, Du R, Day AL. The natural history of intracranial cavernous malformations. Neurosurgical focus. 2011 Jun:30(6):E24. doi: 10.3171/2011.3.FOCUS1165. Epub [PubMed PMID: 21631226]
Level 3 (low-level) evidenceLi D, Jiao YM, Wang L, Lin FX, Wu J, Tong XZ, Wang S, Cao Y. Surgical outcome of motor deficits and neurological status in brainstem cavernous malformations based on preoperative diffusion tensor imaging: a prospective randomized clinical trial. Journal of neurosurgery. 2019 Jan 1:130(1):286-301. doi: 10.3171/2017.8.JNS17854. Epub 2018 Mar 16 [PubMed PMID: 29547081]
Level 1 (high-level) evidencePouratian N, Bookheimer SY, Rex DE, Martin NA, Toga AW. Utility of preoperative functional magnetic resonance imaging for identifying language cortices in patients with vascular malformations. Neurosurgical focus. 2002 Oct 15:13(4):e4 [PubMed PMID: 15771403]
Mikati AG, Tan H, Shenkar R, Li L, Zhang L, Guo X, Larsson HB, Shi C, Liu T, Wang Y, Shah A, Edelman RR, Christoforidis G, Awad I. Dynamic permeability and quantitative susceptibility: related imaging biomarkers in cerebral cavernous malformations. Stroke. 2014 Feb:45(2):598-601. doi: 10.1161/STROKEAHA.113.003548. Epub 2013 Dec 3 [PubMed PMID: 24302484]
Dammann P, Wrede K, Zhu Y, Matsushige T, Maderwald S, Umutlu L, Quick HH, Hehr U, Rath M, Ladd ME, Felbor U, Sure U. Correlation of the venous angioarchitecture of multiple cerebral cavernous malformations with familial or sporadic disease: a susceptibility-weighted imaging study with 7-Tesla MRI. Journal of neurosurgery. 2017 Feb:126(2):570-577. doi: 10.3171/2016.2.JNS152322. Epub 2016 May 6 [PubMed PMID: 27153162]
Bitoh S, Hasegawa H, Fujiwara M, Sakurai M. Angiographically occult vascular malformations causing intracranial hemorrhage. Surgical neurology. 1982 Jan:17(1):35-42 [PubMed PMID: 7071717]
Level 3 (low-level) evidencePetersen TA, Morrison LA, Schrader RM, Hart BL. Familial versus sporadic cavernous malformations: differences in developmental venous anomaly association and lesion phenotype. AJNR. American journal of neuroradiology. 2010 Feb:31(2):377-82. doi: 10.3174/ajnr.A1822. Epub 2009 Oct 15 [PubMed PMID: 19833796]
Level 2 (mid-level) evidenceZabramski JM, Wascher TM, Spetzler RF, Johnson B, Golfinos J, Drayer BP, Brown B, Rigamonti D, Brown G. The natural history of familial cavernous malformations: results of an ongoing study. Journal of neurosurgery. 1994 Mar:80(3):422-32 [PubMed PMID: 8113854]
Moultrie F, Horne MA, Josephson CB, Hall JM, Counsell CE, Bhattacharya JJ, Papanastassiou V, Sellar RJ, Warlow CP, Murray GD, Al-Shahi Salman R, Scottish Audit of Intracranial Vascular Malformations (SAIVMs) steering committee and collaborators. Outcome after surgical or conservative management of cerebral cavernous malformations. Neurology. 2014 Aug 12:83(7):582-9. doi: 10.1212/WNL.0000000000000684. Epub 2014 Jul 3 [PubMed PMID: 24994841]
Level 1 (high-level) evidenceD'Angelo VA, De Bonis C, Amoroso R, Cali A, D'Agruma L, Guarnieri V, Muscarella LA, Zelante L, Bisceglia M, Scarabino T, Catapano D. Supratentorial cerebral cavernous malformations: clinical, surgical, and genetic involvement. Neurosurgical focus. 2006 Jul 15:21(1):e9 [PubMed PMID: 16859262]
Level 2 (mid-level) evidenceFlores BC, Whittemore AR, Samson DS, Barnett SL. The utility of preoperative diffusion tensor imaging in the surgical management of brainstem cavernous malformations. Journal of neurosurgery. 2015 Mar:122(3):653-62. doi: 10.3171/2014.11.JNS13680. Epub 2015 Jan 9 [PubMed PMID: 25574568]
