Introduction
Atrial septal defect (ASD) is one of the most common types of congenital heart defects, affecting about 25% of children (see Image. Atrial Septal Defect).[1] An ASD occurs when the communication between the right and left atria persists after birth. The condition involves defects in the true septal membrane and other abnormalities that enable communication between the atria. From most to least frequent, the 5 types of ASD are the patent foramen ovale (PFO) and the ostium secundum, ostium primum, sinus venosus, and coronary sinus defects.[2] Small ASDs often close spontaneously during childhood. Larger defects that fail to close may require percutaneous or surgical intervention to prevent complications such as stroke, dysrhythmias, and pulmonary hypertension.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
ASDs occur as singular defects but are associated with Mendelian inheritance, aneuploidy, transcription errors, mutations, and maternal exposures. ASDs are noted in patients with syndromes such as Down, Treacher-Collins, thrombocytopenia-absent radii, Turner, and Noonan. These conditions occur as a result of Mendelian inheritance. Maternal exposure to rubella and drugs like cocaine and alcohol may also predispose the unborn fetus to developing an ASD.
ASDs have also been associated with familial genetic disorders and conduction defects. Key transcription factors involved in atrial septation include GATA4, NKX2-5, and TBX5. Holt-Oram syndrome, also known as "heart-hand" syndrome, is commonly characterized by congenital heart defects, including ASDs in 58% of cases and VSDs in 28%, along with dysrhythmias and upper limb malformations typically affecting the hands. These features are linked to TBX5 mutations. NKX2-5 gene mutations are associated with congenital heart diseases, such as ASDs and tetralogy of Fallot, as well as atrioventricular blocks and juvenile sudden cardiac death.[3]
ASDs occur with other congenital heart defects, particularly ventricular septal defects. Communication between the left and right heart circulations is crucial for survival for some patients with congenital heart disease. Conditions such as tricuspid atresia, transposition of the great vessels, hypoplastic left heart syndrome variants, pulmonic or tricuspid atresia with hypoplastic right heart, and total anomalous pulmonary venous return rely on the alternate blood flow provided by an ASD to sustain survival until a definitive solution is implemented.[4]
Epidemiology
The prevalence of congenital heart disease, including ASDs, has risen over the past 50 years. Congenital heart disease was diagnosed in less than 1 per 1,000 live births in the 1930s. Recent data indicate a prevalence of 9 per 1,000 live births. Similarly, ASDs were identified in less than 0.5 per 1,000 live births between 1945 and 1949, but recent epidemiologic studies estimate their occurrence at 1.6 per 1,000 live births.[5] This apparent increase in prevalence is likely attributed to advancements in imaging technologies and improved practitioner training rather than a true rise in disease incidence.
Factors associated with the higher prevalence of congenital heart disease include advanced maternal age. Economic and geographical differences also influence diagnosis rates, with congenital heart disease more commonly identified in individuals from developed countries with higher incomes.[6]
Pathophysiology
Atrial septation begins during the 4th week of gestation and involves a complex series of developmental events.[7] The process starts with the growth of the primary atrial septum, or septum primum, from the roof of the primitive atrium toward the endocardial cushions. The caudal end of the septum primum becomes covered by mesenchymal cells derived from the embryonic endocardium, forming the mesenchymal cap.
As the septum primum extends and attaches to the atrioventricular endocardial cushions, it closes the ostium primum, the gap between the mesenchymal cap and the atrioventricular cushions. Once the leading edge of the septum primum attaches to the atrioventricular cushions, the primitive atrium is divided into right and left atria. The septum primum also attaches dorsally to the dorsal mesenchymal protrusion. As the ostium primum closes, programmed cell death occurs in the dorsal aspect of the septum primum, creating the ostium secundum.
