Introduction
Barrett esophagus is a premalignant condition characterized by salmon-colored mucosa extending at least 1 cm proximal to the gastroesophageal junction, with biopsies showing columnar epithelium and goblet cells indicative of intestinal metaplasia.[1] Most cases are acquired, with the precipitant usually being long-standing gastroesophageal reflux (GERD). Barrett esophagus can be found in 5% to 12% of patients with chronic symptoms of GERD.[2] Some families have a rare but increased incidence of developing Barrett esophagus through autosomal dominant inheritance of certain susceptibility alleles, known as the familial Barrett esophagus phenotype.
The condition predisposes patients to developing dysplasia and esophageal adenocarcinoma, a cancer with high morbidity and mortality that has been increasing in incidence over the past 50 years.[2] Surveillance programs have been developed to aid management decisions based on the presence of nondysplastic Barrett esophagus; indeterminate, low- and high-grade dysplasia; or invasive adenocarcinoma. These protocols continue to change as new studies and data are evaluated and included in newer guidelines. Specific indications for various endoscopic and surgical therapies have recently changed. Advances in endoscopic eradication technology and further studies for patient risk stratification are research areas of interest with the potential to affect future guidelines for diagnosing and managing Barrett esophagus cases.[3][4][5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Most patients with Barrett esophagus have a known history of chronic GERD, the reflux of gastric acid and bile through the lower esophageal sphincter and into the distal esophagus. The stomach lining is a mucinous columnar epithelium that can withstand the acidic environment required for digestion, and the esophagus is lined by a squamous epithelium; as a response to the acidic irritant, the squamous epithelium becomes inflamed. Continued exposure to acids leads to persistent inflammation and a columnar metaplastic reaction with the eventual development of an intestinal-type phenotype characterized by goblet cells.[6]
Why Barrett esophagus develops in some patients with GERD and not in others remains unclear. However, additional risk factors for developing Barrett esophagus include the following:
- Male genotype
- Non-Hispanic White race
- Age older than 50 years
- Central obesity
- Past or present smoking history
- Family history of either Barrett esophagus or esophageal adenocarcinoma in a first-degree relative
- Genetics: inconclusive evidence that specific genetic mutations may increase the risk of Barrett esophagus
Patients with chronic reflux and at least 3 of these other risk factors are considered to be at high risk and should undergo screening endoscopy.[2]
Epidemiology
Barrett esophagus is usually but not always found in patients with GERD. The prevalence is 0.8% in the general population, 3% in patients with GERD, 12.2% with GERD plus any other risk factor, 23.4% with a family history of Barrett esophagus or esophageal adenocarcinoma, 6.1% in individuals older than 50, 1.9% in people with obesity, and 6.8% in men. The prevalence of Barrett esophagus increases linearly with more risk factors, with each additional risk factor adding 1.2%.[7] The condition is the only known endoscopically identifiable precursor lesion for esophageal adenocarcinoma. Both Barrett esophagus and adenocarcinoma are much more common in men than in women, by ratios of 2:1 and 9:1, respectively.
The overall incidence of GERD, Barrett esophagus, and esophageal adenocarcinoma has been increasing significantly over the last 3 to 5 decades, partially attributable to increased numbers of endoscopies. However, fewer than 5% of patients with Barrett esophagus will develop esophageal adenocarcinoma. Study results indicate the absolute annual risk of adenocarcinoma in nondysplastic Barrett esophagus is 0.1% to 0.5% per year, a highly variable 1% to 43% per year for low-grade dysplasia, and 23% to 60% per year for high-grade dysplasia. A greater extent of dysplasia has a significantly higher risk of cancer, as well as the presence of an endoscopic abnormality, such as a nodule or mass.[8][9]
Pathophysiology
The exact pathogenesis of Barrett esophagus remains to be elucidated; however, it is believed to be a response to long-standing GERD. Some progress has been made in determining the molecular alterations and primary cell types involved. Study results have indicated that exposure to acid induces the squamous epithelial cells to secrete inflammatory cytokines such as interleukin 8 and interleukin 1b, which act to mediate the inflammatory response and signal T lymphocytes and neutrophils to migrate into the epithelium. Bile acids, specifically, have been shown to upregulate CDX2, the intestinal differentiation factor, and MUC2, the goblet cell-specific gene, within normal columnar and esophageal cancer cell lines.
Up to 90% of affected patients have a detectable clonal aberration of the p16 tumor suppressor gene. CDX2 and TP53 mutations are early molecular alterations found in metaplastic columnar epithelium even before morphologic dysplasia is recognized. However, immunohistochemical staining to evaluate these markers is not currently recommended in routine diagnostic cases of Barrett esophagus.
