Introduction
Brainstem stroke is the most lethal form of all strokes. Both hemorrhagic and ischemic brainstem strokes account for a significant cause of morbidity and mortality on the global front. An ischemic form has a higher incidence compared to its hemorrhagic brainstem counterpart.[1] Knowledge of brainstem stroke syndromes is prudent for early diagnosis and timely management to ensure better clinical outcomes.
The brainstem is composed of the midbrain, the pons, and the medulla oblongata, situated in the posterior part of the brain, connecting the cerebrum, the cerebellum, and the spinal cord. Embryologically, the brainstem develops from the mesencephalon and part of the rhombencephalon, originating from the neural ectoderm. The brainstem is organized internally in 3 laminae: tectum, tegmentum, and basis. Gray matter in the brainstem is found in clusters all along the brainstem, mainly forming the cranial nerve nuclei, the pontine nuclei, and the reticular formation. White matter in various ascending and descending tracts is found primarily on the basis lamina, the most anterior part.[2] The brainstem is responsible for multiple critical functions, including respiration, cardiac rhythm, blood pressure control, consciousness, and the sleep-wake cycle. The cranial nerve nuclei in the brainstem have a crucial role in vision, balance, hearing, swallowing, taste, speech, motor, and sensory supply to the face. The white matter of the brainstem carries most of the signals between the brain and the spinal cord and helps with its relay and processing.
The brainstem vasculature is divided by anatomic structures (ie, medulla oblongata, pons, and midbrain), which are further subdivided into the following arterial territories:
- Medulla oblongata
- Anteromedial: from the anterior spinal artery and the vertebral artery
- Anterolateral: from the ASA and VA
- Lateral: from the posterior inferior cerebellar artery
- Posterior: from the posterior spinal artery
- Pons
- Anteromedial: from perforating arteries of the basilar artery
- Anterolateral: from the anterior inferior cerebellar artery
- Lateral zone: from lateral pontine perforators of the basilar artery, anterior inferior cerebellar artery, or superior cerebellar artery
- Midbrain
- Anteromedial: from the posterior cerebral artery
- Anterolateral: from the posterior cerebral artery or a branch of the anterior choroidal artery
- Lateral group: from the collicular, choroidal, and posterior cerebellar arteries
- Posterior group: from the superior cerebellar, follicular, and posteromedial choroidal artery [3]
Brainstem infarction is an area of tissue death resulting from a lack of oxygen supply to any part of the brainstem. The knowledge of anatomy, vascular supply, and physical examination can be life-saving in the setting of an acute infarct and provide precise diagnosis and management. Time becomes an essential factor in management. Early intervention has shown dramatically reduced morbidity and mortality.[4] The brainstem, accounting for almost one-third of all ischemic strokes, leads to high morbidity and mortality on the global front. The pons are predominantly affected.[3] Medullary infarction accounts for 7% of all ischemic brainstem strokes, with lateral subtypes being the most common. There is a male preponderance with a ratio of 3 to 1.
Atherosclerosis and vertebral artery dissections are the most common causes.[3] The pontine infarction can be isolated or present as a subset of a more extensive posterior circulation infarction. Ventral infarcts are the most common subtype. Atherosclerosis of the perforating arteries and occlusion of the basilar artery are the most common causes. This can present as a lacunar variant presenting ubiquitously as pure motor, dysarthria-clumsy hand, ataxic hemiparesis syndrome, and pure sensory stroke patterns.[3] Isolated midbrain infarctions are rare and commonly present with concurrent cerebellum, pons, or thalamus involvement.[3] Dorsal pontine involvement is the most common anatomical site for the location of brainstem hemorrhagic stroke.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Ischemic Brainstem Strokes
Brainstem infarction is the sequelae of ischemia to any part of the brainstem due to the loss of blood supply or bleeding. Posterior circulation occlusion and stenosis cause significant hypoperfusion in the brainstem. The most common etiologies for brainstem infarction are atherosclerosis, thromboembolism, lipohylanosis, tumor, arterial dissection, and trauma. In medulla oblongata infarcts, 73% are due to vertebral artery stenosis, 26% are due to arterial dissection, and cardioembolic causes comprise the remainder.[5] However, the number of infarcts due to cardioembolic etiology increases to 8% in pontine infarcts and 20% to 46% in midbrain infarcts.[6]
Large vessel atherothrombosis is the predominant cause of all ischemic strokes in all anatomical locations. Cardioembolic events are more common in mesencephalic infarcts, whereas dissections are common for medullary infarctions.[3] Approximately 25% of ischemic strokes occur within the posterior circulation, 60% in the brainstem, and 40% in the cerebellum.[3] According to the TOAST classification system, large artery atherothrombosis is the primary underlying etiology of these strokes in all arterial territories (63% overall and 74% in pons). Cardioembolism accounts for 15% to 30 % of the same. Cardioembolic etiology is involved in 26% of the midbrain and 31.5% of multiple concurrent multiple infarctions. Dissection is more commonly involved in the lateral medulla supplied by vertebral arteries.[7]
Risk factors for all types of stroke include hypertension, diabetes mellitus, metabolic syndromes, hyperlipidemia, tobacco use, obesity, history of ischemic heart disease, atrial fibrillation, sleep apnea, lack of physical activity, use of oral contraceptives, fibromuscular dysplasia, trauma, and spinal manipulation.[3][8][9] Ischemic brainstem stroke risk factors include:
- Atherosclerosis
- Hypertension
- Diabetes
- Smoking
- Atrial fibrillation
- Hyperlipidemia
- Ischemic heart disease
- Embolism, and
- Dissections [3]
Hemorrhagic Brainstem Strokes
The etiologies for hemorrhagic brainstem strokes include:
Epidemiology
Globally, there is a rise in lifestyle diseases like cardiovascular disease, stroke, and diabetes mellitus, both in developed and developing nations. The global burden of stroke can be measured at 122 million disease-adjusted life years.[11] In the US, a stroke is reported every 40 seconds.[12] Studies have estimated that 10% to 15% of all strokes occur in the brainstem.[8] The lifetime risk of stroke in individuals aged 25 years and older for males is between 23.3% and 26.0%, and for females is between 23.7% and 26.5%. The incidence varies between regions, with Eastern sub-Saharan Africa having the lowest lifetime risk of 11.8% and East Asia with the highest risk of 38.8%. China has the most significant lifetime estimated risk of 39.3%.[13]
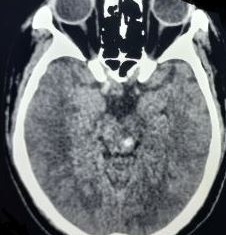
Pontine is the most prevalent site of brainstem strokes, involved with 60% of infarctions (see Image. Pontine Infarction).[14] Ischemic vertebrobasilar strokes account for 23%, whereas ischemic brainstem infarcts comprise 11% of all ischemic strokes. Approximately 27%, 14%, and 7% involve the pons, medulla, and midbrain. The cerebellar is involved in 7%, posterior cerebral artery territory in 36%, and concurrent multiple sites involved in 9%. Isolated pons infarcts were observed in 3% of all ischemic strokes. Lateral medullar infarcts are almost 5 times more common than medial infarcts. The lateral midbrain is the least involved anatomical region in the assessment.[14] The anteromedial (AnM), Anterolateral (AnL), and lateral territories have similar incidences, with posterior infarcts being the least common subtype. AnM variant is frequently involved in the midbrain, AnM and AnL in the pons, and lateral in the medulla.[14] Wallenberg’s syndrome, also known as posterior cerebellar artery syndrome or lateral medullary syndrome, is the most common form of brainstem stroke. Anterior inferior cerebellar artery syndrome (AICAS) or lateral pontine syndrome is the second most common brain stem stroke.[14]
Primary brainstem hemorrhage (PBSH) constitutes 10% of all intracerebral hemorrhages (ICH) and has an annual incidence of approximately 2 to 4 of 100,000 individuals annually.[15] The pons is the most commonly involved area, reportedly in 60% to 80% of brainstem strokes.[15] Furthermore, these strokes occur most frequently in males and individuals aged 40 to 60 years.[15] However, women with brainstem strokes have a better survival rate.[15]
Pathophysiology
The pathophysiology of all infarcts is the lack of oxygen in the tissue, leading to its death. The human brain requires 20% of oxygen consumption even though it accounts for only 2% of the body by weight.[16] The cerebral blood flow is autoregulated to maintain a constant perfusion level and adequate venous drainage for all its needs. The cerebrum is also unique as it has little to no energy stores and uses glucose as its primary energy source, with distally produced ketone bodies being used only in starvation.[17] Dependence on aerobic respiration and low respiratory reserve makes the brain susceptible to ischemia and eventually causes irreversible tissue death (see Table. Brainstem Structures, Deficits, and Vascular Supply). The cellular cascade of events includes:
- Depletion of ATP due to lack of aerobic respiration in the mitochondria
- Loss of function of membrane ion pumps and impaired voltage gradient across membranes, leading to cellular edema
- Neuronal excitotoxicity due to the release of glutamate and synaptosomal-associated protein causes further deterioration of energy levels and membrane ion potentials.; reactive oxygen species and free radical production leads to cell death [18]
While the above apoptotic and necrotic pathway is in process, specific protective pathways are triggered, including:
- Expression of heat shock protein 70, B-cell lymphoma 2 gene family, and prion protein to prevent activation of the apoptotic cascade
- Release of Neurotrophin-3, interleukin-10, and granulocyte-colony stimulating factor, helping activate survival pathways and reduce proinflammatory cytokine activities
The cellular cascade is potentially reversible, which can lead to vasogenic edema over the next few hours. Vasogenic edema causes an increase in pressure in the surrounding tissue, leading to mass effects and worsening the situation.[19] The eventual release of matrix metalloproteinases causes structural integrity loss and the dissolution of the blood-brain barrier.[20]
In the case of hemorrhagic etiology, the rupture of blood vessels causes hypoxia, pressure effects, and chemical irritation of brain tissue due to the disruption of the blood-brain barrier. Animal models for PBSH are mainly obtained through stereotactic collagenase injection or autologous blood.[15]The pathogenesis observed in animal models showed erythrocyte lysis, heme oxygenase expression, iron deposition, and release of reactive oxygen species generation involved in the pathogenesis.[21] Anterior territory involvement is more common with stroke in the mesencephalon and pons, whereas posterior and lateral anatomical involvement occurs more frequently in strokes involving the medulla.[7]
History and Physical
Clinical Symptoms
A loss of about 1.9 million neurons in the brain happens each minute in an untreated stroke.[22] Hence, a targeted approach with clear objectives must be followed. Assessment of airway, breathing, and circulation, and its stabilization as a patient with brainstem stroke can present with trauma, altered mental status, altered respiratory drive, hypoxia, vomiting, and or mechanical airway obstruction. Establishing the time of insult is critical. Patients, family members, attendees, co-workers, first responders, or any reliable witness can determine when the patient was last known to be asymptomatic. If deficits arise in one's sleep, the last known normal is when the patient goes to bed. A clinician needs to distinguish between stroke and other differential diagnoses that can cause neurological deficits. Reliable information about the patient's current medication, especially about oral hypoglycemic, insulin, antiepileptics, neurological or psychological drugs, antiplatelets or blood thinners, drug abuse or overdose, and sleep apnea must be established. Comorbidities and risk factors need to be assessed. Evaluation of signs and symptoms of hemorrhagic stroke is life-saving. Any history of uncontrolled hypertension, sudden onset of headache, vomiting, and signs of raised intracranial pressure must raise a high suspicion of hemorrhage and warrants an immediate noncontrast computed tomographic (CT) scan of the head.
