Introduction
Central retinal artery occlusion (CRAO) is an ophthalmic emergency that can lead to sudden and severe vision loss.[1] CRAO has been defined as interruption of blood flow through the central retinal artery by thromboembolism or vasospasm with or without retinal ischemia. An embolus from the carotid artery, aortic arch, or heart often causes the condition. Giant cell arteritis is another rare but serious cause of CRAO.[2] The condition is similar to a cerebral ischemic event and is associated with an increased risk of subsequent cerebral stroke and ischemic heart disease.[3][4][5][6] Unfortunately, no established therapies exist to improve visual outcomes in CRAO.[7] Therefore, management should focus on preventing further vascular events, such as cerebral ischemia and cardiovascular death.[8] Although visual prognosis is typically poor, early diagnosis and treatment of CRAO can restore visual acuity in some patients.[9][10]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
CRAO is classified into nonarteritic CRAO and arteritic CRAO according to the basic pathophysiology.[11] Nonarteritic CRAO comprises more than 90% of cases.[12] An embolism is the most common cause of nonarteritic CRAO.[3] Atherosclerosis of the carotid artery may be noted on the same side in around 70% of patients with CRAO or branch retinal arterial occlusion.[13][14] The emboli may be composed of solid, liquid, or gaseous material and are carried from a distant source through the blood flow in the arteries.[15] The 3 main types of emboli are cholesterol (Hollenhorst plaques), calcium, and platelet-fibrin.[16] The most common type of retinal embolus is composed of cholesterol, accounting for around 46% to 80% of all retinal emboli. Platelet-fibrin is the second most common type, accounting for 6% to 32%. Calcific embolus is less common, accounting for 6% to 16%.[17][18][19][20][21]
Cholesterol and platelet-fibrin emboli typically arise from atheromas in the carotid arteries, whereas calcium emboli often arise from cardiac valves.[22] On fundoscopy, calcium emboli appear white, cholesterol emboli, also known as Hollenhorst plaques, appear orange, and platelet-fibrin emboli appear dull white.[23] Retinal emboli may comprise other materials, such as talc; malignancy; fat, emboli resulting from long bone fractures; and infectious material commonly observed in patients with septicemia.
| Cholesterol Emboli (Hollenhorst Plaques) | Platelet-Fibrin Emboli | Calcific Emboli | |
| Color | Yellowish orange | Dull gray-white | Chalky white |
| Size | Small | It can be long, involving a segment of a retinal artery or arteriole | Large |
| Number | Multiple in up to 70% of cases | May be multiple | Typically single, may lie over or very near the optic disc |
| Appearance | Refractile, may be mobile | Smooth, may be mobile | May be irregular |
| Shape | Globular (or rectangular) | Concave meniscus at each end | Round or oval |
| Location | At the bifurcation of the retinal artery or arteriole | A segment of a vessel | At the first or second bifurcation |
| Usual source |
Commonly, internal or common carotid artery (sometimes from a brachiocephalic artery or aorta) |
The endothelial wall of an atherosclerotic vessel and heart (valves) | Calcified cardiac valves or an atheromatous plaque |
In-situ thrombosis may also cause CRAO. Thrombi may arise from various disorders, including atherosclerotic diseases, collagen-vascular disease, inflammatory states, and hypercoagulable states.[24] Predisposing conditions of CRAO include but are not limited to polycythemia vera, sickle cell anemia, multiple myeloma, factor V Leiden, prothrombin III mutation, hyperhomocysteinemia, activated protein C resistance, Behçet disease, pyoderma gangrenosum, and syphilis.[10][25] Cosmetic facial injections or fillers can cause CRAO if the agent is inadvertently injected inside an artery communicating with the central retinal artery.[26]
The causes of arteritic CRAO include giant cell arteritis, systemic lupus erythematosus, polyarteritis nodosa, antiphospholipid syndrome, granulomatosis with polyangiitis, and Takayasu arteritis.[27] Rapid differentiation between thromboembolic and arteritic processes is essential as optimal therapy differs, and the rapid administration of steroids for vasculitic causes of CRAO is associated with improved outcomes. Typically, this is completed by checking for inflammatory markers, including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), which are raised in giant cell arteritis.[28]
Hayreh and Zimmerman classified CRAO into 4 groups as follows:
- Nonarteritic CRAO (66%) is caused by a permanent occlusion of the central retinal artery due to an embolus at the narrowest part of its course at its entry into the optic sheath.
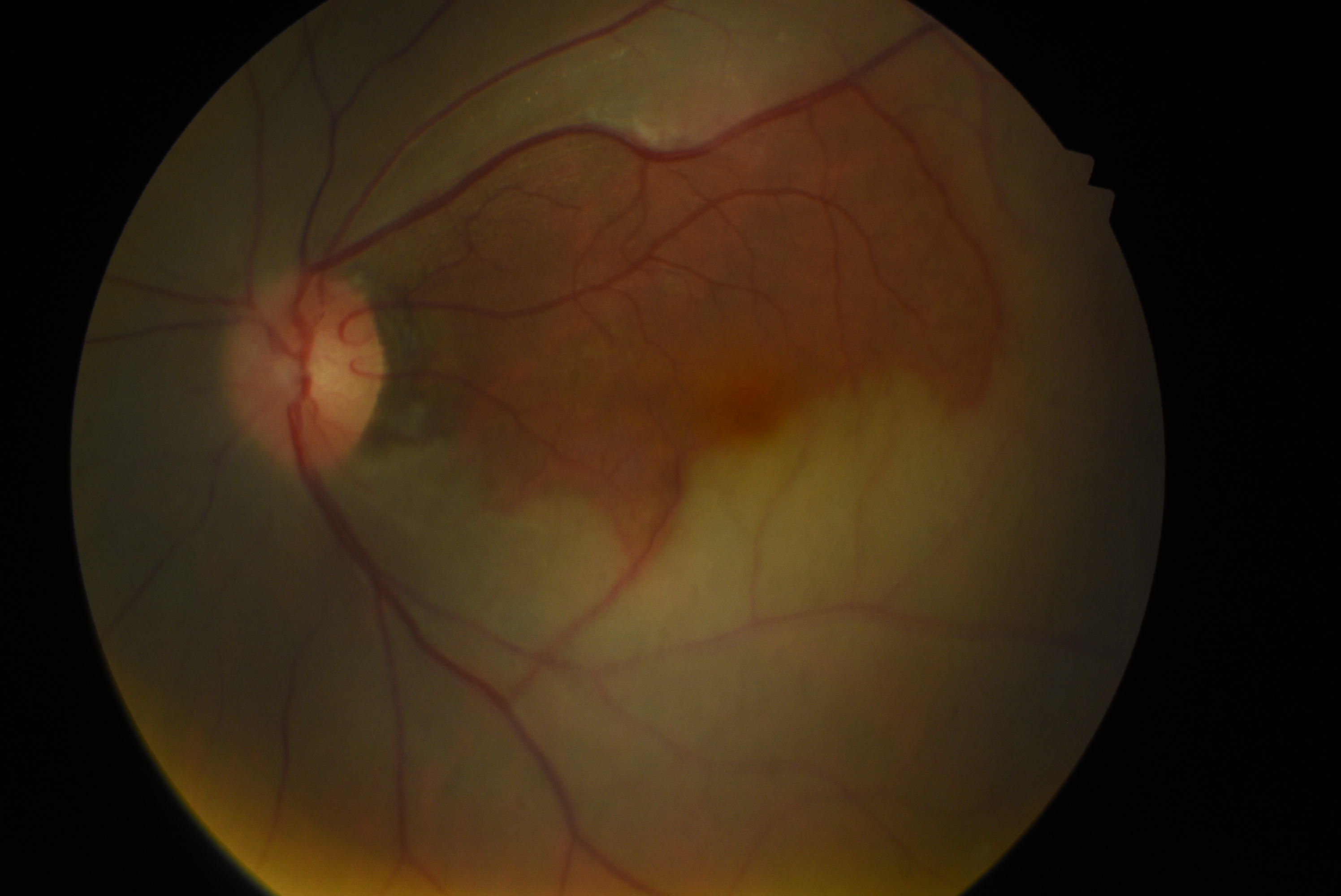
- Nonarteritic CRAO with cilioretinal artery sparing (13%).
- Transient nonarteritic CRAO (16%) is a temporary blockage of the central retinal artery lasting from minutes to hours. This transient occlusion can occur due to various factors, including a migrating embolus, increased intraocular pressure, or a transient severe fall of perfusion pressure of the central retinal artery.