The septum secundum begins to form to the right of the septum primum, growing caudally from the atrial roof and partially covering the ostium secundum. The space between the septum primum and septum secundum forms the foramen ovale. In the fetus, the foramen ovale enables oxygen-rich blood to bypass the lungs by flowing directly from the right atrium to the left atrium. After birth, pulmonary vascular resistance decreases, leading to a reduction in right atrial pressure. This pressure change causes the septum primum to close the foramen ovale, completing the separation of the atria.
PFO is a subclass of the ostium secundum defect and is not considered a defect of the "true septum." PFO is the most common septal anomaly, occurring when the septum primum and septum secundum fail to approximate and close the foramen ovale after the child takes its first breath. Thus, the 4 types of ASDs are as follows:
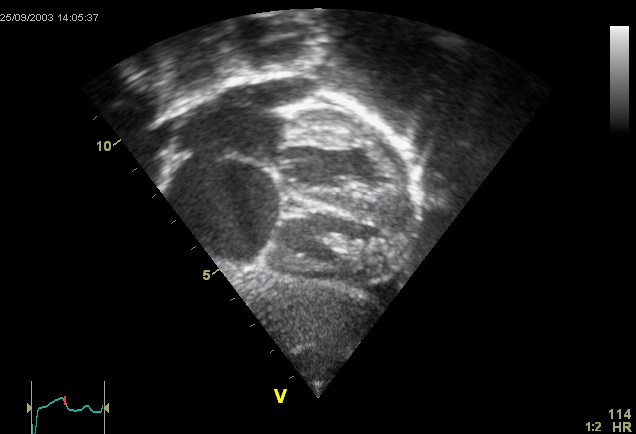
- Ostium secundum defect: This pathology occurs when increased reabsorption of the septum primum in the atrium's roof occurs, or the septum secundum fails to occlude the ostium secundum (see Image. Ostium Secundum Atrial Septal Defect on Ultrasound). This defect is associated with pediatric syndromes such as Noonan, Treacher-Collins, and thrombocytopenia-absent radii syndrome.
- Ostium primum defect: The 3rd most common ASD, it results from the failure of the septum primum to fuse with the endocardial cushions. These defects lead to atrioventricular communications and are best considered part of the spectrum of atrioventricular septal defects.
- Sinus venosus defect: This condition includes both superior and inferior types, neither of which involves the true membranous septum. A superior defect occurs when the orifice of the superior vena cava (SVC) overrides the atrial septum above the oval fossa (the remnant of the foramen ovale) and drains into both the left and right atria. The superior sinus venosus defect is often associated with a partial anomalous connection of the right superior pulmonary vein to the SVC. An inferior defect occurs when the orifice of the inferior vena cava overrides both atria. The inferior sinus venosus defect may be accompanied by an anomalous connection of the right inferior pulmonary vein to the inferior vena cava. The inferior defect is less common than the superior defect.[8]
- Coronary sinus defect: The coronary sinus is a vessel that runs along the groove between the left atrium and left ventricle and drains veins from the heart muscle. This structure normally drains into the floor of the right atrium above the septal leaflet of the tricuspid valve. A defect or hole in the common wall between the left atrium and the coronary sinus, called "unroofing" of the coronary sinus, creates communication between the right and left atria.
Normally, the pressure in the right atrium is significantly lower than in its left counterpart, causing blood to flow from the left atrium to the right atrium, resulting in a left-to-right shunt. The size of the defect determines the significance of the shunt. Significant shunts have a pulmonary (Qp) to systemic (Qs) flow ratio greater than 1.5:1 (Qp/Qs > 1.5). Chronic volume overload from high pulmonary blood flow leads to remodeling of the pulmonary vasculature.
The smooth muscle layer in the vascular wall thickens as the pulmonary vessels remodel. This increase in muscle mass raises the resistance to flow in the pulmonary circuit. Pulmonary hypertension develops due to the rise in vascular resistance and, subsequently, pulmonary pressures. When pulmonary pressures equal systemic pressures, the shunt across the ASD reverses, allowing deoxygenated blood to flow into the left atrium and, ultimately, the systemic circulation. This reversal of the shunt due to pulmonary hypertension leads to Eisenmenger syndrome.
