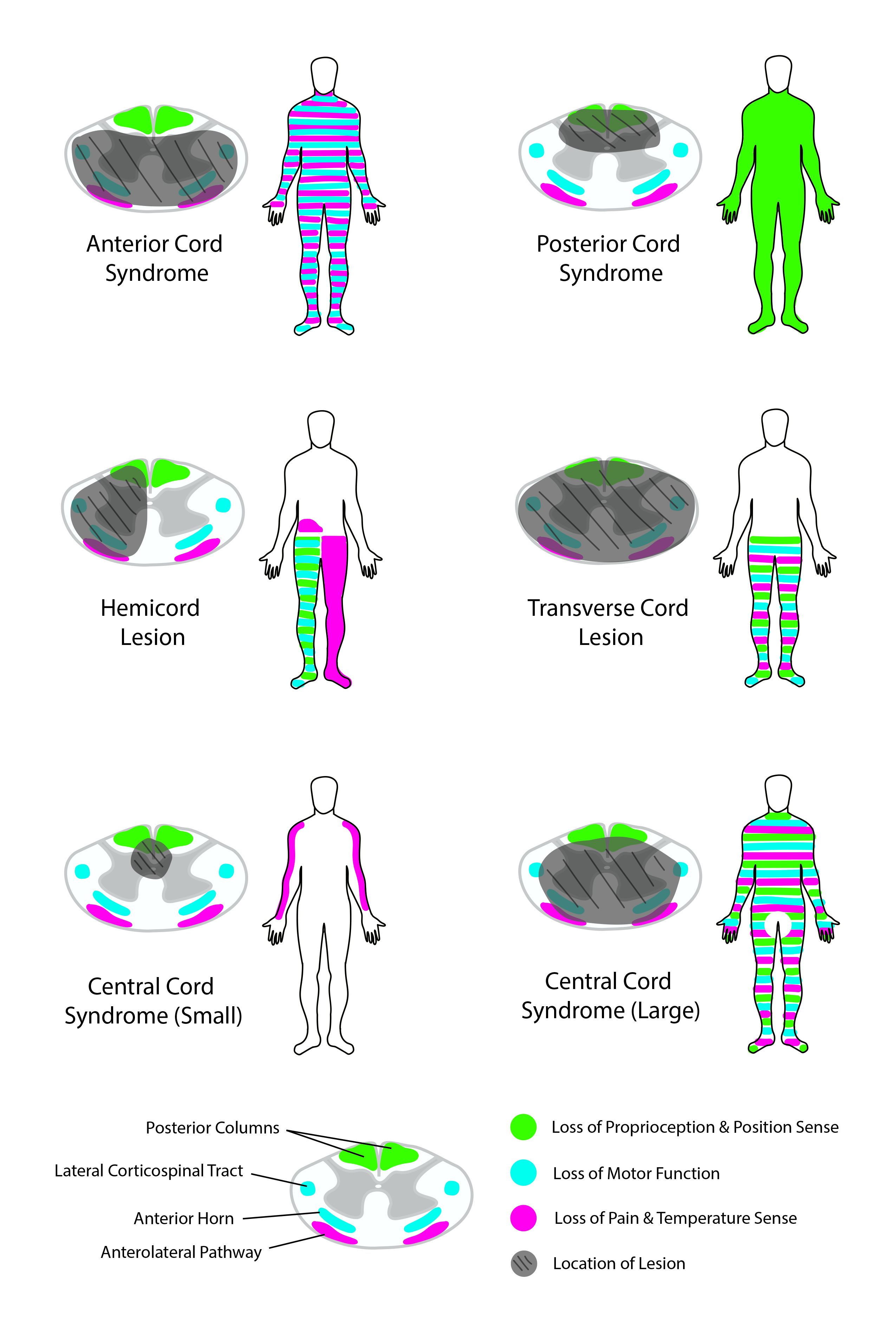
Introduction
There are approximately 300,000 individuals living with spinal cord injury (SCI) in the United States, with approximately 18,000 new cases annually.[1] Central cord syndrome, first described in 1954, is the most common form of incomplete spinal cord injury (SCI) and has an annual incidence of approximately 11,000 cases in the United States.[2][3] It leads to motor deficits that are more pronounced in the upper extremities compared to the lower extremities, as well as bladder dysfunction (retention) with sacral sparing.[4][5][6] Because of its unique clinical presentation, central cord syndrome is also described in the differential diagnosis of "man in a barrel" syndrome.[7] The degree of clinical presentation is quite variable and corresponds to the extent of the injury to the nerve root.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Central cord syndrome is an incomplete cord syndrome that most commonly results following a hyperextension injury of the cervical spine leading to spinal cord impingement. The proposed mechanism of action is hyperextension resulting in anteriorly simultaneous compression of the spinal cord, either by bony spurs or intervertebral disc material and posteriorly by the ligamentum flavum. This theory was aligned with early myelogram studies performed on cadavers.[8][9] Initially, Schneider et al. postulated that spinal cord compression caused central hemorrhaging, resulting in adjacent white matter compression.[10]
Furthermore, Schneider believed that the upper limbs were more affected than the lower limbs because of the somatotropic organization of the lateral corticospinal tract, with arm function lying more medial within the tract. Neuroanatomic studies have since shown a diffuse distribution of upper and lower limb nerve fibers within the corticospinal tract, leading to the possibility that the unique presentation of central cord syndrome is due more to the hand and upper limb being more densely represented within the lateral corticospinal tract.[3] More recent studies have revealed intact gray matter without evidence of hemorrhage within the central canal. Conversely, white matter axonal disruption, particularly in the lateral corticospinal tracts, appears to be the primary feature.[11]
Other less common causes include cervical spondylosis, atlantoaxial instability, tethered cord, osteoporosis, and spinal arthropathies.[12] The impairment of large fibers causes damage to signal conduction and consequently results in motor and sensory problems, especially in the upper limbs and, to some extent, in the lower limbs. Overall, the amount of functional loss is dependent on the extent of the injury to the nerve.[13]
Epidemiology
A retrospective review by McKinley et al. of 839 spinal cord injury patients at a tertiary care level one trauma center found CCS to be the most common clinical spinal cord syndrome.[14] Central cord syndrome mostly affects males in a bimodal distribution (falls/motor vehicle collision in those who are young and hyperextension injury in older patients with likely underlying spinal disease, including osteoarthritis or cervical spondylosis). It has a prevalence of 15 to 25% in the United States and can occasionally be missed if the patient has mild symptoms at the initial presentation.[15] The approximate annual incidence of central cord syndrome is 11,000 cases per year in the United States.[3]
Pathophysiology
Central cord syndrome occurs most commonly in those who suffer a hyperextension injury. This usually happens with a forward fall while striking the chin and having the neck extend backward at the time of the fall. Older patients generally have underlying cervical spondylosis (stress defects). This leads to the posterior cord being either compressed or irritated by the posterior ligamentum flavum or anterior cord compression from underlying spondylosis. These two contusion-type injuries to the cord can lead to clinical symptoms secondary to edema of the cord at the site of injury. It could also lead to bleeding into the cord at the injury site, which understandably has a worse prognosis.[16][17][18]
Usually, young patients often suffer from a higher velocity of trauma, leading to cervical spine subluxation or fracture that leads to the above-described compression, contusion, and bleeding.
The physical examination findings result from the compression of the spinothalamic and corticospinal tracts. The upper limb tracts are medial compared to the lower extremity, while sacral segments lie on the most lateral aspect. The central portion becomes more affected than the outer segments when compression occurs in the cervical cord due to external pressure.[17]
First described by Schneider et al. based on medial somatotopy of the arms within the lateral corticospinal tract as described by Foerster.[3][19] Axonal disruption within the lateral columns of the spinal cord with sparing of the central gray matter was observed in a study pertaining to the radio-pathological analysis.[11] The relative sparing of the extra-pyramidal tracts has recently been postulated in pathogenesis.[20]
History and Physical
Most of these patients will be older and present with symptoms after a fall with hyperextension of their neck. On examination, patients will have more significant strength impairments in the upper extremities (especially the hands) compared to the lower extremities. Patients often complain of sensory deficits below the level of injury, but this is variable. Pain and temperature sensations are typically affected, but the sensation of light touch can also be impaired. The most common sensory deficits are in a "cape-like" distribution across their upper back and down their posterior upper extremities. They will often have neck pain at the site of spinal cord impingement.