Common symptoms associated with brainstem stroke include coma and central hyperthermia. Brainstem lesions can help identify the general region or function of the brainstem affected by the presenting symptoms.[3][23]
- Ascending and descending pathways: Weakness, loss of pain and temperature sensation, ataxia, Horner syndrome, loss of position and vibration sensation, gaze palsy
- Nuclei and cranial nerves: Ocular and extraocular muscle weakness, loss of sensation over the face, autonomic dysregulation, dysphagia, dysarthria, dysphonia, vertigo, alteration in taste and hearing
- Integrative and other functions: Choreoathetosis, tremors, ataxia, central dysautonomia, gaze paresis, lethargy, and locked-in syndrome
Physical Examination
A focused physical examination should be performed to evaluate patients for signs of trauma, meningeal irritation, or neurological deficits. The following areas should be assessed during the neurological examination of patients with a brainstem stroke:
- Levels of consciousness and higher mental function
- Complete evaluation of cranial nerves and their functions
- Motor and sensory system examination, including reflexes, neglect, speech, and language
- Cerebellar signs, coordination, and gait
- Autonomic system
Clinical assessment of the 4 medial structures towards the midline, within the brainstem, including the motor tract (ie, corticospinal tract), medial lemniscus, medial longitudinal fasciculus, and motor nucleus of cranial nerves 3, 4, 6, and 12, and the 4 lateral structures, towards the sidelines, consisting of the spinocerebellar, spinothalamic, and sympathetic tracts as well as the sensory nucleus of the trigeminal nerve can help in the anatomical localization of the brainstem stroke.[24]
Evaluation
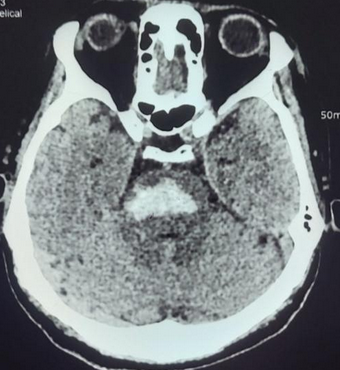
Evaluation of patients presenting with a suspected brainstem stroke includes vital signs, oxygen saturation, blood pressure, pulse rate, respiratory rate, fingerstick blood glucose levels, and noncontrast computed tomography (CT) scan of the head or brain magnetic resonance imaging (MRI). A noncontrast CT scan of the head is the preferred modality to evaluate brainstem stroke due to being a quick and widely available imaging method that is highly sensitive in detecting acute hemorrhage (see Image. Brainstem Stroke MRI). On a head CT scan, blood can be seen as a hyperdense lesion. Brain MRI can detect infarction of brain tissue with diffusion-weighted and fluid-attenuated inversion recovery images being highly sensitive in the hyperacute setting.[25]
Blood workup should include complete blood count, coagulation profile, serum electrolytes, renal function, lipid panel, hemoglobin-A1c level, thyroid function, vitamin B12, and vitamin D levels. Other blood investigations for hypercoagulability states, autoimmune conditions, liver pathologies, and genetic tests can be obtained. A cardiovascular workup for atrial fibrillation with either an electrocardiogram or Holter monitor, echocardiogram, cardiac enzyme levels, and chest x-ray should be obtained. A multi-phase CT angiography can establish the state of vertebral and carotid arteries, along with assessment for any endovascular management. Sleep study or polysomnography is diagnostic for various sleep disorders and must be suspected in stroke cases with unknown etiologies. Evaluation of modifiable and nonmodifiable risk factors for cardiovascular disease must be done.
Due to the high density of nuclei and fibers running through the brainstem, the lesion in various structures gives rise to different signs and symptoms. Variously named stroke and stroke syndromes that have been described in the literature include:
- Top-of-the-basilar syndrome: Also known as the rostral brainstem infarction, this condition results in alternating disorientation, hypersomnolence, unresponsiveness, hallucination, behavioral abnormalities, visual and oculomotor deficits, and cortical blindness. Occurs due to occlusion of the distal basilar artery and its perforators.[26]
- Ondine syndrome: This condition affects the brainstem response centers for automatic breathing, resulting in complete breathing failure during sleep but normal ventilation when awake. The blood supply affected is the pontine perforating arteries, branches of the basilar artery, anterior inferior cerebellar artery, or the superior cerebellar artery.[26]
- One-and-a-half syndrome: The paramedian pontine reticular formation and medial longitudinal fasciculus are affected, resulting in ipsilateral conjugate gaze palsy and internuclear ophthalmoplegia. The blood supply affected is the pontine perforating arteries and branches of the basilar artery.[27]
Brainstem Stroke Syndromes
Several syndromes may be classified based on the area of the brainstem affected.
Midbrain syndromes
The following conditions are associated with abnormalities of the midbrain:
- Claude syndrome: Affects the fibers from CN III, the rubrodentate fibers, corticospinal tract fibers, and corticobulbar fibers, resulting in ipsilateral CN III palsy, contralateral hemiplegia of lower facial muscles, tongue, shoulder, upper and lower limb, and contralateral ataxia. The blood supply involved is from the posterior cerebral artery.
- Dorsal midbrain syndrome: Also known as paramedian midbrain syndrome or Benedikt syndrome, this affects the fibers from CN III and the red nucleus. It results in ipsilateral CN III palsy, contralateral choreoathetosis, tremor, and ataxia. The blood supply involved comes from the posterior cerebral artery and paramedian branches of the basilar artery.
- Nothnagel syndrome: Affects the fibers from CN III and the superior cerebellar peduncle, resulting in ipsilateral CN III palsy and ipsilateral limb ataxia. This syndrome can be due to quadrigeminal neoplasms and is often bilateral.
- Ventral midbrain syndrome: Also known as Weber syndrome, this condition affects the fibers from CN III, cerebral peduncle (ie, corticospinal and corticobulbar tracts), and substantia nigra, resulting in ipsilateral CN III palsy, contralateral hemiplegia of lower facial muscles, tongue, shoulder, and upper and lower limbs. The involvement of substantial nigra is present can result in a contralateral movement disorder. The blood supply affected is the paramedian branches of the posterior cerebral artery.[28][29][30][31][32]
Pontine syndromes
The following conditions are associated with abnormalities of the pons:
- Brissaud-Sicard syndrome: Affects the CN VII nucleus and corticospinal tract, resulting in ipsilateral facial cramps and contralateral upper and lower limb hemiparesis. The blood supply affected is the posterior circulation. Rarely, the syndrome can arise due to brainstem glioma.
- Facial colliculus syndrome: Affects the CN VI nucleus, the CN VII nucleus, fibers, and the medial longitudinal fasciculus, resulting in lower motor neuron CN VII palsy, diplopia, and horizontal conjugate. It can occur due to neoplasm, multiple sclerosis, or viral infection.
- Gasperini syndrome: This syndrome affects the nuclei of CN V, VI, VII, VIII, and the spinothalamic tract, which results in ipsilateral facial sensory loss, ipsilateral impaired eye abduction, ipsilateral impaired eye abduction, ipsilateral nystagmus, vertigo, and contralateral hemi-sensory impairment. The blood supply derives from the basilar artery's pontine branches and the anterior inferior cerebellar artery's long circumferential artery.
- Gellé syndrome: Affects the CN VII, VIII, and corticospinal tract, resulting in ipsilateral facial palsy, hearing loss, and contralateral hemiparesis.
- Grenet syndrome: This syndrome affects the CN V lemniscus, CN VII fibers, and the spinothalamic tract, which results in altered sensation in the ipsilateral face and contralateral upper and lower limbs. It can arise due to neoplasm.
- Inferior medial pontine syndrome: Also known as the lower dorsal pontine syndrome or Foville syndrome, this syndrome affects the corticospinal tract, medial lemniscus, middle cerebellar peduncle, and the nucleus of CN VI and VII, resulting in contralateral hemiparesis, contralateral loss of proprioception and vibration, ipsilateral ataxia, ipsilateral facial palsy, lateral gaze paralysis, and diplopia. The blood supply affected is from branches of the basilar artery.
- Lateral pontine syndrome: Also known as Marie-Foix syndrome, this affects the nuclei of CN VII and VIII, corticospinal tract, spinothalamic tract, and cerebellar tracts, resulting in contralateral hemiparesis, contralateral loss of proprioception and vibration, ipsilateral limb ataxia, ipsilateral facial palsy, lateral hearing loss, vertigo, and nystagmus. The blood supply affected is the perforating branches of the basilar artery and the anterior inferior cerebellar artery.
- Locked-In syndrome: Affects upper ventral pons, including corticospinal tract, corticobulbar tract, and CN VI nuclei, resulting in quadriplegia, bilateral facial palsy, and horizontal eye palsy. The patient can move the eyes vertically, blink, and has intact consciousness. The blood supply affected is the middle and proximal segments of the basilar artery.
- Raymond syndrome: Affects the CN VI fibers, corticospinal tract, and corticofacial fibers, resulting in an ipsilateral lateral gaze palsy, contralateral hemiparesis, and facial palsy. The blood supply involved is from the branches of the basilar artery.