Results from a recent study described a population of possible embryonic stem cells at the squamocolumnar junction. In contrast, other studies suggest that stem cells derived from undifferentiated mesenchymal cells in the lamina propria of the esophagus or the bone marrow may be the cell of origin for Barrett esophagus. Columnar epithelium develops first in the esophagus, possibly beginning as gastric-type mucinous epithelium, reprogramming to intestinal differentiation and, ultimately, goblet cells.[10] There is evidence of a type of epithelium known as multilayered epithelium, which shows combined squamous and columnar features, likely representing an intermediate phase within the metaplastic reaction. The multilayered epithelium is strongly associated with reflux esophagitis and expresses a mucin and cytokeratin profile similar to Barrett esophagus columnar epithelium and intestinal transcription factors. Further studies are needed to understand the complete mechanism of pathogenesis.[6][5]
Histopathology
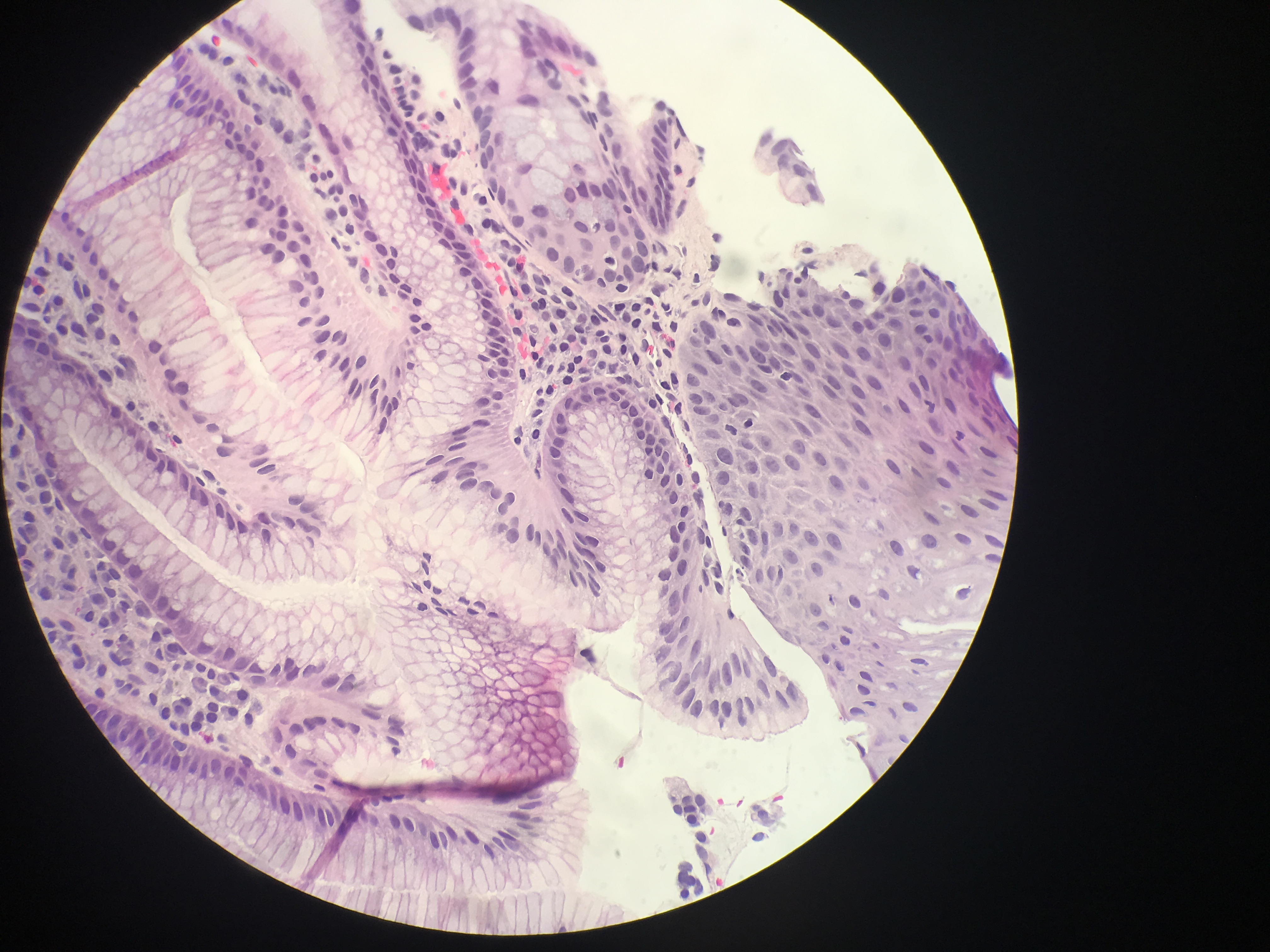
In Barrett esophagus, the normal esophageal squamous epithelium is replaced by intestinal-type mucosa characterized by columnar epithelium and usually with goblet cells (see Image. Barrett Esophagus). Nondysplastic Barrett epithelium may demonstrate nuclear enlargement and stratification in the basal glands, but the surface is mature. Dysplasia is identified by neoplasia limited to the basement membrane. Characteristic pathologic features include:
- Indefinite dysplasia: This type of neoplasia cannot be described as gastric, intestinal, or serrated differentiation. This terminology may be chosen if there is extensive regenerative change so much that the atypia present slightly overlaps with low-grade dysplasia. Still, unequivocal pathologic evidence of dysplasia is not found.
- Low-grade intestinal dysplasia: Goblet cells with enlargement and elongation of nuclei, increased numbers of lymphocytes, an increased nuclear-to-cytoplasmic ratio, and nuclear stratification involving the basal half of cellular cytoplasm characterize this type of dysplasia. Increased mitoses are mostly limited to the crypts, with few, if any, atypical mitoses.
