Introduction
Venous sinus thromboses (VST) are venous blood clots of the major veins of the brain. They can be provoked or unprovoked, and the signs and symptoms thereof will depend on the location and extent of the clot. Common locations for sinus thrombosis include the dural sinuses, the cavernous sinus, and deep sinuses of the cortex.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Venous sinus thromboses occur for similar reasons to other venous thromboses. Anything that promotes clot formation, such as increased coagulability or decreased flow within a localized region of the sinus, predisposes for clot formation. There can be an underlying prothrombotic state, such as inherited coagulopathy, malignancy, oral contraceptive use, pregnancy, infection, or trauma.[1][2] In the case of dural sinus thrombosis, likely antecedent infections would be meningitis, brain abscess, or other central nervous system (CNS) infections adjacent to the sinuses.
Epidemiology
Venous sinus thromboses are uncommon, with current estimates of the incidence of venous sinus thromboses ranging from 13.2 to about 15.7 per million patient-years.[3][4] There is a female preponderance, with the female-to-male ratio ranging from as high as 3:1 in some studies[5] to as low as approximately 1:1 in others.[4] VST occurs on average in the 4th and 5th decades of life but can occur earlier.[4][6] This condition occurs more frequently in patients with conditions that predispose them to other venous clots, such as thrombophilias, acute malignancies, nephrotic syndrome, and COVID-19.[7]
Pathophysiology
Occlusion of dural sinuses leads to decreased venous flow, increased venous pressure, decreased relative cerebral perfusion pressure, and physical dilation of cerebral veins. Some combination of these forces can lead to local areas of hypoperfusion of brain tissue resulting in hypoxic tissue damage. Additionally, the thrombosis itself can damage the vessel wall and predispose to vessel rupture and subarachnoid hemorrhage. The decreased cerebral venous outflow decreases cerebrospinal fluid (CSF) outflow. This leads to elevated intracranial pressure and associated signs and symptoms, e.g., headaches and papilledema.
History and Physical
Far and away, the most common symptom of venous sinus thrombosis is a headache.[8] Other physical symptoms depend heavily on the location, size, and extent of the clot. Common symptoms of occlusion of dural sinus thrombosis include seizure, focal neurologic deficit, and altered mental status. The history of venous sinus thromboses is varied depending on the location, extent, and symptoms of sinus thrombosis. Sinus thrombosis can lead to subarachnoid hemorrhage with a thunderclap headache presentation that is preceded by a gradually worsening headache followed by acute neurologic symptoms.[8] Some common presentations are headaches accompanied by acute neurologic symptoms, e.g., seizures, altered mental status, and elevated intracranial pressure.
For patients presenting due to elevated intracranial pressure, a typical history that is suggestive of dural sinus thrombosis is elevated intracranial pressure in a patient that does not have an offending medication and that does not fit the typical profile for Idiopathic intracranial hypertension. These patients will describe headaches generally worse lying down, pulsatile tinnitus, and transient visual obscurations (blurred vision for several seconds). Patients with idiopathic intracranial hypertension (IIH) will tend to be obese, female predominant, and young. The patients should be screened for the use of medications provoking IIH, such as tetracycline, vitamin A derivative, and growth hormone. On ophthalmic examination, optic nerve edema is usually present. The edema can be symmetric, asymmetric, or unusually absent. The absence of spontaneous retinal venous pulsations in patients with known spontaneous venous pulsations is another sign of ICP.
Cavernous sinus thrombosis results will result in acute cranial nerve (CN) III, IV, V, and VI palsies. The palsies will usually be concurrent, but it is possible to have isolated CN palsies. The pupil may be involved or spared. Cavernous sinus thromboses most commonly occur in the setting of infection, usually orbital cellulitis, sinusitis, or dental infection.
