Introduction
Bowman’s capsule is a part of the nephron that forms a cup-like sack surrounding the glomerulus. Bowman’s capsule encloses a space called “Bowman’s space,” which represents the beginning of the urinary space and is contiguous with the proximal convoluted tubule of the nephron. Bowman’s capsule, Bowman’s space, and the glomerular capillary network and its supporting architecture can collectively be thought of as composing the glomerulus. There are an estimated 900000 glomeruli within the cortex of a mature human kidney.[1][2]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
In the kidney, the glomerulus represents the initial location of the renal filtration of blood. Blood enters the glomerulus through the afferent arteriole at the vascular pole, undergoes filtration in the glomerular capillaries, and exits the glomerulus through the efferent arteriole at the vascular pole.
Bowman’s capsule surrounds the glomerular capillary loops and participates in the filtration of blood from the glomerular capillaries. Bowman’s capsule also has a structural function and creates a urinary space through which filtrate can enter the nephron and pass to the proximal convoluted tubule. Liquid and solutes of the blood must pass through multiple layers to move from the glomerular capillaries into Bowman’s space to ultimately become filtrate within the nephron’s lumen.
The first step of filtration occurs through the endothelial layer of the capillaries, which is composed of fenestrated endothelial cells.[3] These fenestrations, or slits between endothelial cells, are approximately 60 to 80 nm wide and restrict the movement of matter above this size.[4][5][6][7] In addition to filtering based on size, the fenestrated endothelium carries negative charges that preferentially restrict the movement of negatively charged substances into Bowman’s space.[8][9][10]
Filtrate next moves through the glomerular basement membrane (GBM). From the direction of the capillaries and moving towards Bowman’s capsule, three layers compose the GBM – the lamina rara interna, the lamina densa, and the lamina rara externa. Mesangial cells within the glomerulus play a role in creating and maintaining the GBM, as well as holding capillary loops together.[11]
Following the GBM, filtrate must pass through the epithelial layer of Bowman’s capsule, which is composed of podocytes. The podocytes feature finger-like projections of cytoplasm referred to as “foot processes” or “pedicels.” These foot processes interdigitate with one another and create a further barrier through which filtrate must pass. Structures called “slit diaphragms” bridge nearby foot processes and provide structural support. The podocytes are the primary cells of the epithelium adjacent to the capillaries (the visceral epithelium) and play a role in filtration. The parietal epithelium of Bowman’s capsule is the outer layer and is composed of simple squamous epithelial cells called “parietal cells.” The parietal layer is not directly involved with filtration from the capillaries. Parietal cells play a structural role in maintaining Bowman’s capsule and are also speculated to have the ability to differentiate into podocytes to replace damaged or old podocytes.[12][13][14][13] Bowman’s space is the area between the visceral and parietal epithelium of Bowman’s capsule.
In summary, filtrate entering Bowman’s space traverses through glomerular capillaries, the GBM, and the interdigitated foot processes of the podocytes and is filtered based on size and electric charge. The filtrate entering Bowman’s space has a very similar composition to that of the blood in the glomerular capillaries except for the protein, and cell content as these are the components largely prevented from entering Bowman’s space when glomerular filtration is functioning properly.
Embryology
Two embryological precursor structures – the metanephric mesenchyme and the ureteric bud – interact to form the human kidney.[15][16] The metanephric mesenchyme contains, among other progenitor cell types, nephron progenitor cells. The nephron progenitor cells give rise to various cell types of the nephron, including the podocytes and glomerular parietal epithelial cells that compose Bowman’s capsule.[16][17]
The metanephric mesenchyme is also known as metanephric mesoderm, metanephrogenic blastema, and metanephric blastema. The RET (REarranged during Transfection) gene is crucial for the correct formation between the metanephric mesenchyme and renal development. The gene encodes a protein/receptor. The RET receptor tyrosine kinase is activated by a growth factor (glial cell growth factor - GDNF), initiating the development of the cell.
The metanephric mesenchyme appears in the fifth week of gestation from the mesoderm.
Blood Supply and Lymphatics
The blood supply to the glomerulus ultimately comes from the renal arteries (one renal artery supplies each kidney), which comes off of the abdominal aorta. At the renal hilum, the renal artery branches many times as it travels through the kidney. First, it branches into the segmental artery, which branches into various interlobular arteries, which travel to the renal cortex and become arcuate arteries. The afferent arterioles ultimately branch off of these arcuate arteries to supply blood to the glomerular capillaries within the glomerulus.[18] Once blood passes through the glomerular capillaries, it exits the glomerulus through the efferent arteriole. From the efferent arteriole, blood enters a second capillary network, the peritubular capillaries, before exiting the kidney through the renal vein and ultimately entering the inferior vena cava.
