Introduction
First introduced as a technique for providing surgical anesthesia in upper abdominal procedures, the celiac plexus block (CPB) has been used for almost a century. Functioning as a versatile multimodal adjunct in the management of abdominal visceral pain, the CPB targets the intricate celiac plexus, encompassing the celiac, superior mesenteric, and aorticorenal ganglia, which form an extensive neural network, often referred to as the solar plexus.[1] This network orchestrates autonomic innervation for various abdominal organs, including the liver, gallbladder, stomach, pancreas, spleen, kidneys, small bowel, and the initial two-thirds of the large bowel.[2]
Primarily, the CPB is deployed to address afferent nociceptive fibers, rendering it valuable as both a diagnostic and therapeutic tool for managing intraabdominal pain.[1][3] CPB is indicated in cases of intractable abdominal pain refractory to less aggressive analgesic interventions and is mainly used in the palliation of pain related to malignant and benign neoplastic conditions affecting abdominal organs, with pancreatic cancer the most prevalent. However, using CPB in chronic pancreatitis remains a subject of debate.[1][4][5]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
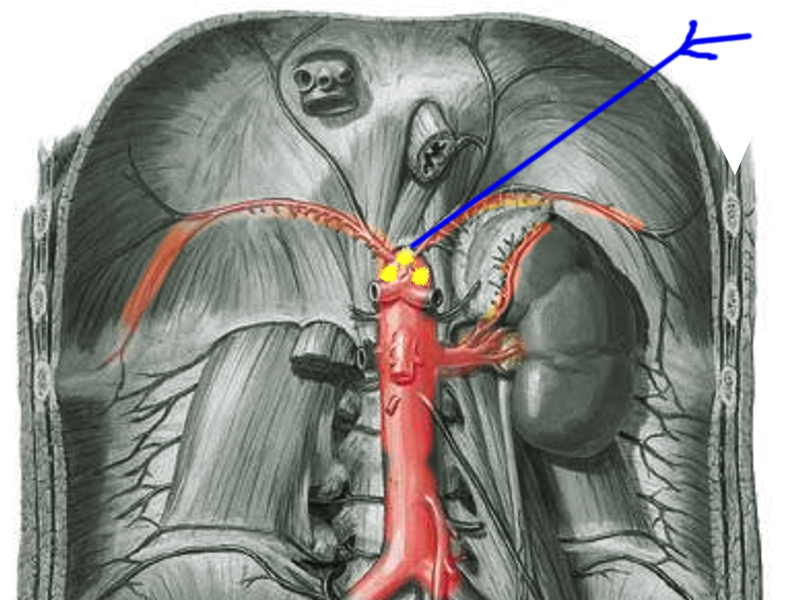
The celiac plexus, or the solar plexus, provides autonomic innervation to a wide range of abdominal organs through a complex network of nerve fibers (see Image. Celiac Plexus Block). These organs include the liver, gallbladder, stomach, pancreas, spleen, kidneys, small intestine, and the first two-thirds of the large intestine. The plexus comprises the celiac, aorticorenal, and superior mesenteric ganglia opposite the aortic diaphragmatic crus at the L1 vertebral level. Further, it contains parasympathetic and sympathetic nerves originating from the anterolateral horns of the spinal cord at T5 to T12 levels.[6][7] As the most extensive plexus of the autonomic nervous system, it plays a crucial role in regulating the functions of these vital organs.[2]
Anatomical Structure
The celiac plexus is anatomically positioned around the celiac artery and superior mesenteric artery, typically spanning the T12 and L1 vertebrae region.[8] The celiac plexus’ location is anterior to the diaphragmatic crura and posterior to the stomach. Comprising a complex network of interconnected nerve fibers and ganglia, the celiac plexus exhibits a mesh-like structure of density. The principal constituents of the celiac plexus encompass the following components:
- Celiac ganglia: The preeminent constituents of the celiac plexus are the right and left celiac ganglia, which represent substantial clusters of nerve cell bodies. These celiac ganglia serve as pivotal centers for both sympathetic and parasympathetic nerve fibers, serving as primary relay stations for bi-directional signals to and from the visceral organs.