History and Physical
ASDs are frequently asymptomatic. However, in symptomatic patients, a soft, systolic ejection murmur over the pulmonic area (2nd intercostal space) combined with a wide, fixed S2 splitting is characteristic.[9] Many ASDs go undiagnosed until adulthood. Therefore, treatment is often delayed, especially of large defects. Untreated large defects can cause exercise intolerance, cardiac dysrhythmias, palpitations, increased risk of pneumonia, pulmonary hypertension, and increased mortality.[10]
Eisenmenger syndrome is a rare but severe complication of untreated ASDs due to vascular remodeling caused by chronic overflow through a left-to-right shunt. Right atrial pressures approach systemic pressures as the vascular resistance increases. Shunt flow reverses when right atrial pressures exceed systemic pressures. Clinically, patients with Eisenmenger syndrome develop chronic cyanosis, increased pulmonary vascular resistance, dyspnea on exertion, syncope, and greater susceptibility to infection.[11]
Patients with smaller heart defects, ie, less than 5 mm, may not develop any symptoms. In contrast, individuals with defects ranging from 5 to 10 mm typically experience symptoms in the 4th or 5th decade of life. Patients with larger defects present earlier, in the 3rd decade of life. Symptoms may include dyspnea, fatigue, exercise intolerance, palpitations, and signs of right-sided heart failure. Approximately 20% of adult patients develop atrial tachydysrhythmias preoperatively. Evidence of stroke or transient ischemic attack, especially following the diagnosis of a peripheral blood clot, should raise suspicion for an ASD.
Evaluation
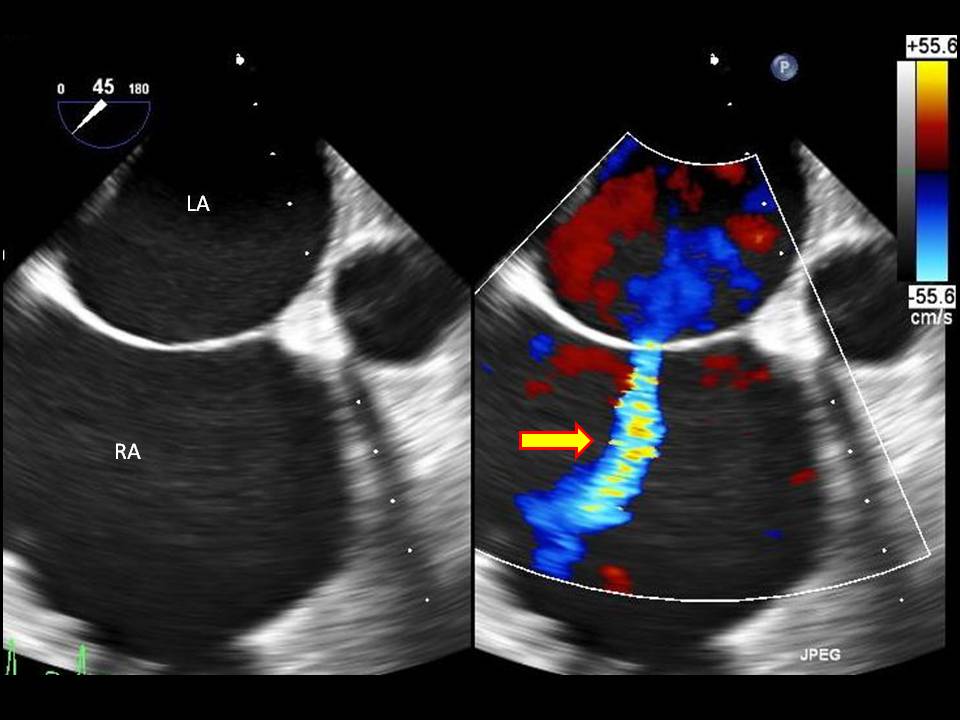
Diagnostic imaging is essential for determining the size of the defect and guiding treatment options. A transthoracic echocardiogram is the gold standard imaging modality, as it helps assess the size of the defect, the direction of blood flow, the presence of associated abnormalities (such as involvement of the endocardial cushions and atrioventricular valves), heart structure and function, pulmonary artery pressure, and the pulmonary/systemic flow ratio (Qp/Qs). A transesophageal echocardiogram is a better tool for diagnosing rarer cardiac defects (see Image. Atrial Septal Defect on Ultrasound with Doppler Study).