- Arteritic CRAO (5%) is caused by the occlusion of the common trunk of the central retinal artery and the posterior ciliary artery, typically due to giant cell arteritis.[2][29]
Epidemiology
The incidence of CRAO is approximately 1 to 1.9 per 100,000 people in the United States, with less than 2% presenting with bilateral involvement.[3][8] The age-standardized incidence of CRAO is 2.53 per 100,000 person-year in Japan, 2.7 per 100,000 person-year in Germany, and 1.8 per 100,000 person-year in Korea.[30][31] The incidence increases with age, with around 10 per 100,000 person-year in adults aged 80 or older.[32] The reported incidence and mean age at presentation may vary according to geographical location, but it typically occurs in individuals aged 60 to 70.[33] However, CRAO can also occur in the pediatric age group related to non-atherosclerotic causes, including trauma, embolism from calcified cardiac valves, and infection.[34] Men have a slightly higher incidence compared to women.[35] Patients diagnosed with CRAO have a life expectancy of 5.5 years compared to 15.4 years for age-matched patients with non-CRAO.[36][37] Risk factors for CRAO are similar to those of other thromboembolic diseases and include hypertension; smoking; hyperlipidemia; diabetes; high body mass index (BMI); dyslipidemia; atrial fibrillation; hypercoagulable states; cardiac disease, including rheumatic heart disease and other cardiac valve diseases; and male gender.[10][25] Approximately 37% to 40% of patients with CRAO have clinically significant ipsilateral carotid artery stenosis.[38][39][40]
Pathophysiology
In the clinical setting where occlusion may be incomplete, the return of vision may be achieved after delays of 8 to 24 hours. Up to 25% of the population receives significant macular collateral circulation from the cilioretinal artery.[41] Patients with this anatomical variant typically have less severe presentations and better long-term prognoses.
Retinal ganglion cell infarction takes an indeterminate amount of time following total CRAO. However, some experts speculate that the process might be less than 15 minutes. In a study by Hayreh and colleagues, the central retinal artery was clamped in rhesus monkeys with hypertension and atherosclerosis aged 38. The monkeys were evaluated using fundus photo, fluorescein angiography, visual evoked potential, and electroretinography. This study found that the retina did not suffer detectable damage in histopathology, electrophysiology, and morphometric analysis in CRAO of 97 minutes in this experimental model. However, after this critical time, irreversible damage increased as the time of CRAO increased.[42] This experimental model may not be extrapolated directly for CRAO in humans.[43][44] The function of the ischemic brain stops within seconds, and tissue necrosis can occur in the brain within 5 minutes of ceasing the supply of oxygen and glucose.[45] Retinal ganglion cells are also recognized as part of the brain tissue.[46] Thus, the exact time for infarction of the retina after CRAO may need further evaluation. However, incomplete or intermittent forms of CRAO exist that can have a satisfactory visual outcome after prompt diagnosis and therapy.
The retina receives blood from 2 vascular systems. The central retinal artery supplies the inner retina, and choriocapillaris supplies the retinal pigment epithelium and outer retina, typically starting from the outer plexiform layer.[47] Short posterior ciliary arteries supply choriocapillaris. The watershed zone of the 2 systems may vary in individuals due to differences in retinal thickness, light exposure, and specific retinal locations. When the central retinal artery is occluded, the inner retina, specifically the nerve fiber and ganglion cell layer, becomes ischemic and loses transparency.[2]
Histopathology
The central retinal artery is the first intraorbital branch of the ophthalmic artery.[48] The artery enters the optic nerve 1 cm posterior to the globe and supplies blood to the retina.[49] The CRAO results in inner retinal ischemia, vision loss, and eventual necrosis. Acute CRAO results in retinal edema and pyknosis of the ganglion cell nuclei. As ischemia progresses, the retina becomes opacified and takes on a yellow-white appearance.
History and Physical
CRAO typically presents with sudden, painless, monocular vision and visual field loss that occurs over seconds. Patients may report an antecedent transient visual loss, also known as amaurosis fugax, and a history of atherosclerotic disease.[50] In patients with no light perception, ophthalmic artery occlusion, abnormalities in the optic nerve, and occluded short posterior ciliary arteries should be excluded.[51]
Patients with CRAO often present with severe monocular vision loss and an afferent pupillary defect, also known as Marcus Gunn pupil.[52] The relative afferent pupillary defect may not be noticeable if the fellow eye has a damaged retina or optic nerve due to preexisting diseases, including glaucoma. The visual acuity can vary from loss of light perception to finger counting. According to Hayreh and Zimmerman, approximately 75% of patients with CRAO present with vision of finger counting or worse.[29] Occasionally, some patients have visual acuity better than 20/40. The causes of good visual acuity in CRAO may include collateral circulation, sparing of fovea due to patent cilioretinal artery, peripheral retinal arterial occlusion, retrograde blood flow through the central retinal artery, paracentral acute middle maculopathy, individual variability, and incomplete or partial CRAO.[53][54] The evolution of CRAO may progress from mild retinal whitening with hyperreflectivity of the inner nuclear layer, which is only visible on optical coherence tomography (OCT) before the full-fledged fundus appearance is observed.[55] Intraocular pressures, anterior chamber examinations, and extraocular eye movements are within normal limits.
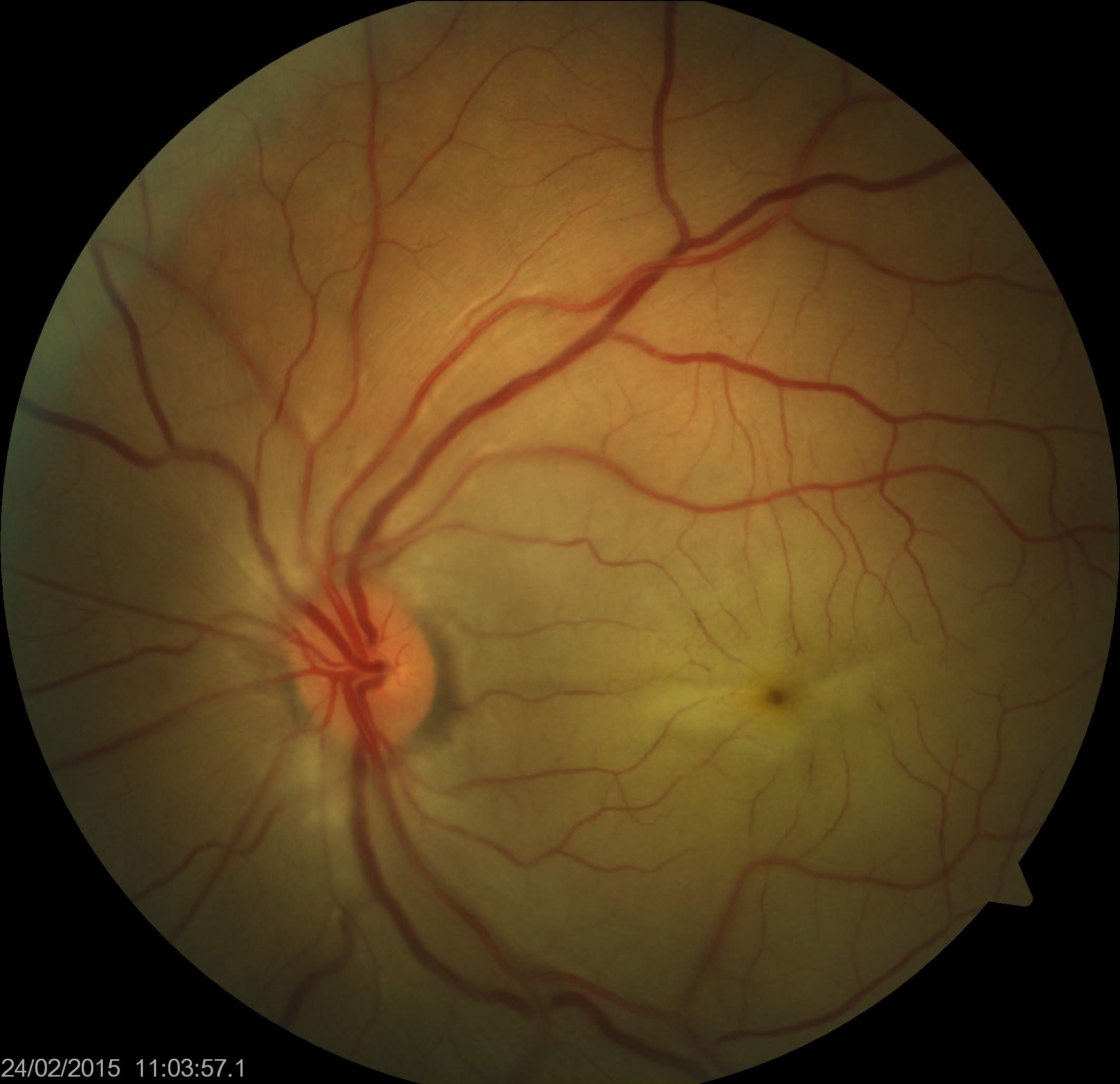
