Introduction
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a relatively rare inherited disease with almost exclusively neurological manifestations. This condition manifests pathologically as the thickening of small and medium-sized arteries, not caused by atherosclerosis or amyloid deposition, resulting in blockades and ischemic damage to the brain. The clinical presentations are primarily migraines with aura and premature onset of recurrent small vessel ischemic disease, mood disorders, and progressive cognitive impairment resulting in early-onset dementia. CADASIL has a relatively characteristic appearance on magnetic resonance imaging, and diagnosis is made via genetic testing or skin biopsy. No disease-modifying therapies are yet available, and treatment is targeted mainly at cardiovascular risk reduction.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Originally, CADASIL was recognized as a heritable early-onset microvascular disease of unknown etiology. The genetic cause of CADASIL was identified by Joutel et al in 1996 as multiple mutations involving genes encoding for the NOTCH3 protein on chromosome 19. CADASIL is the most common inherited small vessel disease caused by mutations involving a single gene. This condition is inherited in an autosomal dominant fashion.[2] Even within families, there is significant variability in presentation.[3]
The abnormal NOTCH3 protein causes accumulation of the ectodomain of this protein within the smooth muscle layer of small- and medium-sized cerebral vessels with accompanying intimal hyperplasia and hyalinization of the vessels. Another hallmark is the presence of granular osmiophilic material between the smooth muscle cells (osmiophilic means reacting with or staining with osmium tetroxide).[4]
Epidemiology
At a minimum, the prevalence of CADASIL mutation has been estimated at approximately 5:100,000. A more recent Italian study found a prevalence of at least 4.1 per 100,000 adults.[5] Actual prevalence is likely higher due to underdiagnosis. There is no sex predilection, though men tend to be slightly more severely affected than women.[6] The prevalence appears to be highest among people of Asian descent.[1] Hypertension and smoking are related to stroke at an early age.[6]
Pathophysiology
CADASIL is caused by NOTCH3 gene mutations on chromosome 19p13. The NOTCH3 protein is a transmembrane receptor expressed in the vascular smooth muscle cells. As a result of the gene mutation, CADASIL causes a vasculopathy predominantly affecting small cerebral vessels. Cells expressing the mutant NOTCH3 gene are also sensitive to stresses such as anoxia or oxidative stress, resulting in the activation of apoptotic cell death.[7] The most common manifestations are migraines, transient ischemic attacks and lacunar infarcts, vascular dementia, and psychiatric diseases such as depression and apathy. The pathophysiology accounts for the clinical findings of lacunar infarcts, vascular dementia, and psychiatric disease. There is not a clear reason on how CADASIL accounts for migraine headaches.[6] This may be the result of cerebral hypoperfusion which triggers cortical spreading depression and migraine with aura.[8]
Histopathology
CADASIL is a systemic microangiopathy, yet vascular manifestations are mainly limited to cerebral vasculature, especially the leptomeningeal and deep penetrating arteries and arterioles. Small arterial vessel wall thickening is caused by marked intimal hyperplasia and smooth muscle cell degeneration, which are the pathological hallmarks of CADASIL. Microhemorrhages are also found in the brains of CADASIL patients, suggesting that microangiopathy causes both ischemic lesions and petechial hemorrhages.[9]
The microvascular changes may be seen in various tissues, including the skin. Skin biopsy becomes the gold standard for diagnosing CADASIL in cases where no genetic mutation is found. The characteristic appearance is the accumulation of non-amyloid granular osmiophilic material (GOM) around smooth muscle in small arteries. GOMs are small 0.2 to 2 mm-sized osmiophilic, periodic acid-Schiff (PAS)-positive, electron-dense granules located around vascular smooth muscle cells. They are best visualized by electron microscopy. Recent studies found that there are lucent halos surrounding many GOMs, suggesting impaired clearance of GOM in patients with CADASIL.[10] This may explain the typical findings in these patients with enlarged perivascular spaces in the brain. These findings are most prevalent in the brain but can also be seen in skin and muscle samples throughout the body; thus, diagnosis is made via skin biopsy. On histopathology, the appearance is typical of any small vessel disease, with degeneration of vascular smooth muscle cells, endothelium, and basal lamina. Notably, atherosclerosis is absent. Again, changes are most prominent in small cerebral vessels as opposed to elsewhere in the body.[11]
History and Physical
