Introduction
A bronchopleural fistula (BPF) is a severe postoperative complication seen in thoracic surgery characterized by a sinus tract between the bronchus (main stem, lobar, or segmental) and the pleural space. This condition can result in serious complications, including respiratory insufficiency, pneumonia in the remaining lung, and empyema.[1] BPF occurs in approximately 1.5% to 12.5% of pneumonectomy cases and about 1% of lobectomy or sublobar resection procedures and has a morbidity ranging between 25% and 71%.[2] Causes include surgical complications, pulmonary infections resulting in necrosis, spontaneous pneumothorax, chemotherapy, radiotherapy, and tuberculosis.
BPF is a complex condition that is often challenging for clinicians to diagnose and manage. Patients with BPF present with a range of symptoms, from acute tension pneumothorax to subacute empyema, typically within the first 2 weeks following lung resection. Diagnosis involves clinical assessment, blood tests, chest computed tomography scans, and flexible bronchoscopy. Treatment strategies vary from medical management and bronchoscopic procedures to surgical interventions for those deemed with the highest risk. Management of BPF requires an interprofessional team approach involving immediate chest tube drainage, supportive care, antibiotics, and potentially surgical or bronchoscopic fistula closure.
The optimal management of BPF lacks consensus due to varying therapeutic success. Varoli et al classified fistulas by the time of their onset following surgical intervention. These classifications were divided into 3 primary time frames: early fistulas occurring within 1 to 7 days, intermediate fistulas occurring in 8 to 30 days, or late fistulas developing after more than 30 days.[3] Although fistulas almost always occur within 3 months after surgery, BPF following pleuropulmonary infection can happen at any point.[4] Also, managing airway pressures is crucial in those who are mechanically ventilated to promote healing and adequate ventilation. Bronchoscopic methods, such as endobronchial injections and sealants, provide temporary closure and can be a bridge to curative surgery.
To reduce BPF risk, preventative measures during initial surgeries (eg, wrapping the trachea or carina with a muscle flap or pericardium), particularly in tracheal resections, carina reconstructions, or pulmonary sleeve lobectomies, are often employed.[5] Monitoring after closure involves clinical assessment, chest tube output, and imaging to detect recurrence or complications. Persistent or complex cases might necessitate repeat interventions or, rarely, open-window thoracostomies.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Right pneumonectomy, right middle lower lobectomy, and right lower lobectomy are generally considered high-risk procedures for developing BPF. Due to impaired blood flow, bronchial stump healing can be delayed following lymph node dissection; additionally, neoadjuvant therapies, particularly chemoradiotherapy, may further hinder the healing process of the bronchial stump.[6] The fistula is commonly found on the stump beside the residual lobe due to the increased risk of ischemic necrosis or the pooling of secretions leading to bacterial overgrowth and colonization. The increased risk of BPF associated with right pneumonectomy is due to the more extensive resection required.[7][8][9][10] Other underlying causes of BPF include:
- Patient factors
- Low nutritional status or poor wound healing
- Heavy smoking
- Age older than 60
- Fever
- Corticosteroid use
- Leukocytosis
- Comorbid conditions
- Gastroesophageal reflux disease with Barrett esophagus
- Diabetes mellitus
- Boerhaave syndrome
- Lung disease or abnormalities
- Bullous lung disease
- Chronic obstructive pulmonary disease
- Spontaneous pneumothorax or other parenchymal abnormalities
- Previous ipsilateral thoracotomy
- A large diameter bronchial stump (>25 mm)
- Residual tumor in the resection margins
- Broncholithiasis
- Infectious disease
- Tuberculosis
- Hemophilus influenza
- Streptococcus viridans
- Staphylococcus aureus
- Pseudomonas aeruginosa
- Klebsiella pneumoniae
- Pneumococcus
- Nonhemolytic streptococcus
- Aspergillus
- Histoplasma capsulatum
- Malignancy
- Lung cancer
- Thyroid cancer
- Esophageal cancer
- Lymphomas
- Traumatic injury
- Lobectomy-right, bilobectomy, and lower lobectomy
- Tracheostomy
- Bronchoscopy for sputum suctioning
- Excessively tight individual sutures
- Excessive peribronchial and paratracheal dissection or lymph node dissection
- Prolonged postoperative mechanical ventilation
- Thoracic trauma with tracheobronchial tree disruption
- Bougie intubation
- Necrotizing lung disease associated with radiation or chemotherapy
- Acute respiratory distress syndrome (especially in patients requiring ventilation with high airway pressures)
- Ventilator-induced barotrauma
- Overzealous manual ventilation
- Central line placement [11]
Epidemiology
BPF is most commonly encountered after lung resection surgery (eg, pneumonectomy, lobectomy, segmentectomy), with a frequency ranging from 4.5% to 20% after pneumonectomy and 0.5% to 1% after lobectomy.[12] BPF is a potentially severe complication of pulmonary tuberculosis, which remains prevalent in developing countries. In one study, the results showed that 69.2% of participants were diagnosed with tuberculosis—a finding consistent with a series conducted at the Maryland School of Medicine involving 77 patients treated for BPF for more than 13 years; in developed regions, postpneumonectomy BPF was more common than postlobectomy BPF.[6]
Pathophysiology
Postoperative BPF may be classified as acute, subacute, or chronic.[13] The acute form is caused by surgical dehiscence and requires prompt surgical intervention. When acute, BPF can be life-threatening due to tension pneumothorax or asphyxiation from pulmonary flooding. Patients with acute BPF typically present with sudden onset dyspnea, hypotension, subcutaneous emphysema, cough with expectoration of purulent fluid, tracheal or mediastinal shift, persistent air leak, and pleural effusion reduction or disappearance on chest radiograph. The subacute and chronic forms are primarily associated with infection and are often seen in patients with immunocompromise or debilitation secondary to multiple comorbidities. The subacute presentation is more insidious, characterized by wasting, malaise, and fever; the chronic form has clinical features of pleural fibrosis.[14]
A BPF can occur throughout the postoperative period but most commonly develops within 8 to 12 days after surgery. If seen within the first 4 postoperative days, BPF is likely secondary to a mechanical failure of bronchial stump closure, requiring reexploration. A BPF may also occur after suppurative pneumonia, massive pulmonary infarction, or spontaneously. Empyema may occur alone or may be associated with a BPF. However, a BPF can be unassociated with empyema; in these cases, the fluid in the pleural space is sterile. Displacement of the mediastinum to the opposite side occurs because of air on the operative side. Clearing fluid from the pleural space and coughing up fluid and blood suggest a BPF. Furthermore, the sudden reappearance of air in an obliterated space suggests either a BPF or a gas-forming infectious process. If a fistula appears in nonsurgical cases or a delayed postoperative period, a BPF diagnosis should be suspected when fever, productive cough, and new or increasing air-fluid levels are seen on the chest radiograph in the pleural space. Esophagotracheal fistulas are associated with coughing and dyspnea during eating and drinking.[14] Moreover, nonresolving pneumonia should be endoscopically investigated.