- High-grade intestinal dysplasia: This type of dysplasia includes a greater degree of nuclear enlargement and number of lymphocytes; nuclear stratification of the full thickness of the mucosa with nuclear pleomorphism, irregular nuclear shapes, and significant loss of nuclear polarity; mitoses are seen on the surface with atypical mitoses.[10]
- Intramucosal carcinoma: In this carcinoma, lamina propria is invaded by individual neoplastic cells with no connection to the crypts, sheeting out of malignant cells, irregular and angulated glands located in the lamina propria or muscularis mucosae—a complex anastomosing pattern of glands within the lamina propria, and/or back-to-back glands or cells in an irregular architectural pattern.[11][12]
Gastric metaplasia of the distal esophagus is distinguished from intestinal metaplasia, and the risk of esophageal adenocarcinoma is much lower with gastric metaplasia than with intestinal metaplasia. There is uncertainty about whether gastric metaplasia increases the risk of esophageal adenocarcinoma significantly or not. This concept is supported by the evidence that intestinal metaplasia demonstrates certain genomic hallmarks of esophageal adenocarcinoma, which is much less significant than gastric metaplasia in the absence of intestinal metaplasia.[13]
History and Physical
Most patients with Barrett esophagus will exhibit symptoms of GERD, such as heartburn (retrosternal burning), especially after eating. Acid regurgitation is another likely symptom. Other possible symptoms, though less common, include dysphagia, a sore throat, hoarseness, chest pain, chronic cough, melena, or weight loss. Some patients are asymptomatic. Typically, patients will have a chronic history of GERD symptoms with no specific physical examination findings.
Evaluation
In 2022, the American College of Gastroenterology (ACG) published new clinical guidelines for diagnosing and managing Barrett esophagus. No randomized controlled trials have confirmed that screening patients with Barrett esophagus reduces mortality from esophageal adenocarcinoma; screening, endoscopic surveillance, and endoscopic eradication therapy have been shown to reduce the incidence of esophageal adenocarcinoma and improve early detection. The ACG now recommends screening for Barrett esophagus in people with chronic symptoms of GERD who also have at least 3 additional risk factors, including male genotype, age older than 50, White race, past or present tobacco smoking, central obesity, or a history of Barrett esophagus or esophageal adenocarcinoma in a first-degree relative.[3][14][15][16]
Esophagogastroduodenoscopy with forceps biopsies is the gold-standard diagnostic test. Wide-area transepithelial sampling with brushing of the entire surface of the Barrett epithelium may add to the yield of standard endoscopy with forceps biopsies; this technique has not yet been incorporated into the ACG guideline. High-definition white light and chromoendoscopy, using topical acetic acid or electronic chromoendoscopy, increase sensitivity and specificity for identifying dysplasia and adenocarcinoma.[17] Artificial intelligence is being evaluated in the United States and the Netherlands to develop a system that will identify neoplasia and the most abnormal areas as the optimal sites for biopsy.[18] However, the cost of sedated endoscopy and the small risk of complications have raised interest in developing alternative diagnostic testing. Such alternatives include unsedated transnasal endoscopy that non-clinicians can perform and the use of one of several commercially available swallowable capsule sponges combined with a biomarker such as a methylated deoxynucleic acid marker or trefoil factor 3.
In cases of Barrett esophagus, the pathologist must determine whether dysplasia or carcinoma is present. Study results suggest that the extent of dysplasia also correlates with the risk of cancer, so it may be valuable for pathologists to comment not only on the presence of but also on the extent of dysplasia (such as focal or diffuse). Sometimes, there may be a specific concern for dysplasia if an area of nodularity or ulceration is noted during endoscopy.
Barrett esophagus-related dysplasia is often flat and not apparent on endoscopy. The major histologic pattern of dysplasia is intestinal, therefore resembling an adenoma of the colon. Non-intestinal dysplasia also exists, including foveolar and serrated patterns; validated criteria for classification as either low- or high-grade dysplasia only exist for intestinal-type dysplasia. The distinction between high-grade dysplasia and intramucosal carcinoma suffers from a high degree of interobserver variability.[11][12]
The diagnosis of Barrett esophagus requires the endoscopic appearance of at least a 1-cm length of "salmon-pink" columnar metaplasia proximal to the esophagogastric junction, defined as the top of the gastric folds, with confirmation of intestinal metaplasia with goblet cells on biopsy evaluation. The British Society of Gastroenterology and the GERD Society Study Committee in Japan do not require the presence of goblet cells to diagnose Barrett esophagus, and they base the diagnosis solely on columnar metaplasia. Due to the controversy over the significance of goblet cells, another alternative classification has been proposed, which allows the pathologist to state that there is columnar metaplasia and then further specify whether goblet cells are present or absent.
To maximize the possibility of finding Barrett esophagus, dysplasia, and adenocarcinoma, the ACG recommends a minimum of 8 biopsies, 2 from each quadrant, at 2 cm intervals for shorter segments of Barrett mucosa.[19][20] The Seattle protocol calls for segments greater than 4 cm long. The Seattle protocol involves taking biopsies of visible lesions, followed by 4-quadrant biopsies every 1 to 2 cm. Mixed results are seen when comparing the Seattle protocol to narrow-band imaging (NBI). Although NBI demonstrated endoscopically visible lesions and dysplasia better than simply utilizing the Seattle protocol, NBI has not yet been incorporated into current guidelines.[21]
The extent of Barrett esophagus is graded by the Prague criteria, specifying the circumferential length of Barrett epithelium at and above the top of the gastric folds (C) and the maximum extent (M) of Barrett mucosa. The M criterion includes finger-like projections of Barrett epithelium proximal to the circumferentially involved segment. Islands of intestinal metaplasia are not considered in the Prague criteria, even if they are proximal to what would otherwise be considered the full length of Barrett mucosa. If the circumferential length is 6 cm and the maximal length is 8 cm, then the Prague classification would describe this lesion as C6M8.[22][23]
Treatment / Management
Multiple study results have initially suggested that endoscopic surveillance of patients with Barrett esophagus is associated with decreased esophageal adenocarcinoma-related deaths; this was not confirmed when adjustments were made for lead and length time biases. Based on a systematic review of the literature and evaluation of the level of evidence, the ACG made several changes to the recommended management in its newest guidelines. High-definition white-light endoscopy is a novel endoscopic technology recommended for routine surveillance beyond electronic chromoendoscopy. When Barrett esophagus is found, the patient should be placed on long-term proton pump inhibitor therapy, regardless of the presence of reflux symptoms, due to evidence of a chemopreventive effect of proton pump inhibitors, where the risk of progression to neoplastic disease is reduced compared to no acid suppression or H2 blocker treatment.