The renal lymphatic system, in general, is much more abundant in the renal cortex than in the medulla.[19] Many lymphatic vessels in the renal cortex appear to begin blindly close to Bowman’s capsule, with some lymphatic vessels either partially or fully surrounding Bowman’s capsule.[20][21] Lymphatic vessels within the kidney generally track the same course as the renal vasculature before leaving the kidney.[19] Lymphatics from the left kidney drain into the paraaortic, preaortic, and the retroaortic lymph nodes.[22] Those from the right kidney drain into the paracaval, precaval, interaortacaval, and retrocaval lymph nodes.[22] Lymph from both kidneys can also drain into lymphatic systems posterior to the aorta.[23] Ultimately, all lymph from the renal system funnels into the thoracic duct.[23]
Nerves
The kidney receives innervation by sympathetic, parasympathetic, and sensory nerves.[24] The effects of renal sympathetic and sensory innervation on renal hemodynamics and filtrate entering Bowman’s space are well described. By contrast, the precise effects of parasympathetic renal innervation, supplied by the Vagus nerve, are less well characterized in the literature.
The sympathetic fibers innervating the kidney arise from the prevertebral and paravertebral ganglia and give rise to postganglionic neurons that largely track with the renal artery towards the kidney and intrarenal vasculature within the kidney.[24][25] Relative to the glomerulus, sympathetic fibers more heavily innervate the afferent arteriole than the efferent arteriole.[24][26] Sympathetic stimulation within the kidney leads to vasoconstriction.[24][27][28] Therefore, increased sympathetic stimulation to the kidney should constrict the afferent arteriole more than the efferent arteriole, yielding a net decrease in glomerular filtration rate and thus a decrease in filtrate entering Bowman’s space.[28]
Sensory nerves within the kidney concentrate in the renal pelvic area.[28][29][30][31][32] These nerves activate upon distention of the renal pelvic wall and have an overall inhibitory effect on renal sympathetic stimulation.[28] As a result, increased renal sensory activation will lead to a reversal of the effects of sympathetic stimulation at the glomerulus leading to relatively more afferent arteriolar dilation than efferent arteriolar dilation, yielding an increase in glomerular filtration pressure, and thus more filtrate entering Bowman’s space.
Muscles
Smooth muscle in the afferent and efferent arterioles play a role mediating the glomerular filtration rate and pressure, forcing filtrate into Bowman’s space. Several important mechanisms regulate smooth muscle in the afferent and efferent arterioles.
One such mechanism, the myogenic response, occurs when the afferent arteriole feels stretch from increased blood flow. In response, the smooth muscles of the afferent arteriole will contract, decreasing blood flow to the glomerulus and ultimately decreasing filtrate into Bowman’s space.[33]
A second mechanism occurs when the afferent arteriole senses less stretch from passing blood flow. In response, the juxtaglomerular cells of the afferent arteriole, a type of specialized smooth muscle cell, secrete renin, which activates the renin-angiotensin-aldosterone system (RAAS), which has many effects; prominent among these is to increase blood pressure. Angiotensin II production occurs through RAAS, which, among other functions, preferentially constricts the efferent arteriole. This constriction increases pressure in the glomerular capillaries and thus filtrate entering Bowman’s space.[33]
A third mechanism is tubuloglomerular feedback. In this process, macula densa cells of the thick ascending limb of the nephron secrete the paracrine mediators ATP, adenosine, and thromboxane in response to increased delivery of electrolytes through the nephron (a proxy for sensing increased glomerular filtration rate). These mediators dilate the efferent arteriole, yielding less glomerular filtration pressure, and thus less filtrate entering Bowman’s space.[33]
As discussed previously, sympathetic stimulation offers a fourth mechanism of regulation, as such stimulation preferentially constricts the afferent arteriole, yielding less filtrate entering Bowman’s space.[28]
In general, constriction of the afferent arteriole or dilation of the efferent arteriole will decrease pressure in the glomerular capillaries, creating less pressure driving filtrate into Bowman’s space. By contrast, dilation of the afferent arteriole or constriction of the efferent arteriole will increase pressure in the glomerular capillaries, creating more pressure driving filtrate into Bowman’s space.