History and Physical
Patients with BPF can present with symptoms that range from acute symptoms of tension pneumothorax to subacute empyema symptoms, typically in the first 2 weeks following lung resection. BPF should be suspected in patients who present with sudden onset dyspnea, chest pain, hemodynamic instability, or subcutaneous emphysema within the postoperative period following a lung resection. Symptoms may be less abrupt if the chest tube is still in place; a large persistent or new air leak through the chest tube drainage system may be the only sign present in these patients.[15] Examination findings are typically nonspecific but may reveal reduced air entry on the affected side and tracheal deviation if tension pneumothorax is present.
BPF associated with empyema is a BPF that is not adequately drained, which results in the infection eroding through the chest wall, leading to a percutaneous opening with drainage of mucopurulent material known as empyema. Patients who present in the late postoperative period (>14 days) or patients with BPF from other causes, including infection or malignancy, commonly present with empyema symptoms and signs, including fever, malaise, muscle wasting, cough with purulent sputum, reduced air entry, and dullness to percussion on the affected side.[13]
Evaluation
BPF evaluation comprises clinical, imaging, and bronchoscopic assessments that confirm air leakage from a significant lobar or segmental bronchus to the pleural space. Although no specific laboratory findings for BPF have been established, some patients with an infected pleural space may exhibit leukocytosis or elevated erythrocyte sedimentation rate. A BPF diagnosis is established by evaluating clinical symptoms (eg, fever, cough, and hypoxemia) alongside blood tests that indicate leukocytosis and elevated C-reactive protein levels. Diagnostic tools include chest computed tomography (CT) scans and flexible bronchoscopy, which typically reveal signs of BPF with fluid-air collections.[16]
Imaging Studies
Radiological features that suggest the presence or development of a BPF include an increase in the intrapleural airspace, the appearance of a new air-fluid level, changes in an already present air-fluid level, development of tension pneumothorax, and a drop in the air-fluid level exceeding 2 cm.[17] With CT imaging, apart from visualization of pneumothorax, pneumomediastinum, and underlying lung pathology, evidence of fistulous communication may be demonstrated in some patients.[18] In those who have not undergone lung resection, features of the underlying etiology (eg, malignancy) may be revealed via CT with a cavitating mass and air-fluid levels.
Using standard and thin section non-contrast CT scans, a study by Westcott and Volpe demonstrated a fistula in 10 out of 20 patients.[18] In almost all of these cases, the fistulous tract was located peripherally, and an air leak was identified in a patient undergoing a lung resection in 1 case. Three-dimensional spiral reconstruction has also been successfully used to demonstrate BPF.[19] Additionally, computed tomography bronchography (CTB) has been used to diagnose a problematic BPF. Following bronchoscopy, Sarkar et al injected 20 to 30 mL of a water-based nonionic low osmolar iodinated contrast medium iohexol at the suspected fistula site either through a catheter or the bronchoscope’s working channel.[20] CT was performed immediately with a targeted reconstruction of images in different planes; the fistula was easily visualized using standard axial and sagittal sections.
Several authors have tried to localize a BPF using radiolabeled aerosol inhalation with planar and single-photon emission tomography imaging.[21] These imaging modalities require substantial time and a patient’s cooperation. This technique has been criticized because the aerosol tends to deposit in areas of turbulence and may lead to false-positive results in patients with obstructive airways. In addition, the estimation of the size of the BPF may be inaccurate as it is an indirect measurement from the kinetics of tracer gas during different phases of the study.[22]
Bronchoscopy and Other Diagnostic Studies
A persistent air leak after pulmonary injury can indicate bronchus disruption or an overdistended alveolus rupture. Several strategies have been used to diagnose BPFs, including injecting methylene blue into the pleural space and bronchography.[23] In addition, small metallic probes introduced through the bronchoscope’s working channel and changes in gas concentration in the pneumonectomy cavity after inhaling different concentrations of oxygen and nitrous oxide also aid with BPF evaluation.[24]
Bronchoscopic exploration allows proper evaluation of the stump and fistula localization. Bronchoscopy also helps exclude tuberculosis or other infectious etiologies and enables the introduction of sealants into the fistulous tract. While a large BPF is more likely to be seen on bronchoscopy, sequential balloon occlusion of the bronchi is occasionally used, particularly for the localization of a smaller or segmental BPF.
