Introduction
Burkitt lymphoma is an aggressive non-Hodgkin B-cell lymphoma. The disease is associated with Epstein Barr Virus (EBV), human immunodeficiency virus (HIV), and chromosomal translocations that cause the overexpression of oncogene C-MYC.[1] The World Health Organization (WHO) classifies Burkitt lymphoma into 3 clinical groups: endemic, sporadic, and immunodeficiency-related. The endemic form is common in Africa and is linked to malaria and EBV. The sporadic form is observed in the United States and Western Europe. The immunodeficiency-related variant is associated with HIV and, to a lesser extent, organ transplantation. With intense chemotherapy treatment, the disease prognosis is excellent in children but poor in adults.[2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
EBV infection is a known risk factor for Burkitt lymphoma. While nearly all endemic cases are associated with EBV infection, only a small proportion of sporadic cases exhibit evidence of EBV infection.[3] EBNA1 protein is an EBV latent protein expressed in endemic Burkitt lymphoma.[1][4] EBNA2 gene deletion leads to the expression of EBNA3 genes (ie, EBNA3A-C). Tumor cells derived from these cell lines are resistant to apoptosis; thus, EBNA2 might confer a survival advantage for the neoplastic cells. In immune-compromised individuals, EBV-driven B-cell proliferation leads to the accumulation of genetic mutations, particularly the MYC translocation. This drives the uncontrolled growth of B-cells, ultimately resulting in the development of Burkitt lymphoma.
Burkitt lymphoma is also endemic to areas where malaria is holoendemic, eg, Brazil, Papua New Guinea, and equatorial Africa.[5] Studies showed a direct correlation between endemic Burkitt lymphoma and increased Plasmodium falciparum antibody titers.[6]
Immunodeficiency-associated Burkitt lymphoma is linked to HIV. Counterintuitively, the incidence of lymphoma is higher when the patient has a CD4 count >200 and no opportunistic infection.[7] The incidence of Burkitt lymphoma in the HIV-positive population has remained unchanged despite the introduction of effective antiretroviral therapy. How HIV affects the risk of endemic Burkitt lymphoma is unclear.
Epidemiology
Burkitt lymphoma accounts for approximately 1% to 5% of all non-Hodgkin lymphomas.[8] Burkitt lymphoma is more common in Caucasians than in persons of African or Asian descent. As with most types of lymphoma, Burkitt lymphoma is more prevalent in males, with a 3 to 4:1 male-to-female ratio.[9]
The distribution of endemic cases of Burkitt lymphoma in Africa and Papua New Guinea corresponds to areas where malaria and Epstein-Barr virus are prevalent. In children younger than 18, the incidence is approximately 3 to 6 cases per 100,000 children annually. The average age of diagnosis is 6 years.[2]
The sporadic form is localized to North America and Europe, with a median diagnosis age of 30 years.[10] Sporadic Burkitt lymphoma has an annual estimated incidence of 4 per 1 million children younger than 16 years of age, whereas the incidence is 2.5 per 1 million in adults. The average age of diagnosis in pediatric patients is 3 to 12 years of age.
The immunodeficiency-associated variant has an incidence of 22 per 100,000 person-years in the United States.[7]
Pathophysiology
The MYC family comprises regulator genes and oncogenes that encode transcription factors that regulate the cell cycle. Translocations of the c-MYC gene on chromosome 8 are the hallmark of Burkitt lymphoma, occurring in approximately 95% of cases. The t(8;14)(q24;q32) is the most common translocation in Burkitt lymphoma, occurring in 70% to 80%.[1] The MYC oncogene is relocated to be juxtaposed to the promoter sequence of the immunoglobulin IgG heavy chain (IgH) gene, leading to constitutive activation of MYC. Other translocations include t(2;8)(p12; q24) and t(8;22)(q24; q11). The collocation of the MYC oncogene to the heavy chain (14q32), kappa light chain (2p12), or lambda light chain (22q11) of the immunoglobulin gene results in the dysregulation of the MYC. The presence of an MYC rearrangement is not specific to Burkitt lymphoma.[11]
In addition to the characteristic translocation of c-MYC, many gene mutations have been identified, including truncating mutations of ARID1A, amplification of MCL1, truncating alterations of PTEN, NOTCH, and ATM; amplifications of RAF1, MDM2, KRAS, IKBKE, deletion of CDKN2A, and CCND3 activating mutations.[9][12] In normal B-cells, MYC overexpression causes apoptosis via a p53-dependent pathway. In the neoplastic cells of Burkitt lymphoma, mutation of tumor suppressor gene TP53 is not uncommon. Classification systems also identify the following 3 aggressive B-cell lymphoma subtypes that resemble Burkitt lymphoma:
- Burkitt-like lymphoma with 11q aberration
- High-grade B-cell lymphoma with MYC and BCL2 rearrangements
- High-grade B-cell lymphoma, not otherwise specified
In endemic Burkitt lymphoma, EBV latent proteins prevent apoptosis in B-cells containing the c-MYC translocation via EBNA1 protein, BHRF1 protein, EBER transcripts, vIL-10, BZLF1, and LMP1. The prevention of apoptosis likely gives rise to the malignant clone of B-cells. EBV is implicated in promoting genomic instability, telomere dysfunction, and DNA damage. Plasmodium falciparum prompts the reactivation of latent EBV. Additionally, P. falciparum can activate toll-like receptor 9 (TLR9), which induces immunoglobulin-MYC translocations.[13] Cytidine deaminase allows for B-cells to switch from IgM expression to the expression of other immunoglobulin subtypes. Aberrant expression of cytidine deaminase is correlated with c-MYC translocation. The enzyme is not detected in HIV-positive patients who do not have Burkitt lymphoma.