- Superior mesenteric ganglion: Situated proximate to the superior mesenteric artery, this ganglion maintains an interconnected relationship with the celiac plexus and innervates the midgut region.
- Inferior mesenteric ganglion: Found near the inferior mesenteric artery, this ganglion is likewise interconnected with the celiac plexus and governs innervation of the hindgut.
In addition to these significant ganglia, the celiac plexus encompasses several smaller ganglia that interconnect with the principal ganglia, collectively contributing to the intricate neural network within this anatomical structure.
Neuroanatomy
The celiac plexus is a complex neural structure encompassing afferent (sensory) and efferent (motor) neural constituents. The efferent components predominantly consist of sympathetic nerve fibers, originating from the spinal levels T5 to T12, coursing through the greater, lesser, and least splanchnic nerves. These sympathetic fibers culminate in synaptic connections with neuronal cell bodies within the celiac ganglia, from which postganglionic fibers intricately innervate the abdominal viscera. In addition to sympathetic elements, the celiac plexus is supplemented by parasympathetic fibers, primarily arising from the vagus nerve (cranial nerve X).
Conversely, the afferent fibers within the celiac plexus serve as conduits for transmitting visceral pain and other sensory inputs from the abdominal organs to the central nervous system. Notably, these afferent fibers traverse analogous anatomical pathways to their efferent counterparts, transmitting neural signals back to the spinal cord and relaying them to the cerebral cortex.
Physiological Functions
The celiac plexus assumes a central role in regulating diverse visceral functions, including:
- Gastrointestinal functions
- The celiac plexus intricately innervates a spectrum of abdominal organs, including the stomach, liver, gallbladder, pancreas, and spleen, as well as segments of the small and large intestines. In doing so, it exercises sophisticated control over vital physiological processes. This control extends to regulating digestive enzyme secretion, modulating gastrointestinal tract motility, and orchestrating blood perfusion to these essential organs.
- Vascular regulation
- The celiac plexus exerts precise governance over the circulatory dynamics within the abdominal milieu; its authority extends to the meticulous oversight of blood flow to the abdominal organs, a function achieved through regulating blood vessel constriction and dilation. This pivotal role in vascular regulation underscores its significance in maintaining the circulatory equilibrium of these critical structures.
- Pain perception
- The celiac plexus is a conduit for transmitting visceral pain signals from the abdominal organs to the central nervous system. This pivotal function endows it with a prominent position in the realm of clinical interventions, particularly in the context of pain management strategies. Notably, in cases characterized by persistent abdominal pain or malignancies, the celiac plexus emerges as a prime target for therapeutic interventions, such as celiac plexus block or neurolysis, designed to alleviate debilitating discomfort and improve the overall quality of life for afflicted individuals.
Indications
CPB emerges as a crucial therapeutic intervention in cases marked by recalcitrant abdominal pain, which remains refractory to less aggressive analgesic measures, often necessitating high-dose opioid therapies.[9][10] The scope of CPB encompasses the selective targeting of visceral afferent pain fibers originating from a spectrum of abdominal structures, including but not limited to the liver, gallbladder, omentum, pancreas, mesentery, and the gastrointestinal tract, spanning from the stomach to the mid-transverse colon. Consequently, CPB holds promise as an efficacious modality for mitigating pain associated with both malignant and benign neoplastic conditions affecting diverse abdominal organs, including the pancreas, biliary tree, retroperitoneal structures, and other abdominal entities. Notably, one of the most prevalent indications for CPB pertains to ameliorating abdominal pain in the context of pancreatic cancer. Recognizing the ongoing debate within the medical community about the use of CPB to alleviate pain secondary to chronic pancreatitis is crucial.
