Introduction
B cells or B lymphocytes (bursa-derived cells) are essential components of adaptive immune response, primarily responsible for humoral immunity in mammals. B-cell production in humans is a lifelong process that starts in the fetal liver, intrauterine, and bone marrow after birth.[1] B-cell development originates from hematopoietic stem cells and involves several stages of early differentiation, progressing through maturation, antigen interaction, and antibody synthesis.[2] Through this process, B cells acquire 2 essential features of adaptive immunity—the ability to distinguish between self and non-self (recognizing foreign antigens rather than self-antigens) and the ability to form a memory of previous antigen encounters. This memory allows for a more effective and rapid response upon subsequent interactions with the same antigens.[3]
B cells acquire their name from early experiments on chickens that demonstrate the synthesis of antibodies. Max Cooper found that antibody production in chickens required an organ called the bursa of Fabricius in the 1960s. Surgical removal of the bursa inhibited antibody production. The cells responsible for antibody production were called bursa-derived or B cells. In contrast to chicken, B-cell development in humans occurs predominantly in the bone marrow.[4] Many B-cell differentiation pathways demonstrate characteristic specific surface markers (CD markers) and immunoglobulin gene arrangements. In addition, developmental checkpoints exist along the pathways to determine whether a cell follows the normal or alternative pathways, resulting in cell death.[5]
B cells are predominantly found in the cortex of lymph nodes and form part of the B-cell follicles, which can be classified into 2 types. Primary follicles are small and uniform, containing naive or resting B cells. In contrast, secondary follicles are characterized by the presence of a germinal center, where B cells undergo activation, proliferation, and differentiation into plasma cells or memory B cells.[6] The germinal center consists of a light zone containing centrocytes and a dark zone containing centroblasts.[7] In the spleen, B cells are located in the white pulp, specifically in periarteriolar lymphoid sheaths and follicles, similar to those observed in lymph nodes.[8] In addition, B cells are prominent in mucosa-associated lymphoid tissue, including in the tonsils and Peyer's patches, where they also form germinal centers.[9]
Structure
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure
Few studies have focused on the composition and internal environment of B lymphocytes; consequently, this section focuses on B lymphocyte external surface and associated structures. B cells have a plasma membrane composed of equal parts of protein glycosphingolipids and carbohydrates. The following paragraphs describe the most critical external B-cell structures responsible for cell activation, antigen recognition, and signal transduction.[10]
Stage-Specific Markers
Different molecules are presented on B cells in various stages of development or maturation and activation. For example, CD10 is expressed in first-stage cells in B-cell lineage–like pro-B, pre-B cell, and germinal centers cells. CD19 and C20 are expressed in all cells of the B-cell lineage except plasma cells. However, CD27 is exclusively expressed on memory and plasma cells. In addition, B-1 cells are characterized by the CD5 molecule.[11]
Antigen-Binding Molecules (Membrane Immunoglobulin)
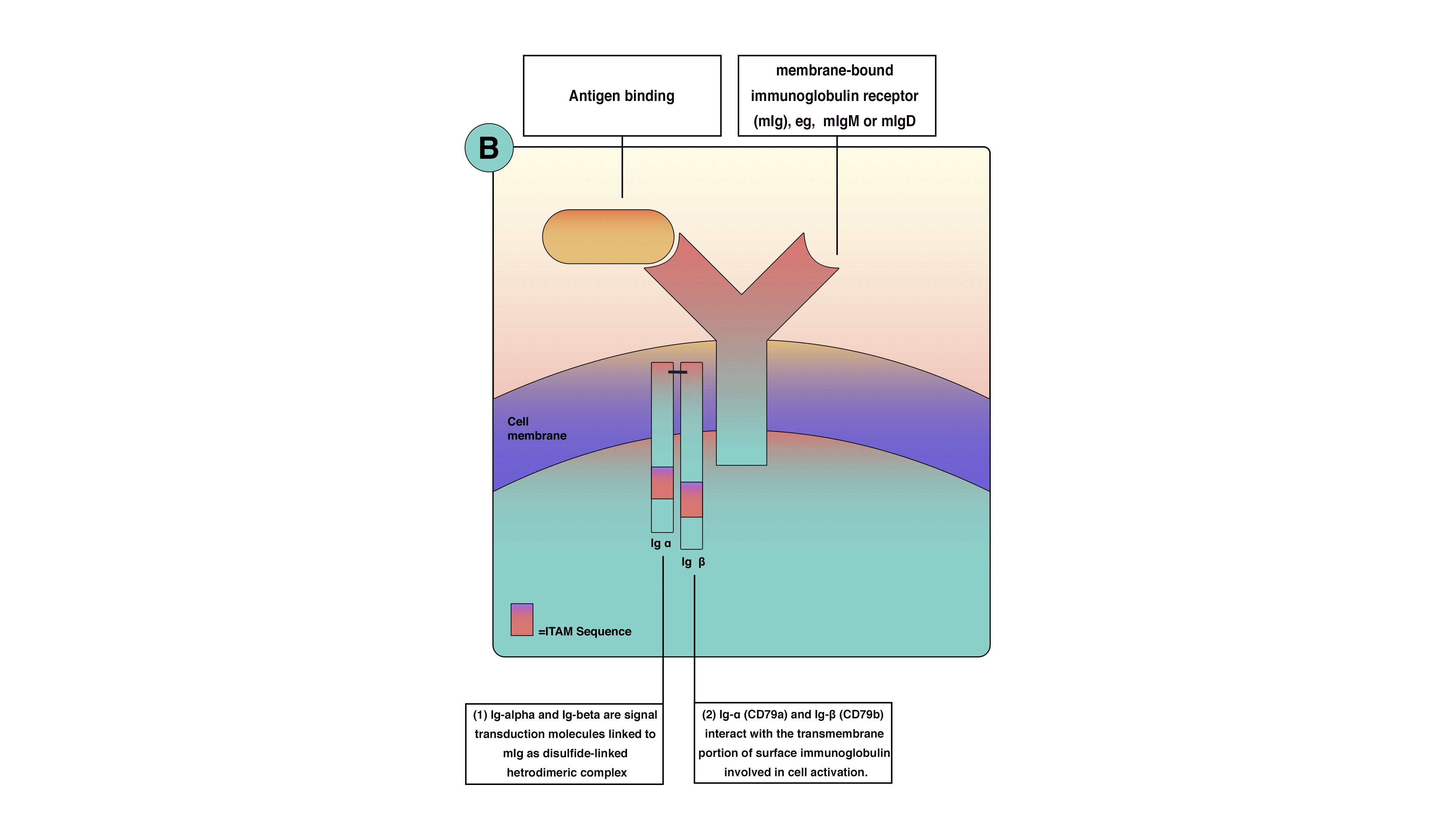
B-cell antigen receptor is functionally part of multimolecular protein complexes at the cell surface. The B-cell antigen receptor is a transmembrane receptor that extends to the cytoplasm but has very short cytoplasmic sequences (tails). These tails are ineffective in transmitting the signals and activating B cells. Consequently, another protein is involved in signal transmission and lymphocyte activation. B-cell receptor (BCR) is a multimolecular protein complex noncovalently associated with other proteins. The BCR consists of membrane-bound immunoglobulin (Ig) receptors—Ig-alpha (CD79a) and Ig-beta (CD79b). Ig-alpha and Ig-beta are