A thorough fundoscopic examination is crucial for accurate diagnosis of CRAO, and a dilated examination should be performed on any patient without contradictions to mydriatic medications who presents with symptoms suggestive of CRAO. The patient may have a normal-looking fundus on a cursory examination. The typical white retina and cherry red spot may take hours to be present on ophthalmoscopy. However, inner retinal hyperreflectivity may be observed on OCT in the early stages of CRAO.[56] Severe sudden onset vision loss and relative afferent pupillary defect in the eye are other indicators. The retina appears diffusely pale on fundoscopy with a cherry red macular spot.[9] The whitening of the retina results from ischemia of the nerve fiber layer around the fovea, which becomes opaque and swollen. A cherry red spot at the macula results from the preserved choroidal circulation (perfused and red vascular choroid) visible beneath the thin fovea with a surrounding white retina that blocks the view of the vascular choroid. The retinal whitening may be more appreciable when comparing the fundus picture of the involved eye with the fellow normal eye. Some cases of CRAO may not show a cherry red spot at the macula.[57]
Narrowing, boxcarring, also known as cattle-tracking, or slow segmental blood flow in the arterioles or venules may also be observed (see Image. Central Retinal Arterial Occlusion). The blood flow through the retinal artery is slow and can be visualized by the movement of the segments of blood during the slit-lamp biomicroscopic examination using a 90D lens. The slow blood flow through the arterioles is also prominently observed during fluorescein angiography. In this procedure, the dye filling of the arteriole progresses slowly. This observation is better appreciated by comparing early images of the involved eye taken in rapid succession or using video angiography.[58] Retinal arteriolar caliber may be irregularly narrowed. Cilioretinal arteries arising from short posterior ciliary arteries are present in around 15% to 25% of patients, and these typically supply the fovea. Thus, in CRAO with the patent cilioretinal artery (cilioretinal artery sparing), the central vision may be maintained. However, the visual field is severely reduced (see Image. Central Retinal Artery Occlusion with Cilioretinal Artery Sparing). Typically, no retinal hemorrhage of retinal venous dilation and tortuosity occurs, as noted in central retinal venous occlusion. Multiple cotton wool spots characterize transient nonarteritic CRAO and may appear similar to Purtscher retinopathy.[59]
In up to 40% of cases, arteriolar emboli may be visualized.[60] Other conditions, such as commotio retinae, Tay-Sachs disease, and Niemann-Pick disease, may present with a cherry red spot on fundoscopic examinations but can be easily differentiated based on clinical presentation.[61][62] Arteritic CRAO almost always has a pale edematous disc suggestive of arteritic anterior ischemic optic neuropathy with characteristics of CRAO.[63][64][65] Arteritic CRAO should be suspected in older patients with a history of jaw claudication, polymyalgia rheumatica, and scalp tenderness. Ipsilateral carotid artery disease may cause a contralateral motor or sensory deficit.[66] The carotid pulse should be checked.
Evaluation
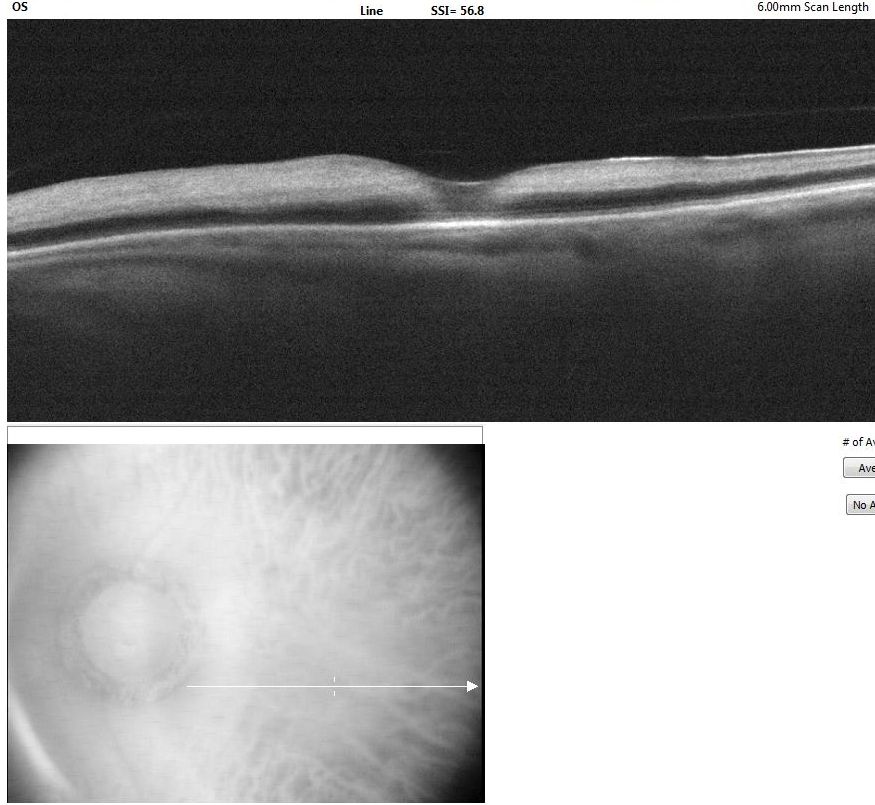
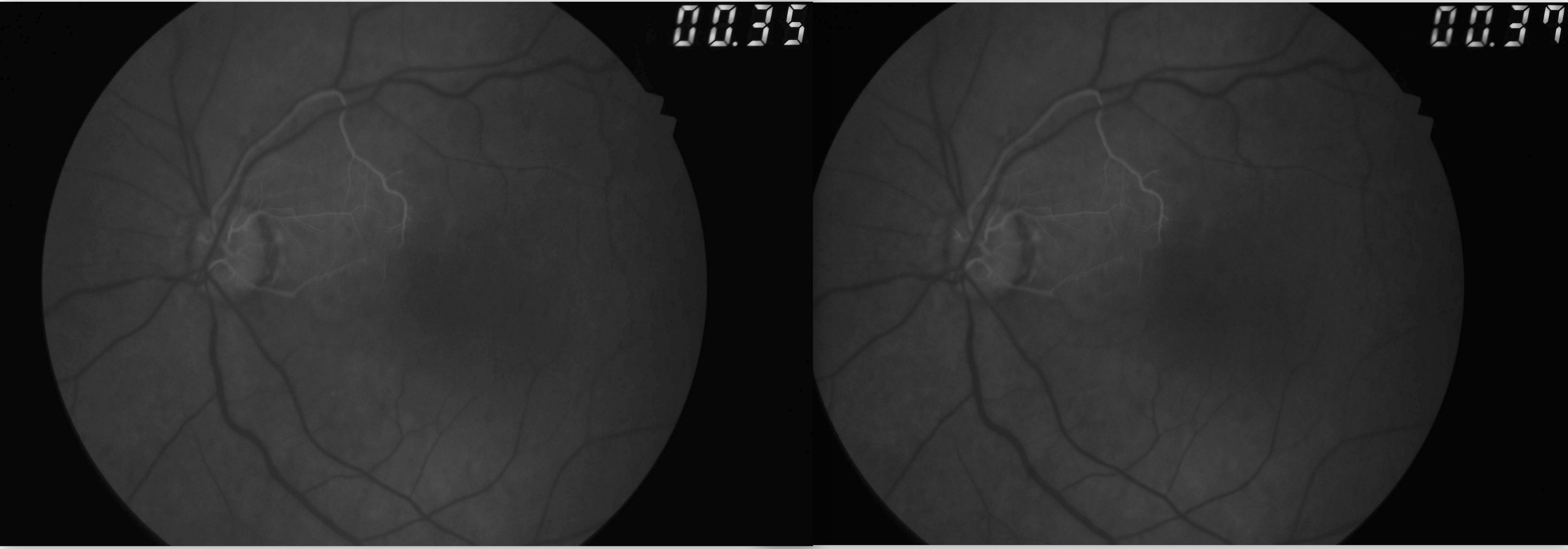
CRAO is analogous to a cerebral vascular accident involving the retina. As such, the workup for CRAO closely parallels the workup for stroke or transient ischemic attacks.[12] The ophthalmic workup includes a photo of the color fundus and an OCT of the macula. The color fundus photograph typically reveals retinal whitening in the involved eye compared to the contralateral eye. Cherry red spots at the macula may or may not be visible. The OCT of the macula shows inner retinal hyperreflectivity in the acute phase (see Image. Optical Coherence Tomography of Macula in Central Retinal Artery Occlusion). Some cases of CRAO may have paracentral acute middle maculopathy characterized by hyperreflectivity of the inner nuclear layer.[67] Over time, the thinning of the inner retina becomes apparent. A fundus fluorescein angiogram (FFA) is not required for the diagnosis. However, the FFA shows a slow progression of the dye in the arterioles (see Image. Fundus Fluorescein Angiogram in Central Retinal Artery Occlusion). The choroid fills normally in nonarteritic CRAO. An absent or late filling of the retinal arterioles may occur. The choroidal phase may show hypoperfusion in arteritic CRAO or ophthalmic artery occlusion. In some cases of CRAO, the FFA may be normal.[58][68]
Initial blood work should include point-of-care glucose, a complete blood count with differential, and coagulation assays, including prothrombin time (PT), international normalized ratio (INR), and activated partial thromboplastin time (PTT). An ESR and CRP should be obtained to rule out giant cell arteritis. If inflammatory markers are elevated, and the patient's history and physical examination findings are consistent with giant cell arteritis, high-dose intravenous (IV) steroids should be initiated immediately.[69] Temporal biopsy should be planned in such cases within 2 weeks of the initiation of steroid therapy.[70][71]
If symptom onset occurs within a window of less than 4.5 hours, a computed tomography (CT) head without contrast should be obtained to rule out intracranial hemorrhage and assess the patient's eligibility for thrombolytic therapy.[8] Other tests should be considered based on individual risk factors and medical history. These tests include hemoglobin A1c (glycated hemoglobin), lipid profile, Rh factor (rheumatoid factor), antinuclear antibody, fluorescent treponemal antibody absorption test, hypercoagulability labs, carotid artery imaging, electrocardiogram, echocardiogram, and outpatient Holter monitoring.[72] Carotid artery imaging is a crucial part of systemic evaluation as the most common source of embolus. The imaging modalities include carotid duplex ultrasound, CT, magnetic resonance, and digital subtraction angiography. If carotid imaging is normal, cardiac evaluation should include modalities such as echocardiography. Patients with CRAO require interprofessional collaboration among ophthalmologists, cardiologists, primary care clinicians or internists, and neurologists.
Treatment / Management