- Upper dorsal pontine syndrome: This syndrome, also known as Raymond-Cestan syndrome, affects the longitudinal medial fasciculus, medial lemniscus, spinothalamic tract, CN V fibers and nuclei, and superior and middle cerebellar peduncle. It results in ipsilateral ataxia, coarse intension tremors, sensory loss in the face, weakness of mastication, and contralateral loss of all sensory modalities. The blood supply involved is from the circumferential branches of the basilar artery.
- Ventral pontine syndrome: Sometimes referred to as Millard-Gubler syndrome, this affects the CN VI and VII and corticospinal tract, resulting in ipsilateral lateral rectus palsy, diplopia, ipsilateral facial palsy, and contralateral hemiparesis of the upper and lower limbs. The blood supply involved derives from the branches of the basilar artery.[28][29][31][33][34][35][36][37][38]
Medulla oblongata syndromes
The following conditions are associated with abnormalities of the medulla oblongata:
- Avellis syndrome: Affects the pyramidal tract and nucleus ambiguus. It results in ipsilateral palatopharyngeal palsy, contralateral hemiparesis, and contralateral hemi-sensory impairment. The blood supply affected is the vertebral arteries.
- Babinski-Nageotte syndrome: Also known as the Wallenberg with hemiparesis, affects the spinal fiber and nucleus of CN V, nucleus ambiguus, lateral spinothalamic tract, sympathetic fibers, afferent spinocerebellar tracts, and corticospinal tract, resulting in ipsilateral facial loss of pain and temperature, ipsilateral palsy of the soft palate, larynx and pharynx, ipsilateral Horner syndrome, ipsilateral cerebellar hemi-ataxia, contralateral hemiparesis, and contralateral loss of body pain and temperature. The blood supply involved is from the intracranial portion of the vertebral artery and branches from the posterior inferior cerebellar artery.
- Cestan-Chenais syndrome: The condition affects the spinal fiber and nucleus of CN V, nucleus ambiguus, lateral spinothalamic tract, sympathetic fibers, and corticospinal tract and results in ipsilateral facial loss of pain and temperature, ipsilateral palsy of the soft palate, larynx and pharynx, ipsilateral Horner's syndrome, contralateral hemiparesis, contralateral loss of body pain and temperature, and contralateral tactile hypesthesia. The blood supply affected is the intracranial portion of the vertebral artery and branches from the posterior inferior cerebellar artery.
- Hemimedullary syndrome: This condition, also known as Reinhold syndrome, affects the nucleus and fiber of CN V, CN XII nucleus ambiguus, lateral spinothalamic tract, sympathetic fibers, afferent spinocerebellar tracts, corticospinal tract, and medial lemniscus. It results in ipsilateral Horner's syndrome, ipsilateral facial loss of pain and temperature, ipsilateral palsy of soft palate, larynx and pharynx, ipsilateral tongue weakness, ipsilateral cerebellar hemi-ataxia, contralateral hemiparesis, and contralateral face sparing hemihypesthesia. The blood supply is from the ipsilateral vertebral artery, the posterior inferior cerebellar artery, and branches from the anterior spinal artery.
- Jackson syndrome: Affects CN XII and pyramidal tract, resulting in ipsilateral palsy of the tongue and contralateral hemiparesis. The blood supply involved is from the branches of the anterior spinal artery.
- Lateral medullary syndrome: Also known as Wallenberg syndrome, this affects the spinal nucleus and fiber of CN V, nucleus ambiguus, lateral spinothalamic tract, sympathetic fibers, inferior cerebellar peduncle, and vestibular nuclei. It results in ipsilateral Horner's syndrome, ipsilateral facial loss of pain and temperature, ipsilateral palsy of soft palate, larynx and pharynx, ipsilateral cerebellar hemi-ataxia, contralateral loss of body pain and temperature, nystagmus, dysarthria, dysphagia, and hyperacusis. The blood supply affected is the vertebral artery and branches from the posterior inferior cerebellar artery.
- Medial medullary syndrome: Sometimes referred to as Dejerine syndrome, this condition affects the fibers of CN XII, corticospinal tract, and medial lemniscus spinal, resulting in ipsilateral tongue weakness, ipsilateral loss of proprioception and vibration, contralateral hemiparesis, and contralateral face-sparing hemihypesthesia. The blood supply affected is the branches from the vertebral artery and the anterior spinal artery.
- Schmidt syndrome: This syndrome affects the fibers and nuclei of CN IX, X, XI, and pyramidal systems, resulting in ipsilateral palsy of the vocal cords, soft palate, trapezius, and sternocleidomastoid muscle, and contralateral spastic hemiparesis. The blood supply involves branches from the vertebral artery, the posterior inferior cerebellar, and the anterior spinal artery.
- Spiller syndrome: The fibers and nucleus of CN XII, corticospinal tract, medial lemniscus spinal, and medial hemi-medulla are affected, resulting in ipsilateral tongue weakness, ipsilateral loss of proprioception and vibration, contralateral hemiparesis, and contralateral face-sparing hemihypesthesia. The blood supply is from the vertebral artery and anterior spinal artery branches.
- Tapia syndrome: Affects the nucleus ambiguus, CN XII, and pyramidal tract, resulting in ipsilateral palsy of the trapezius, sternocleidomastoid muscle, and half of the tongue, dysphagia, dysphonia, and contralateral spasmodic hemiparesis. The blood supply is from the vertebral artery branches, the posterior inferior cerebellar, and the anterior spinal artery.
- Vernet syndrome: Affects the CN IX, X, and XI and occurs due to compression in the jugular foramen.[28][29][39][40][41][42][43]
Brainstem stroke subgroups differ significantly only in the incidence of hemiparesis (74% in pontine but 30% with medullary or cerebellar strokes) and ataxia (97% and 95% of cerebellar and medullary but 74% in pontine) strokes.[14] Convulsive-like movements have been observed in pontine strokes due to ischemia of the corticospinal tracts.[44] Restless leg syndrome has been observed in anteromedial pontine infarction.[45]
Diagnostic Imaging
CT scanning is the preferred method for radio imaging owing to its general availability. CT findings also closely correlate with prognosis in PBSH.[46] Computed tomography (CT) may paradoxically appear normal in the early course of the stroke. The "hyperdense basilar artery sign" may be observed. CT shows a nonenhancing hyperdense lesion. The formula ABC/2 can be used to calculate the volume of an ICH on CT, where "A" is the greatest hemorrhage diameter, "B" is the diameter 90 degrees to A, and "C" is the approximate amount of CT slices containing hemorrhage multiplied by the slice thickness.[47] However, the 2/3SH method is more accurate than ABC/2, with "S" representing the area of the largest axial hemorrhagic slice and "H" representing the height of the hematoma.[46] CT angiography (CTA) helps to locate the occlusion level and delineate the infarct's size. The "spot sign" is not significantly related to hematoma expansion.[48] CT perfusion delineates the core and penumbra zones.[3]
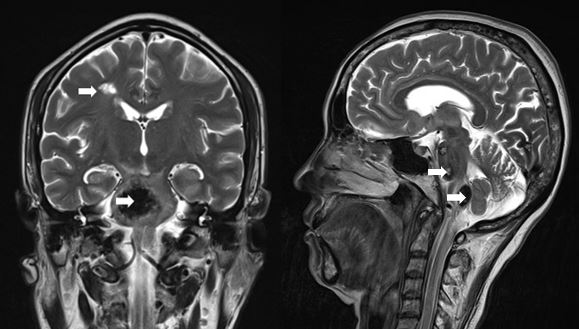
MRI helps to depict the location and extension of the stroke accurately.[3] Diffusion-weighted magnetic resonance sequence is the recommended modality to visualize the irreversibly infarcted regions and also has rapid acquisition. Magnetic resonance perfusion images are paramount in evaluating the penumbra zone.[3] Hemosiderin deposition on T2-weighted (T2W) and gradient-echo magnetic resonance images alongside characteristic "popcorn" appearance are radiological hallmarks for cavernomas (see Image. Cavernoma Imaging Study).[3]
Classifications of Primary Brainstem Hemorrhage
Several methods have classified PBSH. Chung and Park categorized pontine hemorrhage by the following anatomical landmarks:
- Dorsal group involving unilateral or bilateral tegmentum
- A ventral group involving the ventral basis pontis
- A massive group involving the basis pontis and tegmentum with extension into the midbrain.[3]
However, Kase and Caplan classified pontine hemorrhages as large paramedian, unilateral basal or basotegmental, or lateral tegmental subtypes.[3] The TOAST Classification System categorizes strokes according to etiologies, including:
- Large arterial atherothrombosis: >50% narrowing in CTA/MRA
- Cardioembolic: Atrial flutter on ECG, paroxysmal atrial flutter on Holter monitoring, akinetic segments or thrombus on transthoracic echocardiograms and transesophageal echocardiograms (TEE), history of rheumatic valve disease or prosthetic valves, monitoring, myocardial infarction within a month, or the presence of patent foramen ovale seen in TEE or Bubble test
- Small vessel disease: vessels <2 cm presenting as pure motor, pure sensory, ataxic, and dysarthria with clumsy hand patterns
- Other causes: vasculitis, dissection, paraneoplastic syndrome, or stroke of unknown cause [3]
Treatment / Management
After the patient's airway, breathing, and circulation have been stabilized, a timeframe of the patient's symptoms is obtained. Vitals and fluid status must be stabilized. Hypo or hyperglycemia must be corrected. Fever, if present, should be managed accordingly. Blood pressure must not be aggressively controlled to allow permissive hypertension only in the case of ischemic injury. Patients with the last known normal within 4.5 hours can be considered for thrombolysis, whereas a 24-hour last known normal can be a candidate for mechanical thrombectomy. If a case presents earlier than 4.5 hours of onset, thrombolysis with intravenous recombinant tissue plasminogen activator (tPA) significantly improves the clinical outcome.[49](A1)
Tissue Plasminogen Activator Inclusion Criteria
The following clinical criteria should be met before tPA is administered:
- Clinical diagnosis of ischemic stroke
- <4.5 hours of symptom onset
- Age older than 18 and younger than 80 years
- Symptoms of stroke presenting for more than 30 minutes [49][50] (A1)
Tissue Plasminogen Activator Exclusion Criteria
If the following clinical factors are present, tPA is not recommended:
- Unknown timeline of onset of patient symptoms
- Intracranial hemorrhage or any active bleeding
- Persistently elevated blood pressure ≥185 mm Hg systolic and ≥110 mm Hg diastolic
- Low platelets <100,000 cells/microliter, altered international normalized ratio (INR) >1.7, prothrombin time >15 s or partial thromboplastin time >40 s
- Current use of anticoagulant
- Severe hypoglycemia <50 mg/dL
- History of previous intracranial hemorrhage
- History of gastrointestinal bleeding in the past 21 days
- History of intracranial or intraspinal surgery in the past 90 days
- History of intra-axial intracranial neoplasm or gastrointestinal malignancy [50][51]