Though echocardiography is the gold standard for evaluating ASDs, other diagnostic modalities include cardiac computed tomography (CT) and magnetic resonance imaging (MRI). Both CT and MRI assess structures surrounding the heart and within the thoracic cavity. Chest x-ray findings are less helpful diagnostically, though they assist providers in monitoring clinical status by identifying cardiomegaly and pulmonary artery enlargement. Exercise testing can help determine the reversibility of shunt flow and assess the response of patients with pulmonary artery hypertension to activity. Cardiac catheterization is contraindicated in young patients with small, uncomplicated ASDs.[12]
Treatment / Management
Patients with ASDs smaller than 5 mm often experience spontaneous closure of the defect within the 1st year of life. Defects larger than 1 cm usually require medical or surgical intervention for closure.[13] Patients with atrial dysrhythmias initially require control of the abnormal rhythm and anticoagulation, but definitive intervention can occur once the dysrhythmia is under control. Monitoring adult patients with small defects and no signs of right heart failure is appropriate. An echocardiogram every 2 to 3 years evaluates right heart function and structure.[14] A history of transient ischemic attack or stroke requires more aggressive monitoring and possibly surgical intervention. (A1)
Options for ASDs requiring closure include percutaneous and surgical interventions. Indications for treatment include stroke, a hemodynamically significant shunt greater than 1.5:1, and evidence of systemic oxygen desaturation. Percutaneous transcatheter closure poses less risk for the patient but is only suitable for closing ostium secundum defects. The postprocedural complication risk for percutaneous transcatheter ASD closure is 7.2%, compared to 24% for postsurgical complications.
Complications associated with percutaneous closure include arrhythmias, atrioventricular blocks, cardiac erosion, and thromboembolism. Contraindications to percutaneous closure include small, hemodynamically insignificant ASDs, ostium primum defects, sinus venosus defects, coronary sinus defects, and secundum defects with advanced pulmonary hypertension. When ASDs are closed percutaneously, patients require antiplatelet therapy for the next 6 months.[15] Women with large ASDs and Eisenmenger syndrome should avoid pregnancy due to the risk of aggravating pulmonary artery hypertension and the increased occurrence of dysrhythmias.
Surgical repair of ASDs, including secundum, sinus venosus, and primum ASDs, involves various approaches tailored to the specific anatomical characteristics of the defect and associated anomalies. For secundum ASD and PFO, a median sternotomy allows access to harvest autologous pericardium for patch closure, ensuring a tension-free repair. Cardiopulmonary bypass is initiated, and diastolic arrest is achieved with cardioplegia. The intraoperative assessment ensures accurate localization and correction without compromising surrounding structures like the tricuspid valve, coronary sinus, and atrioventricular node. Postoperative transesophageal echocardiography with a bubble study confirms repair integrity and checks for residual shunting.