signal transduction molecules that link to membrane-bound immunoglobulin receptors as a disulfide-linked heterodimeric complex. Ig-alpha and Ig-beta contain an immune receptor tyrosine-based activation motif, essential for signal transduction in both B and T lymphocytes. The immune receptor tyrosine-based activation motif passes the activation signals from the cell surface to the cytoplasm through tyrosine amino acid, which becomes phosphorylated by protein tyrosine kinases during cellular activation to interact with cytoplasmic signaling proteins. Because of genetic arrangement and diversity, the thinking is that there is enough BCR antigen for every microbe. BCRs are inherited as gene fragments; these fragments are joined differently in each developing cell to create a diverse array of receptors. Theoretically, B lymphocytes can make up to 10 to 11 antibodies in an individual. Besides, BCRs undergo somatic hypermutation to create unique receptors.[12][10][13][11][14]
Co-Receptor Molecules of B Cells
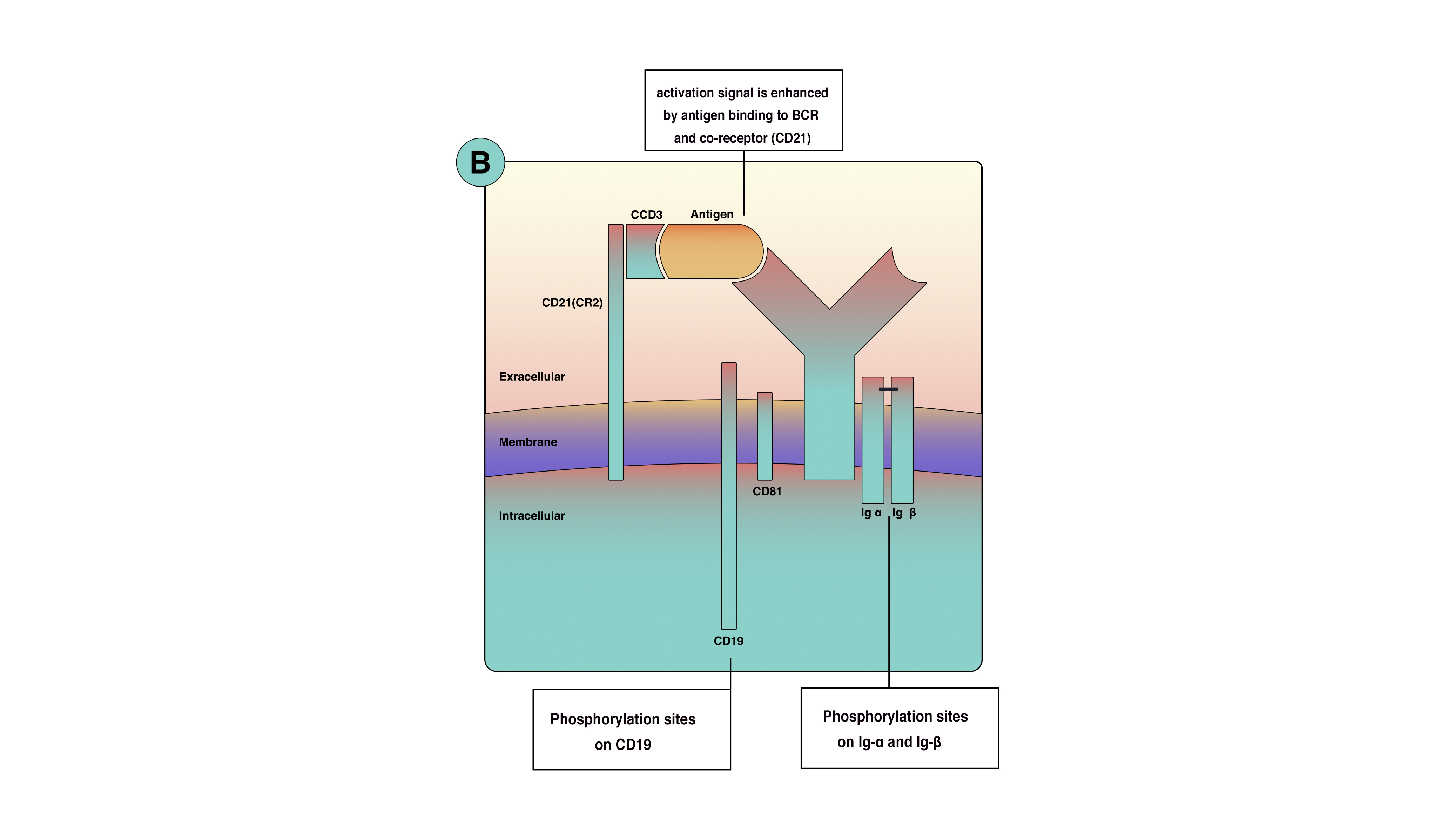
Optimal signaling in B-cell activation requires more than the BCR. These co-receptors are clusters of molecules on the cell membrane that increase signaling efficacy up to 1000-fold. The primary B-cell co-receptors are complement receptor 2 (CD21), CD19, CD81, and CD225. Although these proteins are located close to the BCR, they are not part of it. The phosphorylation of co-receptors proteins, along with Ig-alpha and Ig-beta, amplifies activation singles from the cell surface to the cytoplasm (see Image. B-cell activation). Moreover, co-receptors reduce the BCR stimulation threshold, which means fewer antigens are necessary for BCR stimulation. The function of co-receptors is best demonstrated when microbial pathogens activate complement and then bind to B cells. When the antigen binds to complement protein C3d, this process allows the antigen to bind to CD21 and the BCR simultaneously to prevent the co-receptor complex from clustering and cross-linking with BCR, leading to the phosphorylation of the CD19 tail (see Image. Antigen-Binding). This process increases signal concentration around the BCR.[11]
Signal Transduction Molecules (Molecules Involved in Interactions Between T Cells and B Cells)
In thymus-dependent B-cell activation, the interaction between T and B cells requires specific surface molecules, which include:
- Major histocompatibility complex class II molecule: These molecules present peptides derived from T-dependent protein antigens to T-helper cells (CD4+). All B-cell lineage cells express major histocompatibility complex class (MHC) II except pro-B cells.[15]
- Co-stimulator molecules: These molecules are required as a second signal to accompany the first signal after antigen binding. Co-stimulators are expressed in high quantities in the activated cells rather than naive B cells. Several co-stimulatory studies have been conducted; the most well-known are B7 and CD40. B7 is a family of molecules interacting with CD28 on the T-cell surface. CD40 on B cells interact with CD40 ligand (CD40L or CD154) on activated T cells. This interaction is crucial in somatic hypermutation and class switch. Moreover, the inducible co-stimulatory ligand on B cells interacts with the inducible T-cell co-stimulator on activated T cells, which is critical and necessary for germinal center formation. Therefore, individuals lacking the functional inducible co-stimulatory ligand or inducible T-cell co-stimulator have deficient levels of IgG, IgA, and IgE.[11]
- Cytokines receptors: Cytokines produced by activated T lymphocytes (CD4+) mediate B-cell response to protein antigens. The CD40 ligand on the surface of T-helper lymphocytes interacts with the CD40 molecule and functions to allow B-lymphocyte development into antibody-secreting plasma cells.[11]
Function
Generally, B cells are key regulatory cells in the immune system, producing antibodies and antigen-presenting cells, supporting other mononuclear cells, and directly contributing to inflammatory pathways. However, to understand the nature, function, and subsequent dysfunction of B cells, a brief discussion of B-cell development is essential.