The visual prognosis of CRAO remains poor despite treatment, and ongoing research aims to identify evidence-based, effective, and safe therapy. No consensus is available regarding the optimal treatment for CRAO, although early IV thrombolytic administration shows promise.[73] Time is crucial in management, and treatment should be started before the diagnosis is confirmed. Proposed therapies are aimed at restoring retinal perfusion or oxygenation. Basic strategies include vasodilation, causing ocular hypotony so that the thrombus or embolus dislodges, increasing oxygen tension, surgical removal of the clot, or thrombolysis. Lifestyle modification and control of systemic factors are essential for secondary prevention.(A1)
Various therapies have been tried with variable success:
- Immediate ocular massage: Ocular massage may induce oscillations of intraocular pressure and dislodge the offending thrombus. The patient is seated on a slit-lamp comfortably. Pressure is applied on the globe with a 3-mirror Goldmann lens for 10 seconds until retinal arterial pulsations are visible. If the pulsations are not visible, cessation of arterial flow should be obtained, followed by the release of pressure for 5 seconds. In the absence of a slit-lamp or Goldmann lens, digital ocular massage may be tried. However, ocular massage is not a standard treatment for CRAO. Research on the effectiveness is limited and inconclusive, although described since the 1880s.
- Drugs to reduce intraocular pressure: IV acetazolamide 500 mg, mannitol (1.5 to 2 g/kg over 30 to 60 min), and topical timolol (0.5%, 1 drop twice daily) may be started to reduce intraocular pressure, which can increase the perfusion pressure (mean arterial pressure or intraocular pressure).
- Anterior chamber paracentesis: Paracentesis is often recommended in conjunction with or after failed digital ocular massage. A 26- or 27-gauge needle is inserted into the anterior chamber anteriorly to the limbus, and around 0.1 to 0.2 mL of aqueous liquid is drained. This action is expected to cause sudden ocular hypotony and possible dilation of retinal arteries. However, anterior chamber paracentesis caused a maximum 20% rise in retinal arterial flow in animal studies. Complications of this procedure include hyphema and endophthalmitis. A study on 74 patients with CRAO found no added benefit of anterior chamber paracentesis compared to conservative treatment. The authors recommended against use in CRAO, considering the risks of ocular injury and infection.[74][75][76] (B3)
- Carbogen: A mixture of 95% oxygen and 5% carbon dioxide inhalation is thought to cause vasodilation and increased oxygen supply to the retina. Carbogen is given 10 minutes per 1 hour during waking and 10 minutes per 4 hours during sleep. The therapy is continued for 2 to 3 days. A study involving 89 cases of patients with acute CRAO failed to show the benefit of combined anterior chamber paracentesis with carbogen inhalation compared to no interventions.[77]
- Hyperventilation into a paper bag: Respiratory acidosis and vasodilation are induced by either hyperventilation into a paper bag or by inhaling 10% carbon dioxide.
- Pentoxifylline: The recommended dosage for this hemorheological agent is three 600 mg tablets daily. The hemorheological agent works by increasing erythrocyte flexibility, reducing blood viscosity, and improving perfusion. The medication has been tested in patients with CRAO and shown to increase retinal flow, although studies evaluating this drug have multiple limitations.[78][79] (A1)
- Sublingual isosorbide dinitrate: 10 mg of isosorbide dinitrate causes vasodilation and has been utilized in managing CRAO, although comprehensive studies on its efficacy are currently insufficient.[80] (A1)
- Supplemental oxygen
- IV methylprednisolone: The mainstay of therapy to manage arteritic CRAO caused by giant cell arteritis is 1 g/d for 1 to 3 days. This dose reduces inflammatory causes and retinal edema.
- IV thrombolysis (fibrinolysis): The most commonly used therapy is the IV infusion of alteplase, also known as tissue plasminogen activator, 0.9 mg/kg. The initial 10% dose is delivered rapidly in 1 minute, and the rest is delivered through slow infusion for 59 minutes. Around 5.8% of patients with CRAO in the United States receive tissue plasminogen activator. A patient-level meta-analysis found that thrombolysis was more beneficial compared to the natural history group when administered within 4.5 hours of onset. Larger randomized controlled trials are necessary to further evaluate the role of IV fibrinolysis in treating patients with CRAO. The American Heart Association recommends considering IV thrombolysis in nonarteritic CRAO within 4.5 hours without any systemic contraindications of IV thrombolysis.[81][82] (A1)
- Intra-arterial thrombolysis: In the EAGLE study, a catheter was super selectively inserted into the ophthalmic artery, and a maximum of 50 mg of recombinant tissue plasminogen activator was injected. This study was prematurely stopped due to safety concerns as intra-arterial thrombolysis had similar efficacy but higher adverse events compared to conservative standard treatment. Adverse events of this procedure include dislodgment of atheromatous plaque, arterial dissection, and spasms. The American Heart Association recommends considering intra-arterial thrombolysis in centers with this facility for patients who are not candidates for IV thrombolysis and who have presented within 6 hours.[8][39] (A1)
- Hyperbaric oxygen therapy: This therapy aims to increase blood oxygen tension. Typically, 2 to 2.5 atm is administered for 90 minutes within 8 hours of symptom onset.[1][83]
- Neodymium:yttrium-aluminum-garnet laser embolectomy: The neodymium:yttrium-aluminum-garnet laser (1064 nm) is used to lyse the embolus applying power up to 2 mJ. This procedure is performed using a fundus contact lens such as Goldmann 3-mirror or Volk Quadraspheric lens. A meta-analysis noted that although the procedure was associated with visual recovery in most cases, vitreous hemorrhage was frequent. Higher pulse energy may be associated with more vitrectomies and cause subretinal or vitreous hemorrhage.[84][85] (A1)
Differential Diagnosis
The differential diagnosis of CRAO includes several conditions.
