Catheter Management of Patent Foramen Ovale
Catheter Management of Patent Foramen Ovale
Introduction
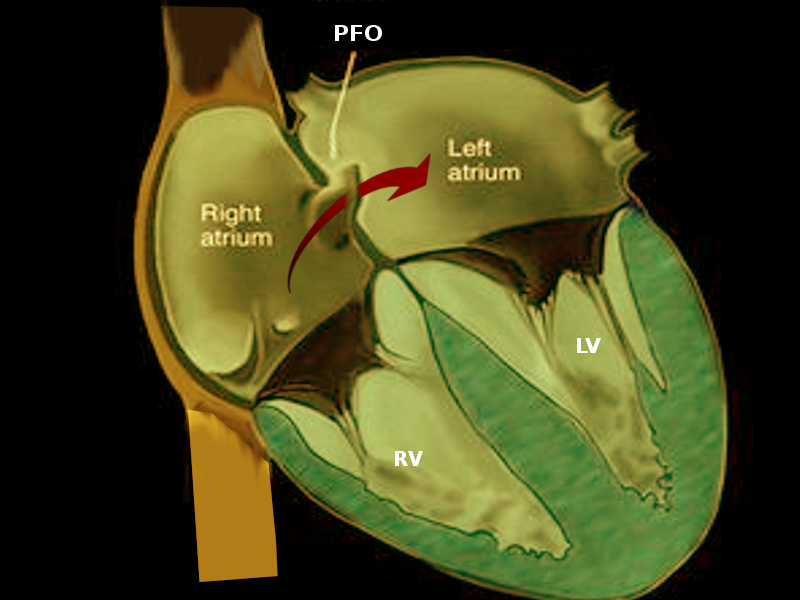
Patent foramen ovale (PFO) is a small opening between the top two chambers of the heart (see Image. Patent Foramen Ovale). However, it is one of the most common cardiac defects. Several large, population-based studies estimated no differences in incidence (at about 9.2%) among patients with stroke and patients without a stroke.[1] Patent foramen ovale is present in one-fifth of the population. They affect males and females equally. The size grows with age whereas the prevalence diminishes with increasing age.[2] PFO has correlations with both cardiac and extra-cardiac disorders. Its strongest association seen in the literature is with cryptogenic stroke. The PFO provides a conduit for emboli to shunt from right to left.[3] Most present incidentally on echocardiogram following stroke workup. The contribution of the PFO to the burden of cryptogenic stroke is well-characterized. Several large, population-based studies estimate that transcatheter closure of PFO in addition to medical therapy is superior to medical therapy alone in preventing recurrent cryptogenic stroke; it bears mention that PFO closure in these patients has not demonstrated a reduction in the risk of recurrent TIA or all-cause mortality. Studies have found a higher rate of postoperative atrial fibrillation following PFO closure device placement, the long-term effects of which have yet to be studied.[4]
Data exists showing that PFO closure for cryptogenic stroke can be cost-effective.[5] One study demonstrated the effectiveness of PFO closure depends on the device used.[6] The gold standard for diagnosis of PFO remains to be a transoesophageal echocardiogram with a bubble test after a sustained Valsalva maneuver (see Video. Transesophageal Echocardiogram). Once identified, the structure can undergo assessment for the need for treatment. There are surgical, transcatheter, and medical therapies available for treatment. The percutaneous transcatheter approach is coming more into favor, as most patients prefer the less invasive procedure.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
A patent foramen ovale is a remnant opening of the fetal foramen ovale. The foramen ovale is a conduit for blood to flow from the right atrium to the left atrium in the fetal state. Usually, the PFO closes at birth, but in about 25% percent of the population, it does not close. Within this subset, a few people experience symptoms or sequelae related to PFO patency. It is these patients who should receive an evaluation for possible PFO closure.