Surgical Considerations
Healthy glomeruli, including Bowman’s capsule and Bowman’s space, are necessary for the proper functioning of the kidney. All glomeruli exist within the cortex of the kidney. Thus those performing renal operations should strive to maintain as much renal cortex as possible to preserve glomeruli and renal function.[34]
Clinical Significance
The glomerulus is clinically significant because it is the location where filtration in the kidney begins. All actions of the nephron downstream of the glomeruli rely on the passage of filtrate from the glomerular capillaries into Bowman’s space.
Several diseases can affect the glomerulus. These diseases broadly divided into those presenting as nephrotic syndrome and those presenting as nephritic syndrome. The nephrotic syndrome is characterized by proteinuria of greater than 3.5 grams of protein per day, hypoalbuminemia, edema, and hyperlipidemia.[34][35] The nephritic syndrome is characterized by oliguria, hematuria, red blood cell casts in the urine, proteinuria under 3.5 grams of protein per day, and hypertension.[35]
Some of the major causes of the nephrotic syndrome are minimal change disease, focal segmental glomerulosclerosis, diabetic nephropathy membranous nephropathy, membranoproliferative glomerulonephritis, and amyloidosis.[35] Nephrotic syndromes are generally the result of damage to the foot processes of the podocytes or the GBM. Some of the major causes of the nephritic syndrome include post-infectious glomerulonephritis, infective endocarditis, IgA nephropathy, Lupus nephritis, Goodpasture disease, and vasculitis.[35] Nephritic syndromes generally result from damage to the glomerular capillary endothelium or the GBM.
Other Issues
Dysfunction of podocytes (cells in close contact with Bowman's capsules) causes a deterioration of glomerular function. Bowman's capsules and Bowman's space are essential to protect the function of the glomerulus because they prevent the infiltration of leukocytes (macrophages and CD4 + and CD8 + T cells). Preventing the accumulation of leukocytes protects the function of the podocytes.[36]
In Bowman's capsules, there are cells with self-renewal properties, which can transform into podocytes. This event is more frequent when the structure needs repair, for example, in the presence of diabetes, such as a safety valve. The mechanism becomes stimulated by the decrease in Gas1 (Growth Arrest-Specific 1).[37]
One of the causes of the presence of focal segmental glomerulosclerosis and crescentic glomerulonephritis is the accumulation of cuboidal cells or cuboidal parietal epithelial cells (PECs) in Bowman's capsule. These cuboidal PECS can create a metabolic environment that leads to the formation of sclerotic lesions, leading to kidney damage.[38]
Media
(Click Image to Enlarge)
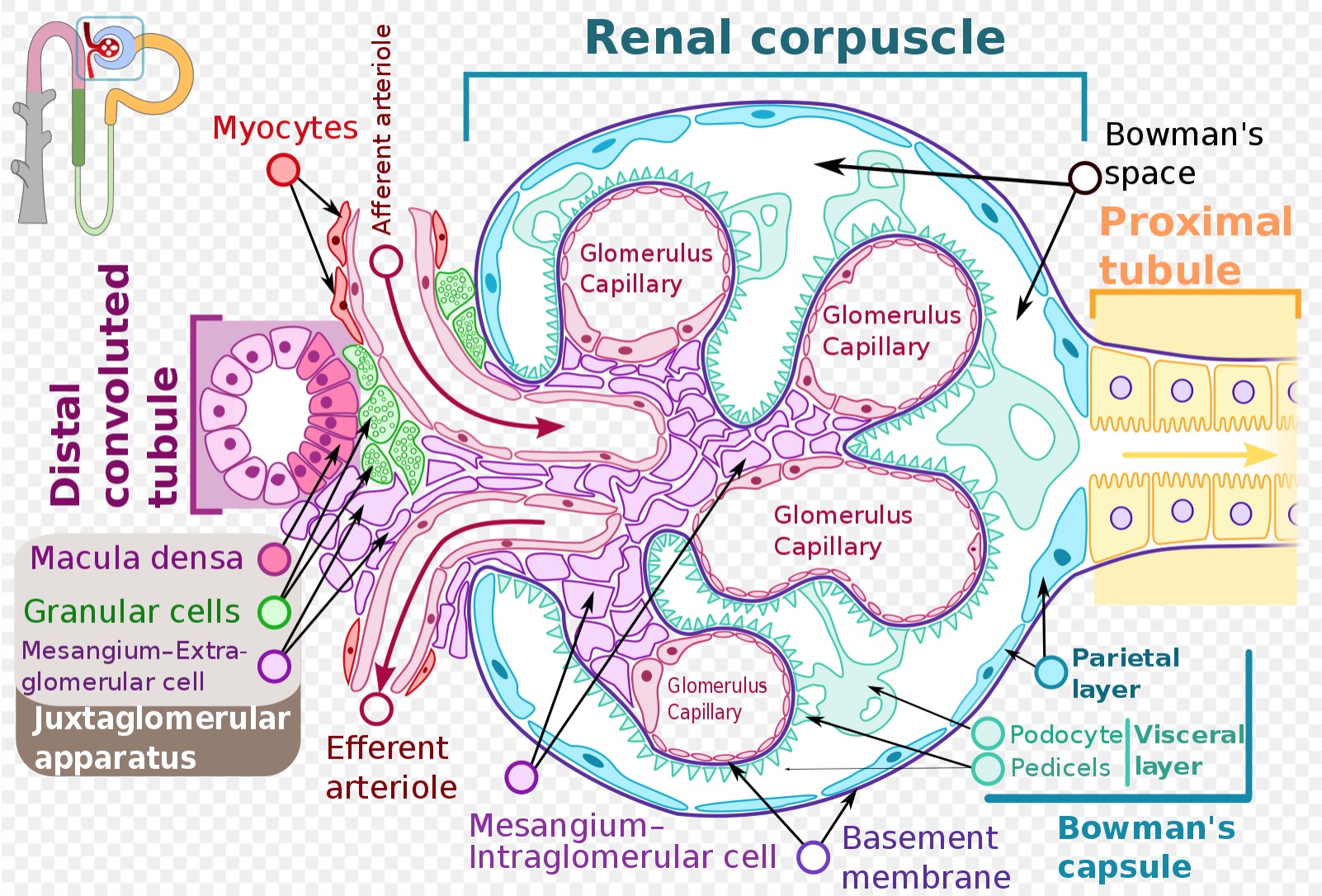