- Ophthalmic artery occlusion: Ophthalmic artery occlusion is characterized by incomplete or complete occlusion of the ophthalmic artery, resulting in severe ischemia or infarction of the globe and surrounding structures. Visual acuity typically means no perception of light, and the cherry red spot at the macula may not be visible due to choroidal ischemia.
- Paracentral acute middle maculopathy: Paracentral acute middle maculopathy is characterized by hyperreflectivity of the inner nuclear layer on the OCT macula. Some cases with aracentral acute middle maculopathy may progress to CRAO, and this may denote a spectrum of ischemic disorders.
- Purtscher retinopathy: Purtscher flecken may simulate cotton wool spots observed in transient nonarteritic CRAO.
- Hypertensive retinopathy: The retinopathy is bilateral with arteriovenous changes, papilledema, cotton wool spots around the optic disc, and subretinal fluid around the disc extending up to the nasal side of the fovea. The blood pressure is raised severely.
- Differential diagnoses of a cherry red spot at the macula include lysosomal storage disorders, commotion retinae, retinitis around the optic disc, and adverse relations of drugs.[9][59]
Prognosis
The visual prognosis of CRAO remains poor despite the recent advances in the medical field. Less than 10% to 20% of patients with CRAO regain functional vision.[82][89] A large study involving 130 patients with CRAO showed that visual improvement was most notable in cases of transient nonarteritic CRAO, with approximately 82% of affected eyes showing improvement from baseline vision of finger counting or worse. Around 67% of eyes with nonarteritic CRAO with patent cilioretinal artery had experienced visual gain compared to 22% with nonarteritic CRAO. The vision typically improved within the first 7 days.[29]
CRAO has been included in the definition of acute ischemic stroke, which encompasses an episode of neurological dysfunction caused by focal cerebral, spinal, or retinal infarction, as outlined by the American Heart Association.[8] Similar to acute ischemic stroke, CRAO is associated with an increased risk of recurrent vascular events.[90][91] Retinal arterial occlusion may result in higher mortality rates compared to the general population.[92]
Complications
Patients with CRAO eventually develop inner retinal thinning and optic atrophy. Around 20% of cases develop neovascularization of the iris, typically after 30 to 60 days, although this may occur within 1 week. Such cases need panretinal photocoagulation with or without intravitreal anti-vascular endothelial growth factor (anti-VEGF agents) injection.[93] The chances of the other eye involvement are high with arteritic CRAO in giant cells, and arteritis and IV high-dose steroids may prevent the involvement of the other eye.[94] As mentioned, the risk of arterial thromboembolic events and mortality may be high after CRAO, and patients should be informed.
Deterrence and Patient Education
Patients should be aware of the signs and symptoms of CRAO, such as sudden, painless vision loss. The urgency of seeking immediate medical attention in the event of sudden vision loss should be emphasized, along with the recommendation to regularly check their vision by closing alternate eyes to detect any sudden visual decline. Time is critical in preserving vision, and delays may result in irreversible damage. CRAO is a medical emergency, and although no therapy is effective in such cases, early diagnosis and prompt initiation of available treatment may improve visual outcomes.
Common risk factors for CRAO, such as hypertension, diabetes, cardiovascular disease, and hyperlipidemia, should be discussed. Patients should be encouraged to manage and control these risk factors through lifestyle modifications, including avoidance of smoking, and medications. Patients should be advised about the necessity of undergoing a thorough examination by healthcare professionals, including eye specialists and possibly vascular specialists, to determine the underlying cause of CRAO and guide appropriate treatment decisions. The uncertain prognosis for visual recovery in CRAO should be discussed. Although some individuals may experience partial or complete recovery, most of the patients (around 80%) may have permanent vision loss.
The outcome depends on factors such as the extent of ischemia and the effectiveness of interventions. Follow-up with eye care professionals and other relevant specialists should be emphasized. Regular monitoring is essential to assess the patient's visual function, manage risk factors, and address potential complications. The emotional impact of sudden vision loss should be acknowledged, and information should be provided about available support services, including counseling or support groups, to help patients cope with the psychological aspects of the condition. To reduce the risk of vascular events, the patients should be educated on lifestyle modifications, such as maintaining a healthy diet, exercising regularly, managing blood pressure and cholesterol levels, and refraining from smoking.[95]
Enhancing Healthcare Team Outcomes
Healthcare professionals should promptly refer patients exhibiting signs of CRAO to an ophthalmologist. CRAO is the sudden blockage of the central retinal artery, resulting in retinal hypoperfusion, rapidly progressive cellular damage, and vision loss. Retina survival depends on the degree of collateralization and the duration of retinal ischemia. Prompt diagnosis and early treatment to dislodge or lyse the offending embolus or thrombus is crucial to avoid irreversible retinal damage and blindness.
The prognosis for these patients depends on the timing of diagnosis and initiation of treatment. Individuals treated promptly often regain their sight, but delay can cause some degree of vision loss and, in some cases, permanent blindness. The team treating a patient with CRAO may include ophthalmologists, cardiologists, primary care physicians or internists, neurologists, nurses, and pharmacists. Ophthalmologists help confirm the diagnosis and follow up on the ocular condition. The cardiologists perform a detailed search for the source of the embolus and ensure optimal cardiovascular health. The internists or the primary care clinicians refer to the ophthalmologists urgently without delay and in following up on the patient's systemic condition. Neurologists help exclude cerebrovascular accidents and follow up on such cases. The nursing staff monitors the patient. Pharmacists help patients with medication management. Effective communication and collaboration among team members facilitate a swift response, improving patient care. This holistic approach ensures that the patient receives prompt management, leading to a potentially better outcome that includes preserving vision.[96]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Cugati S, Varma DD, Chen CS, Lee AW. Treatment options for central retinal artery occlusion. Current treatment options in neurology. 2013 Feb:15(1):63-77. doi: 10.1007/s11940-012-0202-9. Epub [PubMed PMID: 23070637]
Hayreh SS. Central retinal artery occlusion. Indian journal of ophthalmology. 2018 Dec:66(12):1684-1694. doi: 10.4103/ijo.IJO_1446_18. Epub [PubMed PMID: 30451166]