In selecting an appropriate injectate for CPB, a judicious approach dictates the utilization of local anesthetics and steroids for patients afflicted by benign pathologies. In contrast, neurolytic celiac plexus blocks are predominantly applied in managing pain associated with neoplastic conditions.[4][11]
Pancreatic cancer, the second most prevalent abdominal cancer in the United States, often subjects individuals to substantial and inadequately controlled pain despite conventional medication regimens. CPB, particularly when coupled with neurolysis, emerges as a transformative intervention capable of not only alleviating the pain experienced by these patients but also curtailing overall opioid consumption and enhancing patient satisfaction.[3]
The CPB is an invaluable therapeutic approach for managing chronic abdominal pain, particularly in malignant and benign neoplastic disorders affecting abdominal organs, exemplified by pancreatic cancer. By selectively targeting afferent nociceptive fibers within the celiac plexus, CPB affords substantial pain relief and offers an enhanced quality of life for patients with otherwise intractable pain.[1][3]
Contraindications
Administering a CPB necessitates meticulous consideration of patient-related factors, particularly in cases where individuals harbor comorbidities and underlying medical conditions that may influence the suitability of this procedure.[12][13] Patients undergoing CPB may often exhibit a constellation of health concerns that mandate careful evaluation before undertaking the block. Recognizing certain patient populations, such as those undergoing cancer treatments like chemotherapy or radiation, may exhibit compromised immune function, rendering them particularly vulnerable to post-injection infections is imperative. Moreover, individuals may present with coagulopathies or thrombocytopenia, elevating the risk of significant hemorrhagic complications. Even with the use of imaging modalities such as computed tomography (CT) guidance or fluoroscopy, the potential for vascular injury or accidental trauma to adjacent tissues remains an inherent risk, particularly when dealing with patients who have cancer—where the possibility of underlying metastatic disease always warrants consideration.[1] A comprehensive understanding of contraindications, encompassing patient consent, coagulation status, infectious processes, and anatomical considerations, is imperative to ensure the judicious application of this therapeutic modality.[1]
Equipment
Recent advancements in imaging have improved the safety and efficacy of CPB. CT-guided single celiac plexus neurolysis was shown to be efficacious and safe in a systematic review and meta-analysis (see Image. Neurolytic Celiac Plexus Block).[14] CT or fluoroscopic guidance can help minimize the risk of complications, though vascular injury or trauma to surrounding tissues may still occur.[1]
The following is needed:
- C-arm
- Lead apron and thyroid shield
- OR table capable of allowing a C-arm underneath
- Step stool: some patients (especially those with chronic pain and limited mobility) might need step stools to get on the table
- A pointer that can be identified in “scout” fluoro images to identify entry points when starting procedures
- A single fenestrated drape to maintain sterile conditions
- Prep solution to sterilize the operative area
- 5 mL 1% lidocaine in a 5 mL syringe for local anesthetic
- 25 g x 1.5″ hypodermic needle for subcutaneous local anesthetic injection
- 3 mL contrast in a 3 mL syringe
- 22 g x 7″ quincke needle x 2 as your primary needles
- 10 mL 0.25% bupivacaine in a 10 mL syringe for injectate
Personnel
Personnel necessary to perform a cervical plexus block:
- Clinician adequately trained and experienced to perform the block
- Surgical tech or assistant can be used at the clinician's discretion
- Radiology technician to run the C-arm
- Circulating nurse to monitor the patient and their vitals
Technique or Treatment
When performing a CPB, initiating the procedure with a diagnostic block involving a local anesthetic is imperative. This preliminary step corroborates the achievement of pain relief consequent to the block. Notably, within the extant medical literature, alcohol stands as the preferred agent for effectuating neurolytic celiac plexus blocks, owing to its purported superior efficacy.[5]
Various approaches to CPB have been described, with the 2 most widely employed methodologies being the posterior and anterior para-aortic techniques, each elaborated upon below. An alternative technique known as cryoneurolysis, integrated within the framework of CPB, has been documented.[15] Alternatively, the execution of CPB can be facilitated via endoscopic ultrasound guidance, offering an alternate approach to the procedure.[16][17][18]
A CPB must be performed with some form of imaging guidance to facilitate the identification of the target and surrounding structures. Most providers perform the CPB under CT or fluoroscopic guidance. However, some study results show that endoscopic ultrasound guidance has better outcomes, a longer duration of pain control, and reduced costs.[19][20]
Posterior Para-Aortic Approach
Position the patient in a prone posture with adequate abdominal support to enhance thoracic kyphosis. Take note of the following anatomical landmarks for reference: the midline spine and vertebral bodies, iliac crests, 12th rib, and the lateral border of the paraspinal muscles. Subsequently, proceed to disinfect the skin and drape the area following established standard protocols.