B lymphocytes arise from hematopoietic stem cells that are considered the precursor of B-cell lineage. Early development occurs in fetal liver and bone marrow after birth and throughout life; hence, bone marrow is the primary lymphoid organ in humans and many mammals. Continuous differentiation of B cells ensures that B-cell repertoires are continuously replenished for limitless antigen recognitions. Non-lymphoid cells called stroma make up the matrix of the bone marrow, providing essential molecules such as interleukin-7 (IL-7), cytokines, and adhesion molecules critical for B-cell survival and differentiation. During development, B cells undergo a negative selection process to eliminate self-reactive cells, preventing autoimmunity. Surviving B cells circulate to peripheral lymphoid organs, where they await encountering antigens to differentiate into antibody-secreting plasma cells. If they do not encounter antigens, they undergo programmed cell death.[16]
Tolerance to self-antigens must include all self-antigens. However, not all self-antigens are present in the bone marrow. Therefore, another tolerance mechanism ensures that B cells do not cause autoimmunity. Generally, mature B cells require T cells to help produce antibodies. B cells that encounter antigens and do not receive help from T-helper cells specific to that particular antigen undergo anergy or clonal deletion. However, a subset of mature B cells has developed another mechanism to respond to antigens without the help of T cells; this population is called thymus-independent B cells (T-independent B cells).[16]
Lymphoid follicles in secondary lymphoid organs provide a specialized environment to concentrate antigens for proper B-cell function. These follicles contain follicular dendritic cells to display antigens to naive B cells. Secondary lymphoid tissue traps antigens from different sources according to their location and related environment—the spleen collects blood-borne antigens, lymphatic nodes collect antigens trapped in the lymphatic system, and mucosa-associated lymphoid tissue acquires antigens from the surrounding mucosal epithelium.[17][18]
B cells mediate the production of antigen-specific immunoglobulin directed against invasive pathogens (antibodies). B cells recognize antigens through a membrane-bound BCR and accessory cell-surface receptors. B cells are capable of recognizing a variety of structural motifs (epitopes) on antigens that rely on the enormous sequence and structural diversity of BCR repertoires due to genetic rearrangement in V(D)J segments responsible for the variable regions (heavy and light chains) of BCR. Upon stimulation by antigens, B cells mature into plasma cells, synthesizing five different classes of antibodies such as IgA, IgG, IgD, IgM, and IgE. Activated B cells undergo mitotic division after activation, producing a clone of cells that can produce immunoglobulin of the same antigen specificity. These cells mature mostly into plasma cells. The primary immune response is generated when B cells encounter antigens for the first time. However, a few subsets of this clone mature into memory cells, which respond rapidly upon subsequent exposures to the same antigen, leading to a secondary immune response. The secondary immune response is of high magnitude, occurs much more rapidly, and produces IgG rather than IgM. This phenomenon is the key concept of lifetime immunity and vaccines.[19][12][20][21][22][13]
In addition to their crucial role in humoral immunity, B cells mediate and regulate many other functions essential for immune homeostasis. Experimental studies have shown that depletion of B cells during mice development leads to severe consequences and congenital abnormalities within the immune system, including a generalized decrease in the number and diversity of T cells, an absence of Peyer patch organogenesis, and defects within dendritic cells. Moreover, B cells are necessary for immune system maintenance. For example, B cells release immunomodulatory cytokines that influence immune cell functions of T cells and dendritic cells and regulate lymphoid tissue organogenesis, wound healing, and transplanted tissue rejection. Furthermore, regulatory B cells have been discovered as crucial in regulating T-cell-mediated inflammatory responses by producing IL-10.[20][23]
B cells proliferate rapidly after antigenic stimulation in germinal centers, rating about 1 division every 6 hours. The germinal center is a lightly stained region within lymphoid follicles containing follicular dendritic cells. During the proliferation process, point mutations are introduced at a high rate without repair into immunoglobulin genes; this unique process is called somatic hypermutation. Another critical step in B-cell development within the germinal center is immunoglobulin class switching. Initially, B cells express surface IgM and IgD but can only secrete IgM. In many situations, such as mucosal infection, where IgA is the cornerstone in fighting antigens, IgM is not enough; therefore, class switching is necessary to allow B cells to secret all classes of antibodies. Ultimately, mature B cells encounter antigens and differentiate into plasma cells that secret large amounts of antibodies and memory cells that rapidly respond to antigens upon subsequent exposers.[19][12][20][21][22]
B-Cell Activation
The activation of B cells by protein antigens requires them to function as APCs, presenting the protein epitopes on MHC II to helper T cells; hence, this mechanism is called T–cell-dependent activation. However, polysaccharides, lipopolysaccharides, and other non-protein antigens are considered T-independent because they can activate B cells without antigen processing and presentation to T cells.
- Activation of T–cell-independent B cells:
- This type of activation occurs when B cells interact with T–cell-independent antigens. The activation process is composed of 2 signals. The first signal is the cross-linkage of multiple BCRs with repetitive epitopes united on the antigen surface. The second signal is the interaction of toll-like receptors with pathogen-associated molecular patterns or interactions with factors from the complement system. After B-cell activation, B cells undergo clonal proliferation and ultimate differentiation of daughter cells into plasma cells. Ultimately, BCRs disappear. However, plasma cells dominate the antibody production of IgM type with the same specificity as BCRs (pentameric IgM). This process has a short life and cannot produce memory cells.