Several other modalities can also be used in the diagnostic evaluation of BPF. The return of continuous bubbles suggests the presence of BPF during bronchial washing. A bronchoscope with inflation of a balloon-tipped catheter into selected airways can aid in localization, as balloon occlusion decreases or eliminates an air leak. Capnography, in which end-tidal carbon dioxide is measured by connecting a capnograph to a polyethylene catheter, can be used to identify the bronchial segment related to BPF. Capnography has a reported sensitivity of 83% and a specificity of 100% in diagnosing BPF.[25]
Treatment / Management
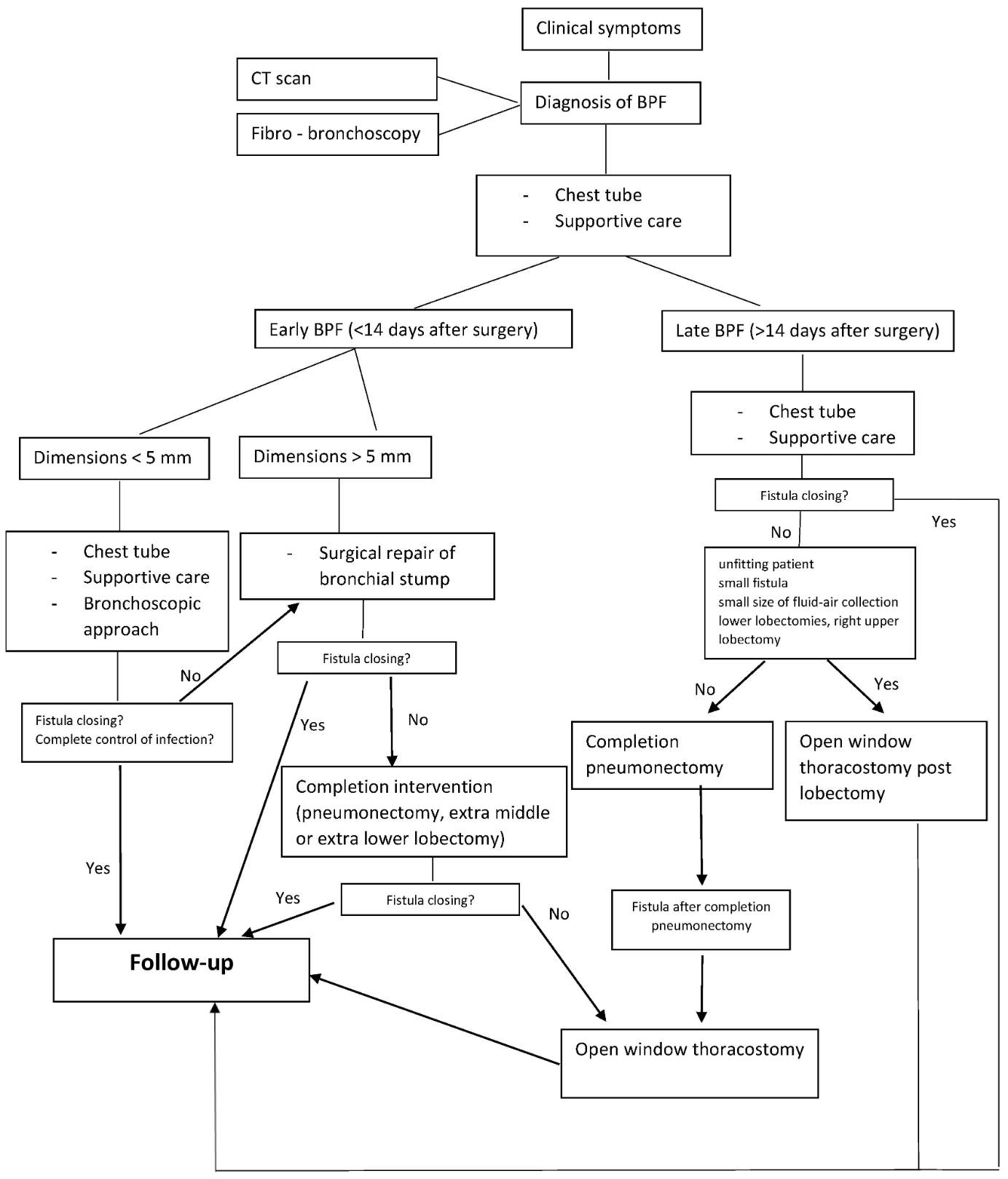
Various management approaches have been proposed for BPF. The initial treatment for all patients using the European Institute of Oncologies algorithm (see Image. European Institute of Oncologies Algorithm) consists of placing a chest tube to evacuate the collection, facilitate pleural washing, and prevent aspiration into the remaining lung parenchyma when fluid-air collections or empyema are detected. Subsequent steps focus on providing supportive care to enhance the patient's clinical condition, followed by a targeted antibiotic regimen that is initiated and adjusted based on results from the microbiological analysis.[16]
Generally, first-line therapy should address any immediate, life-threatening conditions, such as endobronchial contamination, pulmonary flooding, and tension pneumothorax. Patients can successfully undergo surgical repair since most BPFs occur early in the postoperative period and are not infected. Bronchoscopic approaches have variable success rates and are appropriate for those who are not suitable for surgical intervention.