Using radiographic tools, precisely locate and identify the T12 and L1 vertebral bodies. Also, identify and mark the inferior border of the 12th rib. Create a skin wheal by injecting a local anesthetic solution at the inferior border of the 12th rib, typically positioned approximately 6 to 8 cm lateral to the midline. Employ an appropriately designed spinal-type needle and insert it at a 45-degree angle, commencing from the posterior aspect and advancing towards the anterior surface of the T12 to the L1 intervertebral space until it makes contact with the vertebral body.
Upon establishing contact with the vertebral body, advance the spinal needle approximately 1 cm further, traversing into the prevertebral fascial plane. Subsequently, ascertain the precise needle placement by visualizing the contrast spread under the guidance of CT or fluoroscopic imaging. With the needle appropriately positioned, perform a diagnostic block with a local anesthetic or a therapeutic neurolytic block.
A unilateral block approach, particularly on the left side, has demonstrated reliability for single-sided interventions. However, in cases where a unilateral block proves insufficient, a bilateral approach may be warranted to achieve the desired therapeutic effect.
Anterior Para-Aortic Approach
With the patient in a supine position, chart the intended needle trajectory meticulously, employing the guidance of either CT or fluoroscopic imaging. Subsequently, adhere to standard protocols for skin disinfection and draping. Administer a skin wheal by injecting a local anesthetic into the abdominal wall anterior to the T12 vertebral body. Then, proceed by inserting an appropriately designed spinal-type needle and directing it towards the abdominal aorta, which typically traverses through abdominal viscera, including the bowel, stomach, and liver. Ensure the final needle tip placement is anterior to the aorta and the diaphragmatic crura. Subsequently, introduce the neurolytic agent into the antecrural space.
Considering the anterior approach, particularly in patients afflicted by advanced disease or those who may experience abdominal discomfort, cannot maintain a prone position, or have recently undergone abdominal surgery, is imperative.
This approach often offers expeditious execution compared to the posterior para-aortic method and is generally well-tolerated by patients. Nevertheless, it is essential to exercise caution due to the potential risk of accidental organ injury, which restricts its applicability.