Image of a glomerulus including Bowman's capsule. This image is used under a Creative Commons Attribution-Share Alike 4.0 International license. No changes were made to the original image authored by Shypoetess. Page URL: https://commons.wikimedia.org/wiki/File:Renal_corpuscle-en.svg File URL: https://upload.wikimedia.org/wikipedia/commons/6/69/Renal_corpuscle-en.svg Attribution: Shypoetess [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)]
References
Puelles VG, Hoy WE, Hughson MD, Diouf B, Douglas-Denton RN, Bertram JF. Glomerular number and size variability and risk for kidney disease. Current opinion in nephrology and hypertension. 2011 Jan:20(1):7-15. doi: 10.1097/MNH.0b013e3283410a7d. Epub [PubMed PMID: 21099687]
Level 3 (low-level) evidenceWhite KE. Research into the structure of the kidney glomerulus--making it count. Micron (Oxford, England : 1993). 2012 Oct:43(10):1001-9. doi: 10.1016/j.micron.2012.04.013. Epub 2012 May 2 [PubMed PMID: 22607953]
Level 3 (low-level) evidenceAbrahamson DR. Structure and development of the glomerular capillary wall and basement membrane. The American journal of physiology. 1987 Nov:253(5 Pt 2):F783-94 [PubMed PMID: 3318497]
Level 3 (low-level) evidenceAvasthi PS, Evan AP, Hay D. Glomerular endothelial cells in uranyl nitrate-induced acute renal failure in rats. The Journal of clinical investigation. 1980 Jan:65(1):121-7 [PubMed PMID: 7350192]
Level 3 (low-level) evidenceJorgensen F, Bentzon MW. The ultastructure of the normal human glomerulus. Thickness of glomerular basement membrane. Laboratory investigation; a journal of technical methods and pathology. 1968 Jan:18(1):42-8 [PubMed PMID: 5643970]
Lea PJ, Silverman M, Hegele R, Hollenberg MJ. Tridimensional ultrastructure of glomerular capillary endothelium revealed by high-resolution scanning electron microscopy. Microvascular research. 1989 Nov:38(3):296-308 [PubMed PMID: 2607999]
Level 3 (low-level) evidenceRostgaard J, Qvortrup K. Electron microscopic demonstrations of filamentous molecular sieve plugs in capillary fenestrae. Microvascular research. 1997 Jan:53(1):1-13 [PubMed PMID: 9056471]
Level 3 (low-level) evidenceScott RP, Quaggin SE. Review series: The cell biology of renal filtration. The Journal of cell biology. 2015 Apr 27:209(2):199-210. doi: 10.1083/jcb.201410017. Epub [PubMed PMID: 25918223]
Rostgaard J, Qvortrup K. Sieve plugs in fenestrae of glomerular capillaries--site of the filtration barrier? Cells, tissues, organs. 2002:170(2-3):132-8 [PubMed PMID: 11731701]
Level 3 (low-level) evidenceCurry FE, Adamson RH. Endothelial glycocalyx: permeability barrier and mechanosensor. Annals of biomedical engineering. 2012 Apr:40(4):828-39. doi: 10.1007/s10439-011-0429-8. Epub 2011 Oct 19 [PubMed PMID: 22009311]
Level 3 (low-level) evidenceZhao JH. Mesangial Cells and Renal Fibrosis. Advances in experimental medicine and biology. 2019:1165():165-194. doi: 10.1007/978-981-13-8871-2_9. Epub [PubMed PMID: 31399966]
Level 3 (low-level) evidenceBussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. The American journal of pathology. 2005 Feb:166(2):545-55 [PubMed PMID: 15681837]