Age of onset and disease course are widely variable, with case reports describing onset between 20 and 70 years old. Nonetheless, the average age of onset is around 30 years. A migraine with aura is the most common initial symptom, present in about 55% of people diagnosed with CADASIL; migraine is slightly more common in women than men and less common in Asian populations.[6] Cerebral ischemic events, notably transient ischemic attacks, and lacunar infarcts are the most frequent manifestation of the disease, occurring in 60% to 85% of patients with symptoms. TIAs and subcortical/lacunar infarcts manifest with typical clinical lacunar syndromes such as pure motor or sensory deficits or brainstem infarcts. Less commonly, CADASIL may present as an acute encephalopathy, which can evolve from a migraine presentation.[12] Cognitive impairment is the second most frequent manifestation of CADASIL, occurring in about 60% of patients. Cognitive impairment gets progressively worse and finally manifests with vascular dementia. Psychiatric diagnoses such as depression, panic disorder, bipolar disorder, schizophrenia, and apathy are also common, occurring in about 25% to 30% of patients.[6]
Evaluation
CADASIL should be suspected in patients with a strong family history of early strokes and dementia, keeping in mind that CADASIL is likely underdiagnosed. Though definitive diagnosis is via genetic testing or skin biopsy, a CADASIL scale has been proposed by Pescini et al. as a less expensive initial clinical screening measure.[13] This scale includes typical symptoms of the disease, such as migraines and TIA, typical imaging features, and family history, all with various weightings. In patients where CADASIL is strongly suspected, definitive diagnosis begins with serum genetic testing for a NOTCH3 mutation. Approximately 4% of patients with CADASIL will have a negative genetic test, likely related to unidentified genetic mutations. Thus, for those patients with a high clinical suspicion, the next step is a skin biopsy with histopathologic examination for granular osmiophilic material (GOM) accumulation.[14] Skin biopsy has a specificity of 100% and sensitivity of 45% to 100% due to variations in disease progression and focality of the disease process.
Alternatively, CADASIL has a somewhat characteristic appearance on MRI, and a radiologist may suggest the diagnosis. Abnormal T2/FLAIR white matter hyperintensities, similar in appearance to those seen with chronic microvascular disease, are present in CADASIL in the anterior temporal, external capsule, and paramedian superior frontal subcortical white matter. Please see Image. Axial FLAIR Image, Cerebral Autosomal Dominant Ateriopathy. Importantly, these findings are present in younger patients—by a mean age of 30, and almost all patients aged older than 35 years.[4] Though this distribution is reported to be specific for CADASIL, older patients with severe microvascular disease may have lesions in these locations because they have diffuse disease. As expected, lacunar or subcortical infarcts can also be seen with CADASIL, manifesting as foci of restricted diffusion on DWI/ADC sequences (bright on diffusion, dark on ADC), typically measuring less than 1.5 cm. Finally, microbleeds are seen with a greater prevalence in CADASIL patients. These are best seen on a T2*-GRE MRI sequence as multiple punctate foci of susceptibility/hypointensity. These findings of lacunar infarcts and microbleeds in younger patients are less specific but suggestive MRI findings.[6]
Treatment / Management
CADASIL is a progressive and fatal disease. No disease-modifying treatment is available. Migraine attacks are treated with common analgesics. Triptans and ergots should be avoided due to their vasoconstrictor properties. Acute ischemic stroke may be treated with intravenous tissue plasminogen activator if criteria are met. Long-term therapy is very similar to that of traditional chronic microvascular ischemic disease, targeting cardiovascular risk reduction with blood pressure control, smoking cessation, statins, healthy diet and lifestyle, weight control, and antiplatelet therapy. Smoking cessation is especially important. However, given that CADASIL is non-atherosclerotic, the role of thrombosis in causing strokes with CADASIL is in question. Thus, antiplatelet therapy may fall out of favor. Moreover, given that CADASIL patients have a higher incidence of microhemorrhages, any anticoagulation therapy must be considered with caution. In addition, therapy for other disease manifestations, such as migraines and depression, is similar to the general population. Clinical symptoms progress from migraines to infarcts to vascular dementia over decades.[6] Life expectancy is significantly reduced for both men and women (see Prognosis section).[15] As an additional note, genetic counseling should be offered to patients diagnosed with CADASIL.[16][17][18][19](B2)
Due to the discovery of NOTCH3 cysteine-altering mutation in CADASIL. There are some recent experimental approaches. Antisense oligonucleotide(ASO) exon-skipping technology is being studied.[20] Another therapeutic approach is immunotherapy with a monoclonal antibody targeting a domain of the NOTCH3 protein.[21](B2)
Differential Diagnosis
There are numerous differential considerations to keep in mind.