Complications
Complications arising from CPB, although infrequent, are recognized within the medical literature. Nevertheless, there remains a potential for the procedure to yield suboptimal outcomes. The most common complication is orthostatic hypotension, which can be mitigated by implementing appropriate hydration strategies. Beyond orthostatic hypotension, many complications may manifest, even when guided by image-assisted techniques. These complications may encompass, but are not limited to, the following:
- Intravascular injection of the anesthetic agent
- Injury to surrounding tissues, including nerve roots, vasculature, muscles, and nearby organs
- Paresthesias
- Intrathecal or epidural injection of the anesthetic agent
- Bleeding, including retroperitoneal hematoma formation
- Pneumothorax
- Infection with or without abscess formation
- Paraplegia
- Local anesthetic toxicity with the central nervous system and cardiovascular compromise [21]
Furthermore, diarrhea represents a recognized complication of CPB; this arises from the profound sympathetic denervation of the gastrointestinal tract, which, in turn, engenders heightened peristalsis due to unopposed parasympathetic nervous system activity.[22]
Clinical Significance
Pancreatic cancer is the second most prevalent abdominal cancer in the United States. Those with pancreatic cancer often endure significant pain that medication alone cannot alleviate. Utilizing a CPB with neurolysis has proven successful in minimizing pain, reducing opioid usage, and enhancing patient contentment.[23]
Enhancing Healthcare Team Outcomes
When administering a CPB, collaborating with a multidisciplinary healthcare team comprising physicians, nurses, pharmacists, and other health professionals is pivotal in ensuring patient-centered care and optimizing treatment outcomes. Effective interprofessional communication and collaboration are essential components of this endeavor. The primary care providers, including physicians, physician assistants, and nurse practitioners, must consult the appropriate clinicians who perform this procedure for patients who would benefit from a CPB. Physicians, primarily interventional radiologists or anesthesiologists, must possess the technical skills and a thorough understanding of the CPB procedure, including patient selection, anatomical landmarks, and the choice of anesthetic agents or neurolytic substances. Nurses are tasked with patient assessment, preparation, and monitoring during and after the procedure, emphasizing patient comfort and safety. They do this partly by ensuring all consents are signed after specific risks, benefits, and expectations have been explained in detail to the patient and a proper procedural timeout is performed. The patient’s name, date of birth, pertinent allergies, and procedure should be confirmed by all providers and the patient. Site verification and laterality must be confirmed. Implementing guidelines and checklists has proven to reduce the occurrence of adverse outcomes such as surgeon-anesthesiologist miscommunications and wrong-site or wrong-side regional anesthesia procedures.[24][25] Pharmacists contribute by ensuring appropriate medication management and minimizing the risk of drug interactions or complications.