Level 3 (low-level) evidenceSagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. Journal of the American Society of Nephrology : JASN. 2006 Sep:17(9):2443-56 [PubMed PMID: 16885410]
Level 3 (low-level) evidenceRonconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. Journal of the American Society of Nephrology : JASN. 2009 Feb:20(2):322-32. doi: 10.1681/ASN.2008070709. Epub 2008 Dec 17 [PubMed PMID: 19092120]
Level 3 (low-level) evidenceCostantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Developmental cell. 2010 May 18:18(5):698-712. doi: 10.1016/j.devcel.2010.04.008. Epub [PubMed PMID: 20493806]
Level 3 (low-level) evidenceYoshimura Y, Nishinakamura R. Podocyte development, disease, and stem cell research. Kidney international. 2019 Nov:96(5):1077-1082. doi: 10.1016/j.kint.2019.04.044. Epub 2019 Jun 3 [PubMed PMID: 31420196]
Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell stem cell. 2008 Aug 7:3(2):169-81. doi: 10.1016/j.stem.2008.05.020. Epub [PubMed PMID: 18682239]
Level 3 (low-level) evidenceDalal R, Bruss ZS, Sehdev JS. Physiology, Renal Blood Flow and Filtration. StatPearls. 2023 Jan:(): [PubMed PMID: 29489242]
Russell PS, Hong J, Windsor JA, Itkin M, Phillips ARJ. Renal Lymphatics: Anatomy, Physiology, and Clinical Implications. Frontiers in physiology. 2019:10():251. doi: 10.3389/fphys.2019.00251. Epub 2019 Mar 14 [PubMed PMID: 30923503]
RAWSON AJ. Distribution of the lymphatics of the human kidney as shown in a case of carcinomatous permeation. Archives of pathology. 1949 Mar:47(3):283-92 [PubMed PMID: 18111390]
Level 3 (low-level) evidenceBell RD, Keyl MJ, Shrader FR, Jones EW, Henry LP. Renal lymphatics: the internal distribution. Nephron. 1968:5(6):454-63 [PubMed PMID: 5706255]
Level 3 (low-level) evidenceWakiya K, Fukushima T, Miyazaki S. [Results of microvascular decompression in 16 cases of glossopharyngeal neuralgia]. Neurologia medico-chirurgica. 1989 Dec:29(12):1113-8 [PubMed PMID: 2484190]
Level 3 (low-level) evidenceAssouad J, Riquet M, Foucault C, Hidden G, Delmas V. Renal lymphatic drainage and thoracic duct connections: implications for cancer spread. Lymphology. 2006 Mar:39(1):26-32 [PubMed PMID: 16724507]
Burnstock G, Loesch A. Sympathetic innervation of the kidney in health and disease: Emphasis on the role of purinergic cotransmission. Autonomic neuroscience : basic & clinical. 2017 May:204():4-16. doi: 10.1016/j.autneu.2016.05.007. Epub 2016 May 31 [PubMed PMID: 27270214]
Ferguson M, Ryan GB, Bell C. Localization of sympathetic and sensory neurons innervating the rat kidney. Journal of the autonomic nervous system. 1986 Aug:16(4):279-88 [PubMed PMID: 2427560]
Level 3 (low-level) evidenceLuff SE, Hengstberger SG, McLachlan EM, Anderson WP. Distribution of sympathetic neuroeffector junctions in the juxtaglomerular region of the rabbit kidney. Journal of the autonomic nervous system. 1992 Oct:40(3):239-53 [PubMed PMID: 1360993]