Age-advanced traditional microvascular disease in the setting of diabetes or hypertension should be the first consideration. The MRI distribution of lesions may help differentiate this.
Multiple sclerosis is another consideration, but a different MRI appearance and involvement of the optic nerves and spinal cord would not be expected with CADASIL.
Fabry disease is another heritable cause of early-onset microvascular disease and strokes that also feature basal ganglia calcifications and has X-linked inheritence.
Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL) is a similar entity to CADASIL that presents with early-onset spinal degenerative disease and hair loss but typically does not feature migraines.
Cerebral amyloid angiopathy has a similar clinical presentation to CADASIL, with both disorders conferring an increased risk of dementia, stroke, and intracerebral hemorrhage. In addition, both result from protein accumulation causing degeneration of the smooth muscle layer in cerebral vasculature. However, CADASIL will show accumulation of the NOTCH3 protein ectodomain and extracellular granular osmiophilic material, while cerebral amyloid angiopathy will show accumulation of amyloidogenic protein (often Aβ).[4]
Other heritable conditions, such as leukoencephalopathies and mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS), should also be considered.
More broadly, other causes of strokes and hemorrhage in younger individuals include primary CNS vasculitis, reversible cerebral vasoconstriction syndrome (RCVS), inflammatory amyloid angiopathy, venous thrombosis, septic emboli, metastatic disease, and toxic/metabolic exposures.
Other causes of white matter abnormalities seen on imaging include the following:
- Acute disseminated encephalomyelitis
- Behçet disease
- HIV encephalopathy and AIDS dementia complex
- Lyme disease
- Neurosarcoidosis
- Neurosyphilis
Prognosis
The clinical manifestations of CADASIL vary tremendously yet the progression is inevitable. Most patients older than age 58 are unable to ambulate. About 65% of them require total personal care by the age of 65.[22] Life expectancy is reduced significantly with a mean age of death of 64.4 in men and 70.7 in women.[6]
Complications
As a result of non-amyloid intimal and medial hyperplasia, and the resultant occlusion of deep white matter small arteries, patients with CADASIL present with recurrent small vessel deep white matter ischemic infarctions and global cerebral hypoperfusion. With time, these patients will develop progressive cortical atrophy, cognitive impairment, and finally dementia. Similar to other causes of small vessel diseases such as chronic hypertension or amyloid angiopathy, these patients also develop cerebral microhemorrhages visible on susceptibility MRI sequences.[9]
Deterrence and Patient Education
Smoking cessation and hypertension control are two modifiable risk factors that can be addressed.
Genetic counseling is extremely important given the autosomal dominant inheritance pattern of this disease.
Given the progressive nature of the disease and associated disability, end-of-life care goals and long-term care plans should be clarified.
Enhancing Healthcare Team Outcomes
Providing patient-centered care for individuals with CADASIL requires a collaborative effort among healthcare professionals, including physicians, advanced practice practitioners, nurses, pharmacists, and others. First and foremost, healthcare providers must possess the necessary clinical skills and expertise when diagnosing, evaluating, and treating this condition. This includes proficiency in interpreting radiological findings and genetic tests, recognizing potential complications, and understanding the likely disease progression. The interprofessional team may include primary care providers, neurologists, radiologists, geneticists, and emergency department providers.
Ethical considerations come into play when determining treatment options and respecting patient autonomy in decision-making. Responsibilities within the interprofessional team should be clearly defined, with each member contributing their specialized knowledge and skills to optimize patient care. Effective interprofessional communication fosters a collaborative environment where information is shared, questions are encouraged, and concerns are addressed promptly.