Evaluation
Imaging
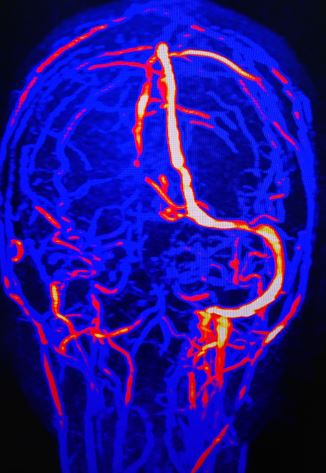
Three imaging modalities are primarily used in the evaluation of venous sinus thrombosis. The first is magnetic resonance imaging (MRI), which is the least sensitive and specific. This is most often used as dural sinus thromboses will often present as undifferentiated neurologic symptoms or elevated intracranial pressure, which prompts an MRI of the brain. Certain findings on MRI can suggest thrombosis depending on the age of the clot and the breakdown stage of hemoglobin being more useful in subacute clots, which have started to break down.[9] Additionally, MRI can show areas of focal edema and intraparenchymal hemorrhage, which can be suggestive of venous sinus thrombosis based on distribution and appearance.[9] Additionally, depending on the amount of local perfusion disruption, there can be areas of restricted diffusion and diminished apparent diffusion coefficient (ADC) values. Magnetic resonance venography or computed tomogram (CT) venography remains much more sensitive and specific modalities for imaging the sinuses. Time-of-flight MR venography, contrast-enhanced MR venography, and CT venography all allow direct visualization of the dural sinuses and can show the presence of a thrombus.
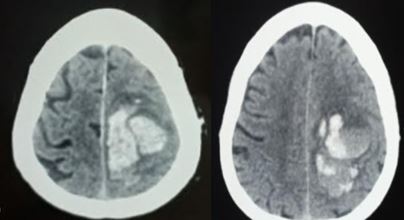
On non-contrast-enhanced head CT, one common sign is hyperdensity within the sinus. This is due to the thrombus being denser than flowing blood. However, sensitivity for non-contrast CT scans is low, around 33% in some studies.[10] Sometimes the cord sign may be present, where the hyperdensity of the vein has a cord-like appearance. The addition of contrast in CT venography enables visualization of the thrombus. This gives rise to the empty delta sign where the thrombus appears dark as a filling defect while the rim around the thrombus enhances. This same lack of flow can be well visualized on 3-D reconstruction, where the absence of flow or focal narrowing can often be seen.
On MRI imaging, several signs can be seen. An acute clot begins as isointense in T1-weighted imaging and will become more hyperintense as oxyhemoglobin converts into deoxyhemoglobin and subsequent breakdown products. On T2-weighted images, the clot will appear hypointense or dark. T2-weighted gradient echo (T2-GRE) sequences can be highly sensitive and specific for venous thrombosis.[11] These will demonstrate signal loss due to the susceptibility effect of hemoglobin and its breakdown products. MR venography, either using time of flight or gadolinium enhancement, will reveal flow defects that can be visualized to demonstrate a clot.
Laboratory
Routine complete blood count, routine coagulation studies - prothrombin time (PT), activated partial thromboplastin time (aPTT), and international normalized ratio (INR) should be performed on patients with suspected venous sinus thrombosis. If no predisposing condition is known, a thorough history should be done to screen for potential predisposing conditions. If none is found, consider workup for occult malignancy or inherited thrombophilia (factor V Leiden, etc.). D-dimer, if normal, indicates less likelihood of venous sinus thrombosis. However, it is possible to have VST in the setting of a normal D-dimer.[12]
Treatment / Management
Treatment of dural sinus thrombosis can be divided into two main components. The first component is treating the clot and the conditions leading to the clot. The second component is treating the sequelae of the clot. The clot itself is usually treated with anticoagulation, like a venous thrombus.[13] Depending on the severity of the illness, sometimes more aggressive therapies to dissolve the clot have been pursued, including thrombolytic therapy - both systemic and catheter-directed, and thrombectomy - both endovascular and surgically. Current guidelines such as those of the American Heart Association (AHA) favor anticoagulation acutely with heparin.[8] There are several small prospective studies (Daif et al. 1995,[14]) and several larger retrospective studies that compared acute anticoagulation with heparin versus no anticoagulation after diagnosis of cerebral venous thrombosis (CVT).(A1)
In both studies, acutely anticoagulated patients had reduced risk of mortality and better functional outcomes at follow-up. The decision to anticoagulate is complicated by the frequent presence of intracerebral hemorrhage. These studies notably did not exclude patients with intracerebral hematoma (ICH) from receiving anticoagulation. Deaths in those studies were not felt to be attributed to bleeding or worsening of existing intracranial bleeds by the study's authors. These studies have led current AHA guidelines to recommend acute anticoagulation with either unfractionated or low molecular weight heparin, even in the setting of ICH. However, any decision to anticoagulate a patient must be made with a careful weighing of the benefits and risks of anticoagulation.