Level 3 (low-level) evidenceTone K, Sakaguchi T, Anzai H, Yoshifuku K, Wada T. Epidemiological and bacteriological studies on an outbreak of Sh. sonnei. The Kitasato archives of experimental medicine. 1975 Mar:48(1):1-8 [PubMed PMID: 1104983]
Level 2 (mid-level) evidenceJohns EJ, Kopp UC, DiBona GF. Neural control of renal function. Comprehensive Physiology. 2011 Apr:1(2):731-67. doi: 10.1002/cphy.c100043. Epub [PubMed PMID: 23737201]
Level 3 (low-level) evidenceKopp UC, Cicha MZ, Smith LA. Endogenous angiotensin modulates PGE(2)-mediated release of substance P from renal mechanosensory nerve fibers. American journal of physiology. Regulatory, integrative and comparative physiology. 2002 Jan:282(1):R19-30 [PubMed PMID: 11742819]
Level 3 (low-level) evidenceKopp UC, Cicha MZ, Nakamura K, Nüsing RM, Smith LA, Hökfelt T. Activation of EP4 receptors contributes to prostaglandin E2-mediated stimulation of renal sensory nerves. American journal of physiology. Renal physiology. 2004 Dec:287(6):F1269-82 [PubMed PMID: 15292051]
Level 3 (low-level) evidenceKopp UC, Cicha MZ, Smith LA, Mulder J, Hökfelt T. Renal sympathetic nerve activity modulates afferent renal nerve activity by PGE2-dependent activation of alpha1- and alpha2-adrenoceptors on renal sensory nerve fibers. American journal of physiology. Regulatory, integrative and comparative physiology. 2007 Oct:293(4):R1561-72 [PubMed PMID: 17699565]
Level 3 (low-level) evidenceLiu L, Barajas L. The rat renal nerves during development. Anatomy and embryology. 1993 Oct:188(4):345-61 [PubMed PMID: 7506501]
Level 3 (low-level) evidenceKaufman DP,Basit H,Knohl SJ, Physiology, Glomerular Filtration Rate (GFR) 2019 Jan; [PubMed PMID: 29763208]
Wang CS, Greenbaum LA. Nephrotic Syndrome. Pediatric clinics of North America. 2019 Feb:66(1):73-85. doi: 10.1016/j.pcl.2018.08.006. Epub [PubMed PMID: 30454752]
Khanna R. Clinical presentation & management of glomerular diseases: hematuria, nephritic & nephrotic syndrome. Missouri medicine. 2011 Jan-Feb:108(1):33-6 [PubMed PMID: 21462608]
Chen A, Lee K, D'Agati VD, Wei C, Fu J, Guan TJ, He JC, Schlondorff D, Agudo J. Bowman's capsule provides a protective niche for podocytes from cytotoxic CD8+ T cells. The Journal of clinical investigation. 2018 Aug 1:128(8):3413-3424. doi: 10.1172/JCI97879. Epub 2018 Jul 9 [PubMed PMID: 29985168]
Luna-Antonio BI, Rodriguez-Muñoz R, Namorado-Tonix C, Vergara P, Segovia J, Reyes JL. Gas1 expression in parietal cells of Bowman's capsule in experimental diabetic nephropathy. Histochemistry and cell biology. 2017 Jul:148(1):33-47. doi: 10.1007/s00418-017-1550-z. Epub 2017 Mar 18 [PubMed PMID: 28315934]
Kuppe C, Leuchtle K, Wagner A, Kabgani N, Saritas T, Puelles VG, Smeets B, Hakroush S, van der Vlag J, Boor P, Schiffer M, Gröne HJ, Fogo A, Floege J, Moeller MJ. Novel parietal epithelial cell subpopulations contribute to focal segmental glomerulosclerosis and glomerular tip lesions. Kidney international. 2019 Jul:96(1):80-93. doi: 10.1016/j.kint.2019.01.037. Epub 2019 Feb 27 [PubMed PMID: 31029503]