- Activation of T–cell-dependent B cells:
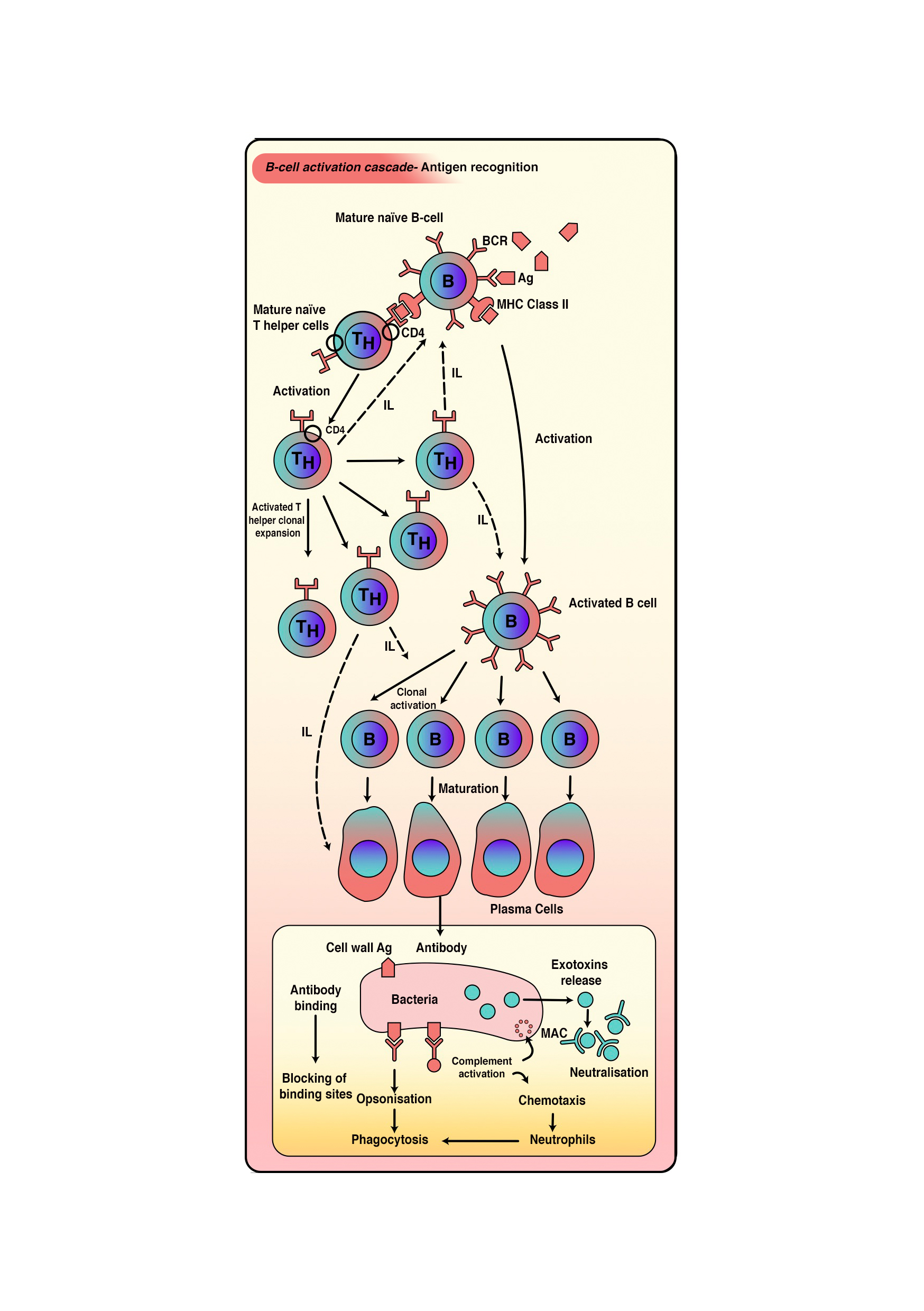
- This process occurs in response to T–cell-dependent antigens, which can be either free or associated with intact pathogens. Free antigen interaction results in direct internalization. However, interaction with intact pathogens leads to antigen separation and extraction from the intact pathogen. Eventually, the antigen is presented on the MHC class II on the external membrane of B cells. T-helper cells recognize this presentation as specific to that particular antigen (see Image. B-Cell Activation Cascade). Linked recognition between T-helper cells and B cells occurs when the TCR of T-helper cells recognizes the antigen presented on B cells, and the CD4 molecule interacts with MHC class II on B cells. Several cytokines secreted by TH2 cells stimulate B cells to proliferate and differentiate into plasma and memory cells.[18][20][21][23][22][17]
Tissue Preparation
Typically, lymphocyte specimens are separated from blood samples through centrifugation. The preparation of samples is then tailored to the specific study method. Please refer to the Microscopy, Light and Microscopy, Electron sections for more information.[24]
Histochemistry and Cytochemistry
Immunohistochemical techniques are commonly used to identify B cells histologically due to the lack of distinct morphological features that differentiate them from other lymphocytes. Common markers used are listed below.
Surface Markers
-
CD19 and CD20 are primary markers for B cells, commonly used in immunohistochemistry to identify B cells in tissues. CD20 is expressed during B-cell development but is absent in plasma cells. CD19 is expressed from the early stages of B-cell development to maturity.[25][26]
-
CD21 and CD23 are markers expressed on follicular dendritic and B cells, particularly in germinal centers. These markers are useful in identifying B-cell follicles and follicular zones within lymphoid tissues.[27][28]
-
Immunoglobulin: B cells express surface immunoglobulins, such as IgM and IgD, in naive B cells, which can be detected in histological sections to help distinguish B cells from T cells. Plasma cells secrete high levels of immunoglobulin, which can be visualized in tissue sections by staining for IgG, IgA, or IgM.[29]
-
CD138, or syndecan-1, is a marker specific to plasma cells and crucial for identifying fully differentiated B cells that produce antibodies. This marker is widely used in diagnosing plasma cell disorders, such as multiple myeloma.[30]
Other Markers
-
PAX5 is a transcription factor essential for B-cell development and is a reliable immunohistochemical marker used to identify B cells at various stages of differentiation. This marker is commonly used to distinguish B cells in diagnostic settings, especially in B-cell lymphomas.[31]
-
BCL-6 is expressed in germinal center B cells and plays a key role in regulating B-cell maturation. This marker is an essential marker for identifying germinal center reactions and is commonly used to diagnose lymphomas of germinal center origin.[32]
-
MUM1/IRF4 is a marker for plasma and late-stage B cells. This marker is combined with BCL-6 and other markers to distinguish different stages of B-cell differentiation in lymphoid tissues, especially in lymphomas.[33]
Microscopy, Light
Lymphocyte types are best differentiated using histochemistry and flow cytometry, as light microscopy cannot distinguish between lymphocyte types based on their appearance. However, light microscopy shows lymphocytes as spherical or oval cells with diameters from 6 to 15 μm when flattened on glass slides. Sample preparation of lymphocytes typically requires staining air-dried films using Romanowsky polychromatic stains, such as Giemsa or Wright.