The patient should be placed with the affected side dependent, and adequate pleural drainage is performed. The first intervention for BPF should be air and fluid drainage from the pleural space by chest tube thoracostomy. Pleural fluid should be sent to assess pleural infection for complete blood cell count, pH, total protein, lactate dehydrogenase, glucose, cytology, triglycerides, Gram stain, and culture. Although integral for drainage, the chest tube can function as a foreign body and predispose to illness at the insertion site and in the pleural space. In mechanically ventilated patients, the chest tube can add positive intrapleural pressure during the expiratory phase or occlusion during the inspiratory phase. These interventions aim to decrease the air leak during expiration to maintain positive end-expiratory pressure and to decrease BPF flow during inspiration. The chest tube can also be used to apply sclerosing agents, such as talc and bleomycin, to promote pleurodesis. All patients should be given broad-spectrum intravenous antibiotics against gram-positive, gram-negative, and anaerobic microorganisms until Gram stain, cultures, and sensitivities are available. Postural drainage can be initiated after specimens have been obtained, as long as a patient can expectorate, the chest cavity and chest tube drainage is less than 30 mL per day, and simultaneous pleural irrigation is performed.[26](B3)
Suture reclosure of the bronchial stump with vascularized flap coverage is curative for the fistula presenting acutely, usually less than 2 weeks after surgery.[27] In most cases, a video-assisted thoracoscopic surgical approach is performed, but rarely a thoracotomy is needed. Surgical fistula closure is done by an anterior, transpericardial approach thoracotomy with a muscle flap to fill the pleural space or with a muscle flap coverage of the fistula with a limited thoracoplasty.(B3)
Medical Management
Cases are typically managed medically initially when patients present with a BPF developing late or in those who develop the fistula as a complication of suppurative pleuropulmonary diseases. Medical management includes dependent drainage and reduction of the pleural space, antibiotic treatment, nutritional supplementation, and adequate ventilator management.[28]
The disease course is more complicated for patients with BPF who are mechanically ventilated. Maintaining airway pressures at or below the critical opening pressure of the fistula to promote healing yet provide adequate alveolar ventilation for sufficient gas exchange can present a significant challenge. Air leaks through BPFs may range from less than 1 L to 16 L per minute. Adverse events from BPF in patients who are mechanically ventilated include incomplete lung expansion, loss of adequate tidal volume or positive end-expiratory pressure, inability to remove carbon dioxide, and prolonged ventilatory support. The large air leak via BPF can also result in auto-triggering the ventilator, leading to severe hyperventilation and inappropriately large doses of sedatives and neuromuscular blockers administered to reduce spontaneous respiration.[29] (B3)
Mean airway pressure should be reduced as much as possible, including minimal positive end-expiratory pressure, low peak airway pressures, and reducing the proportion of minute ventilation provided by the ventilator. Intermittent mandatory ventilation modes with low tidal volumes, respiratory rates, and shorter inspiratory times are appropriate. Other techniques to decrease an air leak include independent lung ventilation with 2 ventilators, differential lung ventilation, and high-frequency jet ventilation (HFJV).[30] Selective intubation of the unaffected lung, double-lumen tube intubation with differential lung ventilation, independent lung ventilation, and patient positioning are all advocated. HFJV with permissive hypercapnia avoids needing a double-lumen tube or single-lumen tube with a bronchial blocker. HFJV avoids barotrauma in the unaffected lung and decreases air leaks. If HFJV is unavailable, BPF is one of the strict indications for split lung ventilation. A double-lumen tube is preferred because it allows physiologic separation of the lungs if an air leak becomes severe enough to require varied ventilator modes or settings for each lung. According to the airway device used, low-frequency jet ventilation or low tidal volume ventilation is applied during resection. Extracorporeal membrane oxygenation, either veno-venous or veno-arterial, is another alternative strategy for gas exchange.[30](B3)
Surgical and Endoscopic Management
Methods to limit airflow across BPFs include direct closure, decortication, thoracoplasty, omental or muscle transposition, and completion pneumonectomy.[31] Localization and size of the fistula may be the decisive factors in choosing between surgical and endoscopic procedures. Suppose a patient is severely debilitated or life expectancy is limited. In that case, palliation can sometimes be provided by a surgically created percutaneous tract to vent the pleural space on a permanent or temporary basis.[32] Patients should be assessed and treated for potential empyema. The development of postpneumonectomy empyema occurs between 2% and 16%of cases. In the setting of postpneumonectomy empyema, BPFs are present in approximately 60% to 80% of patients and carry a high mortality rate ranging from 21% to 71%.[33](B2)
Patients with BPF from causes other than lung resection, including malignancy and infection, are treated with bronchoscopic methods because the bronchial stump is affected by the disease, and stump revision is not feasible. Bronchoscopic management is primarily helpful for temporary fistula closure but can be used as a bridge to curative surgery if the underlying cause is reversible. A flexible bronchoscope provides superior and precise access to a more significant portion of the bronchial tree than a rigid bronchoscope. Outcomes are variable, with successful closure rates ranging from 30% to 80%.[34] Bronchoscopic intervention is a viable first option in small BPFs less than 5 mm in diameter. Endoscopic closure of a BPF is of lower cost, has a reduced rate of trauma, and can be performed in those who are critically ill. The BPF can be closed endoscopically if there is no evidence of infection in the pleural cavity.(A1)
In patients with BPFs equal to 8 mm, fistula closure with airway stents, coils, or Amplatzer devices are viable options. The most prominent case series of 31 patients with BPF reported that the Amplatzer device was effective in 96% of cases, lasting up to 18 months.[35] Silicone and covered metallic stents for BPF closure involving the central airway have also been described.[36] Combined with other occlusive materials, angiographic coils can also successfully treat BPF. Occlusive agents act as a plug, mechanically sealing the leak and later inducing an inflammatory process with mucosal proliferation and fibrosis, creating a permanent seal. Fistula repair also occurs by the organization of granulation tissue.
Several case series and reports of patients with BPF treated with occlusive materials have also been presented, none of which have been compared yet. Bronchoscopic placement of glutaraldehyde sterilized lead shot, gel foam and tetracycline, autologous blood patch, fibrin glue, gelatin-resorcinol mixture, oxidized regenerated cellulose, albumin-glutaraldehyde tissue adhesive, cryoprecipitate fibrin glue, and NN-butyl 2-cyanoacrylate have been attempted at the fistula site.[37][38][39][40][41][42] The 2 component fibrin cryoprecipitate glue (calcium gluconate and cryoprecipitate along with topical thrombin 1000 IU/mL) is delivered at the fistula site through a double lumen catheter inserted into the operative channel of the bronchoscope, creating a fibrin clot that occludes the fistula. Small BPFs can be occluded using an endobronchial injection of ethanol, polidocanol, and tetracycline, followed by autologous blood.[43] Although not often utilized, argon plasma coagulation and neodymium-doped yttrium aluminum garnet laser have been described for patients with small BPFs. Furthermore, transplanted bone marrow-derived mesenchymal stem cells and dehydrated amniotic membrane allografts have succeeded in BPF closure.[44][45](B2)
Patients should be monitored after having a fistula closure for clinical symptoms of recurrence, chest tube output of air, and chest imaging. Patients with a sealed fistula should not have an air leak, and imaging should demonstrate stability or resolution of air in the pleural space. Repeat bronchoscopy is not routine and is only performed if fistula recurrence or a complication is suspected. If valves or stents are used, a chest CT scan and bronchoscopy are repeated at 6 weeks to assess for complications. For patients who fail surgical or bronchoscopic intervention, options include repeat surgery, an alternate bronchoscopic method, or, in rare cases, an open window thoracostomy such as Eloesser flap thoracostomy or a Claggett window.[34](A1)
Differential Diagnosis
In patients with a pneumonectomy, acute findings of tension pneumothorax are essentially pathognomonic for BPF. Although other conditions, such as postoperative tension chylothorax or bleeding into the pleural space, may also cause the findings of tension pneumothorax, the affected hemithorax should be filled with fluid rather than air. Tension pneumothorax may also be due to a displaced or obstructed chest tube; ensuring a patent chest tube while imaging is being obtained may distinguish this phenomenon from true BPF. In patients who present with empyema, BPF is likely if the effusion contains air and there is an air leak after the chest tube placement. The culture of the effusion may help distinguish anaerobic infection from BPF; bronchoscopic findings of a bronchial defect will distinguish BPF from other etiologies.