Intravenous alteplase, a recombinant tissue plasminogen activator, should be given at 0.9 mg/kg with a maximum dose of 90 mg/kg; 10% of the recommended dose should be given as the loading dose in the first minute. The patient must be under continuous observation. Antiplatelet therapy must be withheld for at least 24 hours postthrombolysis and restarted after a head CT scan without evidence of bleeding. The time window for thrombolytic therapy in brainstem ischemic strokes has not been well defined.[52]
Mechanical endovascular thrombectomy in patients with large anterior circulation occlusion is well documented; however, most strokes affecting the brainstem arise from posterior circulation perforating branches. Endovascular thrombectomy is recommended for successful revascularization and favorable outcomes in cases where the occlusion is at the main vertebral or basilar artery.[52][53][54][55][56][57][58][59] Other studies have shown no evidence of a difference in favorable outcomes between endovascular therapy and standard medical therapy alone.[60][61] (B2)
Conservative Therapy
In many patients, PBSH is primarily treated conservatively with antiplatelet therapy using acetylsalicylic acid as monotherapy or dual therapy along with clopidogrel within 24 to 48 hours after the onset of symptoms significantly improved patient outcomes.[48] Conservative treatment of PBSH is generally recommended for patients with the following clinical features:
- Smaller hematomas >5 ml and <10 ml in size
- GCS score of >6 and <8
- Age younger than 65 years
- A unilateral tegmental variant
- No extra-pontine extension [48]
Surgical Management
The surgical treatment of PBSH is controversial. Brainstem hemorrhage has been excluded from previous ICH trials, such as the Surgical Trial in Lobar Intracerebral Hemorrhage (STICH) and Minimally Invasive Surgery Plus Alteplase for Intracerebral Hemorrhage Evacuation (MISTIE).[48] The management of PBSH primarily involves conservative treatment, and surgery is generally not recommended.[46] In patients with the following features, surgery is not advocated:
- Hematomas <3 mL and >15 ml in size
- No evidence of ventricular dilatation and altered level of consciousness
- Severe irreversible brain damage
- Extreme hemodynamic instability [48]
Surgical options for PBSH include craniotomy and microsurgical evacuation, stereotactic hematoma puncture and drainage, and endoscopic hematoma removal (eg, endoscopic endonasal transclival approach and external ventricular drainage).[46][48] Takahama et al performed stereotactic puncture and drainage in 1989. There is a comparatively decreased mortality rate in the aspiration group compared to the microscopic surgery group (24.4% versus 31.6%). Precision critically can be assisted with the application of newer armamentariums such as Stereotaxy, 3D printing, Robotics, and virtual and augmented reality are new armamentariums.[48] Intraoperative neurophysiological monitoring is another helpful adjunct.[48] Hematoma aspiration is justifiable in older, fragile patients.[48] Intraventricular hemorrhage (IVH) occurs in almost 40% of PBSH patients, so external ventricular drainage can effectively prevent the hazards of acute hydrocephalus.[48] CLEAR III trial has, however, shown no significant advantage of ventricular irrigation with alteplase in IVH.[48]
Minimally invasive microsurgery is a rapid, effective, and safe modality with a hematoma volume <10 ml.[48] In 1998, Hong et al first performed craniotomy for evacuation of brainstem hemorrhage. The advantages of microscopic craniotomy include maximum hematoma clearance under direct vision, better hemostasis, and concurrent removal of the ventricular bleed to avoid secondary hydrocephalus can be undertaken.[48] The surgical group has a 2-fold lower mortality risk and a higher odds of recovery. However, there is a 2-fold risk of being in a vegetative state or harbingering moderate to severe disabilities.[48] Early microsurgical clearance reduces mortality and improves prognosis.[48] This, however, requires high surgical skills and precise anatomical knowledge of safe entry zones within the brainstem.[62][63] Animal studies have shown brain edema and arterial necrosis occurring mostly after 6 hours of PBSH. Therefore, in theory, surgery within a 6-hour window appears to be the most logistic approach.[48] Takimoto et al first performed neuroendoscopy in 2003. This surgical approach is ideally suited for ventral hematomas and utilizes natural surgical corridors with minimal brain retraction and direct visualization of the lesion. A longer learning curve is the main limitation of this modality.[48]
Chinese researchers provided indicators for surgical intervention for PBSH to help identify appropriate candidates, including:
- Ictus for 6 to 24 hours
- Hematoma volume of >5 mL
- Hematoma diameter of >2 cm [48]
Chinese surgeons are, however, compelled to operate even on moribund patients owing to the sociological issue of filial piety.[48] However, most of the studies included are of low to moderate quality with minimal follow-up strategies. Moreover, almost 7% of these patients lacked integration of angiographic studies despite being normotensive.[48] None of the studies incorporated any anatomical classification system as well.[48]
Patient Care Considerations
In addition to conservative and surgical therapies, other aspects of patient care in individuals with PBSH are also essential. Class 1 evidence for nursing care and management includes:
- Clinicians should be highly trained in stroke care.
- Frequent thorough neurological assessments should be performed.
- The patient’s head should be in neutral alignment with the body.
- Only non-dextrose and normotonic intravenous fluids (eg, normal saline) should be given.
- Intravenous rtPA should be administered without delay to all eligible candidates.
- Avoid temperatures >99.6 °F, serum glucose concentrations >140 mg/dL, infections, seizures, and constipation.
- Frequent hemodynamic and hydration assessments should be performed
- Measures to prevent aspiration pneumonia, falls, pressure sores, and deep vein thrombosis must be emphasized.
- Nurses should be familiar with bedside swallow assessment, and a swallow screening ideally needs to be performed by the speech-language pathologist.[64] (A1)
Discharge Disposition
Before patients are discharged from the hospital, the following assessments and patient education should be considered:
- Use of statins
- Dysphagia screening
- Stroke education
- Smoking cessation advice and counseling
- Assessment for rehabilitation [64] (A1)
Management of stroke risk factors, including hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, thyroid abnormalities, sleep apnea, malignancies, and hypercoagulable states, should be treated accordingly. Dietary and lifestyle modification must be explained and discussed. Supplementation with vitamin B12 and vitamin D3 should also be considered. Physiotherapy and rehabilitative strategies must start at the earliest and must be aggressively pursued as the brain loses its plasticity within 90 days. The vascular causes for the brainstem hemorrhages (eg, cavernomas) also need to be properly addressed.[10] Nanoparticle and stem cell combined therapy are recent advances in the management of brainstem strokes.[65][66]
Differential Diagnosis
Differential diagnoses that should be considered during the evaluation of brainstem strokes include:
- Neoplastic and metastatic lesions
- Central pontine myelinolysis
- Acute disseminated encephalomyelitis
- Multiple sclerosis
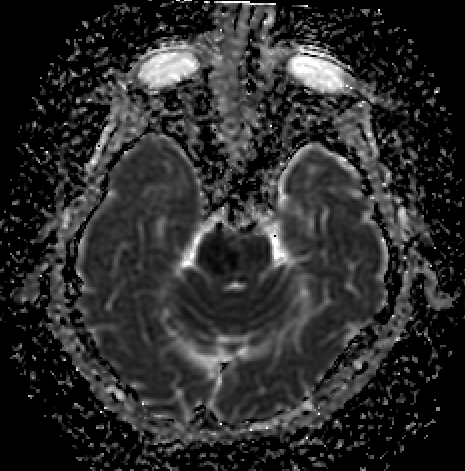
- Diffuse axonal injury (see Image. Diffuse Axonal Injury III)
- Stroke mimics:
- Transient ischemic attack (TIA)
- Subarachnoid hemorrhage
- Seizures
- Basilar migraine
- Basilar meningitis
- Hypoglycemia
- Electrolyte imbalance
- Conversion disorder [48]
Pertinent Studies and Ongoing Trials
The ongoing Safety and Efficacy of Surgical Treatment in Severe Primary Pontine Hemorrhage Evacuation (STIPE) trial should provide newer insights into the role of surgical treatment of PBSH.[46] The inclusion criteria of the trial include:
- GCS <8
- Hematoma volume ≥5 mL
- New PPH score of 2 to 4 points [46]
Prognosis
Stroke is the primary cause of disability and a leading cause of mortality worldwide. Stroke has a burden of 122 million disease-adjusted life years, with gradually increasing incidences.[11] Early diagnosis and management have a lower chance of permanent morbidity. The risk of stroke recurrence is 10% to 15%; hence, regular follow-up is advised. Early initiation of rehabilitative care is also recommended. Patients with significant neurological deficits have a worse prognosis. The final prognosis depends on various factors, including age, the stroke's severity, etiology, location, structures involved, associated risk factors, comorbidities, and management. Variables prognosticating poor outcomes in brainstem strokes include:
- Old age
- Tachycardia (>110 beats/min)
- Systolic blood pressure <100 mm Hg
- Absence of a pupillary light reflex
- Pupillary abnormalities
- Low Glasgow coma scale
- Large hematoma volume
- Central hyperthermia
- A need for mechanical ventilation
- History of diabetes mellitus
- Elevated platelet-to-lymphocyte ratio
- An Elevated Neutrophil-to-lymphocyte ratio [46]
Conventional symptomatic basilar artery occlusion treatment results poorly in almost 80% of cohorts.[67] Death and dependency were observed in nearly 95%. Both vertebral artery occlusion, primarily due to atherosclerosis, and basilar artery occlusion, due to cardio-embolic, have poor outcomes.[68] Time to therapy is better in the intravenous thrombolysis (IVT) group.[68] IVT is safer in posterior circulation ischemic strokes (PCIS) than anterior circulation ischemic strokes (ACIS).[69] This can be considered even in borderline cases even after 4.5 hours of ictus. Time to IVT in PCIS seems to be a less crucial factor than in ACIS.[69] Reportedly, 38–49% have a favorable outcome after IVT. [69] The mortality rate following IVT is not significant between PCIS and ACIS.[69] Moreover, there are no significant differences while comparing time to treatment, mortality, and favorable outcomes between intra-arterial thrombolysis (IAT) and endovascular thrombectomy (EVT), probably owing to collateral flow from posterior communicating arteries towards the posterior circulation.[68]
Recanalization confers a 2-fold decrement in mortality and a 1.5-fold decrease in the futile outcome rates.[68] Better recanalization and improved clinical outcomes in acute basilar occlusion are observed with endovascular thrombectomy compared to thrombolytic therapy (no difference in stent-retrievers and thrombo-aspiration approaches).[68] Significant improvement in the functional outcome and functional independence following thrombectomy, when compared to the best medical therapy alone, was observed. The mortality rate was significantly lower in the intervention group (RR 0.76).[70] A comparative study between different management modalities revealed the following outcomes:
- Mortality: 34.5%, 9.9%, and 28.9% in the mechanical thrombectomy (MT), percutaneous transluminal angioplasty and stenting (PTAS), and MT plus PTAS groups
- Arterial dissection: 3.6% of the MT, 3.1% in the PTAS, and 16.7% of the MT plus PTAS group
- Distal embolization: 3.4%, 5.8%, and 9.5% of the MT, PTAS, and MT plus PTAS groups
- Favorable outcomes: 42.8% of the MT plus PTAS group, 64.7% of the PTAS, and 39.2% of the MT group
- Intracranial hemorrhage: 5.2%, 4.5%, and 15.3% in the MT, PTAS, MT plus PTAS groups
- Successful recanalization: 85.3% of MT, 99.4% of PTAS, and 92.7% of the MT plus PTAS group
PTAS was, therefore, the most effective intervention for vertebrobasilar artery occlusion and was associated with a lower mortality rate than mechanical thrombectomy alone.[71] Almost 30% of these cohorts still possess some disabilities even at 1-year post-stroke. Multiple infarctions and omissions in the use of statins have been associated with poor outcomes at 1 year.[72]
A worse prognosis is observed among patients with PBSH, having dysarthria, pupillary abnormalities, lower cranial nerve involvement, and diminished consciousness on admission.[11] PBSH is the most fatal form of ICH.[46] The mortality rate can be as high as 30% to 88%.[73] In a study comprising 1437 pontine hemorrhages, the overall mortality observed was 48.1%.[74] Massive stroke subtypes with ventral or paramedian locations of involvement have a poor prognosis with a survival rate of only 7.1%. The unilateral tegmental subtype has comparatively more favorable outcomes, with basotegmental variants having an intermediate prognosis (94.1% versus 18.2%).[46][48][73] Bilateral, ventral, and massive hematomas encompass the worst prognosis.[48] A brainstem stroke involving the medulla oblongata is the most serious type, harbingering the risk of rapid death following the ataxic pattern of respiration.[46][48]
Predominant and consistent variables prognosticating outcomes include the presenting Glasgow Coma Scale (GCS) score, location, and hematoma volume.[1][75] Additionally, a low GCS at presentation, hematoma >4 mL in volume and 2 cm diameter, ventral subtype, and need for mechanical ventilation indicate poor outcomes.[1][46][76]
A scoring system by Huang et al incorporating hematoma volume and GCS is the best and the largest population-based and best evidence score in predicting mortality.[48] Almost 90% of patients in the study eventually developed hydrocephalus, causing 100% mortality in the conservative management group. However, early intervention for the same group markedly improved the clinical outcome.[73] In the study, age and intraventricular extension, though important predictive variables, were not independent determinants of early death in PBSH.[46][74] The surgical evacuation group had a mortality of 27.6% compared to 60.6% in the conservative management group.[73] Early tracheostomy ≤7 days after admission had a favorable 30-day functional outcome.[46] Quantitative electroencephalography (EEG) and neurophysiological monitoring like brainstem auditory evoked potential, somatosensory evoked potentials (SEPs), and motor evoked potentials are reliable predictors for recovery and mortality in PBSH patients.[48] Original and modified intracerebral hemorrhage (ICH) scores may not apply well to primary pontine hemorrhage (PPH). Only 10% of PBSH were included in the ICH score.[48] The PPH score also lacks external validation, and the failure to consider early do-not-resuscitate orders (DNRs) was a major flaw.[48]
Complications
Complications associated with brainstem stroke include the following:
- Neurological deficits presenting characteristically as crossed signs as in various brainstem stroke syndromes
- Dysautonomia due to involvement of the sympathetic tract
- Altered level of awareness and coma due to the involvement of the reticular activating system as in the "locked-in" syndrome and the "top-of-the-basilar" syndrome
- Dysregulation of the respiratory control mechanism presents as central hypoventilation syndrome or Ondine syndrome due to the involvement of the dorsal and ventral respiratory group of neurons, pneumotaxic and apnuestic respiratory centers
- Dysregulation of the blood pressure control mechanism due to the involvement of the Nucleus of tractus solitarius and neurons in the ventrolateral medulla
- Acute hydrocephalus
- Dysphagia
- Dysarthria
- Ataxia
- Central pain syndrome in 25% of cases of Wallenberg syndrome
- Restless leg syndrome
- Post-stroke fatigue
- Depression
- Pulmonary aspiration, particularly in patients with medullary and cerebellar strokes
- Deep vein thrombosis and pulmonary embolism
- Bed sores
- Contractures
- Sepsis
- Mortality [14][26][77][78]
Postoperative and Rehabilitation Care
In a cohort study in a rehabilitation unit, ataxia (68%), hemiplegia (70%), and dysphagia (40%) were the most common neurological deficits. However, significant functional gains in all these domains were observed. Aspiration pneumonia and urinary tract infection were observed in 15% and 25% of patients. 96% were ultimately discharged home.[79] Functional recovery and long-term survival with brainstem stroke have been better than that observed in hemispheric stroke.[80] Approximately 35% of brainstem infarction survivors returned to living independently within the first year post-stroke, compared to only 22% with hemispheric stroke survivors.[80]
Dysphagia is seen in approximately 47% of patients with brainstem stroke. Dysphagia is best evaluated by videofluoroscopic modified barium-swallowing. Typically, dysphagia is initially managed by nasogastric tube feeding, though gastrostomy and jejunostomy feeding tubes are required in 20% of patients. Patients with this complication should be given proper swallowing instructions. Furthermore, oral and pharyngeal postures and biofeedback techniques are essential. Speech-language pathologists should also be consulted to help manage these cases. In most patients, the long-term result is favorable.
Ataxia is seen in 86% of patients with brainstem stroke due to gait initiation and patterning being governed by the pons and medulla. Gait training is promoted through the "use it to improve it" technique. Typically, ataxia can be managed by postural training and motor learning, control, and strengthening exercises. The Bobath treatment approach is also effective.
Dysarthria is observed in 49% to 89% of patients. Typically, dysarthria is managed by regaining the tone and strength of facial and buccal muscles. Reducing speech rate, pausing, deep breathing, and over-articulating can also help. Palatal lift and palatal augmentation have been effective. In 94% of patients with brainstem stroke, paresis is noted and is managed by task-related motor training and therapeutic approaches. Diplopia is seen in 38% of patients and can be treated with diachronic mirrors or integration of fogging, occlusion, and suppression. Surgical procedures are utilized only if rehabilitation has failed, usually 6 months post-stroke.[80]
Deterrence and Patient Education
ACT FAST is an acronym suggested by the American Stroke Association to recognize the early symptoms of a stroke and consists of the following components:
- F-Face drooping
- A-Arm Weakness
- S-Speech
- T-Time to call 911
Along with the above symptoms, if the patient experiences any of the following, emergency medical services must be activated:
- Sudden confusion
- Sudden trouble seeing
- Sudden numbness
- Sudden trouble walking
- Sudden severe headache
Managing risk factors can significantly reduce future strokes, including:
- Smoking cessation
- Alcohol use
- Drug addiction and abuse
- Hypertension and diabetes control
- Obesity and a sedentary lifestyle
- Obstructive sleep apnea syndrome [81]
Pearls and Other Issues
Key facts to bear in mind when managing brainstem strokes include the following:
- Early identification of stroke and its management; "time is brain"
- Avoiding pitfalls of stroke-like syndrome and stroke mimics (eg, migraine headache, seizure disorder, transient ischemic attack, and vertigo)
- Consider permissive hypertension to improve perfusion in ischemic stroke
- Patient education with the FAST acronym
Enhancing Healthcare Team Outcomes
The diagnosis and management of brainstem stroke bring a considerable burden to the healthcare system, the patient, the family members, and society at large. The slow increase in the global burden of stroke has been steadily increasing. The enhancement must start with proper patient education about the risk factors and how they can be modified. A simple community educational approach about smoking cessation, a healthy diet, an active lifestyle, regular health screening for diabetes mellitus and hypertension, drug addiction cessation, and rehabilitation can be undertaken. A decentralized model where a community-level assessment of primary and secondary prevention of noncommunicable disease can reduce the incidence of stroke.
Acute management of stroke in a peripheral setting must be managed with skilled individuals; however, a complete and robust interdisciplinary team of neurologists, physicians, psychiatrists, nurses, physiotherapists, and other paramedical staff is necessary for the best patient outcome. A trained first responder who can immediately stabilize the patient and prevent deterioration is critical. The National Institutes of Health Stroke Scale, the Modified Rankin Scale, or other standardized models and scales help clinicians decide the management plan.[82]
Constant root-cause analysis, frequent updates to local hospital protocol, and continued medical education should be implemented. Telemedicine, teleradiology, and a rapid communication system can allow various interprofessionals to deploy rapidly and prevent long-term complications. Examination of the patient must be done as a team, where each member can be delegated certain aspects of evaluation and management. A multi-disciplinary approach has been shown to prevent at least 80% of subsequent strokes.
Post-stroke rehabilitation care must include inputs from clinicians, nurses, and pharmacists to obtain the best outcome for the patient. A healthy support system of dieticians and therapists, along with adequate domiciliary support, must be provided. Proper coordination and communication among the occupational therapist, attending nurses, and the clinician are pivotal. Occupational therapists help to address limitations in activities of daily living (ADLs) by appropriately addressing pertinent impairments.