Two populations of lymphocytes can be observed under light microscopy—large cells with diameters of 9 to 15 μm and small lymphocytes with diameters of 6 to 9 μm. Under the microscope, lymphocytes appear as dark purple cells with a deep bluish nucleus and faint sky-blue cytoplasm. The nucleus occupies a significant portion of the cell's internal environment due to the high density of condensed chromatin.[24][34]
The microscopic appearance of B lymphocytes varies depending on their developmental stage as follows:
- Resting B cells: In their naive or resting state, B cells appear as small- to medium-sized lymphocytes with a round, dense nucleus and a thin rim of basophilic cytoplasm. The chromatin is coarse and clumped, and the nucleolus is not prominent. Without immunohistochemical staining, these cells are often indistinguishable from other small lymphocytes, such as T cells.[35]
- Activated B cells: Upon activation, B cells undergo morphological changes, particularly in germinal centers. In the dark zone of germinal centers, activated B cells, or centroblasts, appear as large cells with open chromatin, multiple nucleoli, and abundant cytoplasm. In the light zone, B cells, known as centrocytes, are smaller and have irregular nuclei with condensed chromatin, indicating a transitional state between active proliferation and differentiation.[36][37]
- Plasma cells: B cells that differentiate into plasma cells show distinct morphological changes. Plasma cells are characterized by an eccentric nucleus with a cartwheel or clock-face appearance due to the radial arrangement of heterochromatin. The cytoplasm is abundant and basophilic, reflecting high protein synthesis, particularly of immunoglobulins. A clear perinuclear Golgi zone is often visible, representing the site of immunoglobulin secretion.[38]
Microscopy, Electron
B lymphocytes can be studied using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). However, each method offers a different perspective. TEM provides detailed internal views of the cells, whereas SEM reveals the outer surface structure of B lymphocytes.
Transmission Electron Microscopy
Under TEM, the nucleus of blood lymphocytes has electron-dense heterochromatin, a feature of nonproliferating cells. The nucleoli of lymphocytes appear round in cross-section. Lymphocytes are arranged concentrically into 3 zones or structural units—the central agranular region, the middle fibrillar region, and the outer granular region composed of intranuclear chromatin. Furthermore, the cytoplasmic organelles of lymphocytes are typical of eukaryotic cells.
Scanning Electron Microscopy
This modality provides three-dimensional information. However, it creates images with less resolution compared to TEM. Normal blood lymphocytes are washed, collected on silver membranes, and fixed in glutaraldehyde for SME analysis. B lymphocytes under SEM range from 5.1 to 6.4 μm in diameter and are identified by their complex surface architecture, with multiple finger-like microvilli covering the entire surface of B cells.[24][34][39]
Pathophysiology
Studies show that intense physical exertion, such as extreme sports or alteration of gravity (space), reduces the number of type B lymphocytes. This decline may result in physiological alterations in the immune response. Furthermore, the circadian rhythms governing the production of these cells must be considered; external stress that alters the body's circadian status could negatively affect the immune response.
Clinical Significance
B-cell dysfunction is pronounced in several disorders, including immunodeficiency, autoimmune diseases, and malignancies. As described earlier, B cells are responsible for antibody production, antigen presentation, immune system regulation, and maintenance. Accumulating evidence from several decades of research shows that disrupting these tightly regulated and controlled pathways could lead to autoimmune diseases, cancer, or other well-known conditions. A fundamental feature of autoimmunity is the inappropriate production of autoantibodies and loss of B-cell tolerance. Genetic mutations in B-cell lineage may lead to intrinsic disorders in B cells and consequent induction of autoimmunity in the T-cell compartment. Therefore, these findings showed the rationale of B cells in depleting B cells as a therapeutic strategy in autoimmune disorders and other diseases. The upcoming paragraphs show the role of B cells in developing or initiating certain disorders.[40][41]
Primary B-Cell Immunodeficiencies or Pure B-Cell Immunodeficiencies
This category includes diseases resulting from abnormal B-cell function, such as antibody production and B-cell interaction with T cells. Classically, patients present with recurrent infections and other complications depending on the disorder and the developmental stage in which the disorder has occurred—the earlier the defect, the more devastating the effect on lymphopoiesis. As B cells begin to express surface receptors, they are subject to positive and negative selection pressure and become dependent on survival signals. Therefore, defects in these processes lead to selective or generalized hypogammaglobulinemia.
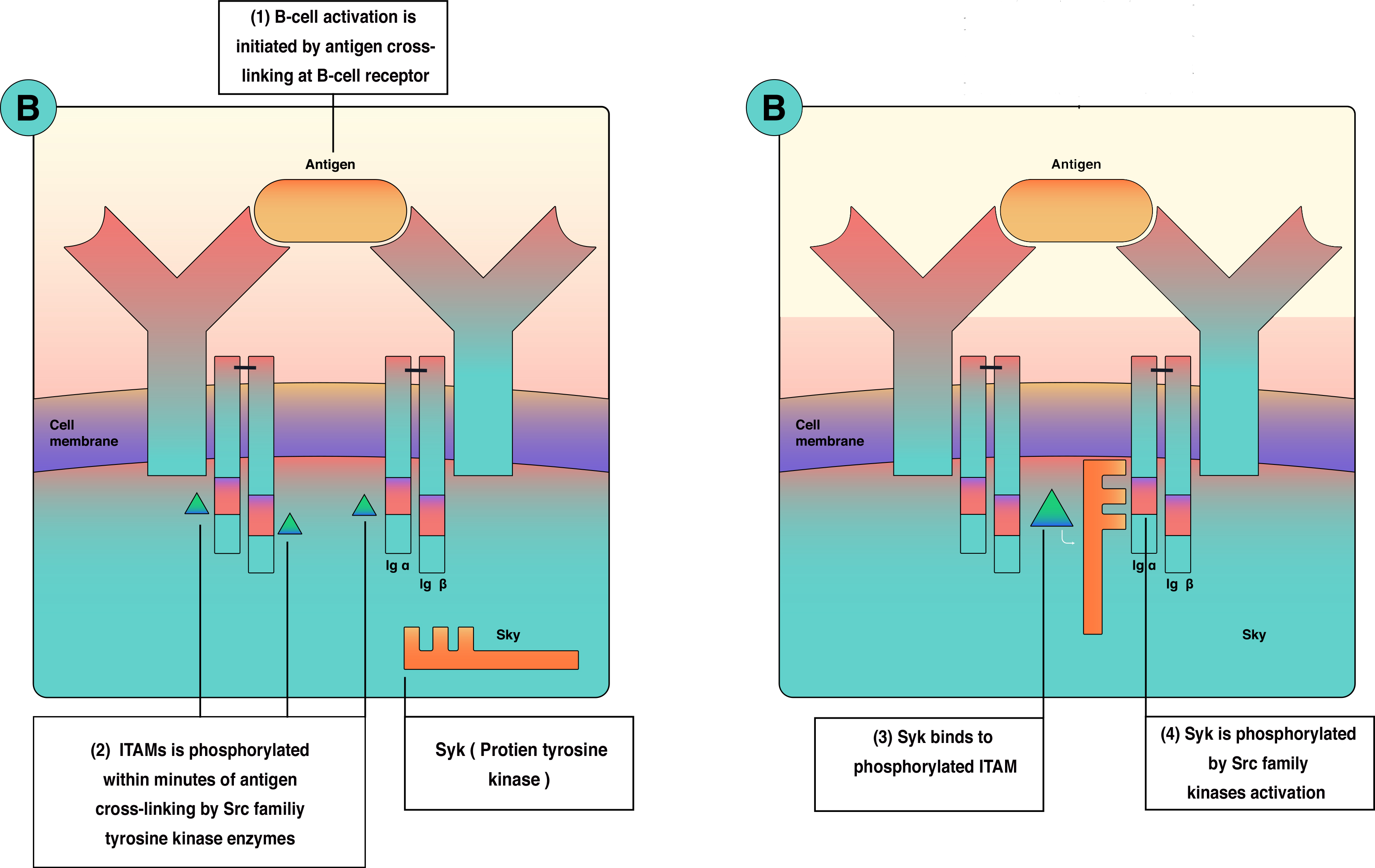