Prognosis
A BPF can cause significant morbidity, prolonged hospitalization, and mortality. Mortality rates vary between 18% to 67%.[46] The most common cause of death is aspiration pneumonia and subsequent acute respiratory distress syndrome or development of tension pneumothorax.[28] Pierson et al reported their experience with all cases of mechanical ventilation at a major trauma center for 4 years.[47] They found that 39 of the 1700 patients receiving mechanical ventilation had BPFs lasting at least 24 hours. Overall mortality in these 39 patients was 67%, and Pierson et al found that mortality was higher when BPF developed late rather than early in the illness (94% versus 45%).[47] Patients with large air leaks also had significant mortality compared to smaller leaks. The authors concluded that BPF during mechanical ventilation identifies patients with high mortality but that unmanageable respiratory acidosis from this complication is rare. Reports using omental and thoracic flaps have also shown decreased mortality.[48][49] Sirbu et al found the mortality rate of patients with BPF to be 27.2% (6 of 22).[50]
Complications
Multiple studies have identified risk factors for developing postoperative BPF. However, information on the adverse factors affecting the success of BPF repair is limited. A multivariable analysis indicated that mechanical ventilation during BPF repair negatively impacted the repair's success. Other factors, including diabetes mellitus, chronic steroid use, type of primary surgery, additional resection during BPF repair, bronchial stump reinforcement with a flap, and the interval between surgery and BPF diagnosis, did not reach statistical significance. Patients on mechanical ventilation at the time of BPF repair were more likely to need postsurgical ventilatory support. Continuous positive pressure ventilation with high airway pressure at the BPF repair site was considered to hinder the healing process.[13]
Deterrence and Patient Education
Results from a recent study provided several key insights; BPF occurred exclusively in men who had undergone right lower lobectomy, despite women making up 47% of the total cohort, with an overall incidence rate of 0.44%. Multivariable analysis in the subgroup of men who underwent right lower lobectomy identified high serum C-reactive protein levels and a history of gastric cancer surgery as significant risk factors for BPF. Conversely, bronchial stump coverage was found to be inversely related to the development of BPF, indicating its potential as a preventive measure for those at high risk.[51]
BPF is one of the most severe complications after thoracic surgery, and no effective prophylactic procedure is available. The evidence for buttressing the bronchial stump leading to a reduction in the incidence of BPF is conflicting. Due to reasonable evidence that an intercostal flap reduces the risk of BPF in those with diabetes, thoracic surgeons should consider covering the bronchial stump with an intercostal muscle pedicle flap in patients at high risk of BPF. Sfyridis et al reported that intercostal muscle flap coverage was effective in a prospective randomized controlled trial of 70 patients who underwent pneumonectomy and had diabetes mellitus.[52] Patients were randomized 1:1 to either intercostal muscle flap reinforcement or no flap coverage; results revealed a significant reduction in BPF risk and empyema in patients with a flap.
Deschamps et al analyzed 713 patients who also underwent pneumonectomy.[53] Univariate analysis revealed bronchial stump reinforcement was associated with an increased incidence of BPF. Surgeons utilized multiple techniques for flap coverage, including a combination of muscle flaps, parietal pleura and pericardium, serratus anterior, and latissimus dorsi. Hamad et al report a series of 50 patients undergoing bronchial stump coverage with pericardium following extrapleural pneumonectomy for malignant mesothelioma.[54] Although 2 patients died secondary to cardiac complications, leading to 4% perioperative mortality, no cases of BPF developed. Lastly, Lardinois et al conducted a nonrandomized comparison study of 26 patients undergoing intercostal muscle or diaphragm flaps.[55] The 30-day mortality rate in both groups was 0%. A higher incidence of complications, including pneumonia, atelectasis, herniation, and BPF, was observed in the diaphragm flap group and 38% versus 8% in the intercostal muscle flap group. Therefore, both methods were deemed effective by the authors.
Pearls and Other Issues
The following key factors should be kept in mind when managing BPF:
- BPF is most commonly encountered after pulmonary resection (pneumonectomy, lobectomy, segmentectomy), with a frequency ranging from 4.5% to 20% after pneumonectomy and 0.5% to 1% after lobectomy.
- Patients with BPF can present with symptoms that range from acute symptoms of tension pneumothorax to subacute symptoms of empyema or a new or persistent air leak via a chest tube.
- Clinical, radiographic, and bronchoscopic findings that confirm an air leak from a bronchus to the pleural space are used to diagnose a BPF.
- BPFs do not typically spontaneously undergo closure and almost always require some surgical or bronchoscopic intervention.
- All patients with a BPF should have a chest tube placed to drain air. In an empyema, infected pleural fluid should be drained, and antibiotics should be initiated. A patient's comorbidities and nutritional status should be optimized in all cases.
- For most patients with postoperative lung resection with BPF who are good surgical candidates, surgical repair is a definitive therapy with excellent success if spontaneous closure is unlikely.
- Bronchoscopic fistula closure is appropriate for patients with BPF who are poor surgical candidates, for those who need a bridge to surgery, or for patients with BPF due to advanced malignancy.
- Bronchoscopic approaches have variable success rates but are typically more successful in those with small fistulas of <8 mm.
- Following fistula closure, patients should be monitored for clinical symptoms of recurrence with chest tube output and chest imaging.
- For patients who fail surgical or bronchoscopic intervention, options include repeat surgery, an alternate bronchoscopic method, or an open window thoracostomy (eg, Eloesser flap thoracostomy or a Claggett window).