Media
(Click Image to Enlarge)
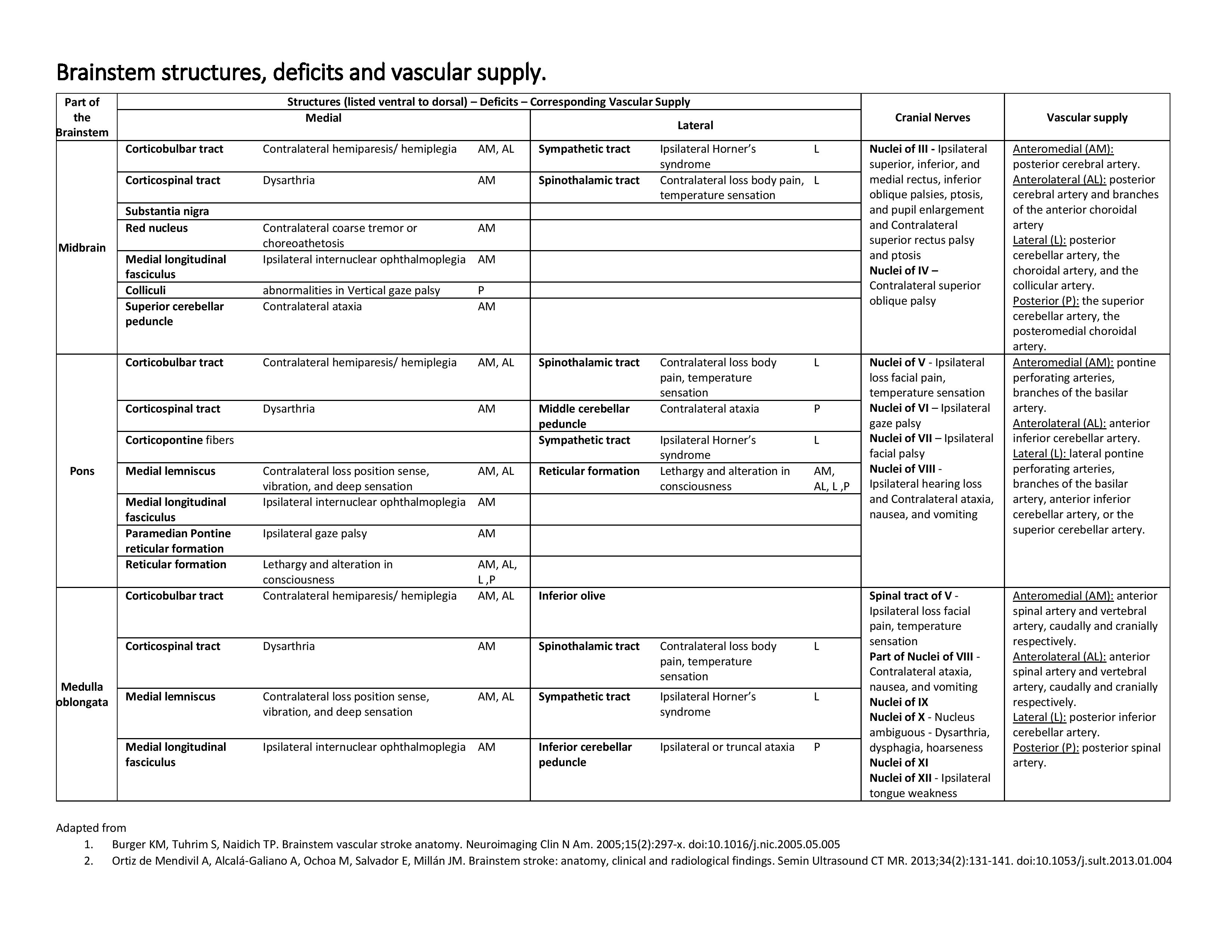
Brainstem Structures, Deficits, and Vascular Supply Table. Brainstem infarction is an area of tissue death resulting from a lack of oxygen supply to any part of the brainstem. The knowledge of anatomy, vascular supply, and physical examination can be life-saving in the setting of an acute infarct and provide precise diagnosis and management. Time becomes an essential factor in management. Early intervention has shown dramatically reduced morbidity and mortality.
Contributed by SN Gowda, MBBS
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
AlMohammedi RM, AlMutairi H, AlHoussien RO, AlOtayan MT, AlMutairi AK, Bafail WO, Khan A, Khatri IA. Brainstem hemorrhage is uncommon and is associated with high morbidity, mortality, and prolonged hospitalization. Neurosciences (Riyadh, Saudi Arabia). 2020 Apr:25(2):91-96. doi: 10.17712/nsj.2020.2.20190102. Epub [PubMed PMID: 32351245]
Angeles Fernández-Gil M, Palacios-Bote R, Leo-Barahona M, Mora-Encinas JP. Anatomy of the brainstem: a gaze into the stem of life. Seminars in ultrasound, CT, and MR. 2010 Jun:31(3):196-219. doi: 10.1053/j.sult.2010.03.006. Epub [PubMed PMID: 20483389]
Ortiz de Mendivil A, Alcalá-Galiano A, Ochoa M, Salvador E, Millán JM. Brainstem stroke: anatomy, clinical and radiological findings. Seminars in ultrasound, CT, and MR. 2013 Apr:34(2):131-41. doi: 10.1053/j.sult.2013.01.004. Epub [PubMed PMID: 23522778]
Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG, DAWN Trial Investigators. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. The New England journal of medicine. 2018 Jan 4:378(1):11-21. doi: 10.1056/NEJMoa1706442. Epub 2017 Nov 11 [PubMed PMID: 29129157]
Kameda W, Kawanami T, Kurita K, Daimon M, Kayama T, Hosoya T, Kato T, Study Group of the Association of Cerebrovascular Disease in Tohoku. Lateral and medial medullary infarction: a comparative analysis of 214 patients. Stroke. 2004 Mar:35(3):694-9 [PubMed PMID: 14963274]
Level 2 (mid-level) evidenceBurger KM, Tuhrim S, Naidich TP. Brainstem vascular stroke anatomy. Neuroimaging clinics of North America. 2005 May:15(2):297-324, x [PubMed PMID: 16198942]
Baran G, Gultekin TO, Baran O, Deniz C, Katar S, Yildiz GB, Asil T. Association between etiology and lesion site in ischemic brainstem infarcts: a retrospective observational study. Neuropsychiatric disease and treatment. 2018:14():757-766. doi: 10.2147/NDT.S154224. Epub 2018 Mar 13 [PubMed PMID: 29559783]
Level 2 (mid-level) evidenceBogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke. 1988 Sep:19(9):1083-92 [PubMed PMID: 3413804]
Guzik A, Bushnell C. Stroke Epidemiology and Risk Factor Management. Continuum (Minneapolis, Minn.). 2017 Feb:23(1, Cerebrovascular Disease):15-39. doi: 10.1212/CON.0000000000000416. Epub [PubMed PMID: 28157742]
Munakomi S, Torregrossa F, Grasso G. Natural Course, Clinical Profile, and Treatment Strategies for Cerebral Cavernous Malformations. World neurosurgery. 2022 Mar:159():373-380. doi: 10.1016/j.wneu.2021.08.134. Epub [PubMed PMID: 35255636]
Kaji R. Global burden of neurological diseases highlights stroke. Nature reviews. Neurology. 2019 Jul:15(7):371-372. doi: 10.1038/s41582-019-0208-y. Epub [PubMed PMID: 31152152]
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020 Mar 3:141(9):e139-e596. doi: 10.1161/CIR.0000000000000757. Epub 2020 Jan 29 [PubMed PMID: 31992061]
GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (London, England). 2017 Sep 16:390(10100):1151-1210. doi: 10.1016/S0140-6736(17)32152-9. Epub [PubMed PMID: 28919116]
Level 1 (high-level) evidenceTeasell R, Foley N, Doherty T, Finestone H. Clinical characteristics of patients with brainstem strokes admitted to a rehabilitation unit. Archives of physical medicine and rehabilitation. 2002 Jul:83(7):1013-6 [PubMed PMID: 12098164]
Level 2 (mid-level) evidenceChen P, Yao H, Tang X, Wang Y, Zhang Q, Liu Y, Hu J, Deng Y. Management of Primary Brainstem Hemorrhage: A Review of Outcome Prediction, Surgical Treatment, and Animal Model. Disease markers. 2022:2022():4293590. doi: 10.1155/2022/4293590. Epub 2022 Jul 12 [PubMed PMID: 35864996]
Level 3 (low-level) evidenceMarkus HS. Cerebral perfusion and stroke. Journal of neurology, neurosurgery, and psychiatry. 2004 Mar:75(3):353-61 [PubMed PMID: 14966145]
Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends in neurosciences. 2013 Oct:36(10):587-97. doi: 10.1016/j.tins.2013.07.001. Epub 2013 Aug 20 [PubMed PMID: 23968694]
Level 3 (low-level) evidenceDeb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology : the official journal of the International Society for Pathophysiology. 2010 Jun:17(3):197-218. doi: 10.1016/j.pathophys.2009.12.001. Epub 2010 Jan 13 [PubMed PMID: 20074922]
Level 3 (low-level) evidenceKlatzo I. Presidental address. Neuropathological aspects of brain edema. Journal of neuropathology and experimental neurology. 1967 Jan:26(1):1-14 [PubMed PMID: 5336776]
Level 3 (low-level) evidenceAdibhatla RM, Hatcher JF. Altered lipid metabolism in brain injury and disorders. Sub-cellular biochemistry. 2008:49():241-68. doi: 10.1007/978-1-4020-8831-5_9. Epub [PubMed PMID: 18751914]
Level 3 (low-level) evidenceGuo X, Ma L, Li H, Qi X, Wei Y, Duan Z, Xu J, Wang C, You C, Tian M. Brainstem iron overload and injury in a rat model of brainstem hemorrhage. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2020 Aug:29(8):104956. doi: 10.1016/j.jstrokecerebrovasdis.2020.104956. Epub 2020 Jun 5 [PubMed PMID: 32689646]
Level 2 (mid-level) evidenceSaver JL. Time is brain--quantified. Stroke. 2006 Jan:37(1):263-6 [PubMed PMID: 16339467]
Querol-Pascual MR. Clinical approach to brainstem lesions. Seminars in ultrasound, CT, and MR. 2010 Jun:31(3):220-9. doi: 10.1053/j.sult.2010.03.004. Epub [PubMed PMID: 20483390]
Gates P. The rule of 4 of the brainstem: a simplified method for understanding brainstem anatomy and brainstem vascular syndromes for the non-neurologist. Internal medicine journal. 2005 Apr:35(4):263-6 [PubMed PMID: 15836511]
Level 3 (low-level) evidenceSrinivasan A, Goyal M, Al Azri F, Lum C. State-of-the-art imaging of acute stroke. Radiographics : a review publication of the Radiological Society of North America, Inc. 2006 Oct:26 Suppl 1():S75-95 [PubMed PMID: 17050521]
Balami JS, Chen RL, Buchan AM. Stroke syndromes and clinical management. QJM : monthly journal of the Association of Physicians. 2013 Jul:106(7):607-15. doi: 10.1093/qjmed/hct057. Epub 2013 Mar 12 [PubMed PMID: 23483140]
Wall M, Wray SH. The one-and-a-half syndrome--a unilateral disorder of the pontine tegmentum: a study of 20 cases and review of the literature. Neurology. 1983 Aug:33(8):971-80 [PubMed PMID: 6683820]
Level 3 (low-level) evidenceSciacca S, Lynch J, Davagnanam I, Barker R. Midbrain, Pons, and Medulla: Anatomy and Syndromes. Radiographics : a review publication of the Radiological Society of North America, Inc. 2019 Jul-Aug:39(4):1110-1125. doi: 10.1148/rg.2019180126. Epub [PubMed PMID: 31283463]
Liu GT, Crenner CW, Logigian EL, Charness ME, Samuels MA. Midbrain syndromes of Benedikt, Claude, and Nothnagel: setting the record straight. Neurology. 1992 Sep:42(9):1820-2 [PubMed PMID: 1513475]
Cormier PJ, Long ER, Russell EJ. MR imaging of posterior fossa infarctions: vascular territories and clinical correlates. Radiographics : a review publication of the Radiological Society of North America, Inc. 1992 Nov:12(6):1079-96 [PubMed PMID: 1439013]