Bladder dysfunction (most commonly urinary retention) and priapism can also be signs of upper motor neuron dysfunction. The sacral sensation is usually preserved, but the clinician should assess the rectal tone to evaluate the severity of the compression.[21]
Evaluation
Radiographic
Patients deemed medically stable, awake, and symptomatic should proceed with a radiographic evaluation of the spinal cord and axial skeleton.[22][23] Computed tomography (CT) is currently the first-line imaging as it provides rapid imaging of the axial skeleton. In suspected cervical spinal cord injury patients, neck flexion and extension images should also be obtained.[23] CT of the cervical spine can show the impingement of the spinal canal to some extent.[24][25] In awake and asymptomatic patients, the American Association of Neurological Surgeons (AANS) does not recommend imaging prior to discontinuing cervical immobilizers.[22] Magnetic resonance imaging (MRI) is the gold standard imaging modality to evaluate the spinal cord and the adjacent soft tissue structures (vasculature, ligaments, intervertebral discs).[23] The Consortium of Spinal Medicine recommends imaging the entire spine for patients with spinal cord injuries.[26]
Clinical
Central cord syndrome is a clinical diagnosis with a presentation that includes disproportionate impairment of the upper limb motor function compared to the lower limbs, along with varying degrees of sensory deficits below the level of the spinal cord lesion and possibly urinary or gastrointestinal dysfunction. Pouw et al. proposed that a difference of 10 between upper and lower limb motor scores on the International Standards for Neurological Classification of SCI (ISNCSCI) may be a more objective diagnostic criterion.[27] The clinical evaluation of central cord syndrome mirrors other spinal cord injuries. Identifying the neurological level of injury is paramount as it can help determine prognosis and guide therapeutic strategies and functional rehabilitation goals. Additionally, a baseline neurological examination can track future improvements as well as future deficits as they arise.
The gold standard examination tool for any spinal cord injury is the ISNCSCI. More severe findings on neurological examination should raise suspicion of a cervical vertebral fracture. When central cord syndrome is associated with a cervical spine fracture, it can prolong recovery.[24][25] Providers must also assess for respiratory compromise, pressure injuries to the skin, neurogenic bowel and bladder, spasticity, autonomic dysreflexia, and temperature dysregulation.
Treatment / Management
Prehospital
Management of spinal cord injuries begins at the scene of the injury with a primary survey involving the airway, breathing, circulation, and disability (ABCD). Disability includes calculating a Glasgow coma scale (GCS), pupil examination, and identifying any lateralizing signs. This should be repeated as often as necessary whenever the patient's status changes. Next, a secondary survey should be performed that involves a focused examination of the patient that includes spinal examination (3-person logroll in order to assess and palpate the spine), assessing distal motor function in both upper and lower limbs, incontinence and skin appearance. Lastly, providers in the field should collect as much patient history as possible, noting any subjective complaints of neck pain. Restricting spinal motion is of the utmost importance in patients with suspected spinal cord injuries.[28]
Immediate Hospitalization Period
In the immediate period following a spinal cord injury, providers should pay particular attention to optimizing tissue perfusion to the penumbra to reduce secondary injury. Hemodynamic stability and mitigating the meta-inflammatory response are the prime initial focus.[3][29]The American Association of Neurosurgeons and Congress of Neurosurgeons recommend preventing hypotension to the injured spine and spinal cord with a target mean arterial pressure (MAP) between 85 and 90 mmHg for at least seven days.[30] However, the current research is inconclusive on the optimal blood pressure range.[3](B3)
The use of corticosteroids in spinal cord injury has a long history of controversy. The goal of intravenous (IV) methylprednisolone therapy is to halt the inflammatory cascade that results in secondary spinal cord injury. The landmark Second National Spinal Cord Injury Study (NASCIS-II) did show statistically significant neurological benefits with the administration of IV methylprednisolone if given within 8 hours of initial injury; however, there was also an increased risk of adverse events. Therefore, the Congress of Neurological Surgeons recommends against IV methylprednisolone administration.[31] On the other hand, in 2016, AO Spine published a position statement recommending a 24-hour infusion of IV methylprednisolone within 8 hours of injury but not offering infusion for patients who present greater than 8 hours after the initial injury. Additionally, AO Spine does not recommend a 48-hour infusion of methylprednisolone.[32](A1)
Any backboards should be removed as soon as possible to reduce the occurrence of pressure ulcers. All patients with concern for spinal cord injury should have a thorough trauma evaluation performed in the emergency department. These patients should have cervical immobilization maintained during the initial evaluation. Providers should utilize the Canadian C-spine Rule and National Emergency X-Radiography Utilization Study (NEXUS) to help guide diagnostic imaging needs, as well as help determine the continued need for cervical immobilization.[28] Hyperintensity on gradient T2 MRI within the cervical cord is the most consistent finding in central cord syndrome.[2]
Conservative management should only be considered in situations without fracture, dislocation, disc herniation, or spinal instability; otherwise, surgical intervention is preferred.[3] Conservative management includes physical and occupational therapy in addition to standard medical care for sequelae of central cord syndrome.(B3)
In patients without instability, the decision for conservative versus surgical management of acute central cord syndrome is highly debated, with no clearly established guidelines. Research studies have produced mixed results comparing conservative management with early surgical intervention. Numerous studies showed that delaying surgery in traumatic central cord syndrome may be beneficial where the odds of mortality decreased within each day of delay. The goal of delayed surgery was to improve the patient's underlying health, especially in older adults, and treat other potential comorbidities before the surgery.[33][34] On the other hand, Godzik et al. performed a retrospective study identifying 2,379 central cord syndrome patients and hypothesized that increased mortality in early surgical patients was more likely related to higher injury severity than surgical timing.[35]
Recently, the Spinal Cord Society (SCS) and Spine Trauma Study Group (STSG) released a position statement after a systematic review and meta-analysis regarding the management of acute central cord syndrome. Their recommendation was patients with acute traumatic central cord syndrome due to hyperextension injury and cervical stenosis without fracture, dislocation, disc herniation, or spinal instability should be given the option of early spinal cord decompression (less than 24 hours after injury) or initial conservative management followed by delayed cord decompression (greater than 24 hours after injury) if there was a neurological decline or a plateau with neurological recovery. The review did not find a difference in complications or outcomes between early surgery and conservative management with delayed surgery.[36](A1)
Surgical Approach
At this time, there are no guidelines for the surgical approach, and it is left to the surgeon to decide the most appropriate course for intervention that will optimize outcomes and reduce complications. Generally, single-level anterior compressive lesions necessitate an anterior approach. A posterior approach should be considered in patients with compression at multiple levels. Patients with spinal fractures or dislocations should undergo reduction and stabilization, which may require a combined anterior and posterior approach.[2]
Differential Diagnosis
When evaluating patients with central cord syndrome, conditions that closely mimic CCS should be ruled out, including cruciate paralysis and the avulsion of cervical roots. Cruciate paralysis is a rare neurological disease involving the cervicomedullary junction. The main precipitating factors of cruciate paralysis are mechanical trauma, metabolic disorders, or post-surgical complication. Typically, the patient with cruciate paralysis presents with bilaterally upper extremity paresis while sparing the lower extremities in most cases.[37][38] Besides other features, the main differentiating feature in cruciate paralysis is that it affects the selective descending fibers of the corticospinal tract as these fibers decussate at the cervicomedullary junction.[39]
A cervical root avulsion is a severe form of nerve root injury that usually results from high-energy trauma to the neck or ipsilateral arm. Neurological deficits from nerve avulsion range from a mild motor function deficit to complete paralysis that requires surgical correction.[40][41]
Prognosis
The natural history of the entity has an inherent potential for recovery even without surgery.[3]The prognosis of central cord syndrome is variable, but most patients have a neurological recovery to some extent. Young trauma patients and those who seek immediate medical attention have better chances of neurological recovery. Prognostic factors include age, the severity of the initial neurologic deficit, and initial MRI findings.[2] Roth et al. found age to be the most important prognostic indicator, as patients younger than 50 have more favorable outcomes.[36] Hohl et al. developed a classification system for CCS that combined initial ISNCSCI motor score and evidence of MRI signal abnormality to predict functional outcomes at one year, but this has not been validated.[42]
Patients with central cord syndrome recover substantial neurological function after the injury; their capacity to walk recovers in most cases. However, some neurological deficits remain.[43][44] Improvement usually occurs in an ascending fashion, with motor leg function recovering first, followed by bladder control, then proximal arms. The hand function appears to return last. Abnormal MRI signals can help predict the likelihood of neurological recovery that may occur later in the course of recovery.[36]
Recovery generally plateaus at two years post-injury.[2] At three years post-injury, patients should recover at least 90% of their motor score regardless of surgical versus conservative management; however, patients who underwent surgical intervention had greater functional scores and were more likely to attain their pre-injury mobility status.[40] Aito et al. performed a retrospective analysis on 82 central cord syndrome patients and found that 47% of patients had persistent neuropathic pain, 68% achieved spontaneous voiding, and 66% reported spasticity at follow-up (at least 1.5 years).[45]
A European multicenter study comparing 110 patients with central cord syndrome with other incomplete spinal cord injury patients revealed that central cord syndrome patients tended to have greater improvement in their ASIA Impairment Scale (AIS) grade as well as better ability to ambulate after one year. However, central cord syndrome patients had lower “self-care” scores from injury onset up to one year based on the Spinal Cord Independence Measure (SCIM), likely due to impaired hand function.[46] Patients admitted to acute inpatient rehabilitation with normal leg strength and those who experienced both upper and lower limb motor recovery had the greatest improvement in completing activities of daily living (ADL) at discharge. Patients who did not demonstrate objective neurologic recovery also made substantial functional improvements after acute inpatient rehabilitation.[42]
A national database study from 2009 through 2012 showed fall as the predominant cause, with a mean age of involvement of 60 years. 55% of cases were managed conservatively, 39% with cervical fusion surgery (62% anterior decompression and fusion), and 6% underwent laminoplasty.[47] The mortality rate was 2.6%, with increased odds among older patients age (OR 1.06, P<0.001) and those with greater comorbidities (OR 1.72, P<0.001).[47]
Early decompression and stabilization have been advocated for cohorts presenting with instability and major or worsening neurological deficits.[3] surgery within 12 hours of the injury can improve neurologic recovery and is associated with fewer post-surgical complications.[48]
No difference has been observed in the overall outcomes between early and late surgical interventions.[3] Early surgery may, however, accelerate recovery in selected cohorts.[49] Long-term follow-up has failed to demonstrate any significant differences among patients undergoing conservative versus surgical treatment.[45]
Lower preoperative Japanese Orthopedic Association (JOA) score, long segment signal changes, and impingement connote poor prognosis.[50],[51] Intramedullary lesion length (IMLL) was the only significant variable predictive of AIS grade conversion to a better grade.[52] The severity of AIS at admission was the strongest predictor of functional outcomes.[53] The majority of American Spinal Injury Association (ASIA) Impairment Scale (AIS) conversion should occur within the first 6 to 9 months, with a peak observed in the first three months.[54]
The neuroanatomical–functional paradox observed among patients with central cord syndrome is due to the interplay between motor synergy encoding (MSE) neurons, as well as lesion-affected and recovery-related networks.[29] The neuronal plasticity, meta-inflammation, and diaschisis also have ripple effects.[29]
Diffusion tensor imaging is a promising radiological armamentarium.[51] Induced pluripotent stem cell therapy might provide a newer therapeutic avenue in the near future.[55]
Complications
Patients with central cord syndrome are at risk for a multitude of complications, like other spinal cord injuries.