Enhancing Healthcare Team Outcomes
An interprofessional team approach involving thoracic surgeons, intensivists, critical care nurses, advanced practitioners, pharmacists, and other healthcare professionals is essential to enhance patient-centered care, outcomes, patient safety, and team performance related to BPF. Physicians and advanced practitioners are crucial in diagnosing and formulating treatment plans, ensuring prompt and individualized care. Nurses, especially those with specialty training, are responsible for continuously monitoring patients, recognizing early signs and symptoms, and promptly reporting status changes to the team. Pharmacists contribute by managing and optimizing medication therapy, including antibiotics. Effective interprofessional communication and coordinated care are vital for the rapid recognition and treatment of BPF, reducing mortality and morbidity. Collaborative efforts and shared responsibilities within the team improve overall patient outcomes, safety, and the quality of care provided.
Media
(Click Image to Enlarge)
References
Skrzypczak PJ, Kasprzyk M, Piwkowski C. The review of the management and prevention methods of bronchopleural fistula in thoracic surgery. Journal of thoracic disease. 2023 Oct 31:15(10):5268-5271. doi: 10.21037/jtd-23-1231. Epub 2023 Sep 14 [PubMed PMID: 37969259]
Nagahiro I, Aoe M, Sano Y, Date H, Andou A, Shimizu N. Bronchopleural fistula after lobectomy for lung cancer. Asian cardiovascular & thoracic annals. 2007 Jan:15(1):45-8 [PubMed PMID: 17244922]
Level 2 (mid-level) evidenceVaroli F, Roviaro G, Grignani F, Vergani C, Maciocco M, Rebuffat C. Endoscopic treatment of bronchopleural fistulas. The Annals of thoracic surgery. 1998 Mar:65(3):807-9 [PubMed PMID: 9527218]
Gaur P, Dunne R, Colson YL, Gill RR. Bronchopleural fistula and the role of contemporary imaging. The Journal of thoracic and cardiovascular surgery. 2014 Jul:148(1):341-7. doi: 10.1016/j.jtcvs.2013.11.009. Epub 2013 Dec 16 [PubMed PMID: 24355543]
Wang Y, Zhu M, Pan Y, Yu K. Long-term follow up and comparison between conservative and interventional therapy in postoperative bronchopleural fistula-a cohort study. Journal of thoracic disease. 2023 Mar 31:15(3):1210-1216. doi: 10.21037/jtd-22-1426. Epub 2023 Mar 9 [PubMed PMID: 37065580]
Tokunaga Y, Kita Y, Okamoto T. Analysis of Risk Factors for Bronchopleural Fistula after Surgical Treatment of Lung Cancer. Annals of thoracic and cardiovascular surgery : official journal of the Association of Thoracic and Cardiovascular Surgeons of Asia. 2020 Dec 20:26(6):311-319. doi: 10.5761/atcs.oa.20-00010. Epub 2020 Mar 27 [PubMed PMID: 32224595]
Wright CD, Wain JC, Mathisen DJ, Grillo HC. Postpneumonectomy bronchopleural fistula after sutured bronchial closure: incidence, risk factors, and management. The Journal of thoracic and cardiovascular surgery. 1996 Nov:112(5):1367-71 [PubMed PMID: 8911336]
Li SJ, Zhou XD, Huang J, Liu J, Tian L, Che GW. A systematic review and meta-analysis-does chronic obstructive pulmonary disease predispose to bronchopleural fistula formation in patients undergoing lung cancer surgery? Journal of thoracic disease. 2016 Jul:8(7):1625-38. doi: 10.21037/jtd.2016.05.78. Epub [PubMed PMID: 27499951]
Level 1 (high-level) evidenceOkuda M, Go T, Yokomise H. Risk factor of bronchopleural fistula after general thoracic surgery: review article. General thoracic and cardiovascular surgery. 2017 Dec:65(12):679-685. doi: 10.1007/s11748-017-0846-1. Epub 2017 Oct 12 [PubMed PMID: 29027099]
Toufektzian L, Patris V, Sepsas E, Konstantinou M. Does postoperative mechanical ventilation predispose to bronchopleural fistula formation in patients undergoing pneumonectomy? Interactive cardiovascular and thoracic surgery. 2015 Sep:21(3):379-82. doi: 10.1093/icvts/ivv149. Epub 2015 Jun 11 [PubMed PMID: 26069338]
Sato M, Saito Y, Fujimura S, Usuda K, Takahashi S, Kanma K, Imai S, Suda H, Nakada T, Hashimoto K. [Study of postoperative bronchopleural fistulas--analysis of factors related to bronchopleural fistulas]. [Zasshi] [Journal]. Nihon Kyobu Geka Gakkai. 1989 Mar:37(3):498-503 [PubMed PMID: 2768924]
Level 2 (mid-level) evidenceAlpert JB, Godoy MC, Degroot PM, Truong MT, Ko JP. Imaging the post-thoracotomy patient: anatomic changes and postoperative complications. Radiologic clinics of North America. 2014 Jan:52(1):85-103. doi: 10.1016/j.rcl.2013.08.008. Epub 2013 Sep 14 [PubMed PMID: 24267712]
Yang YH, Park SY, Kim HE, Park BJ, Lee CY, Lee JG, Kim DJ, Paik HC. Postoperative bronchopleural fistula repair: Surgical outcomes and adverse factors for its success. Thoracic cancer. 2022 May:13(9):1401-1405. doi: 10.1111/1759-7714.14404. Epub 2022 Apr 7 [PubMed PMID: 35393787]
Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest. 2005 Dec:128(6):3955-65 [PubMed PMID: 16354867]
Level 3 (low-level) evidenceLeuzzi G, Facciolo F, Pastorino U, Rocco G. Methods for the postoperative management of the thoracic oncology patients: lessons from the clinic. Expert review of respiratory medicine. 2015:9(6):751-67. doi: 10.1586/17476348.2015.1109453. Epub 2015 Nov 4 [PubMed PMID: 26536136]