Superior sinus venosus ASDs, often associated with anomalous pulmonary venous drainage, require careful dissection to delineate pulmonary vein anatomy and avoid injury to adjacent structures, such as the phrenic nerve. Repairs may involve 1-patch, 2-patch, or Warden procedures, depending on the defect's complexity and anatomical variation. The Warden procedure, which redirects systemic venous return and performs pulmonary venous baffling, provides durable outcomes but requires meticulous attention to tension-free anastomosis and avoidance of complications like SVC obstruction or sinus node dysfunction.[16]
Primum ASDs, characterized by a deficiency of atrial septal tissue between the atrioventricular valves, require direct suturing of a patch to valve tissue while avoiding the conduction system and underlying ventricular septum. Mitral valve cleft repair is often performed concurrently to optimize valve competency.[17][18]
Minimally invasive techniques, such as transxyphoid, ministernotomy, and transaxillary approaches, aim to reduce surgical trauma and improve cosmetic outcomes. These methods use smaller incisions and specialized cannulation techniques, maintaining effective access for defect repair while minimizing risks such as chest wall deformities. Robot-assisted and thoracoscopic approaches offer even less invasive alternatives, though they have limitations in certain populations, such as young children with small femoral vessels.[19][20][21][22]
Despite the evolution of surgical methods, complications such as patch dehiscence, thromboembolism, and arrhythmias remain rare but noteworthy. In patients with pulmonary hypertension, complete defect closure may lead to hemodynamic challenges, occasionally requiring fenestration of the patch. Advances in surgical and minimally invasive techniques continue to refine ASD repair, offering improved outcomes and tailored approaches for a wide spectrum of patients.[23][24]
ASDs are common congenital heart defects and can range from clinically asymptomatic lesions to pathologies that cause pulmonary hypertension, systemic cyanosis, and vascular complications such as strokes. Most small defects spontaneously close in the 1st year of life. However, large defects associated with significant systemic-to-pulmonary shunts and systemic oxygen desaturation require percutaneous or surgical intervention.
Differential Diagnosis
The differential diagnosis of ASDs includes the following:
- Ventricular septal defect
- Cyanotic congenital heart diseases from sinus venosus and coronary sinus defects
- Total anomalous pulmonary venous return
- Pulmonary stenosis
- Truncus arteriosus
- Tricuspid atresia
A thorough clinical evaluation and pattern recognition help differentiate ASDs from these conditions, guide treatment decisions, prevent harmful interventions, ensure efficient resource use, and lead to better outcomes.
Prognosis
The prognosis for individuals with ASDs is primarily influenced by the defect's size, the occurrence of complications, and the age at diagnosis. Small defects often close spontaneously in infancy and may not require intervention, while larger defects can lead to symptoms like right-sided heart failure, arrhythmias, and pulmonary hypertension if untreated. Timely closure of significant ASDs typically results in favorable outcomes, with a reduced risk of long-term complications. However, untreated large defects, particularly those associated with Eisenmenger syndrome, can lead to irreversible damage and reduced life expectancy.
Complications
The complications of ASDs include atrial dysrhythmias, pulmonary arterial hypertension, right-sided congestive heart failure, transient ischemic attack or stroke, and Eisenmenger syndrome. Prevention of complications associated with this condition focuses on early detection and appropriate intervention. Timely closure of significant defects can reduce the risk of developing long-term cardiovascular problems.
Deterrence and Patient Education
Primary prevention of ASDs focuses on reducing the risk of congenital heart defects through measures such as avoiding teratogenic exposures during pregnancy, including alcohol and certain medications, and managing maternal health conditions like diabetes. Genetic counseling may also help families with a history of congenital heart defects understand the risk for future pregnancies.
Secondary prevention involves early detection and monitoring of ASDs in newborns and children, typically through routine pediatric screenings such as echocardiograms when heart murmurs or symptoms are noted. For individuals diagnosed with ASDs, regular follow-up is essential to monitor for complications like atrial dysrhythmias and pulmonary hypertension. Timely interventions, including percutaneous or surgical repair, can prevent progression to more severe complications, such as Eisenmenger syndrome or stroke.
Pearls and Other Issues
Key points to remember about evaluating and managing ASDs include the following:
- ASDs are one of the most common types of congenital heart defects and are present in about 25% of live births.
- The most common type of ASD is the ostium secundum defect.
- The transcutaneous percutaneous approach to ASD closure is only indicated in patients with ostium secundum defects. Surgical intervention is required for the ostium primum, sinus venosus, and coronary sinus defects.