Varma DD, Cugati S, Lee AW, Chen CS. A review of central retinal artery occlusion: clinical presentation and management. Eye (London, England). 2013 Jun:27(6):688-97. doi: 10.1038/eye.2013.25. Epub 2013 Mar 8 [PubMed PMID: 23470793]
Avery MB, Magal I, Kherani A, Mitha AP. Risk of Stroke in Patients With Ocular Arterial Occlusive Disorders: A Retrospective Canadian Study. Journal of the American Heart Association. 2019 Feb 5:8(3):e010509. doi: 10.1161/JAHA.118.010509. Epub [PubMed PMID: 30712440]
Level 2 (mid-level) evidenceMir TA, Arham AZ, Fang W, Alqahtani F, Alkhouli M, Gallo J, Hinkle DM. Acute Vascular Ischemic Events in Patients With Central Retinal Artery Occlusion in the United States: A Nationwide Study 2003-2014. American journal of ophthalmology. 2019 Apr:200():179-186. doi: 10.1016/j.ajo.2019.01.009. Epub 2019 Jan 26 [PubMed PMID: 30689989]
Zhou Y, Zhu W, Wang C. Relationship between retinal vascular occlusions and incident cerebrovascular diseases: A systematic review and meta-analysis. Medicine. 2016 Jun:95(26):e4075. doi: 10.1097/MD.0000000000004075. Epub [PubMed PMID: 27368050]
Level 1 (high-level) evidenceDattilo M, Biousse V, Newman NJ. Update on the Management of Central Retinal Artery Occlusion. Neurologic clinics. 2017 Feb:35(1):83-100. doi: 10.1016/j.ncl.2016.08.013. Epub [PubMed PMID: 27886897]
Mac Grory B, Schrag M, Biousse V, Furie KL, Gerhard-Herman M, Lavin PJ, Sobrin L, Tjoumakaris SI, Weyand CM, Yaghi S, American Heart Association Stroke Council; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Hypertension; and Council on Peripheral Vascular Disease. Management of Central Retinal Artery Occlusion: A Scientific Statement From the American Heart Association. Stroke. 2021 Jun:52(6):e282-e294. doi: 10.1161/STR.0000000000000366. Epub 2021 Mar 8 [PubMed PMID: 33677974]
Tripathy K, Patel BC. Cherry Red Spot. StatPearls. 2024 Jan:(): [PubMed PMID: 30969663]
Tripathy K, Mazumdar S, Sarma B. Central retinal arterial occlusion in a patient with pyoderma gangrenosum. Indian journal of ophthalmology. 2018 Jul:66(7):1019-1021. doi: 10.4103/ijo.IJO_1229_17. Epub [PubMed PMID: 29941761]
Liu W, Bai D, Kou L. Progress in central retinal artery occlusion: a narrative review. The Journal of international medical research. 2023 Sep:51(9):3000605231198388. doi: 10.1177/03000605231198388. Epub [PubMed PMID: 37712755]
Level 3 (low-level) evidenceMadike R, Cugati S, Chen C. A review of the management of central retinal artery occlusion. Taiwan journal of ophthalmology. 2022 Jul-Sep:12(3):273-281. doi: 10.4103/2211-5056.353126. Epub 2022 Aug 18 [PubMed PMID: 36248088]
Babikian V, Wijman CA, Koleini B, Malik SN, Goyal N, Matjucha IC. Retinal ischemia and embolism. Etiologies and outcomes based on a prospective study. Cerebrovascular diseases (Basel, Switzerland). 2001 Aug:12(2):108-13 [PubMed PMID: 11490104]
Ahuja RM, Chaturvedi S, Eliott D, Joshi N, Puklin JE, Abrams GW. Mechanisms of retinal arterial occlusive disease in African American and Caucasian patients. Stroke. 1999 Aug:30(8):1506-9 [PubMed PMID: 10436091]
Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, Ying GS. Retinal and Ophthalmic Artery Occlusions Preferred Practice Pattern®. Ophthalmology. 2020 Feb:127(2):P259-P287. doi: 10.1016/j.ophtha.2019.09.028. Epub 2019 Sep 25 [PubMed PMID: 31757501]
Kaufman EJ, Mahabadi N, Munakomi S, Patel BC. Hollenhorst Plaque. StatPearls. 2024 Jan:(): [PubMed PMID: 29261979]
Cheung N, Lim L, Wang JJ, Islam FM, Mitchell P, Saw SM, Aung T, Wong TY. Prevalence and risk factors of retinal arteriolar emboli: the Singapore Malay Eye Study. American journal of ophthalmology. 2008 Oct:146(4):620-4. doi: 10.1016/j.ajo.2008.05.033. Epub 2008 Jul 21 [PubMed PMID: 18639861]
O'Donnell BA, Mitchell P. The clinical features and associations of retinal emboli. Australian and New Zealand journal of ophthalmology. 1992 Feb:20(1):11-7 [PubMed PMID: 1599661]
Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of retinal emboli in an older population. Stroke. 2006 Mar:37(3):908-10 [PubMed PMID: 16439697]
Mitchell P, Wang JJ, Li W, Leeder SR, Smith W. Prevalence of asymptomatic retinal emboli in an Australian urban community. Stroke. 1997 Jan:28(1):63-6 [PubMed PMID: 8996490]
Hadley G, Earnshaw JJ, Stratton I, Sykes J, Scanlon PH. A potential pathway for managing diabetic patients with arterial emboli detected by retinal screening. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2011 Aug:42(2):153-7. doi: 10.1016/j.ejvs.2011.04.031. Epub 2011 May 25 [PubMed PMID: 21616692]
Ramakrishna G, Malouf JF, Younge BR, Connolly HM, Miller FA. Calcific retinal embolism as an indicator of severe unrecognised cardiovascular disease. Heart (British Cardiac Society). 2005 Sep:91(9):1154-7 [PubMed PMID: 16103545]
Cho KH, Ahn SJ, Cho JH, Jung C, Han MK, Park SJ, Park KH, Woo SJ. The Characteristics of Retinal Emboli and its Association With Vascular Reperfusion in Retinal Artery Occlusion. Investigative ophthalmology & visual science. 2016 Sep 1:57(11):4589-98. doi: 10.1167/iovs.16-19887. Epub [PubMed PMID: 27598864]
Ashorobi D, Ameer MA, Fernandez R. Thrombosis. StatPearls. 2024 Jan:(): [PubMed PMID: 30860701]
Terao R, Fujino R, Ahmed T. Risk Factors and Treatment Strategy for Retinal Vascular Occlusive Diseases. Journal of clinical medicine. 2022 Oct 27:11(21):. doi: 10.3390/jcm11216340. Epub 2022 Oct 27 [PubMed PMID: 36362567]
Asensio-Sánchez VM. Central retinal artery occlusion following facial injection of hyaluronic acid. Archivos de la Sociedad Espanola de Oftalmologia. 2023 Jul:98(7):410-412. doi: 10.1016/j.oftale.2023.05.008. Epub 2023 May 27 [PubMed PMID: 37247664]
Gupta V, Luthra S, Shrinkhal N, Sinha S. Takayasu's arteritis: a unique ophthalmic presentation with CRAO and BRVO. BMJ case reports. 2019 Aug 15:12(8):. doi: 10.1136/bcr-2018-228909. Epub 2019 Aug 15 [PubMed PMID: 31420422]
Level 3 (low-level) evidenceHayreh SS, Podhajsky PA, Raman R, Zimmerman B. Giant cell arteritis: validity and reliability of various diagnostic criteria. American journal of ophthalmology. 1997 Mar:123(3):285-96 [PubMed PMID: 9063237]
Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. American journal of ophthalmology. 2005 Sep:140(3):376-91 [PubMed PMID: 16138997]
Kido A, Tamura H, Ikeda HO, Miyake M, Hiragi S, Tsujikawa A. Nationwide incidence of central retinal artery occlusion in Japan: an exploratory descriptive study using the National Database of Health Insurance Claims (2011-2015). BMJ open. 2020 Sep 24:10(9):e041104. doi: 10.1136/bmjopen-2020-041104. Epub 2020 Sep 24 [PubMed PMID: 32973068]
Pick J, Nickels S, Saalmann F, Finger RP, Schuster AK. Incidence of retinal artery occlusion in Germany. Acta ophthalmologica. 2020 Aug:98(5):e656-e657. doi: 10.1111/aos.14369. Epub 2020 Feb 6 [PubMed PMID: 32026572]
Park SJ, Choi NK, Seo KH, Park KH, Woo SJ. Nationwide incidence of clinically diagnosed central retinal artery occlusion in Korea, 2008 to 2011. Ophthalmology. 2014 Oct:121(10):1933-8. doi: 10.1016/j.ophtha.2014.04.029. Epub 2014 Jun 7 [PubMed PMID: 24913283]
Ivanisević M, Karelović D. The incidence of central retinal artery occlusion in the district of Split, Croatia. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2001 May-Jun:215(3):245-6 [PubMed PMID: 11340401]