Indications
Indications for transcatheter PFO closure [7]:
- Recurrent cryptogenic stroke because of presumed paradoxical embolism
- Recurrent transient ischemic attacks (TIA)
- History of paradoxical peripheral embolism
- Decompression sickness
- Migraine
Contraindications
Contraindications of percutaneous transcatheter PFO closure include but are not limited to the following [6]:
- >25 mm PFO (refer for surgery)
- Active endocarditis
- Active bacteremia
- Active fungemia
- Current sepsis
- Intracardiac mass
- Intracardiac anatomy precluding safe device delivery
Equipment
- Cardiac catheterization laboratory
- Sterile gown, gloves, drapes
- Echocardiogram
- Fluoroscopy
- Selected PFO occluder
- Code cart in the event of arrhythmia
- Balloon-tipped angiographic catheter or pigtail catheter
- Guidewire
Personnel
- Interventional cardiologist trained in PFO closure
- Surgical first assistant
- Cardiac nurse
- Cardiac cath lab technician
Preparation
Preparation for percutaneous transcatheter PFO closure starts with an evaluation of the patient to see if the patient qualifies for catheter closure; this is evaluated first with cranial imaging followed by echocardiogram with bubble study. Additional pre-operative tests may include chest x-ray, electrocardiogram, and blood tests to assess the general health of the patient as well as kidney function. Before the procedure, all patients undergoing PFO closure should be pre-treated with antiplatelet therapy, commonly with both aspirin and Plavix daily. Warfarin therapy alone may be an option in patients who need chronic antithrombotic therapy followed by bridging with low-molecular-weight heparin before and after PFO closure. One hour before percutaneous access an intravenous antibiotic dose should be given, typically cefazolin or vancomycin, if they are allergic to penicillin. To avoid left atrial hypovolemia, patients should also receive IV normal saline before and during the procedure.
Technique or Treatment
The first step in the transcatheter PFO closure procedure is getting access. The femoral venous site is the easiest, safest, and therefore the most used to perform PFO closure. It allows insertion of the occluder vertically from the inferior right atrium to the superior left atrium. After femoral venous sheath insertion, a heparin bolus is given to achieve anticlotting properties. More heparin is used intermittently throughout the procedure to maintain anticlotting activity. Oxygenation is administered via nasal cannula oxygen to achieve hyperoxygenation. Hyperoxygenation is preemptively treats a possible event of air embolus while catheterizing the LA. Using a balloon-tipped angiographic catheter or pigtail catheter is inserted and cineangiography done to outline the left atrial (LA) anatomy. A 6-F multipurpose catheter is then advanced over a guide-wire into the superior vena cava (SVC). The catheter is then positioned posteriorly and withdrawn to the fossa ovalis.
When the catheter is in the fossa ovalis, the guide wire advances across the PFO into the left superior pulmonary vein. The multipurpose catheter then gets advanced into this pulmonary vein, and its position is confirmed by hemodynamic measurement of LA pressure. In some circumstances the guide wire is difficult to pass into the LA. In these circumstances a different guide-wire might be used. The multipurpose catheter is then exchanged over a straight tetrafluoroethylene- coated stainless steel guide wire for the guide or a flex sheath placed in the left atrium. A sheathless approach is undertaken with removal of both the multipurpose catheter and the femoral venous sheath. Many operators attach an accessory adapter to the guiding catheter to administer continuous saline hand flushing while advancing the guide to the left atrium. Tunnel length can be determined with gentle balloon sizing of the PFO. After selecting the appropriate device, the LA disc is placed in the LA. The sheath and device are then drawn back until the LA disc attaches to the LA septum. With slight tension against the LA septum, the RA side of the device is then deployed. Contrast is pushed through the sheath beside the RA disc to confirm proper device positioning. The left anterior oblique projection is then used to view the discs. The space between the two discs is associated with the width of the septum secundum. After device release, RA angiography may be done to assess correct positioning and function of the device implanted.
The data is inconclusive as to whether intracardiac or transesophageal echocardiographic guidance is better between PFO closure as compared to fluoroscopic guidance alone. One study confirms the efficacy and safety of TTE guidance during percutaneous closure of PFO, which shortens the procedural time and removes the need for general anesthesia or endotracheal intubation.[7] However, fluoroscopy guidance has been shown to increase procedural time and cost associated with balloon sizing as opposed to TTE which decreases both of these,
After completion of the procedure, transthoracic echocardiography with bubble study is used to confirm the positioning of the device and to assess for residual shunting. An electrocardiogram and chest x-ray are also useful to evaluate for complications and accuracy of the procedure. Anticoagulation therapy is given for 6 months post-operation. Dental procedures should be postponed for 6 months. If dental procedures are required, antibiotic prophylaxis should be given to prevent endocarditis within the first 6 months. Follow-up with transthoracic echocardiography is at 1 and 6 months following the procedure.