Lastly, care coordination is pivotal in ensuring seamless and efficient patient care, especially given the progressive nature and poor prognosis of the disease. Home health aides, social workers, and long-term care coordination are crucial to improving quality of life for patients and family members. Physicians, advanced practitioners, nurses, pharmacists, and other healthcare professionals must work together to streamline the patient's journey, from diagnosis through treatment and follow-up. This coordination minimizes errors, reduces delays, and enhances patient safety, ultimately leading to improved outcomes and patient-centered care that prioritizes the well-being and satisfaction of those affected by CADASIL.
Media
(Click Image to Enlarge)
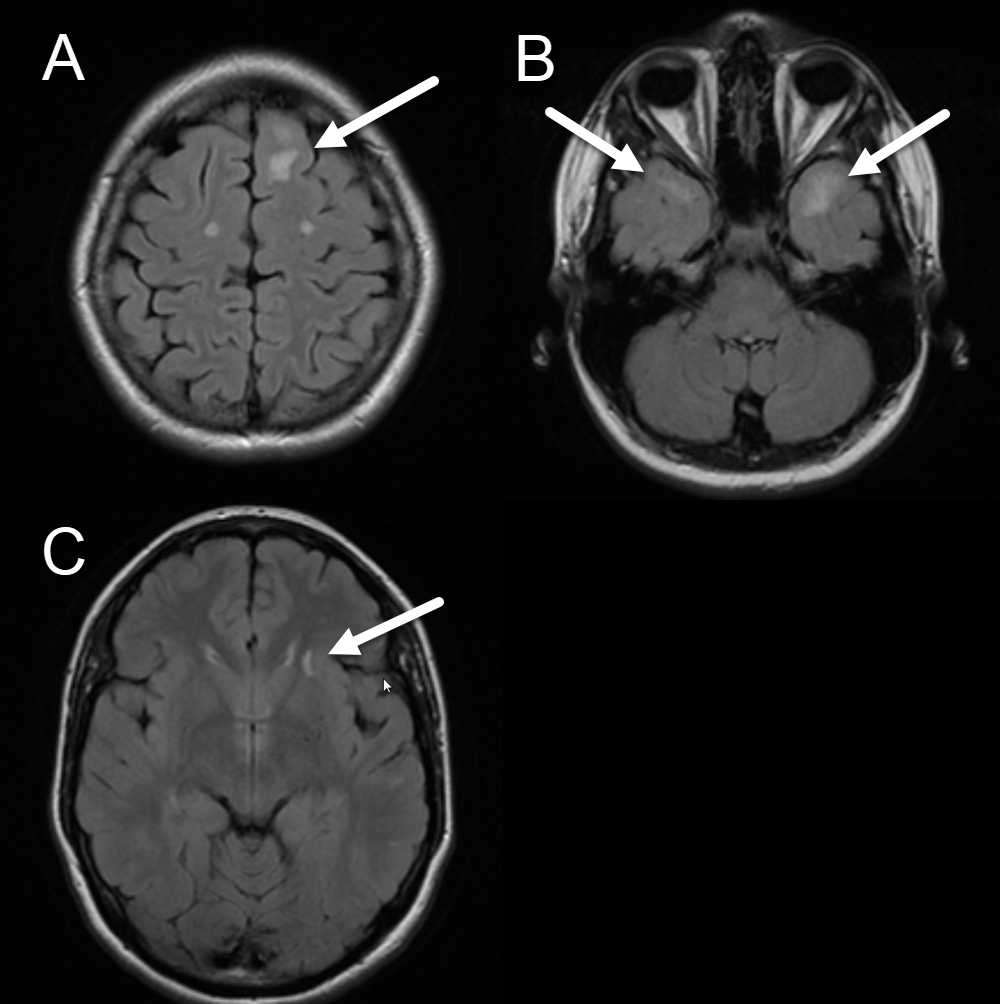
Axial FLAIR Image, Cerebral Autosomal Dominant Ateriopathy. The images demonstrate the characteristic appearance of cerebral autosomal dominant ateriopathy with subcortical infarcts and leukoencephalopathy. White arrows highlight white matter lesions in the paramedian superior frontal (A), anterior temporal (B), and external capsule (C), subcortical white matter.