Level 2 (mid-level) evidenceMarx JJ, Thömke F. Classical crossed brain stem syndromes: myth or reality? Journal of neurology. 2009 Jun:256(6):898-903. doi: 10.1007/s00415-009-5037-2. Epub 2009 Feb 28 [PubMed PMID: 19252797]
Level 2 (mid-level) evidenceMoncayo J. Midbrain infarcts and hemorrhages. Frontiers of neurology and neuroscience. 2012:30():158-61. doi: 10.1159/000333630. Epub 2012 Feb 14 [PubMed PMID: 22377886]
Tacik P, Krasnianski M, Alfieri A, Dressler D. Brissaud-Sicard syndrome caused by a diffuse brainstem glioma. A rare differential diagnosis of hemifacial spasm. Acta neurochirurgica. 2014 Feb:156(2):429-30. doi: 10.1007/s00701-013-1984-6. Epub 2014 Jan 3 [PubMed PMID: 24384991]
Level 3 (low-level) evidenceKrasnianski M, Neudecker S, Zierz S. [Classical crossed pontine syndromes]. Fortschritte der Neurologie-Psychiatrie. 2004 Aug:72(8):460-8 [PubMed PMID: 15305240]
Stalcup ST, Tuan AS, Hesselink JR. Intracranial causes of ophthalmoplegia: the visual reflex pathways. Radiographics : a review publication of the Radiological Society of North America, Inc. 2013 Sep-Oct:33(5):E153-69. doi: 10.1148/rg.335125142. Epub [PubMed PMID: 24025940]
Tacik P, Alfieri A, Kornhuber M, Dressler D. Gasperini's syndrome: its neuroanatomical basis now and then. Journal of the history of the neurosciences. 2012 Jan:21(1):17-30. doi: 10.1080/0964704X.2011.568045. Epub [PubMed PMID: 22239093]
Hubloue I, Laureys S, Michotte A. A rare case of diplopia: medial inferior pontine syndrome or Foville's syndrome. European journal of emergency medicine : official journal of the European Society for Emergency Medicine. 1996 Sep:3(3):194-8 [PubMed PMID: 9023501]
Level 3 (low-level) evidenceZaorsky NG, Luo JJ. A case of classic raymond syndrome. Case reports in neurological medicine. 2012:2012():583123. doi: 10.1155/2012/583123. Epub 2012 Aug 9 [PubMed PMID: 22934209]
Krasnianski M, Neudecker S, Schlüter A, Zierz S. [Avellis' syndrome in brainstem infarctions]. Fortschritte der Neurologie-Psychiatrie. 2003 Dec:71(12):650-3 [PubMed PMID: 14661158]
Level 3 (low-level) evidenceKrasnianski M, Müller T, Stock K, Zierz S. Between Wallenberg syndrome and hemimedullary lesion: Cestan-Chenais and Babinski-Nageotte syndromes in medullary infarctions. Journal of neurology. 2006 Nov:253(11):1442-6 [PubMed PMID: 16775654]
Lui F, Tadi P, Anilkumar AC. Wallenberg Syndrome. StatPearls. 2024 Jan:(): [PubMed PMID: 29262144]
Krasnianski M, Winterholler M, Neudecker S, Zierz S. [Classical crossed syndromes of the medulla oblongata. A historical and topodiagnostic discussion]. Fortschritte der Neurologie-Psychiatrie. 2003 Aug:71(8):397-405 [PubMed PMID: 12910445]
Pergami P, Poloni TE, Imbesi F, Ceroni M, Simonetti F. Dejerine's syndrome or Spiller's syndrome? Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2001 Aug:22(4):333-6 [PubMed PMID: 11808859]
Level 3 (low-level) evidenceSaposnik G, Caplan LR. Convulsive-like movements in brainstem stroke. Archives of neurology. 2001 Apr:58(4):654-7 [PubMed PMID: 11295998]
Level 3 (low-level) evidenceKalampokini S, Poyiadjis S, Vavougios GD, Artemiadis A, Zis P, Hadjigeorgiou GM, Bargiotas P. Restless legs syndrome due to brainstem stroke: A systematic review. Acta neurologica Scandinavica. 2022 Nov:146(5):440-447. doi: 10.1111/ane.13702. Epub 2022 Sep 5 [PubMed PMID: 36063288]
Level 1 (high-level) evidenceChen D, Tang Y, Nie H, Zhang P, Wang W, Dong Q, Wu G, Xue M, Tang Y, Liu W, Pan C, Tang Z. Primary Brainstem Hemorrhage: A Review of Prognostic Factors and Surgical Management. Frontiers in neurology. 2021:12():727962. doi: 10.3389/fneur.2021.727962. Epub 2021 Sep 10 [PubMed PMID: 34566872]
Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, Khoury J. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996 Aug:27(8):1304-5 [PubMed PMID: 8711791]
Musuka TD, Wilton SB, Traboulsi M, Hill MD. Diagnosis and management of acute ischemic stroke: speed is critical. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2015 Sep 8:187(12):887-93. doi: 10.1503/cmaj.140355. Epub 2015 Aug 4 [PubMed PMID: 26243819]
Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. The New England journal of medicine. 2008 Sep 25:359(13):1317-29. doi: 10.1056/NEJMoa0804656. Epub [PubMed PMID: 18815396]
Level 1 (high-level) evidenceRe-examining Acute Eligibility for Thrombolysis (TREAT) Task Force:, Levine SR, Khatri P, Broderick JP, Grotta JC, Kasner SE, Kim D, Meyer BC, Panagos P, Romano J, Scott P, NINDS rt-PA Stroke Trial Investigators. Review, historical context, and clarifications of the NINDS rt-PA stroke trials exclusion criteria: Part 1: rapidly improving stroke symptoms. Stroke. 2013 Sep:44(9):2500-5. doi: 10.1161/STROKEAHA.113.000878. Epub 2013 Jul 11 [PubMed PMID: 23847249]
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019 Dec:50(12):e344-e418. doi: 10.1161/STR.0000000000000211. Epub 2019 Oct 30 [PubMed PMID: 31662037]
Sigurdsson AP, Gunnarsson T, Thorisson HM, Olafsson IH, Gunnarsson GB. [Occlusion of the vertebrobasilar artery. Case presentation and literature review]. Laeknabladid. 2020 Jun:106(6):302-309. doi: 10.17992/lbl.2020.06.586. Epub [PubMed PMID: 32491992]
Diprose WK, Diprose JP, Tarr GP, Sutcliffe J, McFetridge A, Brew S, Caldwell J, McGuinness B, Wang MTM, Barber PA. Vertebrobasilar Artery Calcification and Outcomes in Posterior Circulation Large Vessel Occlusion Thrombectomy. Stroke. 2020 Apr:51(4):1301-1304. doi: 10.1161/STROKEAHA.119.027958. Epub 2020 Feb 14 [PubMed PMID: 32078499]
Kang DH, Jung C, Yoon W, Kim SK, Baek BH, Kim JT, Park MS, Kim YW, Hwang YH, Kim YS, Kim BJ, Han MK, Bae HJ. Endovascular Thrombectomy for Acute Basilar Artery Occlusion: A Multicenter Retrospective Observational Study. Journal of the American Heart Association. 2018 Jul 7:7(14):. doi: 10.1161/JAHA.118.009419. Epub 2018 Jul 7 [PubMed PMID: 29982231]
Level 2 (mid-level) evidenceFan Y, Li Y, Zhang T, Li X, Yang J, Wang B, Jiang C. Endovascular therapy for acute vertebrobasilar occlusion underlying atherosclerosis: A single institution experience. Clinical neurology and neurosurgery. 2019 Jan:176():78-82. doi: 10.1016/j.clineuro.2018.11.016. Epub 2018 Nov 29 [PubMed PMID: 30544008]
Boeckh-Behrens T, Pree D, Lummel N, Friedrich B, Maegerlein C, Kreiser K, Kirschke J, Berndt M, Lehm M, Wunderlich S, Mosimann PJ, Fischer U, Zimmer C, Kaesmacher J. Vertebral Artery Patency and Thrombectomy in Basilar Artery Occlusions. Stroke. 2019 Feb:50(2):389-395. doi: 10.1161/STROKEAHA.118.022466. Epub [PubMed PMID: 30612534]
Level 3 (low-level) evidenceSang HF, Yin CG, Xia WQ, Huang H, Liu KQ, Chen TW, Si XL, Jiang L. Mechanical Thrombectomy Using Solitaire in Acute Ischemic Stroke Patients with Vertebrobasilar Occlusion: A Prospective Observational Study. World neurosurgery. 2019 Aug:128():e355-e361. doi: 10.1016/j.wneu.2019.04.152. Epub 2019 Apr 25 [PubMed PMID: 31029819]
Level 2 (mid-level) evidenceQuan T, Hou H, Xue W, Yu G, Ma H, Sun J, Guan S, Xu Y, Xu H. Endovascular treatment of acute intracranial vertebrobasilar artery occlusion: a multicenter retrospective observational study. Neuroradiology. 2019 Dec:61(12):1477-1484. doi: 10.1007/s00234-019-02282-1. Epub 2019 Sep 4 [PubMed PMID: 31482191]