- Transcutaneous percutaneous closure of ASD is contraindicated in patients with increased pulmonary hypertension.
- Dysrhythmias occurring due to ASDs are treated with cardioversion followed by anticoagulation. If cardioversion is not successful, rate control with medications and anticoagulation are necessary.
- Eisenmenger syndrome is a late complication of ASD that occurs secondary to pulmonary hypertension.
Primary prevention of ASDs includes avoiding teratogenic exposures during pregnancy, managing maternal health conditions, and offering genetic counseling for at-risk families. Secondary prevention involves early detection through screenings and timely interventions, such as repair and regular monitoring, to prevent complications like pulmonary hypertension or stroke.
Enhancing Healthcare Team Outcomes
Improving health outcomes for patients with ASDs requires an interprofessional team, with significant input from the patient's pediatrician, primary care physician, and nurse practitioner. In most cases, ASD is initially diagnosed by the primary provider, who then refers the patient to a cardiologist.
Both adults and children with ASDs require consistent follow-up with cardiologists to monitor heart structure and function, as well as hemodynamic status. Clinicians should monitor asymptomatic patients. All affected individuals should be educated that elective closure reduces the risk of pulmonary hypertension.
When a patient requires intervention, working with cardiac interventionists and thoracic surgeons experienced in caring for children and adults with congenital heart disease provides the best outcomes. Monitoring with echocardiograms assesses the heart's functional health and right heart function. Dysrhythmias require aggressive control. Pregnancy is not recommended for women with large ASDs and pulmonary hypertension.
Due to the challenges of the disease and family concerns, the nurse plays an important role in family education. The family must be taught the signs and symptoms of complications and when the patient should return for further evaluation. The nurse should help ensure the patient has regular follow-up appointments. During follow-up after the procedure, the nurse should monitor the patient for changes in vital signs, new murmurs, or unexplained fever. Open communication between the interprofessional team is recommended to achieve the best outcomes with minimal complications.
Media
(Click Image to Enlarge)
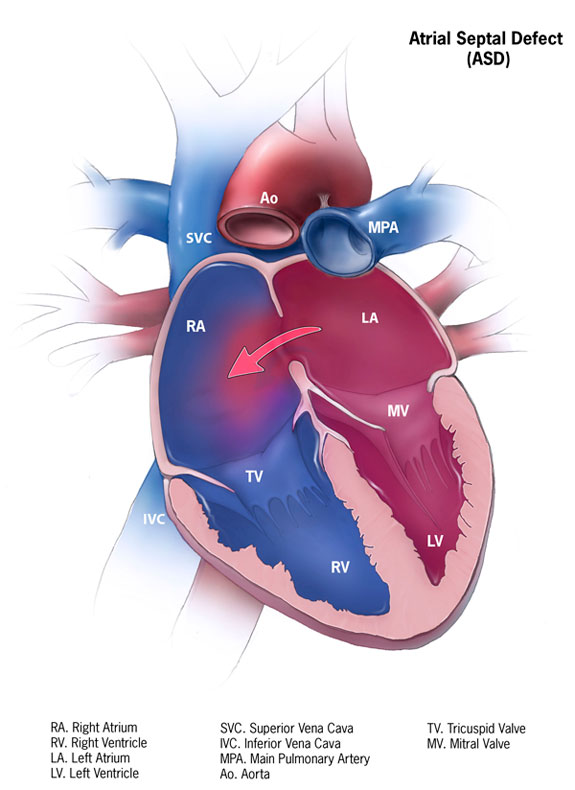
Atrial Septal Defect. This illustration shows the communication between the right and left atria and the shunting direction. Labeled structures include the right and left atria, the right and left ventricles, the superior and inferior vena cavae, the main pulmonary artery, the aorta, and the tricuspid and mitral valves.