Patients with VST should continue to be anticoagulated with warfarin for 3 to 6 months if provoked (pregnancy, trauma, infection) and 6 to 12 months if unprovoked. If any underlying thrombophilia is present or other venous thromboembolic events have occurred, careful consideration should be given to lifelong anticoagulation. At this time, current guidelines do not endorse direct-acting oral anticoagulants, but there are many small studies and case series detailing successful use.[15][16] (A1)
Women with VST while pregnant should be anticoagulated with low molecular weight heparin throughout the pregnancy. For future pregnancies, consideration should be given to prophylactic anticoagulation with low molecular weight (LMWH). If a thrombus is thought to be provoked by a localized CNS infection, e.g., meningitis/brain abscess, then the infectious nidus is treated with appropriate antibiotic coverage and any necessary drainage procedures.
Indications for more aggressive measures to break down the clot are not yet part of clinical guidelines such as the AHA or European Stroke Organization, but they are used in clinical practice, and there is evidence to suggest some benefit in certain cases. In current practice, thrombolytic therapy is usually reserved for patients with clot burdens that are clinically not improving as expected, or the slow rate of improvement is either placing them at risk of vision loss or other significant morbidity or mortality. The decision to pursue systemic versus catheter-directed thrombolytic therapy is guided by the availability of cerebrovascular interventions and provider preference. At this time, the two have not been compared head-to-head prospectively, but retrospective studies of catheter-directed therapy do show some promising results.[17] (A1)
Additionally, if thrombolytic therapy fails to produce the desired clinical improvement and recanalization of the occluded sinus, various types of mechanical thrombectomy have been tried with some success.[18] However, at this time, these therapies have not yet been studied prospectively in large randomized trials. Given the rarity of the clinical situation, performing trials on any intravascular intervention for treatment-resistant VST will be a difficult task. (B3)
Treatments of the sequelae of the venous thrombus are numerous as venous thrombi lead to many complications. For patients with chronically elevated intracranial pressure (ICP), the ICP can be medically lowered with acetazolamide or CSF diversion with ventriculoperitoneal shunts or optic nerve sheath fenestration. In patients with acutely elevated ICP, more common temporizing measures, such as elevation of the head of the bed, hyperventilation, and paralytic agents, can be done as they would in any other condition. For patients with severely elevated ICP, decompressive craniectomies can be performed. For patients with seizures after dural sinus thrombosis, antiepileptic medications are routinely used.[8]
Differential Diagnosis
- Encephalopathy variant: metabolic derangement, medication side effects, delirium, dementia, stroke, sepsis, demyelinating processes
- Focal deficit variant: stroke, hemorrhage stroke, meningitis, tumor, multiple sclerosis, seizure disorder
- Papilledema variant: IIH, meningitis, tumor
Prognosis
If diagnosed and appropriately treated, outcomes from VST are good. In the international study on cerebral vein and dural sinus thrombosis (ISCVT), approximately 57% of patients had no residual symptoms or deficits at a median follow-up time of 16 months.[19] In that same study, about 2% of patients had severe deficits, and 8% of patients died.[19]
Complications
The common complications from venous sinus thromboses include headache, visual loss (optic neuropathy due to papilledema), stroke, seizure, subarachnoid hemorrhage, subdural hemorrhage, and intraparenchymal hemorrhage.
