Introduction
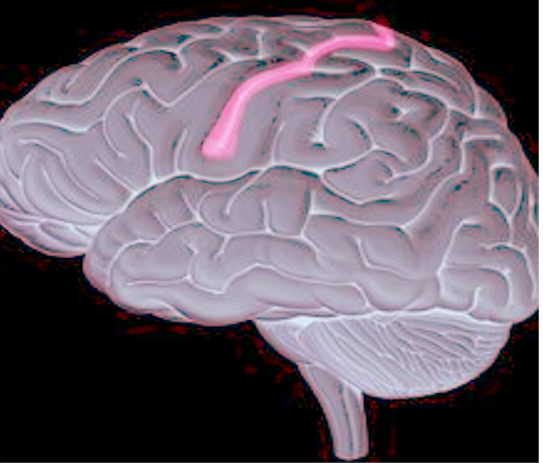
Benign Rolandic epilepsy (BRE), also called benign epilepsy with centrotemporal spikes (BECTS) or benign epilepsy of childhood with centrotemporal spikes (BECCT) is the most common epilepsy syndrome in children.[1] Most of the affected children usually outgrow this condition by puberty, hence the term "benign."[2][3] The seizures originate in the Rolandic area of the brain (situated around the central sulcus of the brain, also called as centrotemporal area, located around the Rolandic fissure). See Figure 1.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Benign Rolandic epilepsy (BRE) is classified as a genetic disorder as approximately 25% of patients have a family history of either febrile seizures or epilepsy.[4] Mode of transmission is thought to be autosomal dominant. However, all studies support the role of genetics.[3][5][6] Although no specific gene has been identified, chromosome 11 (11p13) and chromosome 15 (15q14) are thought to be involved.[5][7] Mutations in KCNQ2,[8] ELP4,[9] and GRIN2A[10] genes have been found in families with BRE.
Epidemiology
Benign Rolandic epilepsy (BRE) can start anywhere between the ages of 1 to 14 years. It peaks around 7 to 10 years when the majority of the cases occur. BRE occurs more often in boys than in girls with a 1.5 to 1 predominance. The incidence of BRE is 10 to 20 per 100,000 children up to age 15 years. BRE makes up about 15% of all epilepsy cases in children[11][12][13][14] which makes it the most common epilepsy syndrome of childhood. Adults are not affected.
History and Physical
Benign Rolandic epilepsy (BRE) affects children until adolescence and usually occur at night or on awakening (greater than 70%).[15] Seizures are infrequent, partial (or focal) as they originate from the Rolandic area (which controls face and oropharynx) with no loss of consciousness.[15][16] Symptoms are generally unilateral and include facial twitching and stiffness, numbness/tingling of the face and throat (tongue, lips, gums, the inner side of the cheek, tooth) that leads to difficulty speaking with gurgling noises, speech arrest, drooling, and hypersalivation.[15][17][18][19] Seizures usually last only 2 to 3 minutes. Facial twitching can spread to the ipsilateral arm and leg. Seizures can spread and occasionally involve both sides (become generalized) and have other manifestations such as generalized shaking, stiffening, bowel or bladder incontinence, loss of consciousness, and a post-ictal state. Since most of the seizures are partial and occur at night, they go unnoticed until a full-blown generalized seizure occurs.[15] Status epilepticus and sudden unexplained death in epilepsy (SUDEP) are rare.[20] BRE can have associated headaches or migraines as well as behavioral and learning difficulties[21][22][23][24] which are more common when the child is having seizures, and there are electroencephalogram (EEG) abnormalities. However, they can improve as the child grows out of the seizures (usually by the age of 15), EEG normalizes, and typically there are no developmental problems seen.[2]
Evaluation
As with other epilepsy syndromes, a diagnosis of benign Rolandic epilepsy (BRE) is based on history and confirmed with characteristic electroencephalogram (EEG) findings.[15] EEG classically shows slow, biphasic (negative discharges in the centrotemporal area and positive discharges in the frontal area), high-voltage, centrotemporal sharp spikes which are often followed by a slow wave. These sharp spikes occur in repetitive bursts, are usually unilateral (corresponding to the focal nature of the seizures) but sometimes can be bilateral. EEG should always include awake and sleep recording. Sleep recording is very important as the spikes sometimes can be seen only during sleep. Non-rapid eye movement (non-REM) sleep recording offers the highest value as it accentuates the epileptiform activity.[25] The background EEG activity, as well as sleep architecture on a polysomnogram, otherwise remains normal. Neurological and developmental assessment is generally normal. Imaging studies including an MRI brain with and without contrast can be considered (to rule out a structural lesion) but are often unnecessary. Diagnosis can be established from history and characteristic EEG findings.[15] Further workup with imaging, laboratory studies may be indicated if history (atypical features) and EEG is nonconclusive. Although the centrotemporal spikes on EEG are characteristic for BRE, they can rarely be seen in asymptomatic children (as an incidental finding) or other epilepsy syndromes.[3]