Mazzella A, Casiraghi M, Uslenghi C, Orlandi R, Lo Iacono G, Bertolaccini L, Varano GM, Orsi F, Spaggiari L. Bronchopleural Fistula after Lobectomy for Lung Cancer: How to Manage This Life-Threatening Complication Using Both Old and Innovative Solutions. Cancers. 2024 Mar 14:16(6):. doi: 10.3390/cancers16061146. Epub 2024 Mar 14 [PubMed PMID: 38539481]
Kim EA, Lee KS, Shim YM, Kim J, Kim K, Kim TS, Yang PS. Radiographic and CT findings in complications following pulmonary resection. Radiographics : a review publication of the Radiological Society of North America, Inc. 2002 Jan-Feb:22(1):67-86 [PubMed PMID: 11796900]
Westcott JL, Volpe JP. Peripheral bronchopleural fistula: CT evaluation in 20 patients with pneumonia, empyema, or postoperative air leak. Radiology. 1995 Jul:196(1):175-81 [PubMed PMID: 7784563]
Vogel N, Wolcke B, Kauczor HU, Kelbel C, Mildenberger P. [Detection of a bronchopleural fistula with spiral CT and 3D reconstruction]. Aktuelle Radiologie. 1995 May:5(3):176-8 [PubMed PMID: 7605817]
Level 3 (low-level) evidenceSarkar P, Patel N, Chusid J, Shah R, Talwar A. The role of computed tomography bronchography in the management of bronchopleural fistulas. Journal of thoracic imaging. 2010 Feb:25(1):W10-3. doi: 10.1097/RTI.0b013e31819d12f1. Epub [PubMed PMID: 20160584]
Level 3 (low-level) evidenceNielsen KR, Blake LM, Mark JB, DeCampli W, McDougall IR. Localization of bronchopleural fistula using ventilation scintigraphy. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1994 May:35(5):867-9 [PubMed PMID: 8176473]
Level 3 (low-level) evidenceRaja S, Rice TW, Neumann DR, Saha GB, Khandekar S, MacIntyre WJ, Go RT. Scintigraphic detection of post-pneumonectomy bronchopleural fistulae. European journal of nuclear medicine. 1999 Mar:26(3):215-9 [PubMed PMID: 10079310]
Hsu JT, Bennett GM, Wolff E. Radiologic assessment of bronchopleural fistula with empyema. Radiology. 1972 Apr:103(1):41-5 [PubMed PMID: 5015836]
Mulot A, Sepulveda S, Haberer JP, Alifano M. Diagnosis of postpneumonectomy bronchopleural fistula using inhalation of oxygen or nitrous oxide. Anesthesia and analgesia. 2002 Oct:95(4):1122-3 [PubMed PMID: 12351312]
Level 3 (low-level) evidenceYork EL, Lewall DB, Hirji M, Gelfand ET, Modry DL. Endoscopic diagnosis and treatment of postoperative bronchopleural fistula. Chest. 1990 Jun:97(6):1390-2 [PubMed PMID: 2347224]
Level 3 (low-level) evidenceMao R, Ying PQ, Xie D, Dai CY, Zha JY, Chen T, Jiang GN, Fei K, Chen C. Conservative management of empyema-complicated post-lobectomy bronchopleural fistulas: experience of consecutive 13 cases in 9 years. Journal of thoracic disease. 2016 Jul:8(7):1577-86. doi: 10.21037/jtd.2016.06.23. Epub [PubMed PMID: 27499946]
Level 3 (low-level) evidenceBaldwin JC, Mark JB. Treatment of bronchopleural fistula after pneumonectomy. The Journal of thoracic and cardiovascular surgery. 1985 Dec:90(6):813-7 [PubMed PMID: 4068731]
Level 3 (low-level) evidenceBaumann MH, Sahn SA. Medical management and therapy of bronchopleural fistulas in the mechanically ventilated patient. Chest. 1990 Mar:97(3):721-8 [PubMed PMID: 2407455]
Sager JS, Eiger G, Fuchs BD. Ventilator auto-triggering in a patient with tuberculous bronchopleural fistula. Respiratory care. 2003 May:48(5):519-21 [PubMed PMID: 12729469]
Level 3 (low-level) evidenceHa DV, Johnson D. High frequency oscillatory ventilation in the management of a high output bronchopleural fistula: a case report. Canadian journal of anaesthesia = Journal canadien d'anesthesie. 2004 Jan:51(1):78-83 [PubMed PMID: 14709467]
Level 3 (low-level) evidenceMassera F, Robustellini M, Pona CD, Rossi G, Rizzi A, Rocco G. Predictors of successful closure of open window thoracostomy for postpneumonectomy empyema. The Annals of thoracic surgery. 2006 Jul:82(1):288-92 [PubMed PMID: 16798231]
Level 2 (mid-level) evidenceMarwah V, Ravikumar R, Rajput AK, Singh A. Transcutaneous closure of chronic broncho-pleuro-cutaneous fistula by duct occluder device. Lung India : official organ of Indian Chest Society. 2016 Mar-Apr:33(2):216-8. doi: 10.4103/0970-2113.177440. Epub [PubMed PMID: 27051115]
Wain JC. Management of late postpneumonectomy empyema and bronchopleural fistula. Chest surgery clinics of North America. 1996 Aug:6(3):529-41 [PubMed PMID: 8818420]
West D, Togo A, Kirk AJ. Are bronchoscopic approaches to post-pneumonectomy bronchopleural fistula an effective alternative to repeat thoracotomy? Interactive cardiovascular and thoracic surgery. 2007 Aug:6(4):547-50 [PubMed PMID: 17669932]
Level 1 (high-level) evidenceFruchter O, El Raouf BA, Abdel-Rahman N, Saute M, Bruckheimer E, Kramer MR. Efficacy of bronchoscopic closure of a bronchopleural fistula with amplatzer devices: long-term follow-up. Respiration; international review of thoracic diseases. 2014:87(3):227-33. doi: 10.1159/000357074. Epub 2014 Jan 14 [PubMed PMID: 24434610]
Battistoni P, Caterino U, Batzella S, Dello Iacono R, Lucantoni G, Galluccio G. The Use of Polyvinyl Alcohol Sponge and Cyanoacrylate Glue in the Treatment of Large and Chronic Bronchopleural Fistulae following Lung Cancer Resection. Respiration; international review of thoracic diseases. 2017:94(1):58-61. doi: 10.1159/000477350. Epub 2017 Jan 25 [PubMed PMID: 28538215]