Level 2 (mid-level) evidenceUno J, Kameda K, Otsuji R, Ren N, Nagaoka S, Maeda K, Ikai Y, Gi H. Mechanical Thrombectomy for Basilar Artery Occlusion Compared with Anterior Circulation Stroke. World neurosurgery. 2020 Feb:134():e469-e475. doi: 10.1016/j.wneu.2019.10.097. Epub 2019 Oct 24 [PubMed PMID: 31669246]
Liu X, Dai Q, Ye R, Zi W, Liu Y, Wang H, Zhu W, Ma M, Yin Q, Li M, Fan X, Sun W, Han Y, Lv Q, Liu R, Yang D, Shi Z, Zheng D, Deng X, Wan Y, Wang Z, Geng Y, Chen X, Zhou Z, Liao G, Jin P, Liu Y, Liu X, Zhang M, Zhou F, Shi H, Zhang Y, Guo F, Yin C, Niu G, Zhang M, Cai X, Zhu Q, Chen Z, Liang Y, Li B, Lin M, Wang W, Xu H, Fu X, Liu W, Tian X, Gong Z, Shi H, Wang C, Lv P, Tao Z, Zhu L, Yang S, Hu W, Jiang P, Liebeskind DS, Pereira VM, Leung T, Yan B, Davis S, Xu G, Nogueira RG, BEST Trial Investigators. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. The Lancet. Neurology. 2020 Feb:19(2):115-122. doi: 10.1016/S1474-4422(19)30395-3. Epub 2019 Dec 9 [PubMed PMID: 31831388]
Baek JH, Kim BM, Heo JH, Kim DJ, Nam HS, Kim YD. Endovascular and Clinical Outcomes of Vertebrobasilar Intracranial Atherosclerosis-Related Large Vessel Occlusion. Frontiers in neurology. 2019:10():215. doi: 10.3389/fneur.2019.00215. Epub 2019 Mar 19 [PubMed PMID: 30941084]
Level 2 (mid-level) evidenceCavalcanti DD, Preul MC, Kalani MY, Spetzler RF. Microsurgical anatomy of safe entry zones to the brainstem. Journal of neurosurgery. 2016 May:124(5):1359-76. doi: 10.3171/2015.4.JNS141945. Epub 2015 Oct 9 [PubMed PMID: 26452114]
Yang Y, van Niftrik B, Ma X, Velz J, Wang S, Regli L, Bozinov O. Analysis of safe entry zones into the brainstem. Neurosurgical review. 2019 Sep:42(3):721-729. doi: 10.1007/s10143-019-01081-9. Epub 2019 Feb 6 [PubMed PMID: 30726522]
Summers D, Leonard A, Wentworth D, Saver JL, Simpson J, Spilker JA, Hock N, Miller E, Mitchell PH, American Heart Association Council on Cardiovascular Nursing and the Stroke Council. Comprehensive overview of nursing and interdisciplinary care of the acute ischemic stroke patient: a scientific statement from the American Heart Association. Stroke. 2009 Aug:40(8):2911-44. doi: 10.1161/STROKEAHA.109.192362. Epub 2009 May 28 [PubMed PMID: 19478222]
Level 1 (high-level) evidenceFarhoudi M, Sadigh-Eteghad S, Farjami A, Salatin S. Nanoparticle and Stem Cell Combination Therapy for the Management of Stroke. Current pharmaceutical design. 2023:29(1):15-29. doi: 10.2174/1381612829666221213113119. Epub [PubMed PMID: 36515043]
Mohd Satar A, Othman FA, Tan SC. Biomaterial application strategies to enhance stem cell-based therapy for ischemic stroke. World journal of stem cells. 2022 Dec 26:14(12):851-867. doi: 10.4252/wjsc.v14.i12.851. Epub [PubMed PMID: 36619694]
Schonewille WJ, Algra A, Serena J, Molina CA, Kappelle LJ. Outcome in patients with basilar artery occlusion treated conventionally. Journal of neurology, neurosurgery, and psychiatry. 2005 Sep:76(9):1238-41 [PubMed PMID: 16107358]
Level 2 (mid-level) evidenceSheng K, Tong M. Therapy for acute basilar artery occlusion: a systematic review and meta-analysis. F1000Research. 2019:8():165. doi: 10.12688/f1000research.18042.1. Epub 2019 Feb 7 [PubMed PMID: 31016013]
Level 1 (high-level) evidenceDorňák T, Král M, Šaňák D, Kaňovský P. Intravenous Thrombolysis in Posterior Circulation Stroke. Frontiers in neurology. 2019:10():417. doi: 10.3389/fneur.2019.00417. Epub 2019 Apr 26 [PubMed PMID: 31080436]
Malik A, Drumm B, D'Anna L, Brooks I, Low B, Raha O, Shabbir K, Vittay O, Kwan J, Brown Z, Halse O, Jamil S, Kalladka D, Venter M, Jenkins H, Rane N, Singh A, Patel M, Hall C, Fatania G, Roi D, Lobotesis K, Banerjee S. Mechanical thrombectomy in acute basilar artery stroke: a systematic review and Meta-analysis of randomized controlled trials. BMC neurology. 2022 Nov 9:22(1):415. doi: 10.1186/s12883-022-02953-2. Epub 2022 Nov 9 [PubMed PMID: 36352362]
Level 1 (high-level) evidenceNso N, Nassar M, Trimingham M, Mbome Y, Lyonga Ngonge A, Badejoko SO, Akbar S, Azhar A, Lakhdar S, Ghallab M, Guzman Perez LM, Rizzo V, Munira MS. Invasive Management of Vertebrobasilar Artery Stenosis and Occlusion: A Meta-Analysis on Efficacy and Safety Endpoints. Cureus. 2022 May:14(5):e24751. doi: 10.7759/cureus.24751. Epub 2022 May 5 [PubMed PMID: 35686282]
Level 1 (high-level) evidenceZhao FL, Mi DH, Zhang CQ, Song QH, Liu HS, Dai HL, Liu ZM, Ge CQ, Wang YJ, Liu LP, Guo L. A cohort study of isolated brainstem infarction based on head MR imaging and clinical findings. The Journal of international medical research. 2018 Dec:46(12):4974-4984. doi: 10.1177/0300060518788253. Epub 2018 Sep 23 [PubMed PMID: 30246581]
Zheng WJ, Shi SW, Gong J. The truths behind the statistics of surgical treatment for hypertensive brainstem hemorrhage in China: a review. Neurosurgical review. 2022 Apr:45(2):1195-1204. doi: 10.1007/s10143-021-01683-2. Epub 2021 Oct 29 [PubMed PMID: 34716511]
Behrouz R. Prognostic factors in pontine haemorrhage: A systematic review. European stroke journal. 2018 Jun:3(2):101-109. doi: 10.1177/2396987317752729. Epub 2018 Jan 8 [PubMed PMID: 31008342]
Level 1 (high-level) evidenceMeng X, Wang Q, Pei X, Xie F. Prognosis and Influencing Factors of Early Microsurgery for Severe Hypertensive Brainstem Hemorrhage. Disease markers. 2022:2022():5062591. doi: 10.1155/2022/5062591. Epub 2022 Sep 22 [PubMed PMID: 36193500]
Wessels T, Möller-Hartmann W, Noth J, Klötzsch C. CT findings and clinical features as markers for patient outcome in primary pontine hemorrhage. AJNR. American journal of neuroradiology. 2004 Feb:25(2):257-60 [PubMed PMID: 14970027]
Colombari E, Sato MA, Cravo SL, Bergamaschi CT, Campos RR Jr, Lopes OU. Role of the medulla oblongata in hypertension. Hypertension (Dallas, Tex. : 1979). 2001 Sep:38(3 Pt 2):549-54 [PubMed PMID: 11566929]
Level 3 (low-level) evidenceIkeda K, Kawakami K, Onimaru H, Okada Y, Yokota S, Koshiya N, Oku Y, Iizuka M, Koizumi H. The respiratory control mechanisms in the brainstem and spinal cord: integrative views of the neuroanatomy and neurophysiology. The journal of physiological sciences : JPS. 2017 Jan:67(1):45-62. doi: 10.1007/s12576-016-0475-y. Epub 2016 Aug 17 [PubMed PMID: 27535569]
Chua KS, Kong KH. Functional outcome in brain stem stroke patients after rehabilitation. Archives of physical medicine and rehabilitation. 1996 Feb:77(2):194-7 [PubMed PMID: 8607746]
Kruger E, Teasell R, Salter K, Foley N, Hellings C. The rehabilitation of patients recovering from brainstem strokes: case studies and clinical considerations. Topics in stroke rehabilitation. 2007 Sep-Oct:14(5):56-64 [PubMed PMID: 17901016]
Level 3 (low-level) evidenceWein T, Lindsay MP, Côté R, Foley N, Berlingieri J, Bhogal S, Bourgoin A, Buck BH, Cox J, Davidson D, Dowlatshahi D, Douketis J, Falconer J, Field T, Gioia L, Gubitz G, Habert J, Jaspers S, Lum C, McNamara Morse D, Pageau P, Rafay M, Rodgerson A, Semchuk B, Sharma M, Shoamanesh A, Tamayo A, Smitko E, Gladstone DJ, Heart and Stroke Foundation Canadian Stroke Best Practice Committees. Canadian stroke best practice recommendations: Secondary prevention of stroke, sixth edition practice guidelines, update 2017. International journal of stroke : official journal of the International Stroke Society. 2018 Jun:13(4):420-443. doi: 10.1177/1747493017743062. Epub 2017 Nov 24 [PubMed PMID: 29171361]
Level 1 (high-level) evidenceBrott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989 Jul:20(7):864-70 [PubMed PMID: 2749846]