Ethical considerations are paramount, and healthcare professionals must engage in shared decision-making with patients, respecting their autonomy and providing comprehensive information about the procedure’s risks and benefits. Additionally, care coordination is critical to ensure seamless transitions between pre-procedure evaluation, the CPB itself, and post-procedure follow-up. The team collaboratively addresses any complications or adverse events promptly, with a focus on patient-centered care and the overall enhancement of team performance. By synergizing their skills, strategies, ethical principles, and responsibilities, these healthcare professionals work together to facilitate safe, effective, and patient-centered CPB, ultimately improving patient outcomes and fostering a culture of excellence in healthcare delivery.
Media
(Click Image to Enlarge)
References
Urits I, Jones MR, Orhurhu V, Peck J, Corrigan D, Hubble A, Andrews M, Feng R, Manchikanti L, Kaye AD, Kaye RJ, Viswanath O. A Comprehensive Review of the Celiac Plexus Block for the Management of Chronic Abdominal Pain. Current pain and headache reports. 2020 Jun 11:24(8):42. doi: 10.1007/s11916-020-00878-4. Epub 2020 Jun 11 [PubMed PMID: 32529305]
Vig S, Bhan S, Bhatnagar S. Celiac Plexus Block - An Old Technique with New Developments. Pain physician. 2021 Aug:24(5):379-398 [PubMed PMID: 34323439]
Okita M, Otani K, Gibo N, Matsui S. Systematic review and meta-analysis of celiac plexus neurolysis for abdominal pain associated with unresectable pancreatic cancer. Pain practice : the official journal of World Institute of Pain. 2022 Sep:22(7):652-661. doi: 10.1111/papr.13143. Epub 2022 Jul 1 [PubMed PMID: 35748531]
Level 1 (high-level) evidenceGunduz OH, Kenis-Coskun O. Ganglion blocks as a treatment of pain: current perspectives. Journal of pain research. 2017:10():2815-2826. doi: 10.2147/JPR.S134775. Epub 2017 Dec 14 [PubMed PMID: 29276402]
Level 3 (low-level) evidenceCornman-Homonoff J, Holzwanger DJ, Lee KS, Madoff DC, Li D. Celiac Plexus Block and Neurolysis in the Management of Chronic Upper Abdominal Pain. Seminars in interventional radiology. 2017 Dec:34(4):376-386. doi: 10.1055/s-0037-1608861. Epub 2017 Dec 14 [PubMed PMID: 29249862]
Ehrhardt JD, Weber C, Carey FJ, Lopez-Ojeda W. Anatomy, Thorax, Greater Splanchnic Nerves. StatPearls. 2024 Jan:(): [PubMed PMID: 29763202]
Rosland JH, Geitung JT. CT guided neurolytic blockade of the coeliac plexus in patients with advanced and intractably painful pancreatic cancer. Scandinavian journal of pain. 2018 Apr 25:18(2):247-251. doi: 10.1515/sjpain-2017-0185. Epub [PubMed PMID: 29794300]
du Plessis M, Loukas M. A comprehensive study of the abdominal ganglia part 1: Celiac, phrenic and superior mesenteric ganglia. Clinical anatomy (New York, N.Y.). 2022 Oct:35(7):998-1006. doi: 10.1002/ca.23894. Epub 2022 May 12 [PubMed PMID: 35484764]
Ambai VT, Singh V, Boorman DW, Neufeld NJ. Celiac plexus neurolysis for abdominal cancers: going beyond pancreatic cancer pain. Pain reports. 2021:6(1):e930. doi: 10.1097/PR9.0000000000000930. Epub 2021 May 12 [PubMed PMID: 34712884]
Podgorski Iii E, Driver L, Gulati A, Abdi S. Catheter-based Techniques for Terminal Cancer Pain: A Review of Nonneuraxial Interventions with Clinical Implications for End-of-Life Pain Management. Pain physician. 2021 Nov:24(7):E1137-E1146 [PubMed PMID: 34704723]
Wang D. Image Guidance Technologies for Interventional Pain Procedures: Ultrasound, Fluoroscopy, and CT. Current pain and headache reports. 2018 Jan 26:22(1):6. doi: 10.1007/s11916-018-0660-1. Epub 2018 Jan 26 [PubMed PMID: 29374352]
Mercadante S. Commentary: Interpreting Data of Celiac Plexus Block in Patients with Pancreatic Pain: Timing, Patients, Survival. Pain and therapy. 2022 Sep:11(3):747-751. doi: 10.1007/s40122-022-00396-8. Epub 2022 May 27 [PubMed PMID: 35622239]
Level 3 (low-level) evidenceZylberberg HM, Nagula S, Rustgi SD, Aronson A, Kessel E, Kumta NA, DiMaio CJ, Lucas AL. Celiac Plexus Neurolysis Is Associated With Decreased Survival in Patients With Pancreatic Cancer: A Propensity Score Analysis. Pancreas. 2022 Feb 1:51(2):153-158. doi: 10.1097/MPA.0000000000001992. Epub [PubMed PMID: 35404890]