Lan RS, Lee CH, Tsai YH, Wang WJ, Chang CH. Fiberoptic bronchial blockade in a small bronchopleural fistula. Chest. 1987 Nov:92(5):944-6 [PubMed PMID: 3665617]
Level 3 (low-level) evidenceMartin WR, Siefkin AD, Allen R. Closure of a bronchopleural fistula with bronchoscopic instillation of tetracycline. Chest. 1991 Apr:99(4):1040-2 [PubMed PMID: 2009764]
Level 3 (low-level) evidenceSprung J, Krasna MJ, Yun A, Thomas P, Bourke DL. Treatment of a bronchopleural fistula with a Fogarty catheter and oxidized regenerated cellulose (surgicel). Chest. 1994 Jun:105(6):1879-81 [PubMed PMID: 8205897]
Level 3 (low-level) evidencePotaris K, Mihos P, Gakidis I. Preliminary results with the use of an albumin-glutaraldehyde tissue adhesive in lung surgery. Medical science monitor : international medical journal of experimental and clinical research. 2003 Jul:9(7):PI79-83 [PubMed PMID: 12883462]
Finch CK, Pittman AL. Use of fibrin glue to treat a persistent pneumothorax with bronchopleural fistula. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2008 Feb 15:65(4):322-4. doi: 10.2146/ajhp070101. Epub [PubMed PMID: 18238769]
Level 3 (low-level) evidenceScappaticci E, Ardissone F, Ruffini E, Baldi S, Mancuso M. Postoperative bronchopleural fistula: endoscopic closure in 12 patients. The Annals of thoracic surgery. 1994 Jan:57(1):119-22 [PubMed PMID: 8279876]
Level 2 (mid-level) evidenceTakaoka K, Inoue S, Ohira S. Central bronchopleural fistulas closed by bronchoscopic injection of absolute ethanol. Chest. 2002 Jul:122(1):374-8 [PubMed PMID: 12114386]
Level 3 (low-level) evidenceAho JM, Dietz AB, Radel DJ, Butler GW, Thomas M, Nelson TJ, Carlsen BT, Cassivi SD, Resch ZT, Faubion WA, Wigle DA. Closure of a Recurrent Bronchopleural Fistula Using a Matrix Seeded With Patient-Derived Mesenchymal Stem Cells. Stem cells translational medicine. 2016 Oct:5(10):1375-1379 [PubMed PMID: 27343169]
Jana M, Gamanagatti SR, Kumar A, Mishra B. Traumatic esophago-bronchopleural fistula-CT finding and treatment using glue: A procedure not so commonly performed. Lung India : official organ of Indian Chest Society. 2011 Oct:28(4):303-5. doi: 10.4103/0970-2113.85697. Epub [PubMed PMID: 22084549]
Hollaus PH, Lax F, el-Nashef BB, Hauck HH, Lucciarini P, Pridun NS. Natural history of bronchopleural fistula after pneumonectomy: a review of 96 cases. The Annals of thoracic surgery. 1997 May:63(5):1391-6; discussion 1396-7 [PubMed PMID: 9146332]
Level 2 (mid-level) evidenceKempainen RR, Pierson DJ. Persistent air leaks in patients receiving mechanical ventilation. Seminars in respiratory and critical care medicine. 2001 Dec:22(6):675-84 [PubMed PMID: 16088712]
Stamatis G, Freitag L, Wencker M, Greschuchna D. Omentopexy and muscle transposition: two alternative methods in the treatment of pleural empyema and mediastinitis. The Thoracic and cardiovascular surgeon. 1994 Aug:42(4):225-32 [PubMed PMID: 7825161]
Level 1 (high-level) evidenceHollaus PH, Huber M, Lax F, Wurnig PN, Böhm G, Pridun NS. Closure of bronchopleural fistula after pneumonectomy with a pedicled intercostal muscle flap. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 1999 Aug:16(2):181-6 [PubMed PMID: 10485418]
Level 2 (mid-level) evidenceSirbu H, Busch T, Aleksic I, Schreiner W, Oster O, Dalichau H. Bronchopleural fistula in the surgery of non-small cell lung cancer: incidence, risk factors, and management. Annals of thoracic and cardiovascular surgery : official journal of the Association of Thoracic and Cardiovascular Surgeons of Asia. 2001 Dec:7(6):330-6 [PubMed PMID: 11888471]
Level 2 (mid-level) evidenceIchinose J, Hashimoto K, Matsuura Y, Nakao M, Okumura S, Mun M. Risk factors for bronchopleural fistula after lobectomy for lung cancer. Journal of thoracic disease. 2023 Jun 30:15(6):3330-3338. doi: 10.21037/jtd-22-1809. Epub 2023 Jun 5 [PubMed PMID: 37426169]
Sfyridis PG, Kapetanakis EI, Baltayiannis NE, Bolanos NV, Anagnostopoulos DS, Markogiannakis A, Chatzimichalis A. Bronchial stump buttressing with an intercostal muscle flap in diabetic patients. The Annals of thoracic surgery. 2007 Sep:84(3):967-71 [PubMed PMID: 17720409]
Level 1 (high-level) evidenceDeschamps C, Bernard A, Nichols FC 3rd, Allen MS, Miller DL, Trastek VF, Jenkins GD, Pairolero PC. Empyema and bronchopleural fistula after pneumonectomy: factors affecting incidence. The Annals of thoracic surgery. 2001 Jul:72(1):243-7; discussion 248 [PubMed PMID: 11465187]
Hamad AM, Marulli G, Sartori F, Rea F. Pericardial flap for bronchial stump coverage after extrapleural pneumonectomy; is it feasible? European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2008 Dec:34(6):1255-6. doi: 10.1016/j.ejcts.2008.09.004. Epub 2008 Oct 9 [PubMed PMID: 18848462]
Lardinois D, Horsch A, Krueger T, Dusmet M, Ris HB. Mediastinal reinforcement after induction therapy and pneumonectomy: comparison of intercostal muscle versus diaphragm flaps. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2002 Jan:21(1):74-8 [PubMed PMID: 11788261]