Complications
Data shows trans-catheter PFO closure complication rates is about 7%. Studies have shown that the incidence of an adverse event is higher in patients >60 years of age. Furthermore, adverse outcomes are higher in patients with preceding ischemic stroke versus transient ischemic strokes. Complications include the following in order of most common to least:
- Arrhythmia (most commonly atrial fibrillation)
- Vascular complications
- Hemorrhage/hematoma
- Cardiac tamponade/perforation
- Loss of life
- Pneumothorax/hemothorax
- Occluder embolus
- Infection
Clinical Significance
Patent foramen ovale closure is not necessary for all patients. However, there are a select number of patients who benefit significantly from PFO closure. The sequelae in this population are many times more devastating especially in those with debilitating strokes. Therefore, it is essential for primary care providers to recognize the indications for PFO closure and refer appropriately. Knowing that PFOs >25 mm usually are not amenable to percutaneous management and more often than not require surgical closure is critical information to share with the patient.[6] In addition to indications and contraindications for PFO closure, every practitioner should be aware of the common post-op complications; arrhythmia is the most common, but death is also a possibility.
Enhancing Healthcare Team Outcomes
Percutaneous transcatheter closure of PFO, for the most part, is a simple and safe procedure. However, complications do occur. Therefore, it is important to have an interprofessional approach to evaluation and determining if which if any closure procedure the patients should undergo. For patient safety, it is critical to follow guideline recommendations when selected a procedure of therapy. [level 1]
For best results, an interprofessional team should evaluate and treat the patient.
- A cardiology specialty nurse should assist with patient education and monitoring before, during, and after the procedure.
- A cardiology trained pharmacist should assist with evaluating the patient for potential drug-drug interactions that my complicate the procedure.
- The team should include a specialty-trained cardiologist as well as a cardiothoracic surgeon to assist with complications.
Use of an interprofessional team will lead to the best results.
Media
(Click Video to Play)
Transesophageal Echocardiogram. Transesophageal echocardiogram, before and after patent foramen ovale closure, revealing delayed intracardiac shunting and hypoxemia after massive pulmonary embolism.
Weig T, Dolch M, Frey L, et al. Delayed intracardial shunting and hypoxemia after massive pulmonary embolism in a patient with a biventricular assist device. J Cardiothorac Surg. 2011;6:133.
doi: 10.1186/1749-8090-6-133.
(Click Image to Enlarge)
References
Fisher DC, Fisher EA, Budd JH, Rosen SE, Goldman ME. The incidence of patent foramen ovale in 1,000 consecutive patients. A contrast transesophageal echocardiography study. Chest. 1995 Jun:107(6):1504-9 [PubMed PMID: 7781337]
Level 2 (mid-level) evidenceHagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clinic proceedings. 1984 Jan:59(1):17-20 [PubMed PMID: 6694427]
Lamy C, Giannesini C, Zuber M, Arquizan C, Meder JF, Trystram D, Coste J, Mas JL. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale: the PFO-ASA Study. Atrial Septal Aneurysm. Stroke. 2002 Mar:33(3):706-11 [PubMed PMID: 11872892]
Sitwala P, Khalid MF, Khattak F, Bagai J, Bhogal S, Ladia V, Mukherjee D, Daggubati R, Paul TK. Percutaneous Closure of Patent Foramen Ovale in Patients with Cryptogenic Stroke - An Updated Comprehensive Meta-Analysis. Cardiovascular revascularization medicine : including molecular interventions. 2019 Aug:20(8):687-694. doi: 10.1016/j.carrev.2018.09.010. Epub 2018 Sep 19 [PubMed PMID: 30282597]
Level 1 (high-level) evidenceLeppert MH, Poisson SN, Carroll JD, Thaler DE, Kim CH, Orjuela KD, Ho PM, Burke JF, Campbell JD. Cost-Effectiveness of Patent Foramen Ovale Closure Versus Medical Therapy for Secondary Stroke Prevention. Stroke. 2018 Jun:49(6):1443-1450. doi: 10.1161/STROKEAHA.117.020322. Epub 2018 May 2 [PubMed PMID: 29720435]
Stortecky S, da Costa BR, Mattle HP, Carroll J, Hornung M, Sievert H, Trelle S, Windecker S, Meier B, Jüni P. Percutaneous closure of patent foramen ovale in patients with cryptogenic embolism: a network meta-analysis. European heart journal. 2015 Jan 7:36(2):120-8. doi: 10.1093/eurheartj/ehu292. Epub 2014 Aug 11 [PubMed PMID: 25112661]
Level 1 (high-level) evidenceOto A, Aytemir K, Ozkutlu S, Kaya EB, Yorgun H, Canpolat U, Ateş AH, Ozkutlu H. Transthoracic echocardiography guidance during percutaneous closure of patent foramen ovale. Echocardiography (Mount Kisco, N.Y.). 2011 Nov:28(10):1074-80. doi: 10.1111/j.1540-8175.2011.01524.x. Epub 2011 Oct 4 [PubMed PMID: 21967656]