Treatment / Management
As the name suggests, benign Rolandic epilepsy (BRE) is generally a benign condition, seizures almost always resolve by adolescence, and are often not treated. This is especially true if the seizures are partial, infrequent, occur only at night, and the patient and family/parents are agreeable.[26] Treatment is considered if seizures are frequent, severe, happen during daytime, are generalized, associated with language and neurocognitive decline/changes, or learning disorder. The patient and family should decide whether or not to treat the seizures in consultation with their treating physician. If treatment is necessary, a single anti-epileptic drug (AED) is usually sufficient, and multiple AEDs are rarely required.[2] Many physicians lean toward giving only a nighttime dose of the medication. Given the focal nature of seizures, carbamazepine is frequently used as a first-line agent. Other drugs that have been used include oxcarbazepine, gabapentin, levetiracetam, valproate, phenytoin, lacosamide, and zonisamide.[27][28][29] Treatment is of short duration and can be discontinued after 1 to 2 years free of seizures. Electroencephalogram (EEG) findings (normal versus abnormal) can be helpful in the decision of tapering and eventually stopping the AEDs. It is possible that seizures can recur after stopping the AEDs. Patient and the family should be aware of the possibility of recurrence and should maintain vigilance.(B3)
Differential Diagnosis
Differential diagnoses include centrotemporal spikes without seizures, centrotemporal spikes with a cerebral lesion, temporal lobe epilepsy, Panayiotopoulos syndrome, and Landau-Kleffner syndrome.
Prognosis
The prognosis of benign Rolandic epilepsy (BRE) is excellent irrespective of treatment. Seizures occur for only 2 to 4 years and spontaneously resolve by the age of 15 to 16 years (in more than 95% of children).[30][26] Majority of the patients have less than 10 seizures, 10% to 20% having only one seizure in their lifetime.[13] A higher number of seizures and/or a prolonged period of seizure activity occurs in children with early seizure onset.[11] Early seizure onset is also known to cause cognitive, behavioral, and speech abnormalities which resolve by adolescence along with resolution of seizures.[22][31][32] BRE can potentially be an early presentation of other epileptic syndromes.[31]
Deterrence and Patient Education
As with any epilepsy syndrome, patient/family education is of paramount importance for a good outcome. Education plays a vital role when decisions are made about starting or stopping treatment. The patient and family should be educated about the possibility of status epilepticus as well as sudden unexplained death in epilepsy (SUDEP).
Enhancing Healthcare Team Outcomes
An interprofessional approach to Rolandic seizures is recommended.
Rolandic epilepsy is a relatively common diagnosis but the condition is benign. Because of its diverse presentation, healthcare workers including nurses need to be aware of the possible signs and symptoms. By far, the majority of children require no treatment, and the disorder gradually disappears in 2 to 4 years. The few patients with recurrent seizures may require treatment with a single anti-epileptic drug. The pharmacist should educate the patient and family about the benign nature of the disorder. In addition, if drug treatment is undertaken, the family/patient must be educated about the need for compliance and watching for side effects. Patients should be informed that the drug should not be abruptly discontinued nor the dose changed without first speaking to the neurologist. The outcomes for most patients with rolandic epilepsy is excellent.