Histopathology
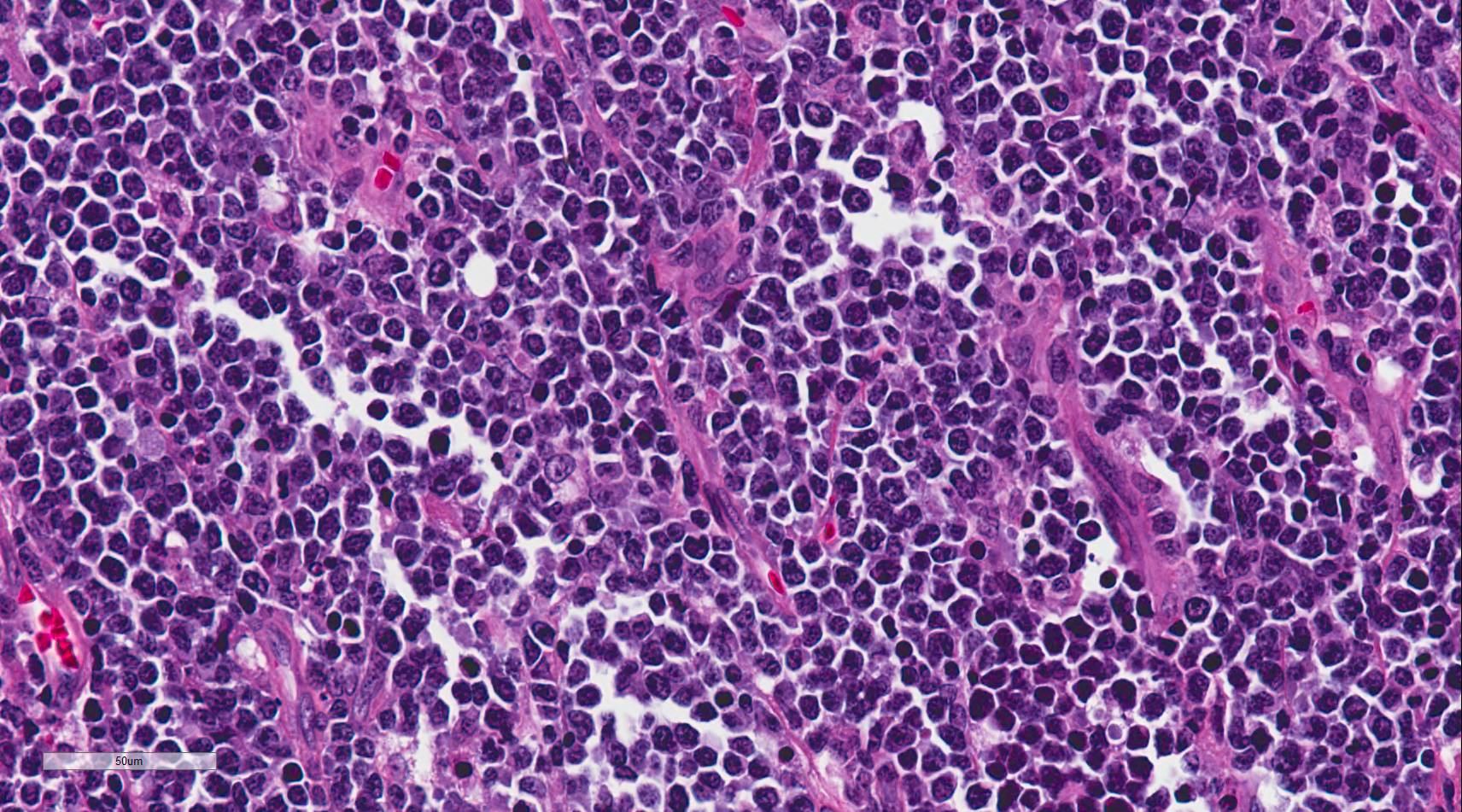
Burkitt lymphoma is an aggressive B-cell lymphoma comprising a monomorphic population of intermediate-sized mature lymphocytes (see Image. Burkitt Lymphoma). The cells contain round nuclei with lacy chromatin and may have ≥1 small nucleoli. The cells characteristically have basophilic cytoplasm with prominent vacuoles.[14] On hematoxylin-eosin-stained sections, the cells have distinct cytoplasmic borders, giving them a molded appearance. Morphologic variants are plasmacytoid or pleomorphic. The neoplastic cells are highly proliferative, which is reflected in the increased number of mitotic figures. Increased cell turnover is countered by increased apoptosis. In tissue, this results in a "starry sky" appearance due to tingible-body macrophages containing cellular debris (see Image. Burkitt Lymphoma, Lymphoblasts).[1][15]
Immunohistochemistry and cytogenetics play a significant role in the diagnosis and management of Burkitt lymphoma. The malignant B-cells express surface IgM and are positive for B-cell markers, including CD19, CD20, CD79a, and PAX5. They are also positive for germinal center markers CD10 and bcl-6 but negative for bcl-2. The neoplastic cells do not express T-cell markers and do not express immature markers TdT or CD34.[16] High Ki67 positivity, nearing 100%, reflects rapid cell turnover, which is an invaluable diagnostic clue.
History and Physical
Patients often present with a rapidly growing mass, elevated lactate dehydrogenase (LDH), and increased uric acid levels because of the tumor’s rapid doubling time.
Endemic Burkitt Lymphoma
Endemic Burkitt lymphoma characteristically presents with an enlarging jaw lesion, periorbital swelling, or genitourinary involvement. Jaw involvement is seen predominantly in children. Bone marrow involvement is present in fewer than 10% of patients at initial diagnosis but frequently occurs as a recurrent or treatment-resistant disease complication.
Sporadic Burkitt Lymphoma
The primary site of sporadic Burkitt lymphoma is typically the abdomen, but the head and neck may also be affected.[1] Patients present with abdominal pain secondary to ileocecal disease, abdominal distention, nausea, vomiting, and gastrointestinal bleeding. Adult patients are more likely to present with constitutional symptoms (ie, fever, weight loss, night sweats). Infrequently, patients have jaw or bone marrow involvement. Burkitt lymphoma rarely involves the mediastinum, central nervous system, testes, skin, thyroid gland, and breast.
Immunodeficiency-Related Burkitt Lymphoma
Immunodeficiency-related Burkitt lymphoma commonly presents with symptoms associated with the underlying immune dysfunction, eg, AIDS, congenital immunodeficiency, or immunosuppression posttransplantation. These cases frequently affect the lymph nodes, bone marrow, and central nervous system.
If the patient has bone marrow involvement of >25% of cellularity, the disease is classified as Burkitt leukemia. A purely leukemic presentation is rare.