Abbati G, Fazi C, Fortunato P, Trapani S. Central retinal artery occlusion in a young child affected by COVID-19: a first case report. BMC pediatrics. 2023 Sep 13:23(1):462. doi: 10.1186/s12887-023-04276-8. Epub 2023 Sep 13 [PubMed PMID: 37704960]
Level 3 (low-level) evidenceLeavitt JA, Larson TA, Hodge DO, Gullerud RE. The incidence of central retinal artery occlusion in Olmsted County, Minnesota. American journal of ophthalmology. 2011 Nov:152(5):820-3.e2. doi: 10.1016/j.ajo.2011.05.005. Epub 2011 Jul 27 [PubMed PMID: 21794842]
Agarwal N, Gala NB, Karimi RJ, Turbin RE, Gandhi CD, Prestigiacomo CJ. Current endovascular treatment options for central retinal arterial occlusion: a review. Neurosurgical focus. 2014 Jan:36(1):E7. doi: 10.3171/2013.11.FOCUS13331. Epub [PubMed PMID: 24380484]
Grzybowski AE, Mimier MK. Evaluation of the Association between the Risk of Central Retinal Artery Occlusion and the Concentration of Environmental Air Pollutants. Journal of clinical medicine. 2019 Feb 7:8(2):. doi: 10.3390/jcm8020206. Epub 2019 Feb 7 [PubMed PMID: 30736427]
Anderson DC, Kappelle LJ, Eliasziw M, Babikian VL, Pearce LA, Barnett HJ. Occurrence of hemispheric and retinal ischemia in atrial fibrillation compared with carotid stenosis. Stroke. 2002 Aug:33(8):1963-7 [PubMed PMID: 12154246]
Schumacher M, Schmidt D, Jurklies B, Gall C, Wanke I, Schmoor C, Maier-Lenz H, Solymosi L, Brueckmann H, Neubauer AS, Wolf A, Feltgen N, EAGLE-Study Group. Central retinal artery occlusion: local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology. 2010 Jul:117(7):1367-75.e1. doi: 10.1016/j.ophtha.2010.03.061. Epub [PubMed PMID: 20609991]
Level 1 (high-level) evidenceLavin P, Patrylo M, Hollar M, Espaillat KB, Kirshner H, Schrag M. Stroke Risk and Risk Factors in Patients With Central Retinal Artery Occlusion. American journal of ophthalmology. 2019 Apr:200():271-272. doi: 10.1016/j.ajo.2019.01.021. Epub 2019 Feb 28 [PubMed PMID: 30827486]
Hayreh SS, Acute retinal arterial occlusive disorders. Progress in retinal and eye research. 2011 Sep; [PubMed PMID: 21620994]
Level 3 (low-level) evidenceHayreh SS, Zimmerman MB, Kimura A, Sanon A. Central retinal artery occlusion. Retinal survival time. Experimental eye research. 2004 Mar:78(3):723-36 [PubMed PMID: 15106952]
Level 3 (low-level) evidenceTobalem S, Schutz JS, Chronopoulos A. Central retinal artery occlusion - rethinking retinal survival time. BMC ophthalmology. 2018 Apr 18:18(1):101. doi: 10.1186/s12886-018-0768-4. Epub 2018 Apr 18 [PubMed PMID: 29669523]
Chronopoulos A, Schutz JS. Central retinal artery occlusion-A new, provisional treatment approach. Survey of ophthalmology. 2019 Jul-Aug:64(4):443-451. doi: 10.1016/j.survophthal.2019.01.011. Epub 2019 Jan 30 [PubMed PMID: 30707925]
Level 3 (low-level) evidenceDeSai C, Hays Shapshak A. Cerebral Ischemia. StatPearls. 2024 Jan:(): [PubMed PMID: 32809345]
Boia R, Ruzafa N, Aires ID, Pereiro X, Ambrósio AF, Vecino E, Santiago AR. Neuroprotective Strategies for Retinal Ganglion Cell Degeneration: Current Status and Challenges Ahead. International journal of molecular sciences. 2020 Mar 25:21(7):. doi: 10.3390/ijms21072262. Epub 2020 Mar 25 [PubMed PMID: 32218163]
Sun Y, Smith LEH. Retinal Vasculature in Development and Diseases. Annual review of vision science. 2018 Sep 15:4():101-122. doi: 10.1146/annurev-vision-091517-034018. Epub [PubMed PMID: 30222533]
Gupta N, Motlagh M, Singh G. Anatomy, Head and Neck, Eye Arteries. StatPearls. 2024 Jan:(): [PubMed PMID: 30725748]
Merriam JC, Casper DS. The entry point of the central retinal artery into the outer meningeal sheath of the optic nerve. Clinical anatomy (New York, N.Y.). 2021 May:34(4):605-608. doi: 10.1002/ca.23637. Epub 2020 Jul 1 [PubMed PMID: 32530060]
Tadi P, Najem K, Margolin E. Amaurosis Fugax. StatPearls. 2025 Jan:(): [PubMed PMID: 29261995]
Brown GC, Magargal LE. Central retinal artery obstruction and visual acuity. Ophthalmology. 1982 Jan:89(1):14-9 [PubMed PMID: 7070767]
Simakurthy S, Tripathy K. Marcus Gunn Pupil. StatPearls. 2025 Jan:(): [PubMed PMID: 32491607]
Carranza-Casas M, Aceves-Velazquez JE, Cano-Hidalgo R, Graue-Wiechers F. Partial Central Retinal Artery Occlusion: An Underrecognized Entity. International medical case reports journal. 2020:13():637-642. doi: 10.2147/IMCRJ.S274409. Epub 2020 Nov 26 [PubMed PMID: 33273866]
Level 3 (low-level) evidenceGong H, Wu B, Xie S. Visual acuity assessment of central retinal artery occlusion patients with or without paracentral acute middle maculopathy via OCT-A. BMC ophthalmology. 2023 Oct 13:23(1):412. doi: 10.1186/s12886-023-03151-5. Epub 2023 Oct 13 [PubMed PMID: 37833625]
Bakhoum MF, Freund KB, Dolz-Marco R, Leong BCS, Baumal CR, Duker JS, Sarraf D. Paracentral Acute Middle Maculopathy and the Ischemic Cascade Associated With Retinal Vascular Occlusion. American journal of ophthalmology. 2018 Nov:195():143-153. doi: 10.1016/j.ajo.2018.07.031. Epub 2018 Aug 3 [PubMed PMID: 30081014]
Wenzel DA, Kromer R, Poli S, Steinhorst NA, Casagrande MK, Spitzer MS, Schultheiss M. Optical coherence tomography-based determination of ischaemia onset - the temporal dynamics of retinal thickness increase in acute central retinal artery occlusion. Acta ophthalmologica. 2021 Mar:99(2):e247-e252. doi: 10.1111/aos.14563. Epub 2020 Aug 6 [PubMed PMID: 32767551]
Fan W, Huang Y, Zhao Y, Yuan R. Central retinal artery occlusion without cherry-red spots. BMC ophthalmology. 2023 Oct 25:23(1):434. doi: 10.1186/s12886-023-03176-w. Epub 2023 Oct 25 [PubMed PMID: 37880636]
Ruia S, Tripathy K. Fluorescein Angiography. StatPearls. 2024 Jan:(): [PubMed PMID: 35015403]
Tripathy K, Patel BC. Purtscher Retinopathy. StatPearls. 2024 Jan:(): [PubMed PMID: 31194324]
Duker JS, Sivalingam A, Brown GC, Reber R. A prospective study of acute central retinal artery obstruction. The incidence of secondary ocular neovascularization. Archives of ophthalmology (Chicago, Ill. : 1960). 1991 Mar:109(3):339-42 [PubMed PMID: 1706177]
Tripathy K, Chawla R. Extensive commotio retinae involving peripheral retina. The National medical journal of India. 2017 Jul-Aug:30(4):242. doi: 10.4103/0970-258X.218686. Epub [PubMed PMID: 29162764]
Ahmed NR, Tripathy K, Kumar V, Gogia V. Choroidal coloboma in a case of tay-sachs disease. Case reports in ophthalmological medicine. 2014:2014():760746. doi: 10.1155/2014/760746. Epub 2014 Sep 10 [PubMed PMID: 25295204]
Level 3 (low-level) evidenceKruszewski AM, Tamhankar MA. Ophthalmic Manifestations of Giant Cell Arteritis. International ophthalmology clinics. 2023 Apr 1:63(2):13-23. doi: 10.1097/IIO.0000000000000465. Epub 2023 Mar 23 [PubMed PMID: 36963824]
Hayreh SS. Anterior ischaemic optic neuropathy. II. Fundus on ophthalmoscopy and fluorescein angiography. The British journal of ophthalmology. 1974 Dec:58(12):964-80 [PubMed PMID: 4376416]
Hayreh SS, Podhajsky PA, Zimmerman B. Ocular manifestations of giant cell arteritis. American journal of ophthalmology. 1998 Apr:125(4):509-20 [PubMed PMID: 9559737]
Qaja E, Tadi P, Theetha Kariyanna P. Symptomatic Carotid Artery Stenosis. StatPearls. 2025 Jan:(): [PubMed PMID: 28723054]
Liang S, Chen Q, Hu C, Chen M. Association of Paracentral Acute Middle Maculopathy with Visual Prognosis in Retinal Artery Occlusion: A Retrospective Cohort Study. Journal of ophthalmology. 2022:2022():9404973. doi: 10.1155/2022/9404973. Epub 2022 May 21 [PubMed PMID: 35637681]