Pulmonary
Respiratory complications are the most common cause of morbidity and mortality in patients with spinal cord injuries.[56] Patients with cervical spinal cord injury are most at risk due to impaired respiratory drive, especially above the C5 vertebral level due to diaphragmatic impairment. Common complications include acute respiratory failure, pneumonia, pleural effusion, pneumothorax, pulmonary embolism, and mucus plug.[56]
Cardiovascular
Cardiovascular complications arise largely due to impaired autonomic function.[57] Central cord syndrome patients should be at lower risk of common cardiovascular complications due to the retained ability of lower limb function and ambulation. However, patients with decreased lower limb function are at higher risk for low baseline blood pressure, orthostatic hypotension, and venous thromboembolism.[58][59]
Low baseline blood pressures in acute injury are managed with fluid resuscitation. Orthostatic hypotension can be managed both with and without medications. Non-pharmacologic strategies include reducing rapid changes in posture, avoiding hot environments, and eating smaller meals to reduce postprandial cardiovascular shunting to gastrointestinal organs.[60][61] Pharmacologic options for orthostatic hypotension include fludrocortisone, ephedrine, and midodrine; however, only midodrine is approved by the Food and Drug Administration(FDA) for treating neurogenic orthostatic hypotension.[60][62][63][64]
Venous thromboembolism (VTE) is a significant problem in spinal cord injury patients. Due to the nature of central cord syndrome, patients are less likely to develop VTE. The incidence of VTE in spinal cord patients ranges from 12 to 100%. While most commonly occurring in the lower limbs, upper limb VTE can also occur.[65] The Consortium of Spinal Medicine recommends VTE prophylaxis for a minimum of 8 weeks after injury and prefers low molecular weight heparin (LMWH) over other anticoagulant medications, though research is needed into the safety and efficacy of direct oral anticoagulants.[59][66] Currently, low-dose subcutaneous heparin and vitamin K antagonists are not recommended therapies.[59]
Patients with central cord syndrome are usually prone to autonomic dysreflexia, a potentially life-threatening emergency that is more common in patients with spinal cord injury above the T6 level. It is an uncoordinated response of the autonomic system to heart rate and the vascular walls. This phenomenon usually occurs in the first month of the injury. Common clinical presentations include headaches, flushing, piloerection, increased blood pressure, anxiety, and nausea.[36] These symptoms are usually episodic, and they are conservatively managed. The initial management strategy is prevention by educating patients, caregivers, and hospital staff about the potential for autonomic dysreflexia as well as warning signs. During an acute episode, management should begin by placing the patient upright, removing all tight clothes, and removing any noxious stimuli such as skin pressure, urinary catheter dysfunction, and bowel impaction. A survey for noxious stimuli should be repeated until the patient’s autonomic dysreflexia episode has subsided.[67]
Blood pressure monitoring may be required in selective cases for the long term, even if the patient is not diagnosed as hypertensive. For blood pressure control, nitrates, hydralazine, and labetalol are used, but prescribers should avoid giving nitrates to patients taking sildenafil.[68]
Lastly, Readdy et al. showed that cardiogenic complications appeared in 68% of patients with no relation to conservative management, surgical management, or surgical timing. Furthermore, they noted that in patients 55 years or older, there was a significant increase in complications with dopamine compared to phenylephrine (83% versus 50%, respectively).[68] The utmost priority in managing cardiometabolic syndrome is essential.[69]
Neuropathic Pain
Patients with central cord syndrome can develop neuropathic pain at the level of the injury and below the level of injury. Neuropathic pain can be caused by damage to either the spinal cord or nerve roots. It is typically described as “hot,” burning,” tingling,” “pins and needles,” “sharp,” “shooting,” or “electric.”[70] First-line treatment for neuropathic pain in spinal cord injury is gabapentinoids (gabapentin and pregabalin) and tricyclic antidepressants (TCA), with additional consideration for selective serotonin reuptake inhibitors (SSRI) and serotonin and norepinephrine reuptake inhibitors (SNRI).[71][72] Guidelines published by the CanPainSCI Working Group recommended tramadol as a second-line medication.[73][74] Lamotrigine was considered a second-line medication, specifically in incomplete spinal cord injury patients. Additional interventions to consider are visual illusion therapy, transcranial direct current stimulation, and physical and occupational therapy.[73] Methylprednisolone (MP) has been advocated for acute neuropathic pain with allodynia.[75]
Spasticity
Spasticity can be defined as “disordered sensory-motor control resulting from an upper motor neuron lesion, presenting as intermittent or sustained involuntary activation of muscles” and is a significant problem in spinal cord injury patients.[76][77] A systematic review by Bovend’Erdt et al. found inconclusive evidence of the beneficial effects of stretching on spasticity.[78] Physical modalities such as cryotherapy and heat therapy (both superficial and deep) provide only short-term effects on spasticity.[79] Neuromuscular stimulation and functional electrical stimulation have shown positive results in reducing spasticity.[80] Transcranial and spinal cord magnetic stimulation has been proposed, but more research is required.[81]
Common oral pharmacologic options include gamma-aminobutyric acid (GABA) agonists baclofen, gabapentinoids and benzodiazepines, alpha-2-adrenergic agonists tizanidine and clonidine, and peripherally acting dantrolene.[82] Alternative medications that are not FDA-approved include cyproheptadine, cannabinoids, and 4-aminopyridine.[83][84][85]
In patients with minimal response to oral medications or have contraindications to oral medications, chemodenervation with botulinum toxin or chemolysis with alcohol are alternative interventions.[86][87] Boviatsis et al. found that intrathecal baclofen pumps improved functional improvement and reduced pain in spinal cord injury and multiple sclerosis.[88] Surgical interventions for spasticity refractory to conservative management include tendon lengthening, tendon transfers, neurotomy, and selective rhizotomy.[89][90] Spinal cord stimulators have shown promising results in the past, but current clinical trials are needed to provide data on their safety and efficacy due to advances in technology and understanding of spasticity pathophysiology.[91]
Chronic Pain, Spasticity, and Autonomic Dysreflexia have been postulated to result from the loss of descending serotonergic fibers. Therefore bolstering 5-HT function and ionic plasticity may provide therapeutic benefits.[92],[93]
Genitourinary
Neurogenic bladder is another prevalent condition among patients with central cord syndrome. In central cord syndrome, the sensation of bladder fullness, sphincter function of the bladder, and motor control of the bladder are impaired. Patients experience urgency, spasms, and frequency, along with urine incontinence.[94] A Foley catheter is used for this purpose, and the majority of the patients regain bladder function. Patients who require long-term catheterization either use a clean intermittent catheter (CIC) or an indwelling catheter. A clean intermittent catheter is preferred over indwelling catheters as the rate of infection is much more with an indwelling catheter.[21]
Pressure Injury
Pressure ulcers are formed due to the tissue damage that typically occurs at bony prominences. Approximately one-third of the patients with central cord syndrome have multiple pressure ulcers. It is usually managed by avoiding immobility, regularly examining the prone parts, applying an emollient on the part exposed to friction, using cushions, and maintaining adequate weight and nutrition.[95]
Additional Complications
Traumatic spinal cord injury (tSCI) patients are at an increased risk of sustaining a concomitant traumatic brain injury (TBI). A meta-analysis by Budisin et al. found that approximately 40 to 50% of tSCI patients were also diagnosed with a TBI. Motor vehicle collisions were more likely to cause comorbid TBI compared to falls (57.6% versus 31.6%).[96] More recently, the prevalence of TBI in patients with a cervical spinal injury is approximately 40%.[97]
Postoperative and Rehabilitation Care
There are currently no studies comparing central cord syndrome patients who received acute inpatient rehabilitative services with those who did not. Central cord syndrome patients should receive occupational and physical therapy after discharge from acute care facilities. While each rehabilitation program should be tailored specifically to the patient and their functional goals, some generalizations can be made. Physical therapy focuses on maintaining an adequate range of motion, increasing strength, and fine-tuning coordination of the lower limbs and trunk to improve ambulation (with or without assistive devices), transfers, and balance.[3][98]
Interventions include stretching, therapeutic exercise, aerobic conditioning, stair and transfer training, gait training, functional electrical stimulation, and aquatic therapy. Since upper limb strength and function are more impacted than lower limbs in central cord syndrome, occupational therapy should focus on hand strength and dexterity to maximize patient function with activities of daily living (ADLs) such as feeding, dressing, bathing, and self-hygiene.[24][98] Splinting may be utilized when not in therapy to prevent the development of contractures.[21]
Deterrence and Patient Education
Patients with central cord syndrome are challenged not only physically but also psychologically as well. Effective communication between interprofessional teams, including neurologists, internists, neurosurgeons, psychiatrists, physiatrists, physical and occupational therapists, and nurses, can lead to better outcomes. These patients are prone to depression and suicidal ideation. Furthermore, the lack of motivation can delay the treatment progress and may lead to permanent disability.