Due to the significant impact on management, if Barrett esophagus is associated with dysplasia, the ACG emphasizes the importance of confirming any dysplasia diagnosis with a second expert pathologist with extensive experience in Barrett esophagus-related neoplasia. Surveillance protocols differ for the level of dysplasia seen as follows:
- No dysplasia: An endoscopy is recommended every 5 years if the Barrett segment is <3 cm long or every 3 years if the segment is at least 3 cm in length.
- Indefinite dysplasia: Guidelines recommend that endoscopy should be repeated in 6 months on twice-daily proton pump inhibitor therapy, with doses taken 30 to 45 minutes before a meal.[24] If the repeat endoscopy is unchanged, then the endoscopy should be repeated annually. If there is progression to low-grade dysplasia (LGD), then the LGD management guideline should be followed.[2]
- LGD: If endoscopic eradication therapy (EET) is declined or cannot be performed for any reason, endoscopy should be repeated in 6 months, then again 6 months later, and then annually if the assessment of dysplasia does not change. EET for LGD is the same as for high-grade dysplasia (HGD), as described below.
- HGD: If there is flat mucosa with HGD (no mucosal abnormality is visible), EET is done to eliminate the entire area of metaplasia and dysplasia. Esophagectomy is no longer the preferred initial approach to managing HGD or intramucosal cancer, as the procedure has a high risk of morbidity. However, it should be considered if intramucosal cancer has poor prognostic factors, such as lymphovascular invasion or poor differentiation, or if cancer involves the submucosa. Patients with significant comorbidities that limit their likely survival may not benefit from endoscopic therapies, with risks of the procedures being greater than potential gains.[2][25][26]
Endoscopic eradication therapy is used to treat LGD, HGD, and early-stage esophageal adenocarcinoma and is preferable to endoscopic surveillance and acid-suppressive medication alone.[27] These therapeutic techniques include:(A1)
- Radiofrequency ablation: Several (usually 3-4) applications of radiofrequency ablation are applied to the Barrett mucosa using devices that ablate 90°, 180°, or 360° of the affected area at a time. Complications of radiofrequency ablation include chest pain for up to 14 days, and less common but more serious complications include aspiration, bleeding, surface lacerations or deeper perforation, and strictures. This technique has effectively eradicated Barrett mucosa and dysplasia and is the preferred ablative modality for EET of flat dysplasia.[28]
- Cryotherapy: This can be performed using either liquid nitrogen cryospray or cryotherapy applied through an inflated balloon that compresses the Barrett segment.
- Hybrid argon plasma coagulation: This technique can be used for small islands of intestinal metaplasia. However, if used circumferentially, it has a relatively high rate of strictures.
- Endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) is used to resect nodules or masses, followed by ablation of the remaining Barrett esophagus mucosa. EMR is preferred for smaller and more superficial neoplasia. At the same time, ESD is needed for more extensive lesions if at least T1b a carcinoma invasion is highly suspected or if EMR has failed to resect the lesion and all neoplastic tissue completely. If there is only a small Barrett esophagus patch around the visible lesion, complete resection using EMR or ESD is preferable to later radiofrequency ablation, which requires multiple endoscopic sessions.
Endoscopic surveillance must be continued after complete eradication of intestinal metaplasia (CEIM) and complete eradication of neoplasia. Biopsies should be taken of any visible lesions randomly from the cardia and the distal 2 cm of the esophagus, where recurrences are most likely to occur.[29] Based on the most advanced histology found before EET, the recommended intervals are as follows:
- LGD: Repeat endoscopy 1 year after CEIM, then 2 years later, then at nondysplastic Barrett esophagus intervals (every 3 to 5 years) if there has been no recurrence of metaplasia or dysplasia.
- HGD: Repeat endoscopy at 3, 6, and 12 months following CEIM. If there has been no recurrence at that point, the endoscopy should be repeated annually.
- Intramucosal esophageal adenocarcinoma: Repeat endoscopy at 3, 6, and 12 months following CEIM. If there has been no recurrence of dysplasia or malignancy at that point, endoscopy should be repeated annually.