Level 2 (mid-level) evidenceHayreh SS, Zimmerman MB. Fundus changes in central retinal artery occlusion. Retina (Philadelphia, Pa.). 2007 Mar:27(3):276-89 [PubMed PMID: 17460582]
Lee WA, Liao IC. Giant cell arteritis presenting as central retinal artery occlusion. QJM : monthly journal of the Association of Physicians. 2022 Jan 21:115(1):32-33. doi: 10.1093/qjmed/hcab196. Epub [PubMed PMID: 34264347]
Ameer MA, Peterfy RJ, Khazaeni B. Giant Cell Arteritis (Temporal Arteritis). StatPearls. 2024 Jan:(): [PubMed PMID: 29083688]
Achkar AA, Lie JT, Hunder GG, O'Fallon WM, Gabriel SE. How does previous corticosteroid treatment affect the biopsy findings in giant cell (temporal) arteritis? Annals of internal medicine. 1994 Jun 15:120(12):987-92 [PubMed PMID: 8185147]
Level 2 (mid-level) evidenceCallizo J, Feltgen N, Pantenburg S, Wolf A, Neubauer AS, Jurklies B, Wachter R, Schmoor C, Schumacher M, Junker B, Pielen A, European Assessment Group for Lysis in the Eye. Cardiovascular Risk Factors in Central Retinal Artery Occlusion: Results of a Prospective and Standardized Medical Examination. Ophthalmology. 2015 Sep:122(9):1881-8. doi: 10.1016/j.ophtha.2015.05.044. Epub 2015 Jul 21 [PubMed PMID: 26231133]
Chen CS, Lee AW, Campbell B, Lee T, Paine M, Fraser C, Grigg J, Markus R. Efficacy of intravenous tissue-type plasminogen activator in central retinal artery occlusion: report from a randomized, controlled trial. Stroke. 2011 Aug:42(8):2229-34. doi: 10.1161/STROKEAHA.111.613653. Epub 2011 Jul 14 [PubMed PMID: 21757667]
Level 1 (high-level) evidenceBeatty S, Au Eong KG. Acute occlusion of the retinal arteries: current concepts and recent advances in diagnosis and management. Journal of accident & emergency medicine. 2000 Sep:17(5):324-9 [PubMed PMID: 11005400]
Level 3 (low-level) evidenceFfytche TJ. A rationalization of treatment of central retinal artery occlusion. Transactions of the ophthalmological societies of the United Kingdom. 1974 Jul:94(2):468-79 [PubMed PMID: 4619853]
Fieß A, Cal Ö, Kehrein S, Halstenberg S, Frisch I, Steinhorst UH. Anterior chamber paracentesis after central retinal artery occlusion: a tenable therapy? BMC ophthalmology. 2014 Mar 10:14():28. doi: 10.1186/1471-2415-14-28. Epub 2014 Mar 10 [PubMed PMID: 24612658]
Atebara NH, Brown GC, Cater J. Efficacy of anterior chamber paracentesis and Carbogen in treating acute nonarteritic central retinal artery occlusion. Ophthalmology. 1995 Dec:102(12):2029-34; discussion 2034-5 [PubMed PMID: 9098313]
Incandela L, Cesarone MR, Belcaro G, Steigerwalt R, De Sanctis MT, Nicolaides AN, Griffin M, Geroulakos G, Ramaswami G. Treatment of vascular retinal disease with pentoxifylline: a controlled, randomized trial. Angiology. 2002 Jan-Feb:53 Suppl 1():S31-4 [PubMed PMID: 11865833]
Level 1 (high-level) evidenceFraser SG, Adams W. Interventions for acute non-arteritic central retinal artery occlusion. The Cochrane database of systematic reviews. 2009 Jan 21:2009(1):CD001989. doi: 10.1002/14651858.CD001989.pub2. Epub 2009 Jan 21 [PubMed PMID: 19160204]
Level 1 (high-level) evidenceRumelt S, Dorenboim Y, Rehany U. Aggressive systematic treatment for central retinal artery occlusion. American journal of ophthalmology. 1999 Dec:128(6):733-8 [PubMed PMID: 10612510]
Level 1 (high-level) evidenceNedelmann M, Graef M, Weinand F, Wassill KH, Kaps M, Lorenz B, Tanislav C. Retrobulbar Spot Sign Predicts Thrombolytic Treatment Effects and Etiology in Central Retinal Artery Occlusion. Stroke. 2015 Aug:46(8):2322-4. doi: 10.1161/STROKEAHA.115.009839. Epub 2015 Jun 25 [PubMed PMID: 26111890]
Schrag M, Youn T, Schindler J, Kirshner H, Greer D. Intravenous Fibrinolytic Therapy in Central Retinal Artery Occlusion: A Patient-Level Meta-analysis. JAMA neurology. 2015 Oct:72(10):1148-54. doi: 10.1001/jamaneurol.2015.1578. Epub [PubMed PMID: 26258861]
Level 1 (high-level) evidenceButler FK Jr, Hagan C, Murphy-Lavoie H. Hyperbaric oxygen therapy and the eye. Undersea & hyperbaric medicine : journal of the Undersea and Hyperbaric Medical Society, Inc. 2008 Sep-Oct:35(5):333-87 [PubMed PMID: 19024664]
Mehboob MA, Khan A, Mukhtar A. Efficacy of YAG Laser Embolysis in Retinal Artery Occlusion. Pakistan journal of medical sciences. 2021 Jan-Feb:37(1):71-75. doi: 10.12669/pjms.37.1.3196. Epub [PubMed PMID: 33437253]
Man V, Hecht I, Talitman M, Hilely A, Midlij M, Burgansky-Eliash Z, Achiron A. Treatment of retinal artery occlusion using transluminal Nd:YAG laser: a systematic review and meta-analysis. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2017 Oct:255(10):1869-1877. doi: 10.1007/s00417-017-3777-8. Epub 2017 Aug 19 [PubMed PMID: 28823062]
Level 1 (high-level) evidenceYoun TS, Lavin P, Patrylo M, Schindler J, Kirshner H, Greer DM, Schrag M. Current treatment of central retinal artery occlusion: a national survey. Journal of neurology. 2018 Feb:265(2):330-335. doi: 10.1007/s00415-017-8702-x. Epub 2017 Dec 13 [PubMed PMID: 29236169]
Level 3 (low-level) evidenceGarcía-Arumí J, Martinez-Castillo V, Boixadera A, Fonollosa A, Corcostegui B. Surgical embolus removal in retinal artery occlusion. The British journal of ophthalmology. 2006 Oct:90(10):1252-5 [PubMed PMID: 16854826]
Tang WM, Topping TM. Vitreous surgery for central retinal artery occlusion. Archives of ophthalmology (Chicago, Ill. : 1960). 2000 Nov:118(11):1586-7 [PubMed PMID: 11074821]
Hayreh SS, Ocular vascular occlusive disorders: natural history of visual outcome. Progress in retinal and eye research. 2014 Jul; [PubMed PMID: 24769221]
Chodnicki KD, Pulido JS, Hodge DO, Klaas JP, Chen JJ. Stroke Risk Before and After Central Retinal Artery Occlusion in a US Cohort. Mayo Clinic proceedings. 2019 Feb:94(2):236-241. doi: 10.1016/j.mayocp.2018.10.018. Epub [PubMed PMID: 30711121]
Shaikh IS, Elsamna ST, Zarbin MA, Bhagat N. Assessing the risk of stroke development following retinal artery occlusion. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2020 Sep:29(9):105002. doi: 10.1016/j.jstrokecerebrovasdis.2020.105002. Epub 2020 Jun 15 [PubMed PMID: 32807420]
Hwang DD, Lee KE, Kim Y, Kim MS, Rim TH, Kim M, Kim H, Kyoung DS, Park JI. Incidence of Retinal Artery Occlusion and Related Mortality in Korea, 2005 to 2018. JAMA network open. 2023 Mar 1:6(3):e233068. doi: 10.1001/jamanetworkopen.2023.3068. Epub 2023 Mar 1 [PubMed PMID: 36897587]
Duker JS, Brown GC. The efficacy of panretinal photocoagulation for neovascularization of the iris after central retinal artery obstruction. Ophthalmology. 1989 Jan:96(1):92-5 [PubMed PMID: 2465523]
Hayreh SS. Giant cell arteritis: Its ophthalmic manifestations. Indian journal of ophthalmology. 2021 Feb:69(2):227-235. doi: 10.4103/ijo.IJO_1681_20. Epub [PubMed PMID: 33463564]
Rippe JM. Lifestyle Strategies for Risk Factor Reduction, Prevention, and Treatment of Cardiovascular Disease. American journal of lifestyle medicine. 2019 Mar-Apr:13(2):204-212. doi: 10.1177/1559827618812395. Epub 2018 Dec 2 [PubMed PMID: 30800027]
Olson EA, Lentz K. Central Retinal Artery Occlusion: A Literature Review and the Rationale for Hyperbaric Oxygen Therapy. Missouri medicine. 2016 Jan-Feb:113(1):53-7 [PubMed PMID: 27039492]