The following strategies can assist in achieving better recovery:
- Close routine follow-up with the neurologist/physiatrist/internist.
- Educate patients on clean intermittent catheterization (CIC) to avoid urinary tract infections and secondary complications.
- Educate patients to avoid immobility and regularly change position after 2 to 4 hours.
- Close follow-up with a physical therapist for muscle and core strengthening exercises to avoid muscle atrophy.
- Blood pressure monitoring at home.
- Close follow-up with the psychiatrist to look for signs of depression and suicidal ideation.
Pearls and Other Issues
Central cord syndrome patients usually require extensive physical therapy for significant neurologic recovery. These patients may develop chronic issues, including autonomic dysregulation with uncontrolled blood pressure, neurogenic bladder requiring catheterization, pressure ulcers, neuropathic pain, and spasticity with chronic pain issues.
Autonomic dysreflexia should be initially managed by sitting the patient upright, loosening tight clothes, and removing any noxious stimuli that may be present. Hypertension due to autonomic dysreflexia may be managed with nifedipine or transdermal nitroglycerin if conservative measures do not resolve the autonomic dysreflexia.
Patients with neurogenic bladder may require intermittent self-catheterization but will remain at risk for iatrogenic bladder infections. The majority of the patients will have the return of bladder function after six to eight months.
Neuropathic pain and spasticity are common. First-line medications for neuropathic pain are gabapentinoids and TCAs. Other considerations include SSRI/SNRI, transcranial electric stimulation, and physiotherapy. Spasticity should be managed with a regular stretching program and antispasmodic medications. Baclofen is the hallmark of spasticity management. Patients may require additional medicinal therapy in consultation with a pain specialist or neurologist for their neuropathic pain and spasticity. Also, providers should consider chemodenervation with botulinum toxin or alcohol injections. Lastly, baclofen pumps can be implanted as well.
Enhancing Healthcare Team Outcomes
The diagnosis and management of central cord syndrome are with an interprofessional team consisting of a neurologist, physiatrist, emergency department physician, neurosurgeon, trauma physician, intensive care unit nurses, physical therapist, internist, and neurology specialty nurses. Once diagnosed, high-dose corticosteroids are the first-line treatment if indicated. Surgery may be an option for select patients.
Most patients show recovery with physical therapy, but the recovery is not always complete. Recovery typically begins with lower limb function, then the proximal arms, and finally, hands and bladder/bowel function. Besides, the recovery may take months or even years, and these patients are prone to pressure ulcers, deep vein thrombosis, urinary tract infections, and muscle wasting. The key is to have the surgeons involved early in the care because, in some cases, surgical decompression may help boost recovery.[4][99][100] Interprofessional communication and collaboration will help achieve optimal outcomes. [Level 5]
Media
(Click Image to Enlarge)
References
Lo J, Chan L, Flynn S. A Systematic Review of the Incidence, Prevalence, Costs, and Activity and Work Limitations of Amputation, Osteoarthritis, Rheumatoid Arthritis, Back Pain, Multiple Sclerosis, Spinal Cord Injury, Stroke, and Traumatic Brain Injury in the United States: A 2019 Update. Archives of physical medicine and rehabilitation. 2021 Jan:102(1):115-131. doi: 10.1016/j.apmr.2020.04.001. Epub 2020 Apr 24 [PubMed PMID: 32339483]
Level 1 (high-level) evidenceBrooks NP. Central Cord Syndrome. Neurosurgery clinics of North America. 2017 Jan:28(1):41-47. doi: 10.1016/j.nec.2016.08.002. Epub 2016 Nov 1 [PubMed PMID: 27886881]
Divi SN, Schroeder GD, Mangan JJ, Tadley M, Ramey WL, Badhiwala JH, Fehlings MG, Oner FC, Kandziora F, Benneker LM, Vialle EN, Rajasekaran S, Chapman JR, Vaccaro AR. Management of Acute Traumatic Central Cord Syndrome: A Narrative Review. Global spine journal. 2019 May:9(1 Suppl):89S-97S. doi: 10.1177/2192568219830943. Epub 2019 May 8 [PubMed PMID: 31157150]
Level 3 (low-level) evidenceKhorasanizadeh M, Yousefifard M, Eskian M, Lu Y, Chalangari M, Harrop JS, Jazayeri SB, Seyedpour S, Khodaei B, Hosseini M, Rahimi-Movaghar V. Neurological recovery following traumatic spinal cord injury: a systematic review and meta-analysis. Journal of neurosurgery. Spine. 2019 Feb 15:():1-17. doi: 10.3171/2018.10.SPINE18802. Epub 2019 Feb 15 [PubMed PMID: 30771786]
Level 1 (high-level) evidenceEmos MC, Agarwal S. Neuroanatomy, Upper Motor Neuron Lesion. StatPearls. 2023 Jan:(): [PubMed PMID: 30725990]
Dimova V, Birklein F. [Complex regional pain syndrome (CRPS) : An update]. Der Anaesthesist. 2019 Feb:68(2):115-128. doi: 10.1007/s00101-019-0539-5. Epub [PubMed PMID: 30719529]
Butterfield MC, DeBlieux P, Palacios E. Man in a barrel: acute central cord syndrome after minor injury. The Journal of emergency medicine. 2015 Mar:48(3):333-4. doi: 10.1016/j.jemermed.2014.10.024. Epub 2014 Dec 17 [PubMed PMID: 25534252]
Level 3 (low-level) evidenceSCHNEIDER RC, THOMPSON JM, BEBIN J. The syndrome of acute central cervical spinal cord injury. Journal of neurology, neurosurgery, and psychiatry. 1958 Aug:21(3):216-27 [PubMed PMID: 13576174]
TAYLOR AR. The mechanism of injury to the spinal cord in the neck without damage to vertebral column. The Journal of bone and joint surgery. British volume. 1951 Nov:33-B(4):543-7 [PubMed PMID: 14880573]
SCHNEIDER RC, CHERRY G, PANTEK H. The syndrome of acute central cervical spinal cord injury; with special reference to the mechanisms involved in hyperextension injuries of cervical spine. Journal of neurosurgery. 1954 Nov:11(6):546-77 [PubMed PMID: 13222164]
Quencer RM, Bunge RP, Egnor M, Green BA, Puckett W, Naidich TP, Post MJ, Norenberg M. Acute traumatic central cord syndrome: MRI-pathological correlations. Neuroradiology. 1992:34(2):85-94 [PubMed PMID: 1603319]
Dydyk AM, Givler A. Central Pain Syndrome. StatPearls. 2023 Jan:(): [PubMed PMID: 31971703]
Arul K, Ge L, Ikpeze T, Baldwin A, Mesfin A. Traumatic spinal cord injuries in geriatric population: etiology, management, and complications. Journal of spine surgery (Hong Kong). 2019 Mar:5(1):38-45. doi: 10.21037/jss.2019.02.02. Epub [PubMed PMID: 31032437]
McKinley W, Santos K, Meade M, Brooke K. Incidence and outcomes of spinal cord injury clinical syndromes. The journal of spinal cord medicine. 2007:30(3):215-24 [PubMed PMID: 17684887]