EET is more cost-effective than endoscopic surveillance.[30] When well-experienced endoscopists perform EET, intestinal metaplasia and neoplasia recurrence rates are lower.[31] If EET fails to achieve CEIM, management options include improving reflux control with a higher proton pump inhibitor dose, changing to a potassium-competitive acid blocker (P-CAB), fundoplication surgery, or repeating EET. However, P-CAB medications have yet to be tested for this indication.[32]
Many case series report short-term Barrett outcomes; there are very few studies on long-term outcomes. Even though there are several types of treatments for Barrett and GERD, there is no solid evidence to indicate which one is the best or most effective. With the availability of EET, more long-term studies are needed to determine the safety of these procedures and their effectiveness in preventing the progression of Barrett esophagus to a malignancy.[33](A1)
Differential Diagnosis
When symptoms are present (eg, GERD) the differential diagnosis of Barrett esophagus is based on endoscopic findings and includes the following:
- Esophageal mucosa with gastric metaplasia: This is identical in endoscopic appearance to Barrett esophagus (intestinal metaplasia), but biopsies show gastric fundic- or junctional-type epithelium instead. These types of gastric metaplasia are not risk factors for esophageal cancer.
- Acute gastritis
- Chronic gastritis with or without Helicobacter pylori infection
- Coronary artery atherosclerosis
- Reflux esophagitis
- Esophageal cancer
- Esophageal motility disorders
- Gallstones
Prognosis
The prognosis of Barrett esophagus largely depends on the degree of dysplasia present at the time of diagnosis and the effectiveness of surveillance and treatment strategies. While Barrett esophagus itself is a premalignant condition, the risk of progression to esophageal adenocarcinoma increases with the severity of dysplasia. Patients with nondysplastic Barrett esophagus have a relatively low risk of cancer progression, estimated at less than 0.5% per year. However, the presence of low- or high-grade dysplasia significantly raises this risk, with high-grade dysplasia carrying the highest potential for malignancy.
Early detection and regular surveillance, along with timely endoscopic or surgical intervention, can improve the prognosis by preventing the progression to esophageal adenocarcinoma, a cancer associated with high morbidity and mortality. Advances in endoscopic eradication techniques have further enhanced outcomes, offering less invasive options with curative potential for patients with dysplastic Barrett esophagus. Therefore, the overall prognosis is favorable when Barrett esophagus is closely monitored and managed according to current guidelines. Average life expectancy with Barrett esophagus should be anticipated in the absence of malignancy or other life-threatening conditions.
Complications
Untreated Barrett esophagus may progress into adenocarcinoma. However, the rate of progression is prolonged, and more than 95% of patients with Barrett esophagus will never develop malignancy. The number of adenocarcinoma cases has steadily increased over the past several decades.[34] There is an increasing risk of malignancy if Barrett mucosa is associated with greater degrees of dysplasia and with longer lengths of the Barrett segment. Complications of reflux esophagitis, such as bleeding or ulceration, may be somewhat more likely with Barrett esophagus than without it. Still, strictures and aspiration are no more frequent with Barrett esophagus than without it.
Deterrence and Patient Education
Smoking and obesity are risk factors for esophageal adenocarcinoma, so smoking cessation and weight loss, if necessary, should be advised. The role of diet in the etiology of Barrett esophagus remains inconclusive, but it should be no different from dietary recommendations for patients with GERD. Following a low-fat and high-fiber diet is best.
Further recommendations include:
- Avoid fried or fatty foods, spicy foods, alcohol, caffeinated and carbonated beverages, chocolate, acidic juices, and tomato sauce.
- Have multiple small meals throughout the day rather than 2 or 3 large meals.
- Avoid eating or drinking for 4 hours before bedtime.
- Weight loss is beneficial in patients who are overweight.[35][36]
Pearls and Other Issues
Because not all patients will progress from Barrett esophagus to cancer, an essential area of research is the development of reliable risk-profiling methods to identify patients at higher risk and those who will benefit more from therapies. The use of biomarkers to risk-stratify patients is currently undergoing extensive research, with deoxyribonucleic acid content abnormalities and TP53 and CDKN2A alterations showing the most promise to date. Newer alternatives to endoscopy for screening are being assessed to reduce cost and risk, including transnasal endoscopy and capsule sponges. Other ongoing and future studies should add to our current knowledge base regarding Barrett esophagus and its best evaluation and management.
Enhancing Healthcare Team Outcomes
The incidence of adenocarcinoma of the esophagus is increasing in most Western nations, and this change is undoubtedly due to Barrett esophagus. The progression of Barrett esophagus to cancer is slow, but once cancer occurs, the prognosis is very poor. Over the years, multiple societies have developed guidelines for screening, surveillance, and managing Barrett esophagus.[15][37] Since most patients may have no symptoms or symptoms of reflux, suspicion of Barrett esophagus must always be increased, especially in high-risk populations. Unfortunately, many gastroenterologists and surgeons have been performing upper endoscopies without clear-cut evidence that this procedure can detect esophageal cancer early or prevent cancer. Unnecessary endoscopies also lead to high healthcare costs.
Thus, the recommendations are that one follow strict guidelines for surveillance based on patient risk factors rather than empirically performing endoscopy in all patients with chronic GERD. An interprofessional approach with a team of pathologists, thoracic surgeons, radiologists, gastroenterologists, and general surgeons will permit individualized care and the best results. The nurse's role is vital in educating the patient about reflux and ways to prevent it. The pharmacist should ensure that patients are adequately treated for reflux and have a referral to a gastroenterologist or primary care clinicians to manage therapy.[38][39] Pharmacists will verify all acid-reducing medication strategies (eg, proton pump inhibitors or H2-receptor blockers), verify dosing and drug interactions, counsel patients on administration and adverse events, and report back to the prescriber with any concerns.