Evaluation
Adequate tissue availability is paramount to the diagnosis. Fine-needle aspiration might not provide enough tissue for the diagnosis; thus, excisional biopsy is preferred. Superficial lymph nodes or pleural fluid may be sampled for diagnosis. In developed countries, Burkitt lymphoma is suspected by microscopy or flow cytometry and confirmed by immunohistochemistry and cytogenetics. In areas without access to confirmatory testing, the diagnosis of Burkitt may be made with fine-needle aspiration and clinical correlation.
After a tissue diagnosis is made, bone marrow aspiration, biopsy, and cerebrospinal fluid (CSF) evaluation should be performed to assess the extent of involvement. Computerized tomography (CT) scans of the chest, abdomen, and pelvis are performed initially. Whole-body PET/CT scans should be performed but should not delay therapy. Required lab tests include complete blood count (CBC) with differential, ESR, complete metabolic panel (CMP), PT, PTT, serum lactate dehydrogenase, uric acid, hepatitis B serology, pregnancy testing in women, and HIV testing.[1] In areas with limited resources, ultrasonography may aid in staging.[17]
Treatment / Management
The optimal standard of care for Burkitt lymphoma remains undefined; enrolling patients in well-controlled cooperative clinical trials is strongly preferred. For individuals ineligible for or unwilling to participate in such trials, an intensive combination chemotherapy regimen with CNS prophylaxis is the recommended approach. Given the rapid response to chemotherapy and the diffuse nature of the disease, radiation therapy has no role in Burkitt lymphoma management, even for localized cases. Similarly, surgical intervention is no longer utilized in Burkitt lymphoma treatment. Prompt initiation of therapy, ideally within 48 hours of diagnosis, is crucial, and dose reductions should be avoided whenever possible.
Chemotherapy Management
In developed countries, the overall cure rate for sporadic Burkitt lymphoma approaches 90% in pediatric and young adult populations. In pediatric patients with complete surgical resection of disease, 2 cycles of chemotherapy of moderate intensity (ie, cyclophosphamide, vincristine, prednisolone, doxorubicin) are recommended. In pediatric patients with stage I and II disease, overall survival is greater than 98%.[1] (B2)
For patients with residual disease or those presenting with stage III Burkitt lymphoma, more intensive therapy is required. A minimum of 4 cycles of dose-intensive chemotherapy is recommended, with 2 cycles consisting of cyclophosphamide, vincristine, prednisolone, doxorubicin, and high-dose methotrexate, followed by 2 additional cycles of cytarabine and high-dose methotrexate. In these cases, intrathecal chemotherapy is concurrently administered to address potential CNS involvement, which is common in Burkitt lymphoma.
For pediatric patients with stage IV disease or those with CNS or bone marrow involvement, a more aggressive approach is warranted. These patients typically undergo up to 8 cycles of intensive chemotherapy, starting with 2 cycles of cyclophosphamide, vincristine, prednisolone, doxorubicin, and high-dose methotrexate, followed by 2 cycles of cytarabine and etoposide. Maintenance therapy consisting of 4 courses of chemotherapy, including vincristine, prednisolone, high-dose methotrexate, cyclophosphamide, doxorubicin, cytarabine, and etoposide, is administered to help prevent relapse. Intrathecal chemotherapy is also incorporated throughout treatment.
Standard chemotherapy regimens, eg, cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), are inadequate in treating adult Burkitt lymphoma. The National Comprehensive Cancer Network offers current chemotherapy recommendations with multiagent regimens and CNS prophylaxis, including:
- R-hyper-CVAD [18]
- CODOX-M/IVACA [19] with or without rituximab [20]
- Dose-adjusted EPOCH with rituximab [21] (B2)
Alternative Management Approaches
Immunotherapy has a role in treatment. Rituximab (anti-CD20) should be a component of all treatment regimens as it is correlated with a favorable prognosis. Newer anti-CD20 agents, ofatumumab and obinutuzumab, are under investigation. Blinatumomab, an anti-CD19 monoclonal antibody, and inotuzumab, an anti-CD22 monoclonal antibody, are under investigation. Experimental drugs to inhibit the growth of Burkitt lymphoma B-cells by inducing apoptosis include histone acetylase inhibitors (ie, rapamycin, valproic acid, tubacin) and mTOR inhibitors (ie, temsirolimus). Anti-PD1 agents prevent tumor cells from evading the immune system via the PD1 pathway. Therapies inhibiting the MYC oncogene are under investigation.[9]
Highly active antiretroviral therapy (HAART) has allowed for the management of immunodeficiency-related Burkitt lymphoma using high-dose chemotherapy in patients with HIV. In this patient population, it is advised that less toxic chemotherapy is used because of the susceptibility to organ failure and infection.[14]
Relapsing Disease Management
In patients with relapsing disease, the prognosis is typically poor, and treatment will depend on the specific clinical scenario. Salvage regimens may include R-IVAC, R-GDP, R-ICE, and high-dose cytarabine + rituximab.[9] Autologous and allogeneic hematopoietic cell transplants may be considered.[22] In elderly patients who cannot endure intense chemotherapy, treatment is often palliative.
Differential Diagnosis
The differential diagnosis of Burkitt lymphoma primarily includes other CD10 positive B-cell lymphomas: diffuse large B-cell lymphoma (DLBCL), high-grade B cell lymphoma, high-grade follicular lymphoma, and B-cell acute lymphoblastic leukemia/lymphoma (B-ALL). Both DLBCL and high-grade B cell lymphomas typically have larger cells with more pleomorphism than is expected with Burkitt lymphoma. BCL2 positivity and a Ki67 proliferation index <90% favor a diagnosis other than Burkitt lymphoma. Finding an MYC translocation is not diagnostic of Burkitt lymphoma; approximately 10% of DLBCL have an MYC translocation.[14] B-ALL may resemble Burkitt lymphoma in size, but it typically has finer chromatin. By immunohistochemistry or flow cytometry, B-ALL will often express markers of immaturity, eg, CD34 and TdT.
Some uncommonly encountered entities should also be considered. Cases with Burkitt lymphoma-type morphology that lack an MYC translocation should be examined for abnormalities of chromosome 11q for the diagnosis of Burkitt-like lymphoma with 11q aberration.[23] The prognosis for this recently described entity appears similar to that of Burkitt lymphoma.