Level 2 (mid-level) evidencePeterson MD, Kamdar N, Whitney DG, Ng S, Chiodo A, Tate DG. Psychological morbidity and chronic disease among adults with nontraumatic spinal cord injuries: a cohort study of privately insured beneficiaries. The spine journal : official journal of the North American Spine Society. 2019 Oct:19(10):1680-1686. doi: 10.1016/j.spinee.2019.05.591. Epub 2019 May 31 [PubMed PMID: 31153961]
Badhiwala JH, Ahuja CS, Fehlings MG. Time is spine: a review of translational advances in spinal cord injury. Journal of neurosurgery. Spine. 2018 Dec 20:30(1):1-18. doi: 10.3171/2018.9.SPINE18682. Epub [PubMed PMID: 30611186]
Level 3 (low-level) evidenceHashmi SZ, Marra A, Jenis LG, Patel AA. Current Concepts: Central Cord Syndrome. Clinical spine surgery. 2018 Dec:31(10):407-412. doi: 10.1097/BSD.0000000000000731. Epub [PubMed PMID: 30346310]
Baude M, Nielsen JB, Gracies JM. The neurophysiology of deforming spastic paresis: A revised taxonomy. Annals of physical and rehabilitation medicine. 2019 Nov:62(6):426-430. doi: 10.1016/j.rehab.2018.10.004. Epub 2018 Nov 28 [PubMed PMID: 30500361]
Wang TY, Park C, Zhang H, Rahimpour S, Murphy KR, Goodwin CR, Karikari IO, Than KD, Shaffrey CI, Foster N, Abd-El-Barr MM. Management of Acute Traumatic Spinal Cord Injury: A Review of the Literature. Frontiers in surgery. 2021:8():698736. doi: 10.3389/fsurg.2021.698736. Epub 2021 Dec 13 [PubMed PMID: 34966774]
Jimenez O, Marcillo A, Levi AD. A histopathological analysis of the human cervical spinal cord in patients with acute traumatic central cord syndrome. Spinal cord. 2000 Sep:38(9):532-7 [PubMed PMID: 11035473]
Level 3 (low-level) evidenceSacco E. [Physiopathology of overactive bladder syndrome]. Urologia. 2012:79(1):24-35. doi: 10.5301/RU.2012.8972. Epub [PubMed PMID: 22287269]
Level 3 (low-level) evidenceRyken TC, Hadley MN, Walters BC, Aarabi B, Dhall SS, Gelb DE, Hurlbert RJ, Rozzelle CJ, Theodore N. Radiographic assessment. Neurosurgery. 2013 Mar:72 Suppl 2():54-72. doi: 10.1227/NEU.0b013e318276edee. Epub [PubMed PMID: 23417179]
Yue JK, Winkler EA, Rick JW, Deng H, Partow CP, Upadhyayula PS, Birk HS, Chan AK, Dhall SS. Update on critical care for acute spinal cord injury in the setting of polytrauma. Neurosurgical focus. 2017 Nov:43(5):E19. doi: 10.3171/2017.7.FOCUS17396. Epub [PubMed PMID: 29088951]
Fehlings MG, Tetreault LA, Wilson JR, Aarabi B, Anderson P, Arnold PM, Brodke DS, Burns AS, Chiba K, Dettori JR, Furlan JC, Hawryluk G, Holly LT, Howley S, Jeji T, Kalsi-Ryan S, Kotter M, Kurpad S, Marino RJ, Martin AR, Massicotte E, Merli G, Middleton JW, Nakashima H, Nagoshi N, Palmieri K, Singh A, Skelly AC, Tsai EC, Vaccaro A, Yee A, Harrop JS. A Clinical Practice Guideline for the Management of Patients With Acute Spinal Cord Injury and Central Cord Syndrome: Recommendations on the Timing (≤24 Hours Versus }24 Hours) of Decompressive Surgery. Global spine journal. 2017 Sep:7(3 Suppl):195S-202S. doi: 10.1177/2192568217706367. Epub 2017 Sep 5 [PubMed PMID: 29164024]
Level 1 (high-level) evidenceAhuja CS, Schroeder GD, Vaccaro AR, Fehlings MG. Spinal Cord Injury-What Are the Controversies? Journal of orthopaedic trauma. 2017 Sep:31 Suppl 4():S7-S13. doi: 10.1097/BOT.0000000000000943. Epub [PubMed PMID: 28816870]
Consortium for Spinal Cord Medicine. Early acute management in adults with spinal cord injury: a clinical practice guideline for health-care professionals. The journal of spinal cord medicine. 2008:31(4):403-79 [PubMed PMID: 18959359]
Level 1 (high-level) evidencePouw MH, van Middendorp JJ, van Kampen A, Hirschfeld S, Veth RP, EM-SCI study group, Curt A, Hosman AJ, van de Meent H. Diagnostic criteria of traumatic central cord syndrome. Part 1: a systematic review of clinical descriptors and scores. Spinal cord. 2010 Sep:48(9):652-6. doi: 10.1038/sc.2009.155. Epub 2010 Jan 5 [PubMed PMID: 20048754]
Level 1 (high-level) evidenceGalvagno SM Jr, Nahmias JT, Young DA. Advanced Trauma Life Support(®) Update 2019: Management and Applications for Adults and Special Populations. Anesthesiology clinics. 2019 Mar:37(1):13-32. doi: 10.1016/j.anclin.2018.09.009. Epub 2018 Dec 27 [PubMed PMID: 30711226]
Fouad K, Popovich PG, Kopp MA, Schwab JM. The neuroanatomical-functional paradox in spinal cord injury. Nature reviews. Neurology. 2021 Jan:17(1):53-62. doi: 10.1038/s41582-020-00436-x. Epub 2020 Dec 11 [PubMed PMID: 33311711]
Lee YS, Kim KT, Kwon BK. Hemodynamic Management of Acute Spinal Cord Injury: A Literature Review. Neurospine. 2021 Mar:18(1):7-14. doi: 10.14245/ns.2040144.072. Epub 2020 Nov 17 [PubMed PMID: 33211951]
Evaniew N, Noonan VK, Fallah N, Kwon BK, Rivers CS, Ahn H, Bailey CS, Christie SD, Fourney DR, Hurlbert RJ, Linassi AG, Fehlings MG, Dvorak MF, RHSCIR Network. Methylprednisolone for the Treatment of Patients with Acute Spinal Cord Injuries: A Propensity Score-Matched Cohort Study from a Canadian Multi-Center Spinal Cord Injury Registry. Journal of neurotrauma. 2015 Nov 1:32(21):1674-83. doi: 10.1089/neu.2015.3963. Epub 2015 Jul 17 [PubMed PMID: 26065706]
Fehlings MG, Tetreault LA, Wilson JR, Kwon BK, Burns AS, Martin AR, Hawryluk G, Harrop JS. A Clinical Practice Guideline for the Management of Acute Spinal Cord Injury: Introduction, Rationale, and Scope. Global spine journal. 2017 Sep:7(3 Suppl):84S-94S. doi: 10.1177/2192568217703387. Epub 2017 Sep 5 [PubMed PMID: 29164036]
Level 1 (high-level) evidenceWilson JR,Witiw CD,Badhiwala J,Kwon BK,Fehlings MG,Harrop JS, Early Surgery for Traumatic Spinal Cord Injury: Where Are We Now? Global spine journal. 2020 Jan; [PubMed PMID: 31934526]
Zheng C, Yu Q, Shan X, Zhu Y, Lyu F, Ma X, Zhou S, Jiang J. Early Surgical Decompression Ameliorates Dysfunction of Spinal Motor Neuron in Patients With Acute Traumatic Central Cord Syndrome: An Ambispective Cohort Analysis. Spine. 2020 Jul 15:45(14):E829-E838. doi: 10.1097/BRS.0000000000003447. Epub [PubMed PMID: 32097277]
Godzik J, Dalton J, Hemphill C, Walker C, Chapple K, Cook A, Uribe JS, Turner JD. Early surgical intervention among patients with acute central cord syndrome is not associated with higher mortality and morbidity. Journal of spine surgery (Hong Kong). 2019 Dec:5(4):466-474. doi: 10.21037/jss.2019.09.26. Epub [PubMed PMID: 32042997]
Yelamarthy PKK, Chhabra HS, Vaccaro A, Vishwakarma G, Kluger P, Nanda A, Abel R, Tan WF, Gardner B, Chandra PS, Chatterjee S, Kahraman S, Naderi S, Basu S, Theron F. Management and prognosis of acute traumatic cervical central cord syndrome: systematic review and Spinal Cord Society-Spine Trauma Study Group position statement. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2019 Oct:28(10):2390-2407. doi: 10.1007/s00586-019-06085-z. Epub 2019 Jul 31 [PubMed PMID: 31367852]