A comprehensive care strategy for patients with Barrett esophagus involves implementing evidence-based guidelines for screening, surveillance, and treatment. This strategy should include risk assessment protocols, personalized treatment plans, and the use of the latest EET techniques. Proactive management and regular surveillance are crucial to preventing the progression of Barrett esophagus to esophageal adenocarcinoma.
By focusing on patient-centered care, health professionals can enhance patient outcomes and safety. This approach involves engaging patients in their care, respecting their preferences, and providing comprehensive education about their condition and treatment options. The multidisciplinary team works together to ensure that care is effective and aligned with the patient's goals and values, ultimately leading to better outcomes and a higher standard of patient safety.
Media
(Click Image to Enlarge)
References
Shaheen NJ, Falk GW, Iyer PG, Gerson LB, American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. The American journal of gastroenterology. 2016 Jan:111(1):30-50; quiz 51. doi: 10.1038/ajg.2015.322. Epub 2015 Nov 3 [PubMed PMID: 26526079]
Shaheen NJ, Falk GW, Iyer PG, Souza RF, Yadlapati RH, Sauer BG, Wani S. Diagnosis and Management of Barrett's Esophagus: An Updated ACG Guideline. The American journal of gastroenterology. 2022 Apr 1:117(4):559-587. doi: 10.14309/ajg.0000000000001680. Epub [PubMed PMID: 35354777]
Michopoulos S. Critical appraisal of guidelines for screening and surveillance of Barrett's esophagus. Annals of translational medicine. 2018 Jul:6(13):259. doi: 10.21037/atm.2018.05.09. Epub [PubMed PMID: 30094245]
Nowicki A, Kula Z, Świerszczyńska A. Barrett's esophagus and gland cancer - the experience of one center. Polski przeglad chirurgiczny. 2018 May 16:90(3):19-24. doi: 10.5604/01.3001.0011.8166. Epub [PubMed PMID: 30015321]
Dewan KR, Patowary BS, Bhattarai S, Shrestha G. Barrett's Esophagus in Patients with Gastroesophageal Reflux Disease. Journal of Nepal Health Research Council. 2018 Jul 3:16(2):144-148 [PubMed PMID: 29983427]
Inadomi J, Alastal H, Bonavina L, Gross S, Hunt RH, Mashimo H, di Pietro M, Rhee H, Shah M, Tolone S, Wang DH, Xie SH. Recent advances in Barrett's esophagus. Annals of the New York Academy of Sciences. 2018 Dec:1434(1):227-238. doi: 10.1111/nyas.13909. Epub 2018 Jul 5 [PubMed PMID: 29974975]
Level 3 (low-level) evidenceQumseya BJ, Bukannan A, Gendy S, Ahemd Y, Sultan S, Bain P, Gross SA, Iyer P, Wani S. Systematic review and meta-analysis of prevalence and risk factors for Barrett's esophagus. Gastrointestinal endoscopy. 2019 Nov:90(5):707-717.e1. doi: 10.1016/j.gie.2019.05.030. Epub 2019 May 29 [PubMed PMID: 31152737]
Level 1 (high-level) evidenceErőss B, Farkas N, Vincze Á, Tinusz B, Szapáry L, Garami A, Balaskó M, Sarlós P, Czopf L, Alizadeh H, Rakonczay Z Jr, Habon T, Hegyi P. Helicobacter pylori infection reduces the risk of Barrett's esophagus: A meta-analysis and systematic review. Helicobacter. 2018 Aug:23(4):e12504. doi: 10.1111/hel.12504. Epub 2018 Jun 25 [PubMed PMID: 29938864]
Level 1 (high-level) evidenceThrift AP. Barrett's Esophagus and Esophageal Adenocarcinoma: How Common Are They Really? Digestive diseases and sciences. 2018 Aug:63(8):1988-1996. doi: 10.1007/s10620-018-5068-6. Epub [PubMed PMID: 29671158]
Naini BV, Souza RF, Odze RD. Barrett's Esophagus: A Comprehensive and Contemporary Review for Pathologists. The American journal of surgical pathology. 2016 May:40(5):e45-66. doi: 10.1097/PAS.0000000000000598. Epub [PubMed PMID: 26813745]
Dunbar KB, Souza RF. Beyond Dysplasia Grade: The Role of Biomarkers in Stratifying Risk. Gastrointestinal endoscopy clinics of North America. 2017 Jul:27(3):447-459. doi: 10.1016/j.giec.2017.02.003. Epub [PubMed PMID: 28577766]
Ooi J, Wilson P, Walker G, Blaker P, DeMartino S, O'Donohue J, Reffitt D, Lanaspre E, Chang F, Meenan J, Dunn JM. Dedicated Barrett's surveillance sessions managed by trained endoscopists improve dysplasia detection rate. Endoscopy. 2017 Jun:49(6):524-528. doi: 10.1055/s-0043-103410. Epub 2017 Apr 11 [PubMed PMID: 28399610]
Black EL, Ococks E, Devonshire G, Ng AWT, O'Donovan M, Malhotra S, Tripathi M, Miremadi A, Freeman A, Coles H, Oesophageal Cancer Clinical and Molecular Stratification (OCCAMS) Consortium, Fitzgerald RC. Understanding the malignant potential of gastric metaplasia of the oesophagus and its relevance to Barrett's oesophagus surveillance: individual-level data analysis. Gut. 2024 Apr 5:73(5):729-740. doi: 10.1136/gutjnl-2023-330721. Epub 2024 Apr 5 [PubMed PMID: 37989565]