Matsumoto T, Yoshimatsu R, Osaki M, Miyatake K, Yamanishi T, Yamagami T. Computed tomography-guided single celiac plexus neurolysis analgesic efficacy and safety: a systematic review and meta-analysis. Abdominal radiology (New York). 2022 Nov:47(11):3892-3906. doi: 10.1007/s00261-022-03670-7. Epub 2022 Sep 10 [PubMed PMID: 36087117]
Level 1 (high-level) evidenceChary A, Edalat F. Celiac Plexus Cryoneurolysis. Seminars in interventional radiology. 2022 Apr:39(2):138-141. doi: 10.1055/s-0042-1745762. Epub 2022 Jun 30 [PubMed PMID: 35781989]
Wyse JM, Sahai AV. EUS-guided celiac plexus neurolysis for pancreas cancer - Finally established or still under review? Best practice & research. Clinical gastroenterology. 2022 Sep-Dec:60-61():101809. doi: 10.1016/j.bpg.2022.101809. Epub 2022 Nov 22 [PubMed PMID: 36577532]
Okita M, Otani K, Matsui S. Efficacy of Endoscopic Ultrasound-guided Celiac Plexus Neurolysis for Abdominal Pain in Patients With Unresectable Pancreatic Cancer: Network Meta-analysis of Randomized Controlled Trials. Journal of clinical gastroenterology. 2023 Nov-Dec 01:57(10):1054-1062. doi: 10.1097/MCG.0000000000001773. Epub 2022 Oct 11 [PubMed PMID: 36227001]
Level 1 (high-level) evidenceLi M, Wang Z, Chen Y, Wu Z, Huang X, Wu C, Tian B. EUS-CGN versus EUS-CPN in pancreatic cancer: A qualitative systematic review. Medicine. 2021 Oct 15:100(41):e27103. doi: 10.1097/MD.0000000000027103. Epub [PubMed PMID: 34731101]
Level 1 (high-level) evidenceBang JY, Sutton B, Hawes RH, Varadarajulu S. EUS-guided celiac ganglion radiofrequency ablation versus celiac plexus neurolysis for palliation of pain in pancreatic cancer: a randomized controlled trial (with videos). Gastrointestinal endoscopy. 2019 Jan:89(1):58-66.e3. doi: 10.1016/j.gie.2018.08.005. Epub 2018 Aug 16 [PubMed PMID: 30120957]
Level 1 (high-level) evidenceMinaga K, Takenaka M, Kamata K, Yoshikawa T, Nakai A, Omoto S, Miyata T, Yamao K, Imai H, Sakamoto H, Kitano M, Kudo M. Alleviating Pancreatic Cancer-Associated Pain Using Endoscopic Ultrasound-Guided Neurolysis. Cancers. 2018 Feb 15:10(2):. doi: 10.3390/cancers10020050. Epub 2018 Feb 15 [PubMed PMID: 29462851]
Dos Santos Silva RP, Lopes AJM, Bezerra RB, Andrade RA, Andrade RG, da Costa LMF, de Albuquerque da Cunha Andrade CA. Persistent hypotension and other complications of celiac plexus neurolysis: A case report and literature review. Clinical case reports. 2023 Jun:11(6):e7505. doi: 10.1002/ccr3.7505. Epub 2023 Jun 8 [PubMed PMID: 37305872]
Level 3 (low-level) evidenceYousefshahi F, Tahmasebi M. Long-Lasting Orthostatic Hypotension and Constipation After Celiac Plexus Block; A Case Report. Anesthesiology and pain medicine. 2018 Feb:8(1):e63221. doi: 10.5812/aapm.63221. Epub 2018 Feb 21 [PubMed PMID: 29868459]
Level 3 (low-level) evidenceHochberg U, Ingelmo P, Solé E, Miró J, Rivera G, Perez J. Early Interventional Treatments for Patients with Cancer Pain: A Narrative Review. Journal of pain research. 2023:16():1663-1671. doi: 10.2147/JPR.S405808. Epub 2023 May 18 [PubMed PMID: 37223437]
Level 3 (low-level) evidenceSlocombe P, Pattullo S. A site check prior to regional anaesthesia to prevent wrong-sided blocks. Anaesthesia and intensive care. 2016 Jul:44(4):513-6 [PubMed PMID: 27456184]
Hopping M, Merry AF, Pandit JJ. Exploring performance of, and attitudes to, Stop- and Mock-Before-You-Block in preventing wrong-side blocks. Anaesthesia. 2018 Apr:73(4):421-427. doi: 10.1111/anae.14167. Epub 2017 Dec 27 [PubMed PMID: 29280131]