Toxicity and Adverse Effect Management
Tumor lysis syndrome is the cytolysis of tumor cells causing hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia, and is frequently encountered before and during Burkitt lymphoma treatment.[9] Calcium can precipitate in the kidneys, causing acute kidney injury (AKI). Calcium, phosphate, and potassium fluctuation are associated with cardiac arrhythmias, CNS toxicity, and death. Because TLS can occur before treatment is initiated, prophylaxis should be started promptly. Tumor lysis syndrome is more likely to occur in male patients, as well as patients with a history of renal disease, elevated LDH or uric acid levels, or splenomegaly. Rigorous hydration before and after chemotherapy treatment, as well as allopurinol and rasburicase, have improved outcomes. Hemodialysis is advised if the patient develops severe kidney injury.[24]
High-intensity chemotherapy regimens used in Burkitt lymphoma are associated with other significant adverse effects, namely hematologic toxicity and severe infection risk. Gastrointestinal toxicities, including mucositis, nausea, and vomiting, are frequently observed, necessitating prophylactic antiemetic therapy and supportive measures. The use of high-dose methotrexate can cause nephrotoxicity, requiring careful hydration and monitoring of renal function, along with the use of leucovorin rescue to mitigate toxicity.
Cardiovascular toxicity, particularly due to anthracyclines like doxorubicin, poses a risk for cardiomyopathy, especially in patients with preexisting heart conditions. Careful cardiac monitoring, including ejection fraction assessment, is essential in these patients, and alternative regimens may be considered if necessary. Additionally, the use of intrathecal chemotherapy carries risks of neurotoxicity, including myelopathy and arachnoiditis, which require vigilant neurological monitoring.
Medical Oncology
Burkitt lymphoma requires an intensive, multi-agent chemotherapy regimen with adequate CNS prophylaxis to prevent relapse. Without CNS prophylaxis, approximately 30% to 50% of patients experience CNS relapse, but with its incorporation, this rate has dropped to around 6% to 11%.[25] Treatment strategies for adult Burkitt lymphoma are primarily adapted from pediatric protocols, although no randomized trials exist for direct comparison due to variability in diagnostic criteria, staging, and patient populations.
The following 3 primary chemotherapy approaches have been used in adults with Burkitt lymphoma:
- Intensive, short-duration combination therapy (eg, rituximab, cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate with ifosfamide, cytarabine, and etoposide (R-CODOX-M/IVAC))[26]
- Acute lymphoblastic leukemia (ALL)-like stepwise therapy with induction, consolidation, and maintenance (eg, rituximab with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone, alternating with high-dose methotrexate and cytarabine (R-HyperCVAD))[27]
- 3. Infusional chemotherapy with dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R).[21]
The intensive short-duration approach is generally preferred due to its quicker administration and clinical familiarity, whereas dose-adjusted EPOCH-R is an alternative for older or less fit patients. While data in adult populations are limited, retrospective analyses suggest that intensive short-duration regimens provide superior outcomes.[28]
Among intensive regimens, cyclophosphamide, vincristine, doxorubicin, and high-dose methotrexate with ifosfamide, cytarabine, etoposide, and intrathecal methotrexate (CODOX-M/IVAC), also known as the Magrath regimen, has been widely used. This regimen involves a risk-adapted approach, treating low-risk patients with 3 cycles of CODOX-M and high-risk patients with alternating cycles of CODOX-M and IVAC. Although effective, this regimen is highly toxic, necessitating prolonged hospitalization and supportive care. Studies have reported a 2-year overall survival rate of approximately 75% in adults, with common toxicities including neutropenia, mucositis, and sepsis.[29][19]
Other regimens, eg, the Cancer and Leukemia Group B (CALGB) protocol 9251, based on adult B-cell ALL protocols, consist of alternating cycles of cyclophosphamide, prednisone, ifosfamide, high-dose methotrexate, vincristine, dexamethasone, doxorubicin, etoposide, cytarabine, and triple intrathecal therapy with methotrexate, cytarabine, and hydrocortisone. This regimen has shown promising survival rates when combined with rituximab.[30]
HyperCVAD is effective but associated with severe hematologic toxicity and high rates of febrile neutropenia. Dose-adjusted EPOCH-R, initially used in AIDS-related Burkitt lymphoma, provides a less intensive alternative, particularly for older adult patients unable to tolerate aggressive chemotherapy. Although it does not penetrate the CNS, intrathecal methotrexate is included for CNS prophylaxis. Cardiac disease patients may face complications with anthracycline-based therapies, requiring alternative regimens if their ejection fraction is low.
The incorporation of rituximab has significantly improved outcomes for Burkitt lymphoma patients. Both randomized and nonrandomized trials suggest that rituximab enhances event-free and overall survival without increasing toxicity. Given its benefits, rituximab is now routinely added to chemotherapy regimens, typically starting with the second cycle to minimize tumor lysis risk.[31][32] Despite variations in protocol, the preferred treatment for most patients remains intensive, short-duration chemotherapy with CNS prophylaxis, tailored based on patient risk stratification.
Staging
The St. Jude staging system is routinely used for pediatric patients, whereas the Ann Arbor and Murphy staging systems are commonly used for adults. The Ann Arbor system accounts for the patient’s symptoms. The Murphy staging system emphasizes extranodal disease and distinguishes bone marrow involvement from central nervous system disease. Because of the aggressive nature of the disease in adult patients, staging should be performed using bone marrow and lumbar puncture samples, and treatment should start immediately after confirmation of diagnosis.
St. Jude/Murphy Staging System
The following staging system is utilized in children:
- Stage I: A single tumor (extranodal) or a single anatomical area (nodal), excluding mediastinum or abdomen or a tumor (extranodal) with regional node involvement, on the same side of the diaphragm.
- Stage II: A single tumor (extranodal) with regional node involvement, lymph node involvement on the same side of the diaphragm (two or more nodal areas or 2 single extranodal tumors, with or without regional node involvement), or a primary gastrointestinal tract tumor (usually ileocecal) with or without associated mesenteric node involvement, grossly completely resected.
- Stage III: On both sides of the diaphragm (two or more nodal areas or 2 single extranodal tumors), all primary intrathoracic tumors (eg, mediastinal or pleural thymic), all extensive primary intraabdominal disease; unresectable abdominal disease, even if only in 1 area, or all primary paraspinal or epidural tumors, irrespective of other sites.