As outlined in the Physiology section, B-cell activation requires 2 waves of activation signals—the first involves the Src family, and the second involves Bruton tyrosine kinase (BTK) and Syk (see Image. B-Cell Activation and Signal Transduction). Consequently, a mutation in the BTK gene leads to X-linked agammaglobulinemia. In addition, a failure to express the CD40 ligand due to mutation in gp39 prevents isotype class switching, resulting in X-linked immunodeficiency with hyper-IgM, which can be associated with liver disease, sclerosing cholangitis, and liver or gastrointestinal malignancies.[12][42][41][43][44][45]
Autoimmune Diseases
B-cell dysregulation leads to the production of autoantibodies, contributing to diseases such as systemic lupus erythematosus, rheumatoid arthritis, and multiple sclerosis. B cells contribute to autoimmune diseases by producing pathogenic antibodies, presenting autoantigens to T cells, and exacerbating inflammation in these diseases. In addition, overactive B cells produce excessive pro-inflammatory cytokines such as IL-6, worsening autoimmune conditions.[46][47][48]
B-Cell Malignancies
B-cell lymphomas result from the abnormal proliferation of B cells, observed in conditions such as diffuse large B-cell lymphoma (DLBCL), Hodgkin's and non-Hodgkin's lymphoma, chronic lymphocytic leukemia, and plasma cell cancers, including multiple myeloma. These malignancies stem from genetic mutations in B-cell development, leading to unchecked growth and impaired apoptosis. These conditions disrupt normal immune functions and cause systemic symptoms such as fever, weight loss, and night sweats.[49][50][51][40][52]
Hyperactive B-Cell Responses
Overactive B cells can primarily trigger hypersensitivity and allergic reactions by producing IgE antibodies, leading to allergic responses such as asthma, rhinitis, or anaphylaxis. In chronic inflammatory conditions, hyperactive B cells produce excessive cytokines and antibodies, contributing to tissue damage.[53]
Role in Chronic Inflammatory Diseases
B cells play a role in chronic inflammatory diseases such as atherosclerosis and type 2 diabetes. In atherosclerosis, B cells contribute to plaque formation by secreting pro-inflammatory cytokines. In type 2 diabetes, dysfunctional B cells exacerbate inflammation in metabolic tissues, promoting insulin resistance and accelerating disease progression.[54][55]
Inadequate B-Cell Responses in Aging
As individuals age, B cells undergo functional decline, known as immunosenescence, which weakens immune responses and reduces vaccine efficacy in older adults. This decline includes diminished antibody production, impaired class-switching, reduced memory B-cell function, and increased infection vulnerability.[56][57]
Monoclonal Gammopathies
Multiple myeloma and monoclonal gammopathy of undetermined significance are conditions in which B cells or plasma cells produce abnormal monoclonal antibodies. In multiple myeloma, these abnormal antibodies can cause organ damage, particularly to the kidneys and bones, and suppress normal immune functions.[58][59]
Transplant Rejection
B cells contribute to transplant rejection through various effector pathways, including antigen presentation to T cells and dysregulated autoimmune antibody synthesis; they also produce donor-specific antibodies that target the transplanted organ, leading to antibody-mediated rejection. This type of rejection is complicated to manage and can result in chronic graft loss.[60][61][62]
Therapeutic Challenges
B-cell depletion therapies, such as rituximab (anti-CD20 monoclonal antibody), are used in treating autoimmune diseases and B-cell malignancies. However, these therapies also increase the risk of infections due to the depletion of normal B cells. Striking a balance between effective immune suppression and avoiding excessive immunosuppression is a significant challenge in B-cell-targeted therapies.[63][64][65]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Pieper K, Grimbacher B, Eibel H. B-cell biology and development. The Journal of allergy and clinical immunology. 2013 Apr:131(4):959-71. doi: 10.1016/j.jaci.2013.01.046. Epub 2013 Mar 5 [PubMed PMID: 23465663]
Clark MR, Mandal M, Ochiai K, Singh H. Orchestrating B cell lymphopoiesis through interplay of IL-7 receptor and pre-B cell receptor signalling. Nature reviews. Immunology. 2014 Feb:14(2):69-80. doi: 10.1038/nri3570. Epub 2013 Dec 31 [PubMed PMID: 24378843]
Khodadadi L, Cheng Q, Radbruch A, Hiepe F. The Maintenance of Memory Plasma Cells. Frontiers in immunology. 2019:10():721. doi: 10.3389/fimmu.2019.00721. Epub 2019 Apr 5 [PubMed PMID: 31024553]
Nandiwada SL. Overview of human B-cell development and antibody deficiencies. Journal of immunological methods. 2023 Aug:519():113485. doi: 10.1016/j.jim.2023.113485. Epub 2023 May 5 [PubMed PMID: 37150477]
Level 3 (low-level) evidenceSchatz DG, Swanson PC. V(D)J recombination: mechanisms of initiation. Annual review of genetics. 2011:45():167-202. doi: 10.1146/annurev-genet-110410-132552. Epub 2011 Aug 19 [PubMed PMID: 21854230]
Inoue T, Kurosaki T. Memory B cells. Nature reviews. Immunology. 2024 Jan:24(1):5-17. doi: 10.1038/s41577-023-00897-3. Epub 2023 Jul 3 [PubMed PMID: 37400644]
Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007 Aug:27(2):190-202 [PubMed PMID: 17723214]
Level 3 (low-level) evidenceCyster JG, Allen CDC. B Cell Responses: Cell Interaction Dynamics and Decisions. Cell. 2019 Apr 18:177(3):524-540. doi: 10.1016/j.cell.2019.03.016. Epub [PubMed PMID: 31002794]
Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nature reviews. Immunology. 2011 Nov 11:11(12):823-36. doi: 10.1038/nri3084. Epub 2011 Nov 11 [PubMed PMID: 22076556]
Level 3 (low-level) evidenceSathe A, Cusick JK. Biochemistry, Immunoglobulin M. StatPearls. 2024 Jan:(): [PubMed PMID: 32310455]