Media
References
Kramer U. Atypical presentations of benign childhood epilepsy with centrotemporal spikes: a review. Journal of child neurology. 2008 Jul:23(7):785-90. doi: 10.1177/0883073808316363. Epub [PubMed PMID: 18658078]
Wirrell EC. Benign epilepsy of childhood with centrotemporal spikes. Epilepsia. 1998:39 Suppl 4():S32-41 [PubMed PMID: 9637591]
Chahine LM, Mikati MA. Benign pediatric localization-related epilepsies. Epileptic disorders : international epilepsy journal with videotape. 2006 Dec:8(4):243-58 [PubMed PMID: 17150437]
Vears DF, Tsai MH, Sadleir LG, Grinton BE, Lillywhite LM, Carney PW, Harvey AS, Berkovic SF, Scheffer IE. Clinical genetic studies in benign childhood epilepsy with centrotemporal spikes. Epilepsia. 2012 Feb:53(2):319-24. doi: 10.1111/j.1528-1167.2011.03368.x. Epub 2012 Jan 5 [PubMed PMID: 22220564]
Neubauer BA. The genetics of rolandic epilepsy. Epileptic disorders : international epilepsy journal with videotape. 2000:2 Suppl 1():S67-8 [PubMed PMID: 11231229]
Bali B, Kull LL, Strug LJ, Clarke T, Murphy PL, Akman CI, Greenberg DA, Pal DK. Autosomal dominant inheritance of centrotemporal sharp waves in rolandic epilepsy families. Epilepsia. 2007 Dec:48(12):2266-72 [PubMed PMID: 17662063]
Neubauer BA, Fiedler B, Himmelein B, Kämpfer F, Lässker U, Schwabe G, Spanier I, Tams D, Bretscher C, Moldenhauer K, Kurlemann G, Weise S, Tedroff K, Eeg-Olofsson O, Wadelius C, Stephani U. Centrotemporal spikes in families with rolandic epilepsy: linkage to chromosome 15q14. Neurology. 1998 Dec:51(6):1608-12 [PubMed PMID: 9855510]
Neubauer BA, Waldegger S, Heinzinger J, Hahn A, Kurlemann G, Fiedler B, Eberhard F, Muhle H, Stephani U, Garkisch S, Eeg-Olofsson O, Müller U, Sander T. KCNQ2 and KCNQ3 mutations contribute to different idiopathic epilepsy syndromes. Neurology. 2008 Jul 15:71(3):177-83. doi: 10.1212/01.wnl.0000317090.92185.ec. Epub [PubMed PMID: 18625963]
Level 3 (low-level) evidenceStrug LJ, Clarke T, Chiang T, Chien M, Baskurt Z, Li W, Dorfman R, Bali B, Wirrell E, Kugler SL, Mandelbaum DE, Wolf SM, McGoldrick P, Hardison H, Novotny EJ, Ju J, Greenberg DA, Russo JJ, Pal DK. Centrotemporal sharp wave EEG trait in rolandic epilepsy maps to Elongator Protein Complex 4 (ELP4). European journal of human genetics : EJHG. 2009 Sep:17(9):1171-81. doi: 10.1038/ejhg.2008.267. Epub 2009 Jan 28 [PubMed PMID: 19172991]
Lemke JR, Lal D, Reinthaler EM, Steiner I, Nothnagel M, Alber M, Geider K, Laube B, Schwake M, Finsterwalder K, Franke A, Schilhabel M, Jähn JA, Muhle H, Boor R, Van Paesschen W, Caraballo R, Fejerman N, Weckhuysen S, De Jonghe P, Larsen J, Møller RS, Hjalgrim H, Addis L, Tang S, Hughes E, Pal DK, Veri K, Vaher U, Talvik T, Dimova P, Guerrero López R, Serratosa JM, Linnankivi T, Lehesjoki AE, Ruf S, Wolff M, Buerki S, Wohlrab G, Kroell J, Datta AN, Fiedler B, Kurlemann G, Kluger G, Hahn A, Haberlandt DE, Kutzer C, Sperner J, Becker F, Weber YG, Feucht M, Steinböck H, Neophythou B, Ronen GM, Gruber-Sedlmayr U, Geldner J, Harvey RJ, Hoffmann P, Herms S, Altmüller J, Toliat MR, Thiele H, Nürnberg P, Wilhelm C, Stephani U, Helbig I, Lerche H, Zimprich F, Neubauer BA, Biskup S, von Spiczak S. Mutations in GRIN2A cause idiopathic focal epilepsy with rolandic spikes. Nature genetics. 2013 Sep:45(9):1067-72. doi: 10.1038/ng.2728. Epub 2013 Aug 11 [PubMed PMID: 23933819]
Kramer U, Zelnik N, Lerman-Sagie T, Shahar E. Benign childhood epilepsy with centrotemporal spikes: clinical characteristics and identification of patients at risk for multiple seizures. Journal of child neurology. 2002 Jan:17(1):17-9 [PubMed PMID: 11913563]
Level 2 (mid-level) evidenceKramer U, Nevo Y, Neufeld MY, Fatal A, Leitner Y, Harel S. Epidemiology of epilepsy in childhood: a cohort of 440 consecutive patients. Pediatric neurology. 1998 Jan:18(1):46-50 [PubMed PMID: 9492091]
Level 2 (mid-level) evidenceBouma PA, Bovenkerk AC, Westendorp RG, Brouwer OF. The course of benign partial epilepsy of childhood with centrotemporal spikes: a meta-analysis. Neurology. 1997 Feb:48(2):430-7 [PubMed PMID: 9040734]
Level 2 (mid-level) evidenceBerg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia. 1999 Apr:40(4):445-52 [PubMed PMID: 10219270]