- Stage IV: Any of the above with initial CNS or bone marrow involvement (only if <25% of the marrow comprises Burkitt cells).[1]
Murphy Staging System
The following staging system is utilized in adults:
- Stage I: Single nodal or extranodal site excluding the mediastinum or abdomen.
- Stage II: ≥2 nodal areas on 1 side of the diaphragm.
- Stage IIR: Completely resectable abdominal disease
- Stage III: ≥2 nodal areas on opposite sides of the diaphragm, or a primary intrathoracic tumor, paraspinal or epidural tumors, extensive intra-abdominal disease
- Stage IIIA: Completely nonresectable abdominal disease
- Stage IIIB: Widespread multiorgan intra-abdominal disease
- Stage IV: Central nervous system or bone marrow involvement
Stage I or IIR are considered favorable prognoses.[14]
Ann Arbor System (Adult)
The Ann Arbor system also uses the following staging system in adults and considers patient symptoms:
- Stage I: Single nodal or extranodal site
- Stage II: ≥2 nodal areas on 1 side of the diaphragm, or localized involvement of an extra-lymphatic site, and ≥1 site on the same side of the diaphragm (IIE)
- Stage III: ≥2 nodal areas on opposite sides of the diaphragm, which may include involvement of the spleen (IIIs), or localized involvement of an extranodal site (IIIE)
- Stage IV: Diffuse or disseminated involvement of ≥1 extra-lymphatic sites or 2 single extranodal tumors on opposite sides of the diaphragm
Stage I, II, and III are considered favorable prognoses.[14]
Prognosis
The prognosis is dependent on clinical and histopathologic staging, particularly the extent of the disease. In general, younger patients have a better prognosis. Pediatric patients may be better able to tolerate intense chemotherapy. According to a recent study, overall survival of Burkitt lymphoma was approximately 64% for patients 2 years of age; however, overall survival was the lowest in Black and elderly individuals.[33]
Prognostic factors in Burkitt lymphoma include clinical, laboratory, and molecular markers. Clinical factors, including advanced age, impaired performance status, advanced-stage disease, bulky disease, and involvement of multiple extranodal sites, as well as CNS or bone marrow involvement, are associated with worse outcomes. Laboratory markers, including elevated serum LDH, decreased hemoglobin levels, and low serum albumin, further correlate with poorer prognosis. The Burkitt lymphoma international prognostic index (BL-IPI) helps assess prognosis, considering factors like age ≥40, impaired performance status, elevated LDH, and CNS involvement. The BL-IPI classifies patients into low, intermediate, and high-risk categories, with distinct survival outcomes: 96%, 76%, and 59% 3-year overall survival, respectively.[4]
The finding of some additional cytogenetic findings beyond the MYC translocation, including deletion of 13q, a gain of 7q, or complex cytogenetics, may portend a worse prognosis.[9] Double hit mutations in ID3, CCND3, and mutations in 18q21 CN-LOH indicate a poor response to therapy and poor prognosis. Malnourishment, particularly in low-income countries, is associated with neutropenia secondary to chemotherapy. In immunodeficiency-related Burkitt lymphoma, low CD4 count is a poor prognostic indicator.
The prognostic significance of early PET or CT scans to assess for residual or recurrent disease is an area of investigation. Research is being conducted to elucidate whether minimal residual disease (MRD) is a risk factor for a worse prognosis in Burkitt lymphoma. Polymerase chain reaction can assess MRD by detecting MYC/IgH fusion.[34]
In underdeveloped nations, treatment must be catered to account for socioeconomic factors.[1] Because of the high cost, lack of access to medical care, and health illiteracy, many patients do not complete treatment. In patients with endemic Burkitt lymphoma, 1-year event-free survival is a gauge of long-term survival (risk of relapse is ≤5% after 1 year). When Burkitt lymphoma relapses within 6 months of treatment completion, the prognosis is poor because of the limited availability of new drugs for treatment, as most were exhausted during initial therapy. Conversely, in developing nations where treatment is less aggressive, a greater number of children can be treated after relapse.
Complications
Burkitt lymphoma can lead to various complications due to its aggressive nature and rapid progression. One of the most common complications is TLS, which results from the rapid breakdown of tumor cells during treatment, leading to elevated levels of potassium, phosphate, and uric acid in the blood, potentially causing renal failure, cardiac arrhythmias, and seizures.
Another significant complication is CNS involvement, which occurs in a substantial proportion of patients, particularly in advanced stages. This can result in neurologic deficits, eg, headaches, confusion, and paralysis, and often requires intensive therapy, including intrathecal chemotherapy. In addition, due to the involvement of the bone marrow, patients may experience myelosuppression, leading to neutropenia, anemia, and thrombocytopenia, increasing the risk of infection and bleeding. Moreover, the intense chemotherapy regimens used to treat Burkitt lymphoma carry risks of cardiotoxicity, particularly with anthracyclines, and other long-term complications, such as secondary malignancies.
Postoperative and Rehabilitation Care
After completing the initial treatment for Burkitt lymphoma, patients should undergo a comprehensive response evaluation, including history, physical examination, and laboratory tests, with CT scans as the preferred imaging tool. Following complete remission, regular follow-up visits are recommended at intervals of 3 to 4 months during the first year, with continued monitoring for relapse. The relapsed disease must be confirmed with a biopsy. Survivors should also be monitored for long-term complications related to both the disease and its treatment, particularly after 5 years.
Deterrence and Patient Education
Successful management requires educating patients and their families about the aggressive nature of Burkitt lymphoma, treatment regimens, potential adverse effects, and the importance of adherence to therapy. Discussions should address the need for regular follow-ups, surveillance for relapse, and recognition of early warning signs, such as symptoms of infection or organ dysfunction. Additionally, patients should be informed about the potential long-term effects of treatment, including fertility concerns and the risk of secondary malignancies due to chemotherapy.