Level 2 (mid-level) evidenceAbla AA, Lekovic GP, Turner JD, de Oliveira JG, Porter R, Spetzler RF. Advances in the treatment and outcome of brainstem cavernous malformation surgery: a single-center case series of 300 surgically treated patients. Neurosurgery. 2011 Feb:68(2):403-14; discussion 414-5. doi: 10.1227/NEU.0b013e3181ff9cde. Epub [PubMed PMID: 21654575]
Level 2 (mid-level) evidenceAtwal GS, Sarris CE, Spetzler RF. Brainstem and cerebellar cavernous malformations. Handbook of clinical neurology. 2017:143():291-295. doi: 10.1016/B978-0-444-63640-9.00028-X. Epub [PubMed PMID: 28552152]
Yeon JY, Kim JS, Choi SJ, Seo DW, Hong SB, Hong SC. Supratentorial cavernous angiomas presenting with seizures: surgical outcomes in 60 consecutive patients. Seizure. 2009 Jan:18(1):14-20. doi: 10.1016/j.seizure.2008.05.010. Epub 2008 Jul 24 [PubMed PMID: 18656386]
Level 2 (mid-level) evidenceLu XY, Sun H, Xu JG, Li QY. Stereotactic radiosurgery of brainstem cavernous malformations: a systematic review and meta-analysis. Journal of neurosurgery. 2014 Apr:120(4):982-7. doi: 10.3171/2013.12.JNS13990. Epub 2014 Feb 7 [PubMed PMID: 24506243]
Level 1 (high-level) evidenceHasegawa T, McInerney J, Kondziolka D, Lee JY, Flickinger JC, Lunsford LD. Long-term results after stereotactic radiosurgery for patients with cavernous malformations. Neurosurgery. 2002 Jun:50(6):1190-7; discussion 1197-8 [PubMed PMID: 12015835]
Nimjee SM, Powers CJ, Bulsara KR. Review of the literature on de novo formation of cavernous malformations of the central nervous system after radiation therapy . Neurosurgical focus. 2006 Jul 15:21(1):e4 [PubMed PMID: 16859257]
McCracken DJ, Willie JT, Fernald BA, Saindane AM, Drane DL, Barrow DL, Gross RE. Magnetic Resonance Thermometry-Guided Stereotactic Laser Ablation of Cavernous Malformations in Drug-Resistant Epilepsy: Imaging and Clinical Results. Operative neurosurgery (Hagerstown, Md.). 2016 Mar:12(1):39-48. doi: 10.1227/NEU.0000000000001033. Epub 2015 Sep 25 [PubMed PMID: 27959970]
Shenkar R, Shi C, Austin C, Moore T, Lightle R, Cao Y, Zhang L, Wu M, Zeineddine HA, Girard R, McDonald DA, Rorrer A, Gallione C, Pytel P, Liao JK, Marchuk DA, Awad IA. RhoA Kinase Inhibition With Fasudil Versus Simvastatin in Murine Models of Cerebral Cavernous Malformations. Stroke. 2017 Jan:48(1):187-194. doi: 10.1161/STROKEAHA.116.015013. Epub 2016 Nov 22 [PubMed PMID: 27879448]
Awad IA, Polster SP. Cavernous angiomas: deconstructing a neurosurgical disease. Journal of neurosurgery. 2019 Jul 1:131(1):1-13. doi: 10.3171/2019.3.JNS181724. Epub 2019 Jul 1 [PubMed PMID: 31261134]
Schneble HM, Soumare A, Hervé D, Bresson D, Guichard JP, Riant F, Tournier-Lasserve E, Tzourio C, Chabriat H, Stapf C. Antithrombotic therapy and bleeding risk in a prospective cohort study of patients with cerebral cavernous malformations. Stroke. 2012 Dec:43(12):3196-9. doi: 10.1161/STROKEAHA.112.668533. Epub 2012 Nov 13 [PubMed PMID: 23150651]
Level 2 (mid-level) evidenceTaslimi S, Modabbernia A, Amin-Hanjani S, Barker FG 2nd, Macdonald RL. Natural history of cavernous malformation: Systematic review and meta-analysis of 25 studies. Neurology. 2016 May 24:86(21):1984-91. doi: 10.1212/WNL.0000000000002701. Epub 2016 Apr 22 [PubMed PMID: 27164680]
Level 1 (high-level) evidenceBarker FG 2nd, Amin-Hanjani S, Butler WE, Lyons S, Ojemann RG, Chapman PH, Ogilvy CS. Temporal clustering of hemorrhages from untreated cavernous malformations of the central nervous system. Neurosurgery. 2001 Jul:49(1):15-24; discussion 24-5 [PubMed PMID: 11440436]
Level 2 (mid-level) evidenceKashefiolasl S, Bruder M, Brawanski N, Herrmann E, Seifert V, Tritt S, Konczalla J. A benchmark approach to hemorrhage risk management of cavernous malformations. Neurology. 2018 Mar 6:90(10):e856-e863. doi: 10.1212/WNL.0000000000005066. Epub 2018 Feb 2 [PubMed PMID: 29429974]