Level 2 (mid-level) evidenceLoiseau P, Beaussart M. The seizures of benign childhood epilepsy with Rolandic paroxysmal discharges. Epilepsia. 1973 Dec:14(4):381-9 [PubMed PMID: 4521094]
Gregory DL, Wong PK. Topographical analysis of the centrotemporal discharges in benign rolandic epilepsy of childhood. Epilepsia. 1984 Dec:25(6):705-11 [PubMed PMID: 6510378]
Beaussart M. Benign epilepsy of children with Rolandic (centro-temporal) paroxysmal foci. A clinical entity. Study of 221 cases. Epilepsia. 1972 Dec:13(6):795-811 [PubMed PMID: 4509173]
Level 3 (low-level) evidenceLombroso CT. Sylvian seizures and midtemporal spike foci in children. Archives of neurology. 1967 Jul:17(1):52-9 [PubMed PMID: 6026172]
Lerman P, Kivity S. Benign focal epilepsy of childhood. A follow-up study of 100 recovered patients. Archives of neurology. 1975 Apr:32(4):261-4 [PubMed PMID: 804895]
Level 2 (mid-level) evidenceGregory DL, Farrell K, Wong PK. Partial status epilepticus in benign childhood epilepsy with centrotemporal spikes: are independent right and left seizures a risk factor? Epilepsia. 2002 Aug:43(8):936-40 [PubMed PMID: 12181016]
Level 3 (low-level) evidenceBaglietto MG, Battaglia FM, Nobili L, Tortorelli S, De Negri E, Calevo MG, Veneselli E, De Negri M. Neuropsychological disorders related to interictal epileptic discharges during sleep in benign epilepsy of childhood with centrotemporal or Rolandic spikes. Developmental medicine and child neurology. 2001 Jun:43(6):407-12 [PubMed PMID: 11409830]
Level 2 (mid-level) evidenceVannest J, Tenney JR, Gelineau-Morel R, Maloney T, Glauser TA. Cognitive and behavioral outcomes in benign childhood epilepsy with centrotemporal spikes. Epilepsy & behavior : E&B. 2015 Apr:45():85-91. doi: 10.1016/j.yebeh.2015.01.041. Epub 2015 Mar 13 [PubMed PMID: 25775975]
Northcott E, Connolly AM, Berroya A, Sabaz M, McIntyre J, Christie J, Taylor A, Batchelor J, Bleasel AF, Lawson JA, Bye AM. The neuropsychological and language profile of children with benign rolandic epilepsy. Epilepsia. 2005 Jun:46(6):924-30 [PubMed PMID: 15946332]
Level 2 (mid-level) evidenceVago C, Bulgheroni S, Franceschetti S, Usilla A, Riva D. Memory performance on the California Verbal Learning Test of children with benign childhood epilepsy with centrotemporal spikes. Epilepsy & behavior : E&B. 2008 Nov:13(4):600-6. doi: 10.1016/j.yebeh.2008.07.003. Epub 2008 Aug 15 [PubMed PMID: 18655847]
Level 2 (mid-level) evidenceBlom S, Heijbel J. Benign epilepsy of children with centro-temporal EEG foci. Discharge rate during sleep. Epilepsia. 1975 Mar:16(1):133-40 [PubMed PMID: 1122897]
Peters JM, Camfield CS, Camfield PR. Population study of benign rolandic epilepsy: is treatment needed? Neurology. 2001 Aug 14:57(3):537-9 [PubMed PMID: 11502931]
Hughes JR. Benign epilepsy of childhood with centrotemporal spikes (BECTS): to treat or not to treat, that is the question. Epilepsy & behavior : E&B. 2010 Nov:19(3):197-203. doi: 10.1016/j.yebeh.2010.07.018. Epub 2010 Aug 24 [PubMed PMID: 20797913]
Gastaut H. A new type of epilepsy: benign partial epilepsy of childhood with occipital spike-waves. Clinical EEG (electroencephalography). 1982 Jan:13(1):13-22 [PubMed PMID: 6802526]
Level 3 (low-level) evidenceBello-Espinosa LE, Roberts SL. Levetiracetam for benign epilepsy of childhood with centrotemporal spikes-three cases. Seizure. 2003 Apr:12(3):157-9 [PubMed PMID: 12651081]
Level 3 (low-level) evidenceLoiseau P, Duché B, Cordova S, Dartigues JF, Cohadon S. Prognosis of benign childhood epilepsy with centrotemporal spikes: a follow-up study of 168 patients. Epilepsia. 1988 May-Jun:29(3):229-35 [PubMed PMID: 3371279]
Level 3 (low-level) evidenceSmith AB, Bajomo O, Pal DK. A meta-analysis of literacy and language in children with rolandic epilepsy. Developmental medicine and child neurology. 2015 Nov:57(11):1019-26. doi: 10.1111/dmcn.12856. Epub 2015 Jul 28 [PubMed PMID: 26219529]
Level 1 (high-level) evidenceLindgren S, Kihlgren M, Melin L, Croona C, Lundberg S, Eeg-Olofsson O. Development of cognitive functions in children with rolandic epilepsy. Epilepsy & behavior : E&B. 2004 Dec:5(6):903-10 [PubMed PMID: 15582839]
Level 2 (mid-level) evidence