Clark EA, Ledbetter JA. Structure, function, and genetics of human B cell-associated surface molecules. Advances in cancer research. 1989:52():81-149 [PubMed PMID: 2662716]
Level 3 (low-level) evidenceJustiz Vaillant AA, Jamal Z, Patel P, Ramphul K. Immunoglobulin. StatPearls. 2024 Jan:(): [PubMed PMID: 30035936]
Tanaka S, Baba Y. B Cell Receptor Signaling. Advances in experimental medicine and biology. 2020:1254():23-36. doi: 10.1007/978-981-15-3532-1_2. Epub [PubMed PMID: 32323266]
Level 3 (low-level) evidenceBrezski RJ, Monroe JG. B-cell receptor. Advances in experimental medicine and biology. 2008:640():12-21. doi: 10.1007/978-0-387-09789-3_2. Epub [PubMed PMID: 19065780]
Level 3 (low-level) evidenceTumer G, Simpson B, Roberts TK. Genetics, Human Major Histocompatibility Complex (MHC). StatPearls. 2024 Jan:(): [PubMed PMID: 30855806]
Chapman J, Zhang Y. Histology, Hematopoiesis. StatPearls. 2024 Jan:(): [PubMed PMID: 30480979]
Null M, Arbor TC, Agarwal M. Anatomy, Lymphatic System. StatPearls. 2024 Jan:(): [PubMed PMID: 30020619]
Ozdowski L, Gupta V. Physiology, Lymphatic System. StatPearls. 2024 Jan:(): [PubMed PMID: 32491765]
Aziz M, Iheanacho F, Hashmi MF. Physiology, Antibody. StatPearls. 2024 Jan:(): [PubMed PMID: 31536276]
Grubbs H, Kahwaji CI. Physiology, Active Immunity. StatPearls. 2024 Jan:(): [PubMed PMID: 30020652]
Allen HC, Sharma P. Histology, Plasma Cells. StatPearls. 2024 Jan:(): [PubMed PMID: 32310542]
Justiz Vaillant AA, Sabir S, Jan A. Physiology, Immune Response. StatPearls. 2024 Jan:(): [PubMed PMID: 30969623]
Sauls RS, McCausland C, Taylor BN. Histology, T-Cell Lymphocyte. StatPearls. 2024 Jan:(): [PubMed PMID: 30571054]
Gurina TS, Simms L. Histology, Staining. StatPearls. 2024 Jan:(): [PubMed PMID: 32491595]
Pavlasova G, Mraz M. The regulation and function of CD20: an "enigma" of B-cell biology and targeted therapy. Haematologica. 2020 Jun:105(6):1494-1506. doi: 10.3324/haematol.2019.243543. Epub [PubMed PMID: 32482755]
Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Experimental hematology & oncology. 2012 Nov 29:1(1):36. doi: 10.1186/2162-3619-1-36. Epub 2012 Nov 29 [PubMed PMID: 23210908]
Kurshumliu F, Sadiku-Zehri F, Qerimi A, Vela Z, Jashari F, Bytyci S, Rashiti V, Sadiku S. Divergent immunohistochemical expression of CD21 and CD23 by follicular dendritic cells with increasing grade of follicular lymphoma. World journal of surgical oncology. 2019 Jul 3:17(1):115. doi: 10.1186/s12957-019-1659-8. Epub 2019 Jul 3 [PubMed PMID: 31269981]
Meng QH, White HN. CD21(int) CD23(+) follicular B cells express antigen-specific secretory IgM mRNA as primary and memory responses. Immunology. 2017 Jun:151(2):211-218. doi: 10.1111/imm.12724. Epub 2017 Mar 16 [PubMed PMID: 28190261]
Noviski M, Mueller JL, Satterthwaite A, Garrett-Sinha LA, Brombacher F, Zikherman J. IgM and IgD B cell receptors differentially respond to endogenous antigens and control B cell fate. eLife. 2018 Mar 9:7():. doi: 10.7554/eLife.35074. Epub 2018 Mar 9 [PubMed PMID: 29521626]
O'Connell FP, Pinkus JL, Pinkus GS. CD138 (syndecan-1), a plasma cell marker immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. American journal of clinical pathology. 2004 Feb:121(2):254-63 [PubMed PMID: 14983940]
Desouki MM, Post GR, Cherry D, Lazarchick J. PAX-5: a valuable immunohistochemical marker in the differential diagnosis of lymphoid neoplasms. Clinical medicine & research. 2010 Jul:8(2):84-8. doi: 10.3121/cmr.2010.891. Epub [PubMed PMID: 20660931]
Yang H, Green MR. Epigenetic Programing of B-Cell Lymphoma by BCL6 and Its Genetic Deregulation. Frontiers in cell and developmental biology. 2019:7():272. doi: 10.3389/fcell.2019.00272. Epub 2019 Nov 7 [PubMed PMID: 31788471]
Gualco G, Queiroga EM, Weiss LM, Klumb CE, Harrington WJ Jr, Bacchi CE. Frequent expression of multiple myeloma 1/interferon regulatory factor 4 in Burkitt lymphoma. Human pathology. 2009 Apr:40(4):565-71. doi: 10.1016/j.humpath.2008.07.021. Epub 2009 Jan 13 [PubMed PMID: 19144381]
Polliack A, Siegal FP, Clarkson BD, Fu SM, Winchester RJ, Lampen N, Siegal M, De Harven E. A scanning electron microscopy and immunological study of 84 cases of lymphocytic leukaemia and related lymphoproliferative disorders. Scandinavian journal of haematology. 1975 Dec:15(5):359-76 [PubMed PMID: 812174]
Level 3 (low-level) evidenceHoffman W, Lakkis FG, Chalasani G. B Cells, Antibodies, and More. Clinical journal of the American Society of Nephrology : CJASN. 2016 Jan 7:11(1):137-54. doi: 10.2215/CJN.09430915. Epub 2015 Dec 23 [PubMed PMID: 26700440]
De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nature reviews. Immunology. 2015 Mar:15(3):137-48. doi: 10.1038/nri3804. Epub 2015 Feb 6 [PubMed PMID: 25656706]
Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nature reviews. Immunology. 2015 Mar:15(3):160-71. doi: 10.1038/nri3795. Epub 2015 Feb 20 [PubMed PMID: 25698678]
Jourdan M, Caraux A, De Vos J, Fiol G, Larroque M, Cognot C, Bret C, Duperray C, Hose D, Klein B. An in vitro model of differentiation of memory B cells into plasmablasts and plasma cells including detailed phenotypic and molecular characterization. Blood. 2009 Dec 10:114(25):5173-81. doi: 10.1182/blood-2009-07-235960. Epub [PubMed PMID: 19846886]
Polliack A, Lampen N, Clarkson BD, De Harven E, Bentwich Z, Siegal FP, Kunkel HG. Identification of human B and T lymphocytes by scanning electron microscopy. The Journal of experimental medicine. 1973 Sep 1:138(3):607-24 [PubMed PMID: 4542254]