Porter PJ, Willinsky RA, Harper W, Wallace MC. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. Journal of neurosurgery. 1997 Aug:87(2):190-7 [PubMed PMID: 9254081]
Denier C, Labauge P, Bergametti F, Marchelli F, Riant F, Arnoult M, Maciazek J, Vicaut E, Brunereau L, Tournier-Lasserve E. Genotype-phenotype correlations in cerebral cavernous malformations patients. Annals of neurology. 2006 Nov:60(5):550-556. doi: 10.1002/ana.20947. Epub [PubMed PMID: 17041941]
Gross BA, Batjer HH, Awad IA, Bendok BR, Du R. Brainstem cavernous malformations: 1390 surgical cases from the literature. World neurosurgery. 2013 Jul-Aug:80(1-2):89-93. doi: 10.1016/j.wneu.2012.04.002. Epub 2012 Apr 5 [PubMed PMID: 22484766]
Level 1 (high-level) evidenceRudy RF, Du R. Pharmacotherapy for cavernous malformations. Handbook of clinical neurology. 2017:143():309-316. doi: 10.1016/B978-0-444-63640-9.00031-X. Epub [PubMed PMID: 28552155]
Englot DJ, Han SJ, Lawton MT, Chang EF. Predictors of seizure freedom in the surgical treatment of supratentorial cavernous malformations. Journal of neurosurgery. 2011 Dec:115(6):1169-74. doi: 10.3171/2011.7.JNS11536. Epub 2011 Aug 5 [PubMed PMID: 21819194]
Level 2 (mid-level) evidenceStavrou I, Baumgartner C, Frischer JM, Trattnig S, Knosp E. Long-term seizure control after resection of supratentorial cavernomas: a retrospective single-center study in 53 patients. Neurosurgery. 2008 Nov:63(5):888-96; discussion 897. doi: 10.1227/01.NEU.0000327881.72964.6E. Epub [PubMed PMID: 19005379]
Level 2 (mid-level) evidenceRathore C, Panda S, Sarma PS, Radhakrishnan K. How safe is it to withdraw antiepileptic drugs following successful surgery for mesial temporal lobe epilepsy? Epilepsia. 2011 Mar:52(3):627-35. doi: 10.1111/j.1528-1167.2010.02890.x. Epub 2011 Jan 10 [PubMed PMID: 21219315]
Schmidt D, Baumgartner C, Löscher W. Seizure recurrence after planned discontinuation of antiepileptic drugs in seizure-free patients after epilepsy surgery: a review of current clinical experience. Epilepsia. 2004 Feb:45(2):179-86 [PubMed PMID: 14738426]
Stockton RA, Shenkar R, Awad IA, Ginsberg MH. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. The Journal of experimental medicine. 2010 Apr 12:207(4):881-96. doi: 10.1084/jem.20091258. Epub 2010 Mar 22 [PubMed PMID: 20308363]
Level 3 (low-level) evidenceBorikova AL, Dibble CF, Sciaky N, Welch CM, Abell AN, Bencharit S, Johnson GL. Rho kinase inhibition rescues the endothelial cell cerebral cavernous malformation phenotype. The Journal of biological chemistry. 2010 Apr 16:285(16):11760-4. doi: 10.1074/jbc.C109.097220. Epub 2010 Feb 24 [PubMed PMID: 20181950]
McDonald DA, Shi C, Shenkar R, Stockton RA, Liu F, Ginsberg MH, Marchuk DA, Awad IA. Fasudil decreases lesion burden in a murine model of cerebral cavernous malformation disease. Stroke. 2012 Feb:43(2):571-4. doi: 10.1161/STROKEAHA.111.625467. Epub 2011 Oct 27 [PubMed PMID: 22034008]
Level 3 (low-level) evidenceMaddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, Ferrarini L, Orsenigo F, Papa E, Boulday G, Tournier-Lasserve E, Chapon F, Richichi C, Retta SF, Lampugnani MG, Dejana E. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature. 2013 Jun 27:498(7455):492-6. doi: 10.1038/nature12207. Epub 2013 Jun 9 [PubMed PMID: 23748444]
Level 3 (low-level) evidenceBravi L, Rudini N, Cuttano R, Giampietro C, Maddaluno L, Ferrarini L, Adams RH, Corada M, Boulday G, Tournier-Lasserve E, Dejana E, Lampugnani MG. Sulindac metabolites decrease cerebrovascular malformations in CCM3-knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2015 Jul 7:112(27):8421-6. doi: 10.1073/pnas.1501352112. Epub 2015 Jun 24 [PubMed PMID: 26109568]