Because Burkitt lymphoma is a prevalent childhood cancer in sub-Saharan Africa associated with malaria, some studies have suggested that malaria interventions may contribute to reducing the incidence of this condition. Results have been mixed; however, the distribution of insecticide-treated bed nets was correlated with a significant decline in Burkitt lymphoma cases. While findings support malaria control as a potential strategy for Burkitt lymphoma prevention, limitations in cancer surveillance data highlight the need for improved monitoring and further research.[5]
Enhancing Healthcare Team Outcomes
The management of Burkitt lymphoma requires a highly coordinated, interprofessional approach to ensure optimal patient outcomes, safety, and quality of care. Physicians, including hematologists and oncologists, lead the diagnostic and staging process using advanced imaging and molecular profiling to tailor treatment strategies. Advanced practitioners support patient monitoring, adjusting treatment regimens based on response and adverse effects while ensuring continuity of care. Nurses play a crucial role in patient education, symptom management, and psychosocial support, addressing concerns about adverse effects of treatment and emotional well-being. Pharmacists contribute by ensuring the safe administration of chemotherapy, preventing drug interactions, and managing supportive medications such as those for tumor lysis syndrome prophylaxis. Radiologists and pathologists provide critical diagnostic input, while intensivists may be involved in managing severe complications. Effective interprofessional communication, including regular tumor board meetings, facilitates shared decision-making and improves care coordination, particularly in complex cases with CNS involvement or comorbid conditions.
Beyond clinical management, patient-centered care in Burkitt lymphoma requires strong ethical considerations and care coordination. Informed consent, equitable access to treatment, and respect for patient preferences must be integrated into the care plan. The involvement of case managers and social workers helps address logistical barriers, such as access to specialty care and financial support for high-cost treatments. Public health measures, including malaria control and EBV vaccination in endemic regions, further emphasize the need for a holistic approach beyond direct patient care. By fostering teamwork, clear communication, and shared responsibility, the interprofessional team enhances patient safety, optimizes treatment efficacy, and improves the overall experience for individuals affected by Burkitt lymphoma.
Media
(Click Image to Enlarge)
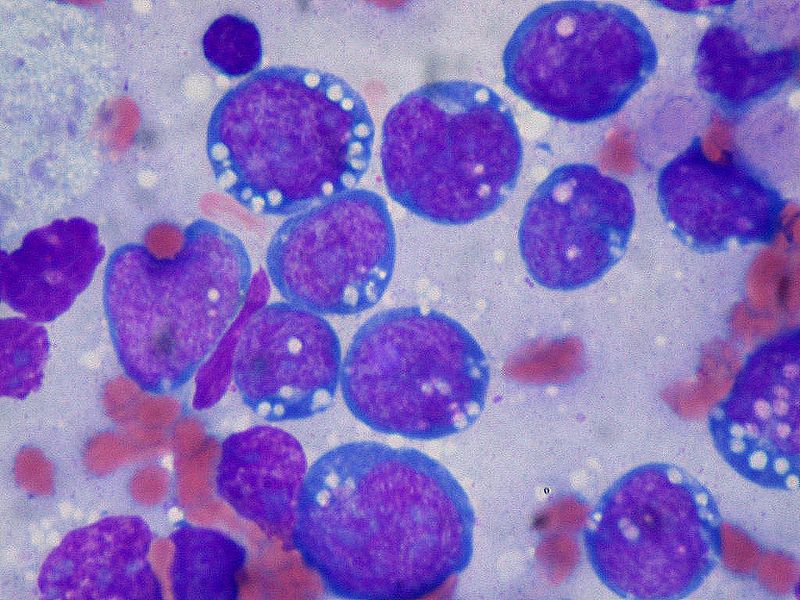
Burkitt Lymphoma, Lymphoblasts. The neoplastic cells are highly proliferative, which is reflected in the increased number of mitotic figures; increased cell turnover is countered by increased apoptosis. In tissue, this results in a "starry sky" appearance due to tingible-body macrophages containing cellular debris.
Ed Uthman, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
References
Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G, Harrison CJ, Israels T, Bailey S. Burkitt's lymphoma. Lancet (London, England). 2012 Mar 31:379(9822):1234-44. doi: 10.1016/S0140-6736(11)61177-X. Epub 2012 Feb 13 [PubMed PMID: 22333947]
Level 2 (mid-level) evidenceMagrath I. Epidemiology: clues to the pathogenesis of Burkitt lymphoma. British journal of haematology. 2012 Mar:156(6):744-56. doi: 10.1111/j.1365-2141.2011.09013.x. Epub 2012 Jan 20 [PubMed PMID: 22260300]
Brady G, MacArthur GJ, Farrell PJ. Epstein-Barr virus and Burkitt lymphoma. Journal of clinical pathology. 2007 Dec:60(12):1397-402 [PubMed PMID: 18042696]
Olszewski AJ, Jakobsen LH, Collins GP, Cwynarski K, Bachanova V, Blum KA, Boughan KM, Bower M, Dalla Pria A, Danilov A, David KA, Diefenbach C, Ellin F, Epperla N, Farooq U, Feldman TA, Gerrie AS, Jagadeesh D, Kamdar M, Karmali R, Kassam S, Kenkre VP, Khan N, Kim SH, Klein AK, Lossos IS, Lunning MA, Martin P, Martinez-Calle N, Montoto S, Naik S, Palmisiano N, Peace D, Phillips EH, Phillips TJ, Portell CA, Reddy N, Santarsieri A, Sarraf Yazdy M, Smeland KB, Smith SE, Smith SD, Sundaram S, Zayac AS, Zhang XY, Zhu C, Cheah CY, El-Galaly TC, Evens AM. Burkitt Lymphoma International Prognostic Index. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2021 Apr 1:39(10):1129-1138. doi: 10.1200/JCO.20.03288. Epub 2021 Jan 27 [PubMed PMID: 33502927]
Schmit N, Kaur J, Aglago EK. Mosquito Bed Net Use and Burkitt Lymphoma Incidence in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. JAMA network open. 2024 Apr 1:7(4):e247351. doi: 10.1001/jamanetworkopen.2024.7351. Epub 2024 Apr 1 [PubMed PMID: 38635267]
Level 1 (high-level) evidenceVelavan TP. Epstein-Barr virus, malaria and endemic Burkitt lymphoma. EBioMedicine. 2019 Jan:39():13-14. doi: 10.1016/j.ebiom.2018.12.041. Epub 2018 Dec 22 [PubMed PMID: 30584007]