Level 1 (high-level) evidenceA MS, V TS, B DS. Cruciate Paralysis in a 20- year -old Male with an Undisplaced Type III Odontoid Fracture. Journal of orthopaedic case reports. 2016 Apr-Jun:6(2):40-42. doi: 10.13107/jocr.2250-0685.424. Epub [PubMed PMID: 28111622]
Level 3 (low-level) evidenceHopkins B, Khanna R, Dahdaleh NS. Revisiting cruciate paralysis: A case report and systematic review. Journal of craniovertebral junction & spine. 2016 Oct-Dec:7(4):265-272 [PubMed PMID: 27891037]
Level 3 (low-level) evidenceLevi AD, Tator CH, Bunge RP. Clinical syndromes associated with disproportionate weakness of the upper versus the lower extremities after cervical spinal cord injury. Neurosurgery. 1996 Jan:38(1):179-83; discussion 183-5 [PubMed PMID: 8747967]
Level 3 (low-level) evidenceNasri A, Kacem I, Sidhom Y, Djebara MB, Gargouri A, Gouider R. Isolated spinal cord compression syndrome revealing delayed extensive superficial siderosis of the central nervous system secondary to cervical root avulsion. The journal of spinal cord medicine. 2018 Jul:41(4):490-495. doi: 10.1080/10790268.2017.1329053. Epub 2017 Jun 5 [PubMed PMID: 28580859]
Edeiken-Monroe B, Wagner LK, Harris JH Jr. Hyperextension dislocation of the cervical spine. AJR. American journal of roentgenology. 1986 Apr:146(4):803-8 [PubMed PMID: 3485356]
Roth EJ, Lawler MH, Yarkony GM. Traumatic central cord syndrome: clinical features and functional outcomes. Archives of physical medicine and rehabilitation. 1990 Jan:71(1):18-23 [PubMed PMID: 2297304]
Level 2 (mid-level) evidenceWiginton JG 4th, Brazdzionis J, Mohrdar C, Sweiss R, Lawandy S. Spinal Cord Reperfusion Injury: Case Report, Review of the Literature, and Future Treatment Strategies. Cureus. 2019 Jul 30:11(7):e5279. doi: 10.7759/cureus.5279. Epub 2019 Jul 30 [PubMed PMID: 31576271]
Level 3 (low-level) evidenceSchlaff CD, Sack KD, Elliott RJ, Rosner MK. Early Experience with Electric Scooter Injuries Requiring Neurosurgical Evaluation in District of Columbia: A Case Series. World neurosurgery. 2019 Dec:132():202-207. doi: 10.1016/j.wneu.2019.08.237. Epub 2019 Sep 7 [PubMed PMID: 31505288]
Level 2 (mid-level) evidenceAito S, D'Andrea M, Werhagen L, Farsetti L, Cappelli S, Bandini B, Di Donna V. Neurological and functional outcome in traumatic central cord syndrome. Spinal cord. 2007 Apr:45(4):292-7 [PubMed PMID: 16773038]
Level 2 (mid-level) evidenceBlasetti G, Pavese C, Maier DD, Weidner N, Rupp R, Abel R, Yorck BK, Jiri K, Curt A, Molinari M, Schubert M, Scivoletto G. Comparison of outcomes between people with and without central cord syndrome. Spinal cord. 2020 Dec:58(12):1263-1273. doi: 10.1038/s41393-020-0491-x. Epub 2020 Jun 2 [PubMed PMID: 32488195]
Segal DN, Grabel ZJ, Heller JG, Rhee JM, Michael KW, Yoon ST, Jain A. Epidemiology and treatment of central cord syndrome in the United States. Journal of spine surgery (Hong Kong). 2018 Dec:4(4):712-716. doi: 10.21037/jss.2018.11.02. Epub [PubMed PMID: 30714002]
Yousefifard M, Rahimi-Movaghar V, Baikpour M, Ghelichkhani P, Hosseini M, Jafari A, Aziznejad H, Tafakhori A. Early versus late spinal decompression surgery in treatment of traumatic spinal cord injuries; a systematic review and meta-analysis. Emergency (Tehran, Iran). 2017:5(1):e37 [PubMed PMID: 28286844]
Level 1 (high-level) evidenceOSCIS investigators, Chikuda H, Koyama Y, Matsubayashi Y, Ogata T, Ohtsu H, Sugita S, Sumitani M, Kadono Y, Miura T, Tanaka S, Akiyama T, Ando K, Anno M, Azuma S, Endo K, Endo T, Fujiyoshi T, Furuya T, Hayashi H, Higashikawa A, Hiyama A, Horii C, Iimoto S, Iizuka Y, Ikuma H, Imagama S, Inokuchi K, Inoue H, Inoue T, Ishii K, Ishii M, Ito T, Itoi A, Iwamoto K, Iwasaki M, Kaito T, Kato T, Katoh H, Kawaguchi Y, Kawano O, Kimura A, Kobayashi K, Koda M, Komatsu M, Kumagai G, Maeda T, Makino T, Mannoji C, Masuda K, Masuda K, Matsumoto K, Matsumoto M, Matsunaga S, Matsuyama Y, Mieda T, Miyoshi K, Mochida J, Moridaira H, Motegi H, Nakagawa Y, Nohara Y, Oae K, Ogawa S, Okazaki R, Okuda A, Onishi E, Ono A, Oshima M, Oshita Y, Saita K, Sasao Y, Sato K, Sawakami K, Seichi A, Seki S, Shigematsu H, Suda K, Takagi Y, Takahashi M, Takahashi R, Takasawa E, Takenaka S, Takeshita K, Takeshita Y, Tokioka T, Tokuhashi Y, Tonosu J, Uei H, Wada K, Watanabe M, Yahata T, Yamada K, Yasuda T, Yasui K, Yoshii T. Effect of Early vs Delayed Surgical Treatment on Motor Recovery in Incomplete Cervical Spinal Cord Injury With Preexisting Cervical Stenosis: A Randomized Clinical Trial. JAMA network open. 2021 Nov 1:4(11):e2133604. doi: 10.1001/jamanetworkopen.2021.33604. Epub 2021 Nov 1 [PubMed PMID: 34751757]
Level 1 (high-level) evidenceLi J, Shi D, Hua Z, Wang L. The Assessment of Dynamic Spinal Cord Impingement by Kinematic Magnetic Resonance Imaging in Patients with Traumatic Central Cord Syndrome. Therapeutics and clinical risk management. 2021:17():23-29. doi: 10.2147/TCRM.S288076. Epub 2021 Jan 7 [PubMed PMID: 33447038]
Parthiban J, Zileli M, Sharif SY. Outcomes of Spinal Cord Injury: WFNS Spine Committee Recommendations. Neurospine. 2020 Dec:17(4):809-819. doi: 10.14245/ns.2040490.245. Epub 2020 Dec 31 [PubMed PMID: 33401858]
Aarabi B, Akhtar-Danesh N, Chryssikos T, Shanmuganathan K, Schwartzbauer GT, Simard JM, Olexa J, Sansur CA, Crandall KM, Mushlin H, Kole MJ, Le EJ, Wessell AP, Pratt N, Cannarsa G, Lomangino C, Scarboro M, Aresco C, Oliver J, Caffes N, Carbine S, Mori K. Efficacy of Ultra-Early ({ 12 h), Early (12-24 h), and Late (}24-138.5 h) Surgery with Magnetic Resonance Imaging-Confirmed Decompression in American Spinal Injury Association Impairment Scale Grades A, B, and C Cervical Spinal Cord Injury. Journal of neurotrauma. 2020 Feb 1:37(3):448-457. doi: 10.1089/neu.2019.6606. Epub 2019 Aug 1 [PubMed PMID: 31310155]
Nakajima H, Yokogawa N, Sasagawa T, Ando K, Segi N, Watanabe K, Nori S, Watanabe S, Honjoh K, Funayama T, Eto F, Terashima Y, Hirota R, Furuya T, Yamada T, Inoue G, Kaito T, Kato S, JASA Study Group. Prognostic Factors for Cervical Spinal Cord Injury without Major Bone Injury in Elderly Patients. Journal of neurotrauma. 2022 May:39(9-10):658-666. doi: 10.1089/neu.2021.0351. Epub 2022 Feb 4 [PubMed PMID: 35044252]
Kirshblum S, Snider B, Eren F, Guest J. Characterizing Natural Recovery after Traumatic Spinal Cord Injury. Journal of neurotrauma. 2021 May 1:38(9):1267-1284. doi: 10.1089/neu.2020.7473. Epub 2021 Jan 22 [PubMed PMID: 33339474]
Doulames VM, Plant GW. Induced Pluripotent Stem Cell Therapies for Cervical Spinal Cord Injury. International journal of molecular sciences. 2016 Apr 9:17(4):530. doi: 10.3390/ijms17040530. Epub 2016 Apr 9 [PubMed PMID: 27070598]