Level 3 (low-level) evidenceBellizzi AM, Hafezi-Bakhtiari S, Westerhoff M, Marginean EC, Riddell RH. Gastrointestinal pathologists' perspective on managing risk in the distal esophagus: convergence on a pragmatic approach. Annals of the New York Academy of Sciences. 2018 Dec:1434(1):35-45. doi: 10.1111/nyas.13680. Epub 2018 May 11 [PubMed PMID: 29749623]
Level 3 (low-level) evidenceClermont M, Falk GW. Clinical Guidelines Update on the Diagnosis and Management of Barrett's Esophagus. Digestive diseases and sciences. 2018 Aug:63(8):2122-2128. doi: 10.1007/s10620-018-5070-z. Epub [PubMed PMID: 29671159]
Muthusamy VR, Wani S, Gyawali CP, Komanduri S, CGIT Barrett’s Esophagus Consensus Conference Participants. AGA Clinical Practice Update on New Technology and Innovation for Surveillance and Screening in Barrett's Esophagus: Expert Review. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2022 Dec:20(12):2696-2706.e1. doi: 10.1016/j.cgh.2022.06.003. Epub 2022 Jul 3 [PubMed PMID: 35788412]
Level 3 (low-level) evidenceColetta M, Sami SS, Nachiappan A, Fraquelli M, Casazza G, Ragunath K. Acetic acid chromoendoscopy for the diagnosis of early neoplasia and specialized intestinal metaplasia in Barrett's esophagus: a meta-analysis. Gastrointestinal endoscopy. 2016 Jan:83(1):57-67.e1. doi: 10.1016/j.gie.2015.07.023. Epub 2015 Sep 12 [PubMed PMID: 26371851]
Level 1 (high-level) evidencede Groof AJ, Struyvenberg MR, van der Putten J, van der Sommen F, Fockens KN, Curvers WL, Zinger S, Pouw RE, Coron E, Baldaque-Silva F, Pech O, Weusten B, Meining A, Neuhaus H, Bisschops R, Dent J, Schoon EJ, de With PH, Bergman JJ. Deep-Learning System Detects Neoplasia in Patients With Barrett's Esophagus With Higher Accuracy Than Endoscopists in a Multistep Training and Validation Study With Benchmarking. Gastroenterology. 2020 Mar:158(4):915-929.e4. doi: 10.1053/j.gastro.2019.11.030. Epub 2019 Nov 22 [PubMed PMID: 31759929]
Level 1 (high-level) evidenceAkın H, Aydın Y. How should we describe, diagnose and observe the Barrett's esophagus? The Turkish journal of gastroenterology : the official journal of Turkish Society of Gastroenterology. 2017 Dec:28(Suppl 1):S26-S30. doi: 10.5152/tjg.2017.08. Epub [PubMed PMID: 29199163]
Beg S, Ragunath K, Wyman A, Banks M, Trudgill N, Pritchard DM, Riley S, Anderson J, Griffiths H, Bhandari P, Kaye P, Veitch A. Quality standards in upper gastrointestinal endoscopy: a position statement of the British Society of Gastroenterology (BSG) and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS). Gut. 2017 Nov:66(11):1886-1899. doi: 10.1136/gutjnl-2017-314109. Epub 2017 Aug 18 [PubMed PMID: 28821598]
Level 2 (mid-level) evidenceSharma P, Hawes RH, Bansal A, Gupta N, Curvers W, Rastogi A, Singh M, Hall M, Mathur SC, Wani SB, Hoffman B, Gaddam S, Fockens P, Bergman JJ. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett's oesophagus: a prospective, international, randomised controlled trial. Gut. 2013 Jan:62(1):15-21. doi: 10.1136/gutjnl-2011-300962. Epub 2012 Feb 7 [PubMed PMID: 22315471]
Level 1 (high-level) evidenceVahabzadeh B, Seetharam AB, Cook MB, Wani S, Rastogi A, Bansal A, Early DS, Sharma P. Validation of the Prague C & M criteria for the endoscopic grading of Barrett's esophagus by gastroenterology trainees: a multicenter study. Gastrointestinal endoscopy. 2012 Feb:75(2):236-41. doi: 10.1016/j.gie.2011.09.017. Epub [PubMed PMID: 22248595]
Level 1 (high-level) evidenceSharma P, Dent J, Armstrong D, Bergman JJ, Gossner L, Hoshihara Y, Jankowski JA, Junghard O, Lundell L, Tytgat GN, Vieth M. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C & M criteria. Gastroenterology. 2006 Nov:131(5):1392-9 [PubMed PMID: 17101315]
Level 1 (high-level) evidenceFitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, Patel P, Kaye PV, Sanders S, O'Donovan M, Bird-Lieberman E, Bhandari P, Jankowski JA, Attwood S, Parsons SL, Loft D, Lagergren J, Moayyedi P, Lyratzopoulos G, de Caestecker J, British Society of Gastroenterology. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2014 Jan:63(1):7-42. doi: 10.1136/gutjnl-2013-305372. Epub 2013 Oct 28 [PubMed PMID: 24165758]
Markar SR, Arhi C, Leusink A, Vidal-Diez A, Karthikesalingam A, Darzi A, Lagergren J, Hanna GB. The Influence of Antireflux Surgery on Esophageal Cancer Risk in England: National Population-based Cohort Study. Annals of surgery. 2018 Nov:268(5):861-867. doi: 10.1097/SLA.0000000000002890. Epub [PubMed PMID: 30048317]