Guech-Ongey M, Simard EP, Anderson WF, Engels EA, Bhatia K, Devesa SS, Mbulaiteye SM. AIDS-related Burkitt lymphoma in the United States: what do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood. 2010 Dec 16:116(25):5600-4. doi: 10.1182/blood-2010-03-275917. Epub 2010 Sep 2 [PubMed PMID: 20813897]
Linch DC. Burkitt lymphoma in adults. British journal of haematology. 2012 Mar:156(6):693-703. doi: 10.1111/j.1365-2141.2011.08877.x. Epub 2011 Sep 19 [PubMed PMID: 21923642]
Dozzo M, Carobolante F, Donisi PM, Scattolin A, Maino E, Sancetta R, Viero P, Bassan R. Burkitt lymphoma in adolescents and young adults: management challenges. Adolescent health, medicine and therapeutics. 2017:8():11-29. doi: 10.2147/AHMT.S94170. Epub 2016 Dec 23 [PubMed PMID: 28096698]
Armitage JO,Weisenburger DD, New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998 Aug; [PubMed PMID: 9704731]
Haberl S, Haferlach T, Stengel A, Jeromin S, Kern W, Haferlach C. MYC rearranged B-cell neoplasms: Impact of genetics on classification. Cancer genetics. 2016 Oct:209(10):431-439. doi: 10.1016/j.cancergen.2016.08.007. Epub 2016 Sep 14 [PubMed PMID: 27810071]
Grande BM, Gerhard DS, Jiang A, Griner NB, Abramson JS, Alexander TB, Allen H, Ayers LW, Bethony JM, Bhatia K, Bowen J, Casper C, Choi JK, Culibrk L, Davidsen TM, Dyer MA, Gastier-Foster JM, Gesuwan P, Greiner TC, Gross TG, Hanf B, Harris NL, He Y, Irvin JD, Jaffe ES, Jones SJM, Kerchan P, Knoetze N, Leal FE, Lichtenberg TM, Ma Y, Martin JP, Martin MR, Mbulaiteye SM, Mullighan CG, Mungall AJ, Namirembe C, Novik K, Noy A, Ogwang MD, Omoding A, Orem J, Reynolds SJ, Rushton CK, Sandlund JT, Schmitz R, Taylor C, Wilson WH, Wright GW, Zhao EY, Marra MA, Morin RD, Staudt LM. Genome-wide discovery of somatic coding and noncoding mutations in pediatric endemic and sporadic Burkitt lymphoma. Blood. 2019 Mar 21:133(12):1313-1324. doi: 10.1182/blood-2018-09-871418. Epub 2019 Jan 7 [PubMed PMID: 30617194]
Torgbor C, Awuah P, Deitsch K, Kalantari P, Duca KA, Thorley-Lawson DA. A multifactorial role for P. falciparum malaria in endemic Burkitt's lymphoma pathogenesis. PLoS pathogens. 2014 May:10(5):e1004170. doi: 10.1371/journal.ppat.1004170. Epub 2014 May 29 [PubMed PMID: 24874410]
Sandlund JT. Burkitt lymphoma: staging and response evaluation. British journal of haematology. 2012 Mar:156(6):761-5. doi: 10.1111/j.1365-2141.2012.09026.x. Epub 2012 Feb 1 [PubMed PMID: 22296338]
Crombie J, LaCasce A. The treatment of Burkitt lymphoma in adults. Blood. 2021 Feb 11:137(6):743-750. doi: 10.1182/blood.2019004099. Epub [PubMed PMID: 33171490]
Chuang SS, Ye H, Du MQ, Lu CL, Dogan A, Hsieh PP, Huang WT, Jung YC. Histopathology and immunohistochemistry in distinguishing Burkitt lymphoma from diffuse large B-cell lymphoma with very high proliferation index and with or without a starry-sky pattern: a comparative study with EBER and FISH. American journal of clinical pathology. 2007 Oct:128(4):558-64 [PubMed PMID: 17875505]
Level 2 (mid-level) evidenceMarjerrison S, Fernandez CV, Price VE, Njume E, Hesseling P. The use of ultrasound in endemic Burkitt lymphoma in Cameroon. Pediatric blood & cancer. 2012 Mar:58(3):352-5. doi: 10.1002/pbc.23050. Epub 2011 Mar 2 [PubMed PMID: 21370431]
Level 2 (mid-level) evidenceThomas DA, Faderl S, O'Brien S, Bueso-Ramos C, Cortes J, Garcia-Manero G, Giles FJ, Verstovsek S, Wierda WG, Pierce SA, Shan J, Brandt M, Hagemeister FB, Keating MJ, Cabanillas F, Kantarjian H. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006 Apr 1:106(7):1569-80 [PubMed PMID: 16502413]
Mead GM, Sydes MR, Walewski J, Grigg A, Hatton CS, Pescosta N, Guarnaccia C, Lewis MS, McKendrick J, Stenning SP, Wright D, UKLG LY06 collaborators. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt's lymphoma: results of United Kingdom Lymphoma Group LY06 study. Annals of oncology : official journal of the European Society for Medical Oncology. 2002 Aug:13(8):1264-74 [PubMed PMID: 12181251]
Barnes JA, Lacasce AS, Feng Y, Toomey CE, Neuberg D, Michaelson JS, Hochberg EP, Abramson JS. Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt's lymphoma: a retrospective analysis. Annals of oncology : official journal of the European Society for Medical Oncology. 2011 Aug:22(8):1859-64. doi: 10.1093/annonc/mdq677. Epub 2011 Feb 21 [PubMed PMID: 21339382]
Level 2 (mid-level) evidenceDunleavy K, Pittaluga S, Shovlin M, Steinberg SM, Cole D, Grant C, Widemann B, Staudt LM, Jaffe ES, Little RF, Wilson WH. Low-intensity therapy in adults with Burkitt's lymphoma. The New England journal of medicine. 2013 Nov 14:369(20):1915-25. doi: 10.1056/NEJMoa1308392. Epub [PubMed PMID: 24224624]