Aarabi B, Harrop JS, Tator CH, Alexander M, Dettori JR, Grossman RG, Fehlings MG, Mirvis SE, Shanmuganathan K, Zacherl KM, Burau KD, Frankowski RF, Toups E, Shaffrey CI, Guest JD, Harkema SJ, Habashi NM, Andrews P, Johnson MM, Rosner MK. Predictors of pulmonary complications in blunt traumatic spinal cord injury. Journal of neurosurgery. Spine. 2012 Sep:17(1 Suppl):38-45. doi: 10.3171/2012.4.AOSPINE1295. Epub [PubMed PMID: 22985369]
Biering-Sørensen F, Biering-Sørensen T, Liu N, Malmqvist L, Wecht JM, Krassioukov A. Alterations in cardiac autonomic control in spinal cord injury. Autonomic neuroscience : basic & clinical. 2018 Jan:209():4-18. doi: 10.1016/j.autneu.2017.02.004. Epub 2017 Feb 15 [PubMed PMID: 28228335]
Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Archives of physical medicine and rehabilitation. 2000 Apr:81(4):506-16 [PubMed PMID: 10768544]
. Prevention of Venous Thromboembolism in Individuals with Spinal Cord Injury: Clinical Practice Guidelines for Health Care Providers, 3rd ed.: Consortium for Spinal Cord Medicine. Topics in spinal cord injury rehabilitation. 2016 Summer:22(3):209-240. doi: 10.1310/sci2203-209. Epub [PubMed PMID: 29339863]
Level 1 (high-level) evidenceGibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, Freeman R, Isaacson S, Karabin B, Kuritzky L, Lew M, Low P, Mehdirad A, Raj SR, Vernino S, Kaufmann H. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. Journal of neurology. 2017 Aug:264(8):1567-1582. doi: 10.1007/s00415-016-8375-x. Epub 2017 Jan 3 [PubMed PMID: 28050656]
Level 3 (low-level) evidenceJansen RW, Lipsitz LA. Postprandial hypotension: epidemiology, pathophysiology, and clinical management. Annals of internal medicine. 1995 Feb 15:122(4):286-95 [PubMed PMID: 7825766]
Barber DB, Rogers SJ, Fredrickson MD, Able AC. Midodrine hydrochloride and the treatment of orthostatic hypotension in tetraplegia: two cases and a review of the literature. Spinal cord. 2000 Feb:38(2):109-11 [PubMed PMID: 10762185]
Level 3 (low-level) evidenceDavies B, Bannister R, Sever P. Pressor amines and monoamine-oxidase inhibitors for treatment of postural hypotension in autonomic failure. Limitations and hazards. Lancet (London, England). 1978 Jan 28:1(8057):172-5 [PubMed PMID: 74603]
Groomes TE, Huang CT. Orthostatic hypotension after spinal cord injury: treatment with fludrocortisone and ergotamine. Archives of physical medicine and rehabilitation. 1991 Jan:72(1):56-8 [PubMed PMID: 1985624]
Level 3 (low-level) evidenceOnmez H, Cingoz HT, Kucuksen S, Anliacık E, Yaşar O, Yilmaz H, Salli A. Bilateral upper-extremity deep vein thrombosis following central cord syndrome. The journal of spinal cord medicine. 2013 May:36(3):243-6. doi: 10.1179/2045772313Y.0000000096. Epub [PubMed PMID: 23809596]
Level 3 (low-level) evidenceFehlings MG, Tetreault LA, Aarabi B, Anderson P, Arnold PM, Brodke DS, Burns AS, Chiba K, Dettori JR, Furlan JC, Hawryluk G, Holly LT, Howley S, Jeji T, Kalsi-Ryan S, Kotter M, Kurpad S, Kwon BK, Marino RJ, Martin AR, Massicotte E, Merli G, Middleton JW, Nakashima H, Nagoshi N, Palmieri K, Singh A, Skelly AC, Tsai EC, Vaccaro A, Wilson JR, Yee A, Harrop JS. A Clinical Practice Guideline for the Management of Patients With Acute Spinal Cord Injury: Recommendations on the Type and Timing of Anticoagulant Thromboprophylaxis. Global spine journal. 2017 Sep:7(3 Suppl):212S-220S. doi: 10.1177/2192568217702107. Epub 2017 Sep 5 [PubMed PMID: 29164026]
Level 1 (high-level) evidenceKrassioukov A, Warburton DE, Teasell R, Eng JJ, Spinal Cord Injury Rehabilitation Evidence Research Team. A systematic review of the management of autonomic dysreflexia after spinal cord injury. Archives of physical medicine and rehabilitation. 2009 Apr:90(4):682-95. doi: 10.1016/j.apmr.2008.10.017. Epub [PubMed PMID: 19345787]
Level 1 (high-level) evidenceReaddy WJ, Whetstone WD, Ferguson AR, Talbott JF, Inoue T, Saigal R, Bresnahan JC, Beattie MS, Pan JZ, Manley GT, Dhall SS. Complications and outcomes of vasopressor usage in acute traumatic central cord syndrome. Journal of neurosurgery. Spine. 2015 Nov:23(5):574-580. doi: 10.3171/2015.2.SPINE14746. Epub 2015 Jul 31 [PubMed PMID: 26230417]
Farkas GJ, Burton AM, McMillan DW, Sneij A, Gater DR Jr. The Diagnosis and Management of Cardiometabolic Risk and Cardiometabolic Syndrome after Spinal Cord Injury. Journal of personalized medicine. 2022 Jun 30:12(7):. doi: 10.3390/jpm12071088. Epub 2022 Jun 30 [PubMed PMID: 35887592]
Gilron I, Baron R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clinic proceedings. 2015 Apr:90(4):532-45. doi: 10.1016/j.mayocp.2015.01.018. Epub [PubMed PMID: 25841257]
Levendoglu F, Ogün CO, Ozerbil O, Ogün TC, Ugurlu H. Gabapentin is a first line drug for the treatment of neuropathic pain in spinal cord injury. Spine. 2004 Apr 1:29(7):743-51 [PubMed PMID: 15087796]
Level 1 (high-level) evidenceBates D, Schultheis BC, Hanes MC, Jolly SM, Chakravarthy KV, Deer TR, Levy RM, Hunter CW. A Comprehensive Algorithm for Management of Neuropathic Pain. Pain medicine (Malden, Mass.). 2019 Jun 1:20(Suppl 1):S2-S12. doi: 10.1093/pm/pnz075. Epub [PubMed PMID: 31152178]
Guy SD, Mehta S, Casalino A, Côté I, Kras-Dupuis A, Moulin DE, Parrent AG, Potter P, Short C, Teasell R, Bradbury CL, Bryce TN, Craven BC, Finnerup NB, Harvey D, Hitzig SL, Lau B, Middleton JW, O'Connell C, Orenczuk S, Siddall PJ, Townson A, Truchon C, Widerström-Noga E, Wolfe D, Loh E. The CanPain SCI Clinical Practice Guidelines for Rehabilitation Management of Neuropathic Pain after Spinal Cord: Recommendations for treatment. Spinal cord. 2016 Aug:54 Suppl 1():S14-23. doi: 10.1038/sc.2016.90. Epub [PubMed PMID: 27444715]
Level 1 (high-level) evidenceNorrbrink C, Lundeberg T. Tramadol in neuropathic pain after spinal cord injury: a randomized, double-blind, placebo-controlled trial. The Clinical journal of pain. 2009 Mar-Apr:25(3):177-84. doi: 10.1097/AJP.0b013e31818a744d. Epub [PubMed PMID: 19333166]
Level 1 (high-level) evidenceLi L, Han Y, Li T, Zhou J, Sun C, Xue Y. The analgesic effect of intravenous methylprednisolone on acute neuropathic pain with allodynia due to central cord syndrome: a retrospective study. Journal of pain research. 2018:11():1231-1238. doi: 10.2147/JPR.S160463. Epub 2018 Jun 25 [PubMed PMID: 29983586]
Level 2 (mid-level) evidencevan den Noort JC,Bar-On L,Aertbeliën E,Bonikowski M,Braendvik SM,Broström EW,Buizer AI,Burridge JH,van Campenhout A,Dan B,Fleuren JF,Grunt S,Heinen F,Horemans HL,Jansen C,Kranzl A,Krautwurst BK,van der Krogt M,Lerma Lara S,Lidbeck CM,Lin JP,Martinez I,Meskers C,Metaxiotis D,Molenaers G,Patikas DA,Rémy-Néris O,Roeleveld K,Shortland AP,Sikkens J,Sloot L,Vermeulen RJ,Wimmer C,Schröder AS,Schless S,Becher JG,Desloovere K,Harlaar J, European consensus on the concepts and measurement of the pathophysiological neuromuscular responses to passive muscle stretch. European journal of neurology. 2017 Jul; [PubMed PMID: 28557247]