Deterrence and Patient Education
In patients with known thrombophilia, treating with long-term anticoagulation can reduce the likelihood of recurrent VST. Patients need to be educated on the importance and rationale of continued anticoagulation. The decision to anticoagulate should be made in consultation with the patient and how their predisposing thrombophilia puts them at risk. Certain syndromes, such as antiphospholipid antibody syndrome, have been shown to have dramatically higher rates of recurrent venous thrombosis than other causes.[20]
Enhancing Healthcare Team Outcomes
Cerebral venous sinus thromboses are rare but cause significant morbidity if not recognized and treated.[2] A high index of suspicion needs to be kept when new neurologic signs are present in the setting of a headache. When atypical patients present with what appears to be idiopathic intracranial hypertension, care should be taken to exclude dural sinus thrombosis. Intracranial hemorrhages in odd locations that span arterial supplies should raise the possibility of venous occlusion. When working up suspected papilledema, one should obtain some form of venography.
Media
(Click Image to Enlarge)
References
Alvis-Miranda HR, Milena Castellar-Leones S, Alcala-Cerra G, Rafael Moscote-Salazar L. Cerebral sinus venous thrombosis. Journal of neurosciences in rural practice. 2013 Oct:4(4):427-38. doi: 10.4103/0976-3147.120236. Epub [PubMed PMID: 24347950]
Das JM, Sapkota R, Shrestha B. Pediatric dural venous sinus thrombosis following closed head injury: an easily overlooked diagnosis with devastating consequences. Ulusal travma ve acil cerrahi dergisi = Turkish journal of trauma & emergency surgery : TJTES. 2018 Jan:24(1):74-77. doi: 10.5505/tjtes.2017.22823. Epub [PubMed PMID: 29350373]
Coutinho JM, Zuurbier SM, Aramideh M, Stam J. The incidence of cerebral venous thrombosis: a cross-sectional study. Stroke. 2012 Dec:43(12):3375-7. doi: 10.1161/STROKEAHA.112.671453. Epub 2012 Sep 20 [PubMed PMID: 22996960]
Level 2 (mid-level) evidenceDevasagayam S, Wyatt B, Leyden J, Kleinig T. Cerebral Venous Sinus Thrombosis Incidence Is Higher Than Previously Thought: A Retrospective Population-Based Study. Stroke. 2016 Sep:47(9):2180-2. doi: 10.1161/STROKEAHA.116.013617. Epub 2016 Jul 19 [PubMed PMID: 27435401]
Level 2 (mid-level) evidenceCoutinho JM, Ferro JM, Canhão P, Barinagarrementeria F, Cantú C, Bousser MG, Stam J. Cerebral venous and sinus thrombosis in women. Stroke. 2009 Jul:40(7):2356-61. doi: 10.1161/STROKEAHA.108.543884. Epub 2009 May 28 [PubMed PMID: 19478226]
Level 2 (mid-level) evidenceFerro JM, Canhão P, Bousser MG, Stam J, Barinagarrementeria F, ISCVT Investigators. Cerebral vein and dural sinus thrombosis in elderly patients. Stroke. 2005 Sep:36(9):1927-32 [PubMed PMID: 16100024]
Cavalcanti DD, Raz E, Shapiro M, Dehkharghani S, Yaghi S, Lillemoe K, Nossek E, Torres J, Jain R, Riina HA, Radmanesh A, Nelson PK. Cerebral Venous Thrombosis Associated with COVID-19. AJNR. American journal of neuroradiology. 2020 Aug:41(8):1370-1376. doi: 10.3174/ajnr.A6644. Epub 2020 Jun 18 [PubMed PMID: 32554424]
Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, deVeber G, Ferro JM, Tsai FY, American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011 Apr:42(4):1158-92. doi: 10.1161/STR.0b013e31820a8364. Epub 2011 Feb 3 [PubMed PMID: 21293023]
Leach JL, Fortuna RB, Jones BV, Gaskill-Shipley MF. Imaging of cerebral venous thrombosis: current techniques, spectrum of findings, and diagnostic pitfalls. Radiographics : a review publication of the Radiological Society of North America, Inc. 2006 Oct:26 Suppl 1():S19-41; discussion S42-3 [PubMed PMID: 17050515]
Poon CS, Chang JK, Swarnkar A, Johnson MH, Wasenko J. Radiologic diagnosis of cerebral venous thrombosis: pictorial review. AJR. American journal of roentgenology. 2007 Dec:189(6 Suppl):S64-75 [PubMed PMID: 18029905]
Bidar F, Faeghi F, Ghorbani A. Assessment of cerebral venous sinus thrombosis using T2 (*)-weighted gradient echo magnetic resonance imaging sequences. Iranian journal of neurology. 2016 Apr 3:15(2):96-9 [PubMed PMID: 27326365]
Crassard I, Soria C, Tzourio C, Woimant F, Drouet L, Ducros A, Bousser MG. A negative D-dimer assay does not rule out cerebral venous thrombosis: a series of seventy-three patients. Stroke. 2005 Aug:36(8):1716-9 [PubMed PMID: 16020765]
de Bruijn SF, Stam J. Randomized, placebo-controlled trial of anticoagulant treatment with low-molecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999 Mar:30(3):484-8 [PubMed PMID: 10066840]
Level 1 (high-level) evidencede Bruijn SF, Budde M, Teunisse S, de Haan RJ, Stam J. Long-term outcome of cognition and functional health after cerebral venous sinus thrombosis. Neurology. 2000 Apr 25:54(8):1687-9 [PubMed PMID: 10762517]
Level 1 (high-level) evidenceBose G, Graveline J, Yogendrakumar V, Fergusson D, Dowlatshahi D. Direct oral anticoagulants in treatment of cerebral venous thrombosis: a systematic review protocol. Systematic reviews. 2019 Apr 18:8(1):99. doi: 10.1186/s13643-019-1022-8. Epub 2019 Apr 18 [PubMed PMID: 30999965]
Level 1 (high-level) evidenceWasay M, Khan M, Rajput HM, Farooq S, Memon MI, AlRukn SA, Malik A, Abd-Allah F, Shoaib RF, Shahid R, Nishat S, Awan S. New Oral Anticoagulants versus Warfarin for Cerebral Venous Thrombosis: A Multi-Center, Observational Study. Journal of stroke. 2019 May:21(2):220-223. doi: 10.5853/jos.2019.00150. Epub 2019 May 31 [PubMed PMID: 31161765]
Level 2 (mid-level) evidenceCanhão P, Falcão F, Ferro JM. Thrombolytics for cerebral sinus thrombosis: a systematic review. Cerebrovascular diseases (Basel, Switzerland). 2003:15(3):159-66 [PubMed PMID: 12646773]
Level 1 (high-level) evidenceChaloupka JC, Mangla S, Huddle DC. Use of mechanical thrombolysis via microballoon percutaneous transluminal angioplasty for the treatment of acute dural sinus thrombosis: case presentation and technical report. Neurosurgery. 1999 Sep:45(3):650-6; discussion 656-7 [PubMed PMID: 10493388]
Level 3 (low-level) evidenceFerro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F, ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004 Mar:35(3):664-70 [PubMed PMID: 14976332]
Level 2 (mid-level) evidenceSchulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. The American journal of medicine. 1998 Apr:104(4):332-8 [PubMed PMID: 9576405]