Gross TG, Hale GA, He W, Camitta BM, Sanders JE, Cairo MS, Hayashi RJ, Termuhlen AM, Zhang MJ, Davies SM, Eapen M. Hematopoietic stem cell transplantation for refractory or recurrent non-Hodgkin lymphoma in children and adolescents. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010 Feb:16(2):223-30. doi: 10.1016/j.bbmt.2009.09.021. Epub 2009 Sep 30 [PubMed PMID: 19800015]
Salaverria I, Martin-Guerrero I, Wagener R, Kreuz M, Kohler CW, Richter J, Pienkowska-Grela B, Adam P, Burkhardt B, Claviez A, Damm-Welk C, Drexler HG, Hummel M, Jaffe ES, Küppers R, Lefebvre C, Lisfeld J, Löffler M, Macleod RA, Nagel I, Oschlies I, Rosolowski M, Russell RB, Rymkiewicz G, Schindler D, Schlesner M, Scholtysik R, Schwaenen C, Spang R, Szczepanowski M, Trümper L, Vater I, Wessendorf S, Klapper W, Siebert R, Molecular Mechanisms in Malignant Lymphoma Network Project, Berlin-Frankfurt-Münster Non-Hodgkin Lymphoma Group. A recurrent 11q aberration pattern characterizes a subset of MYC-negative high-grade B-cell lymphomas resembling Burkitt lymphoma. Blood. 2014 Feb 20:123(8):1187-98. doi: 10.1182/blood-2013-06-507996. Epub 2014 Jan 7 [PubMed PMID: 24398325]
Adeyinka A, Kaur A, Bashir K. Tumor Lysis Syndrome. StatPearls. 2025 Jan:(): [PubMed PMID: 30085527]
Hill QA, Owen RG. CNS prophylaxis in lymphoma: who to target and what therapy to use. Blood reviews. 2006 Nov:20(6):319-32 [PubMed PMID: 16884838]
Bishop PC, Rao VK, Wilson WH. Burkitt's lymphoma: molecular pathogenesis and treatment. Cancer investigation. 2000:18(6):574-83 [PubMed PMID: 10923106]
Hoelzer D, Ludwig WD, Thiel E, Gassmann W, Löffler H, Fonatsch C, Rieder H, Heil G, Heinze B, Arnold R, Hossfeld D, Büchner T, Koch P, Freund M, Hiddemann W, Maschmeyer G, Heyll A, Aul C, Faak T, Kuse R, Ittel TH, Gramatzki M, Diedrich H, Kolbe K, Fuhr HG, Fischer K, Schadeck-Gressel C, Weiss A, Strohscheer I, Metzner B, Fabry U, Gökbuget N, Völkers B, Messerer D, Uberla K. Improved outcome in adult B-cell acute lymphoblastic leukemia. Blood. 1996 Jan 15:87(2):495-508 [PubMed PMID: 8555471]
Roschewski M, Dunleavy K, Abramson JS, Powell BL, Link BK, Patel P, Bierman PJ, Jagadeesh D, Mitsuyasu RT, Peace D, Watson PR, Hanna WT, Melani C, Lucas AN, Steinberg SM, Pittaluga S, Jaffe ES, Friedberg JW, Kahl BS, Little RF, Bartlett NL, Fanale MA, Noy A, Wilson WH. Multicenter Study of Risk-Adapted Therapy With Dose-Adjusted EPOCH-R in Adults With Untreated Burkitt Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020 Aug 1:38(22):2519-2529. doi: 10.1200/JCO.20.00303. Epub 2020 May 26 [PubMed PMID: 32453640]
Level 2 (mid-level) evidenceMagrath I, Adde M, Shad A, Venzon D, Seibel N, Gootenberg J, Neely J, Arndt C, Nieder M, Jaffe E, Wittes RA, Horak ID. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1996 Mar:14(3):925-34 [PubMed PMID: 8622041]
Rizzieri DA, Johnson JL, Byrd JC, Lozanski G, Blum KA, Powell BL, Shea TC, Nattam S, Hoke E, Cheson BD, Larson RA, Alliance for Clinical Trials In Oncology (ACTION). Improved efficacy using rituximab and brief duration, high intensity chemotherapy with filgrastim support for Burkitt or aggressive lymphomas: cancer and Leukemia Group B study 10 002. British journal of haematology. 2014 Apr:165(1):102-11. doi: 10.1111/bjh.12736. Epub 2014 Jan 15 [PubMed PMID: 24428673]
Hoelzer D,Walewski J,Döhner H,Viardot A,Hiddemann W,Spiekermann K,Serve H,Dührsen U,Hüttmann A,Thiel E,Dengler J,Kneba M,Schaich M,Schmidt-Wolf IG,Beck J,Hertenstein B,Reichle A,Domanska-Czyz K,Fietkau R,Horst HA,Rieder H,Schwartz S,Burmeister T,Gökbuget N, Improved outcome of adult Burkitt lymphoma/leukemia with rituximab and chemotherapy: report of a large prospective multicenter trial. Blood. 2014 Dec 18; [PubMed PMID: 25359988]
Level 1 (high-level) evidenceRibera JM, García O, Grande C, Esteve J, Oriol A, Bergua J, González-Campos J, Vall-Llovera F, Tormo M, Hernández-Rivas JM, García D, Brunet S, Alonso N, Barba P, Miralles P, Llorente A, Montesinos P, Moreno MJ, Hernández-Rivas JÁ, Bernal T. Dose-intensive chemotherapy including rituximab in Burkitt's leukemia or lymphoma regardless of human immunodeficiency virus infection status: final results of a phase 2 study (Burkimab). Cancer. 2013 May 1:119(9):1660-8. doi: 10.1002/cncr.27918. Epub 2013 Jan 29 [PubMed PMID: 23361927]
Mburu W, Devesa SS, Check D, Shiels MS, Mbulaiteye SM. Incidence of Burkitt lymphoma in the United States during 2000 to 2019. International journal of cancer. 2023 Sep 15:153(6):1182-1191. doi: 10.1002/ijc.34618. Epub 2023 Jun 6 [PubMed PMID: 37278097]
Shiramizu B, Goldman S, Smith L, Agsalda-Garcia M, Galardy P, Perkins SL, Frazer JK, Sanger W, Anderson JR, Gross TG, Weinstein H, Harrison L, Barth MJ, Mussolin L, Cairo MS. Impact of persistent minimal residual disease post-consolidation therapy in children and adolescents with advanced Burkitt leukaemia: a Children's Oncology Group Pilot Study Report. British journal of haematology. 2015 Aug:170(3):367-71. doi: 10.1111/bjh.13443. Epub 2015 Apr 8 [PubMed PMID: 25858645]
Level 3 (low-level) evidence