Justiz Vaillant AA, Stang CM. Lymphoproliferative Disorders. StatPearls. 2024 Jan:(): [PubMed PMID: 30725847]
Justiz Vaillant AA, Ramphul K. Antibody Deficiency Disorder (Archived). StatPearls. 2024 Jan:(): [PubMed PMID: 29939682]
Mazhar M, Waseem M. Agammaglobulinemia. StatPearls. 2024 Jan:(): [PubMed PMID: 32310401]
Lackey AE, Ahmad F. X-Linked Agammaglobulinemia. StatPearls. 2024 Jan:(): [PubMed PMID: 31751055]
Notarangelo L, Casanova JL, Fischer A, Puck J, Rosen F, Seger R, Geha R, International Union of Immunological Societies Primary Immunodeficiency diseases classification committee. Primary immunodeficiency diseases: an update. The Journal of allergy and clinical immunology. 2004 Sep:114(3):677-87 [PubMed PMID: 15356576]
Abbott JK, Gelfand EW. Common Variable Immunodeficiency: Diagnosis, Management, and Treatment. Immunology and allergy clinics of North America. 2015 Nov:35(4):637-58. doi: 10.1016/j.iac.2015.07.009. Epub 2015 Sep 4 [PubMed PMID: 26454311]
Lou H, Ling GS, Cao X. Autoantibodies in systemic lupus erythematosus: From immunopathology to therapeutic target. Journal of autoimmunity. 2022 Oct:132():102861. doi: 10.1016/j.jaut.2022.102861. Epub 2022 Jul 21 [PubMed PMID: 35872103]
Gharibi T, Babaloo Z, Hosseini A, Marofi F, Ebrahimi-Kalan A, Jahandideh S, Baradaran B. The role of B cells in the immunopathogenesis of multiple sclerosis. Immunology. 2020 Aug:160(4):325-335. doi: 10.1111/imm.13198. Epub 2020 May 10 [PubMed PMID: 32249925]
Hampe CS. B Cell in Autoimmune Diseases. Scientifica. 2012:2012():. pii: 215308. Epub [PubMed PMID: 23807906]
Sehn LH, Salles G. Diffuse Large B-Cell Lymphoma. The New England journal of medicine. 2021 Mar 4:384(9):842-858. doi: 10.1056/NEJMra2027612. Epub [PubMed PMID: 33657296]
Stéphan P, Bouherrou K, Guillermin Y, Michallet AS, Grinberg-Bleyer Y. Immunophenotyping of Peripheral Blood Cells in Patients with Chronic Lymphocytic Leukemia Treated with Ibrutinib. Cells. 2024 Aug 30:13(17):. doi: 10.3390/cells13171458. Epub 2024 Aug 30 [PubMed PMID: 39273028]
Jin H, Liao W, Yang S, Peng L, Xing W. Clinical features and imaging manifestations for intravascular large B-cell lymphoma. Zhong nan da xue xue bao. Yi xue ban = Journal of Central South University. Medical sciences. 2023 Dec 28:48(12):1920-1928. doi: 10.11817/j.issn.1672-7347.2023.230305. Epub [PubMed PMID: 38448386]
Stone WL, Basit H, Zubair M, Burns B. Pathology, Inflammation. StatPearls. 2024 Jan:(): [PubMed PMID: 30521241]
Palomares O, Akdis M, Martín-Fontecha M, Akdis CA. Mechanisms of immune regulation in allergic diseases: the role of regulatory T and B cells. Immunological reviews. 2017 Jul:278(1):219-236. doi: 10.1111/imr.12555. Epub [PubMed PMID: 28658547]
Pattarabanjird T, Li C, McNamara C. B Cells in Atherosclerosis: Mechanisms and Potential Clinical Applications. JACC. Basic to translational science. 2021 Jun:6(6):546-563. doi: 10.1016/j.jacbts.2021.01.006. Epub 2021 Jun 28 [PubMed PMID: 34222726]
DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, Markham D, Strissel KJ, Watkins AA, Zhu M, Allen J, Bouchard J, Toraldo G, Jasuja R, Obin MS, McDonnell ME, Apovian C, Denis GV, Nikolajczyk BS. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proceedings of the National Academy of Sciences of the United States of America. 2013 Mar 26:110(13):5133-8. doi: 10.1073/pnas.1215840110. Epub 2013 Mar 11 [PubMed PMID: 23479618]
Ma S, Wang C, Mao X, Hao Y. B Cell Dysfunction Associated With Aging and Autoimmune Diseases. Frontiers in immunology. 2019:10():318. doi: 10.3389/fimmu.2019.00318. Epub 2019 Feb 27 [PubMed PMID: 30873171]
Hou Y, Chen M, Bian Y, Hu Y, Chuan J, Zhong L, Zhu Y, Tong R. Insights into vaccines for elderly individuals: from the impacts of immunosenescence to delivery strategies. NPJ vaccines. 2024 Apr 10:9(1):77. doi: 10.1038/s41541-024-00874-4. Epub 2024 Apr 10 [PubMed PMID: 38600250]
Atkin C, Richter A, Sapey E. What is the significance of monoclonal gammopathy of undetermined significance? Clinical medicine (London, England). 2018 Oct:18(5):391-396. doi: 10.7861/clinmedicine.18-5-391. Epub [PubMed PMID: 30287433]
Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. American journal of hematology. 2020 May:95(5):548-567. doi: 10.1002/ajh.25791. Epub [PubMed PMID: 32212178]
Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nature reviews. Immunology. 2005 Oct:5(10):807-17 [PubMed PMID: 16175181]
Platt JL, Cascalho M. Donor specific antibodies after transplantation. Pediatric transplantation. 2011 Nov:15(7):686-90. doi: 10.1111/j.1399-3046.2010.01436.x. Epub 2011 Mar 29 [PubMed PMID: 22004543]
Justiz Vaillant AA, Modi P, Mohammadi O. Graft-Versus-Host Disease. StatPearls. 2024 Jan:(): [PubMed PMID: 30855823]
Zhang Z, Xu Q, Huang L. B cell depletion therapies in autoimmune diseases: Monoclonal antibodies or chimeric antigen receptor-based therapy? Frontiers in immunology. 2023:14():1126421. doi: 10.3389/fimmu.2023.1126421. Epub 2023 Feb 10 [PubMed PMID: 36855629]
Salles G, Barrett M, Foà R, Maurer J, O'Brien S, Valente N, Wenger M, Maloney DG. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Advances in therapy. 2017 Oct:34(10):2232-2273. doi: 10.1007/s12325-017-0612-x. Epub 2017 Oct 5 [PubMed PMID: 28983798]
Level 3 (low-level) evidenceLee DSW, Rojas OL, Gommerman JL. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nature reviews. Drug discovery. 2021 Mar:20(3):179-199. doi: 10.1038/s41573-020-00092-2. Epub 2020 Dec 15 [PubMed PMID: 33324003]
Level 3 (low-level) evidence