Contributed by J Cramer
References
Locatelli M, Padovani A, Pezzini A. Pathophysiological Mechanisms and Potential Therapeutic Targets in Cerebral Autosomal Dominant Arteriopathy With Subcortical Infarcts and Leukoencephalopathy (CADASIL). Frontiers in pharmacology. 2020:11():321. doi: 10.3389/fphar.2020.00321. Epub 2020 Mar 13 [PubMed PMID: 32231578]
Yuan L, Chen X, Jankovic J, Deng H. CADASIL: A NOTCH3-associated cerebral small vessel disease. Journal of advanced research. 2024 Jan 2:():. pii: S2090-1232(24)00001-8. doi: 10.1016/j.jare.2024.01.001. Epub 2024 Jan 2 [PubMed PMID: 38176524]
Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cécillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis EA, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996 Oct 24:383(6602):707-10 [PubMed PMID: 8878478]
Level 3 (low-level) evidenceYoung KZ, Xu G, Keep SG, Borjigin J, Wang MM. Overlapping Protein Accumulation Profiles of CADASIL and CAA: Is There a Common Mechanism Driving Cerebral Small-Vessel Disease? The American journal of pathology. 2021 Nov:191(11):1871-1887. doi: 10.1016/j.ajpath.2020.11.015. Epub 2020 Dec 30 [PubMed PMID: 33387456]
Bianchi S, Zicari E, Carluccio A, Di Donato I, Pescini F, Nannucci S, Valenti R, Ragno M, Inzitari D, Pantoni L, Federico A, Dotti MT. CADASIL in central Italy: a retrospective clinical and genetic study in 229 patients. Journal of neurology. 2015 Jan:262(1):134-41. doi: 10.1007/s00415-014-7533-2. Epub 2014 Oct 26 [PubMed PMID: 25344745]
Level 2 (mid-level) evidenceDi Donato I, Bianchi S, De Stefano N, Dichgans M, Dotti MT, Duering M, Jouvent E, Korczyn AD, Lesnik-Oberstein SA, Malandrini A, Markus HS, Pantoni L, Penco S, Rufa A, Sinanović O, Stojanov D, Federico A. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) as a model of small vessel disease: update on clinical, diagnostic, and management aspects. BMC medicine. 2017 Feb 24:15(1):41. doi: 10.1186/s12916-017-0778-8. Epub 2017 Feb 24 [PubMed PMID: 28231783]
Takahashi K, Adachi K, Yoshizaki K, Kunimoto S, Kalaria RN, Watanabe A. Mutations in NOTCH3 cause the formation and retention of aggregates in the endoplasmic reticulum, leading to impaired cell proliferation. Human molecular genetics. 2010 Jan 1:19(1):79-89. doi: 10.1093/hmg/ddp468. Epub [PubMed PMID: 19825845]
Liem MK, Oberstein SA, van der Grond J, Ferrari MD, Haan J. CADASIL and migraine: A narrative review. Cephalalgia : an international journal of headache. 2010 Nov:30(11):1284-9 [PubMed PMID: 21038489]
Level 3 (low-level) evidenceNannucci S, Rinnoci V, Pracucci G, MacKinnon AD, Pescini F, Adib-Samii P, Bianchi S, Dotti MT, Federico A, Inzitari D, Markus HS, Pantoni L. Location, number and factors associated with cerebral microbleeds in an Italian-British cohort of CADASIL patients. PloS one. 2018:13(1):e0190878. doi: 10.1371/journal.pone.0190878. Epub 2018 Jan 25 [PubMed PMID: 29370179]
Lorenzi T, Ragno M, Paolinelli F, Castellucci C, Scarpelli M, Morroni M. CADASIL: Ultrastructural insights into the morphology of granular osmiophilic material. Brain and behavior. 2017 Mar:7(3):e00624. doi: 10.1002/brb3.624. Epub 2017 Feb 22 [PubMed PMID: 28293466]
Craggs LJ, Yamamoto Y, Deramecourt V, Kalaria RN. Microvascular pathology and morphometrics of sporadic and hereditary small vessel diseases of the brain. Brain pathology (Zurich, Switzerland). 2014 Sep:24(5):495-509. doi: 10.1111/bpa.12177. Epub [PubMed PMID: 25323665]