Level 3 (low-level) evidenceHsieh JT, Wolfe DL, Miller WC, Curt A, SCIRE Research Team. Spasticity outcome measures in spinal cord injury: psychometric properties and clinical utility. Spinal cord. 2008 Feb:46(2):86-95 [PubMed PMID: 17909559]
Bovend'Eerdt TJ, Newman M, Barker K, Dawes H, Minelli C, Wade DT. The effects of stretching in spasticity: a systematic review. Archives of physical medicine and rehabilitation. 2008 Jul:89(7):1395-406. doi: 10.1016/j.apmr.2008.02.015. Epub 2008 Jun 13 [PubMed PMID: 18534551]
Level 1 (high-level) evidenceOttoson D. The effects of temperature on the isolated muscle spindle. The Journal of physiology. 1965 Oct:180(3):636-48 [PubMed PMID: 4221242]
Level 3 (low-level) evidenceBekhet AH,Bochkezanian V,Saab IM,Gorgey AS, The Effects of Electrical Stimulation Parameters in Managing Spasticity After Spinal Cord Injury: A Systematic Review. American journal of physical medicine [PubMed PMID: 30300228]
Level 1 (high-level) evidenceKorzhova J, Sinitsyn D, Chervyakov A, Poydasheva A, Zakharova M, Suponeva N, Chernikova L, Piradov M. Transcranial and spinal cord magnetic stimulation in treatment of spasticity: a literature review and meta-analysis. European journal of physical and rehabilitation medicine. 2018 Feb:54(1):75-84. doi: 10.23736/S1973-9087.16.04433-6. Epub 2016 Dec 22 [PubMed PMID: 28004906]
Level 1 (high-level) evidenceCabahug P, Pickard C, Edmiston T, Lieberman JA. A Primary Care Provider's Guide to Spasticity Management in Spinal Cord Injury. Topics in spinal cord injury rehabilitation. 2020 Summer:26(3):157-165. doi: 10.46292/sci2603-157. Epub [PubMed PMID: 33192042]
Barbeau H, Richards CL, Bédard PJ. Action of cyproheptadine in spastic paraparetic patients. Journal of neurology, neurosurgery, and psychiatry. 1982 Oct:45(10):923-6 [PubMed PMID: 7143011]
Level 1 (high-level) evidenceWhiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30:313(24):2456-73. doi: 10.1001/jama.2015.6358. Epub [PubMed PMID: 26103030]
Level 1 (high-level) evidenceWiener J, Hsieh J, McIntyre A, Teasell R. Effectiveness of 4-Aminopyridine for the Management of Spasticity in Spinal Cord Injury: A Systematic Review. Topics in spinal cord injury rehabilitation. 2018 Fall:24(4):353-362. doi: 10.1310/sci17-00048. Epub 2018 May 3 [PubMed PMID: 30459498]
Level 1 (high-level) evidenceMoeini-Naghani I, Hashemi-Zonouz T, Jabbari B. Botulinum Toxin Treatment of Spasticity in Adults and Children. Seminars in neurology. 2016 Feb:36(1):64-72. doi: 10.1055/s-0036-1571847. Epub 2016 Feb 11 [PubMed PMID: 26866498]
Lui J, Sarai M, Mills PB. Chemodenervation for treatment of limb spasticity following spinal cord injury: a systematic review. Spinal cord. 2015 Apr:53(4):252-64. doi: 10.1038/sc.2014.241. Epub 2015 Jan 13 [PubMed PMID: 25582713]
Level 1 (high-level) evidenceBoviatsis EJ, Kouyialis AT, Korfias S, Sakas DE. Functional outcome of intrathecal baclofen administration for severe spasticity. Clinical neurology and neurosurgery. 2005 Jun:107(4):289-95 [PubMed PMID: 15885386]
Jozefczyk PB. The management of focal spasticity. Clinical neuropharmacology. 2002 May-Jun:25(3):158-73 [PubMed PMID: 12023570]
Madsen PJ, Isaac Chen HC, Lang SS. Neurosurgical Approaches. Physical medicine and rehabilitation clinics of North America. 2018 Aug:29(3):553-565. doi: 10.1016/j.pmr.2018.04.002. Epub 2018 May 29 [PubMed PMID: 30626515]
Nagel SJ,Wilson S,Johnson MD,Machado A,Frizon L,Chardon MK,Reddy CG,Gillies GT,Howard MA 3rd, Spinal Cord Stimulation for Spasticity: Historical Approaches, Current Status, and Future Directions. Neuromodulation : journal of the International Neuromodulation Society. 2017 Jun; [PubMed PMID: 28370802]
Level 3 (low-level) evidenceFauss GNK, Hudson KE, Grau JW. Role of Descending Serotonergic Fibers in the Development of Pathophysiology after Spinal Cord Injury (SCI): Contribution to Chronic Pain, Spasticity, and Autonomic Dysreflexia. Biology. 2022 Feb 1:11(2):. doi: 10.3390/biology11020234. Epub 2022 Feb 1 [PubMed PMID: 35205100]
Viswanath O, Urits I, Burns J, Charipova K, Gress K, McNally A, Urman RD, Welschmeyer A, Berger AA, Kassem H, Sanchez MG, Kaye AD, Eubanks TN, Cornett EM, Ngo AL. Central Neuropathic Mechanisms in Pain Signaling Pathways: Current Evidence and Recommendations. Advances in therapy. 2020 May:37(5):1946-1959. doi: 10.1007/s12325-020-01334-w. Epub 2020 Apr 10 [PubMed PMID: 32291648]
Level 3 (low-level) evidenceGrundy L, Caldwell A, Brierley SM. Mechanisms Underlying Overactive Bladder and Interstitial Cystitis/Painful Bladder Syndrome. Frontiers in neuroscience. 2018:12():931. doi: 10.3389/fnins.2018.00931. Epub 2018 Dec 12 [PubMed PMID: 30618560]
Dulamea AO,Sirbu-Boeti MP,Bleotu C,Dragu D,Moldovan L,Lupescu I,Comi G, Autologous mesenchymal stem cells applied on the pressure ulcers had produced a surprising outcome in a severe case of neuromyelitis optica. Neural regeneration research. 2015 Nov; [PubMed PMID: 26807122]
Level 3 (low-level) evidenceBudisin B, Bradbury CC, Sharma B, Hitzig SL, Mikulis D, Craven C, McGilivray C, Corbie J, Green RE. Traumatic Brain Injury in Spinal Cord Injury: Frequency and Risk Factors. The Journal of head trauma rehabilitation. 2016 Jul-Aug:31(4):E33-42. doi: 10.1097/HTR.0000000000000153. Epub [PubMed PMID: 26394288]
Pandrich MJ, Demetriades AK. Prevalence of concomitant traumatic cranio-spinal injury: a systematic review and meta-analysis. Neurosurgical review. 2020 Feb:43(1):69-77. doi: 10.1007/s10143-018-0988-3. Epub 2018 Jun 7 [PubMed PMID: 29882173]
Level 1 (high-level) evidenceVining RD, Gosselin DM, Thurmond J, Case K, Bruch FR. Interdisciplinary rehabilitation for a patient with incomplete cervical spinal cord injury and multimorbidity: A case report. Medicine. 2017 Aug:96(34):e7837. doi: 10.1097/MD.0000000000007837. Epub [PubMed PMID: 28834891]
Level 3 (low-level) evidenceWilson JR,Tetreault LA,Kwon BK,Arnold PM,Mroz TE,Shaffrey C,Harrop JS,Chapman JR,Casha S,Skelly AC,Holmer HK,Brodt ED,Fehlings MG, Timing of Decompression in Patients With Acute Spinal Cord Injury: A Systematic Review. Global spine journal. 2017 Sep; [PubMed PMID: 29164038]
Level 1 (high-level) evidenceBroomfield A, Zuberi K, Mercer J, Moss G, Finnegan N, Hensman P, Walker R, Bukhari S, Wright NB, Stewart F, Jones SA, Ramirez R. Outcomes from 18 years of cervical spine surgery in MPS IVA: a single centre's experience. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2018 Sep:34(9):1705-1716. doi: 10.1007/s00381-018-3823-9. Epub 2018 Jun 26 [PubMed PMID: 29946810]