Ali Khan M, Howden CW. The Role of Proton Pump Inhibitors in the Management of Upper Gastrointestinal Disorders. Gastroenterology & hepatology. 2018 Mar:14(3):169-175 [PubMed PMID: 29928161]
Rubenstein JH, Sawas T, Wani S, Eluri S, Singh S, Chandar AK, Perumpail RB, Inadomi JM, Thrift AP, Piscoya A, Sultan S, Singh S, Katzka D, Davitkov P. AGA Clinical Practice Guideline on Endoscopic Eradication Therapy of Barrett's Esophagus and Related Neoplasia. Gastroenterology. 2024 Jun:166(6):1020-1055. doi: 10.1053/j.gastro.2024.03.019. Epub [PubMed PMID: 38763697]
Level 1 (high-level) evidenceShaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, Galanko JA, Bronner MP, Goldblum JR, Bennett AE, Jobe BA, Eisen GM, Fennerty MB, Hunter JG, Fleischer DE, Sharma VK, Hawes RH, Hoffman BJ, Rothstein RI, Gordon SR, Mashimo H, Chang KJ, Muthusamy VR, Edmundowicz SA, Spechler SJ, Siddiqui AA, Souza RF, Infantolino A, Falk GW, Kimmey MB, Madanick RD, Chak A, Lightdale CJ. Radiofrequency ablation in Barrett's esophagus with dysplasia. The New England journal of medicine. 2009 May 28:360(22):2277-88. doi: 10.1056/NEJMoa0808145. Epub [PubMed PMID: 19474425]
Omar M, Thaker AM, Wani S, Simon V, Ezekwe E, Boniface M, Edmundowicz S, Obuch J, Cinnor B, Brauer BC, Wood M, Early DS, Lang GD, Mullady D, Hollander T, Kushnir V, Komanduri S, Muthusamy VR. Anatomic location of Barrett's esophagus recurrence after endoscopic eradication therapy: development of a simplified surveillance biopsy strategy. Gastrointestinal endoscopy. 2019 Sep:90(3):395-403. doi: 10.1016/j.gie.2019.04.216. Epub 2019 Apr 17 [PubMed PMID: 31004598]
Rubenstein JH, Inadomi JM. Cost-Effectiveness of Screening, Surveillance, and Endoscopic Eradication Therapies for Managing the Burden of Esophageal Adenocarcinoma. Gastrointestinal endoscopy clinics of North America. 2021 Jan:31(1):77-90. doi: 10.1016/j.giec.2020.08.005. Epub 2020 Oct 26 [PubMed PMID: 33213801]
Tan MC, Kanthasamy KA, Yeh AG, Kil D, Pompeii L, Yu X, El-Serag HB, Thrift AP. Factors Associated With Recurrence of Barrett's Esophagus After Radiofrequency Ablation. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2019 Jan:17(1):65-72.e5. doi: 10.1016/j.cgh.2018.05.042. Epub 2018 Jun 11 [PubMed PMID: 29902646]
Rawla P, Sunkara T, Ofosu A, Gaduputi V. Potassium-competitive acid blockers - are they the next generation of proton pump inhibitors? World journal of gastrointestinal pharmacology and therapeutics. 2018 Dec 13:9(7):63-68. doi: 10.4292/wjgpt.v9.i7.63. Epub [PubMed PMID: 30595950]
Codipilly DC, Chandar AK, Singh S, Wani S, Shaheen NJ, Inadomi JM, Chak A, Iyer PG. The Effect of Endoscopic Surveillance in Patients With Barrett's Esophagus: A Systematic Review and Meta-analysis. Gastroenterology. 2018 Jun:154(8):2068-2086.e5. doi: 10.1053/j.gastro.2018.02.022. Epub 2018 Feb 16 [PubMed PMID: 29458154]
Level 1 (high-level) evidenceBresalier RS. Chemoprevention of Barrett's Esophagus and Esophageal Adenocarcinoma. Digestive diseases and sciences. 2018 Aug:63(8):2155-2162. doi: 10.1007/s10620-018-5149-6. Epub [PubMed PMID: 29948566]
Meining A, Classen M. The role of diet and lifestyle measures in the pathogenesis and treatment of gastroesophageal reflux disease. The American journal of gastroenterology. 2000 Oct:95(10):2692-7 [PubMed PMID: 11051337]
Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA Jr. Body-mass index and symptoms of gastroesophageal reflux in women. The New England journal of medicine. 2006 Jun 1:354(22):2340-8 [PubMed PMID: 16738270]
Level 2 (mid-level) evidenceFreedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology. 2017 Mar:152(4):706-715. doi: 10.1053/j.gastro.2017.01.031. Epub [PubMed PMID: 28257716]
Schmidt HM, Mohiuddin K, Bodnar AM, El Lakis M, Kaplan S, Irani S, Gan I, Ross A, Low DE. Multidisciplinary treatment of T1a adenocarcinoma in Barrett's esophagus: contemporary comparison of endoscopic and surgical treatment in physiologically fit patients. Surgical endoscopy. 2016 Aug:30(8):3391-401. doi: 10.1007/s00464-015-4621-z. Epub 2015 Nov 5 [PubMed PMID: 26541725]
Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N. Surveillance of Barrett's oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling. Health technology assessment (Winchester, England). 2006 Mar:10(8):1-142, iii-iv [PubMed PMID: 16545207]
Level 1 (high-level) evidence