Drazyk AM, Tan RYY, Tay J, Traylor M, Das T, Markus HS. Encephalopathy in a Large Cohort of British Cerebral Autosomal Dominant Arteriopathy With Subcortical Infarcts and Leukoencephalopathy Patients. Stroke. 2019 Feb:50(2):283-290. doi: 10.1161/STROKEAHA.118.023661. Epub [PubMed PMID: 30636574]
Pescini F, Nannucci S, Bertaccini B, Salvadori E, Bianchi S, Ragno M, Sarti C, Valenti R, Zicari E, Moretti M, Chiti S, Stromillo ML, De Stefano N, Dotti MT, Federico A, Inzitari D, Pantoni L. The Cerebral Autosomal-Dominant Arteriopathy With Subcortical Infarcts and Leukoencephalopathy (CADASIL) Scale: a screening tool to select patients for NOTCH3 gene analysis. Stroke. 2012 Nov:43(11):2871-6. doi: 10.1161/STROKEAHA.112.665927. Epub 2012 Sep 20 [PubMed PMID: 22996955]
Peters N, Opherk C, Bergmann T, Castro M, Herzog J, Dichgans M. Spectrum of mutations in biopsy-proven CADASIL: implications for diagnostic strategies. Archives of neurology. 2005 Jul:62(7):1091-4 [PubMed PMID: 16009764]
Opherk C, Peters N, Herzog J, Luedtke R, Dichgans M. Long-term prognosis and causes of death in CADASIL: a retrospective study in 411 patients. Brain : a journal of neurology. 2004 Nov:127(Pt 11):2533-9 [PubMed PMID: 15364702]
Level 2 (mid-level) evidenceAnamnart C, Songsaeng D, Chanprasert S. A large number of cerebral microbleeds in CADASIL patients presenting with recurrent seizures: a case report. BMC neurology. 2019 May 30:19(1):106. doi: 10.1186/s12883-019-1342-2. Epub 2019 May 30 [PubMed PMID: 31146726]
Level 3 (low-level) evidenceJokumsen-Cabral A, Aires A, Ferreira S, Azevedo E, Castro P. Primary involvement of neurovascular coupling in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Journal of neurology. 2019 Jul:266(7):1782-1788. doi: 10.1007/s00415-019-09331-y. Epub 2019 Apr 26 [PubMed PMID: 31028544]
Su J, Wang M, Ban S, Wang L, Cheng X, Hua F, Tang Y, Zhou H, Zhai Y, Du X, Liu J. Relationship between changes in resting-state spontaneous brain activity and cognitive impairment in patients with CADASIL. The journal of headache and pain. 2019 Apr 17:20(1):36. doi: 10.1186/s10194-019-0982-3. Epub 2019 Apr 17 [PubMed PMID: 30995925]
Koizumi T, Mizuta I, Watanabe-Hosomi A, Mukai M, Hamano A, Matsuura J, Ohara T, Mizuno T. The CADASIL Scale-J, A Modified Scale to Prioritize Access to Genetic Testing for Japanese CADASIL-Suspected Patients. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2019 Jun:28(6):1431-1439. doi: 10.1016/j.jstrokecerebrovasdis.2019.03.026. Epub 2019 Apr 4 [PubMed PMID: 30956055]
Rutten JW, Dauwerse HG, Peters DJ, Goldfarb A, Venselaar H, Haffner C, van Ommen GJ, Aartsma-Rus AM, Lesnik Oberstein SA. Therapeutic NOTCH3 cysteine correction in CADASIL using exon skipping: in vitro proof of concept. Brain : a journal of neurology. 2016 Apr:139(Pt 4):1123-35. doi: 10.1093/brain/aww011. Epub 2016 Feb 19 [PubMed PMID: 26912635]
Level 2 (mid-level) evidenceGhezali L, Capone C, Baron-Menguy C, Ratelade J, Christensen S, Østergaard Pedersen L, Domenga-Denier V, Pedersen JT, Joutel A. Notch3(ECD) immunotherapy improves cerebrovascular responses in CADASIL mice. Annals of neurology. 2018 Aug:84(2):246-259. doi: 10.1002/ana.25284. Epub 2018 Aug 25 [PubMed PMID: 30014602]
Moreton FC, Razvi SS, Davidson R, Muir KW. Changing clinical patterns and increasing prevalence in CADASIL. Acta neurologica Scandinavica. 2014 Sep:130(3):197-203. doi: 10.1111/ane.12266. Epub 2014 May 19 [PubMed PMID: 24840674]