Contributed by Centers for Disease Control and Prevention (Public Domain)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Meissner I, Whisnant JP, Khandheria BK, Spittell PC, O'Fallon WM, Pascoe RD, Enriquez-Sarano M, Seward JB, Covalt JL, Sicks JD, Wiebers DO. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clinic proceedings. 1999 Sep:74(9):862-9 [PubMed PMID: 10488786]
Celermajer DS. Atrial septal defects: even simple congenital heart diseases can be complicated. European heart journal. 2018 Mar 21:39(12):999-1001. doi: 10.1093/eurheartj/ehx633. Epub [PubMed PMID: 29194471]
Aoki H, Horie M. Electrical disorders in atrial septal defect: genetics and heritability. Journal of thoracic disease. 2018 Sep:10(Suppl 24):S2848-S2853. doi: 10.21037/jtd.2018.02.53. Epub [PubMed PMID: 30305944]
Torres AJ. Hemodynamic assessment of atrial septal defects. Journal of thoracic disease. 2018 Sep:10(Suppl 24):S2882-S2889. doi: 10.21037/jtd.2018.02.17. Epub [PubMed PMID: 30305948]
Chelu RG, Horowitz M, Sucha D, Kardys I, Ingremeau D, Vasanawala S, Nieman K, Paul JF, Hsiao A. Evaluation of atrial septal defects with 4D flow MRI-multilevel and inter-reader reproducibility for quantification of shunt severity. Magma (New York, N.Y.). 2019 Apr:32(2):269-279. doi: 10.1007/s10334-018-0702-z. Epub 2018 Aug 31 [PubMed PMID: 30171383]
van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos-Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. Journal of the American College of Cardiology. 2011 Nov 15:58(21):2241-7. doi: 10.1016/j.jacc.2011.08.025. Epub [PubMed PMID: 22078432]
Level 1 (high-level) evidenceKloesel B, DiNardo JA, Body SC. Cardiac Embryology and Molecular Mechanisms of Congenital Heart Disease: A Primer for Anesthesiologists. Anesthesia and analgesia. 2016 Sep:123(3):551-69. doi: 10.1213/ANE.0000000000001451. Epub [PubMed PMID: 27541719]
Naqvi N, McCarthy KP, Ho SY. Anatomy of the atrial septum and interatrial communications. Journal of thoracic disease. 2018 Sep:10(Suppl 24):S2837-S2847. doi: 10.21037/jtd.2018.02.18. Epub [PubMed PMID: 30305943]
Naik RJ, Shah NC. Teenage heart murmurs. Pediatric clinics of North America. 2014 Feb:61(1):1-16. doi: 10.1016/j.pcl.2013.09.014. Epub 2013 Oct 30 [PubMed PMID: 24267454]
El-Segaier M, Pesonen E, Lukkarinen S, Peters K, Ingemansson J, Sörnmo L, Sepponen R. Atrial septal defect: a diagnostic approach. Medical & biological engineering & computing. 2006 Sep:44(9):739-45 [PubMed PMID: 16941100]
Neema PK. Eisenmenger syndrome: an unsolved malady. Annals of cardiac anaesthesia. 2012 Oct-Dec:15(4):257-8. doi: 10.4103/0971-9784.101844. Epub [PubMed PMID: 23041681]
Martin SS, Shapiro EP, Mukherjee M. Atrial septal defects - clinical manifestations, echo assessment, and intervention. Clinical Medicine Insights. Cardiology. 2014:8(Suppl 1):93-8. doi: 10.4137/CMC.S15715. Epub 2015 Mar 23 [PubMed PMID: 25861226]
Behjati-Ardakani M, Golshan M, Akhavan-Karbasi S, Hosseini SM, Behjati-Ardakani MA, Sarebanhassanabadi M. The Clinical Course of Patients With Atrial Septal Defects. Iranian journal of pediatrics. 2016 Aug:26(4):e4649 [PubMed PMID: 27713810]
Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, Del Nido P, Fasules JW, Graham TP Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines for the management of adults with congenital heart disease). Circulation. 2008 Dec 2:118(23):2395-451. doi: 10.1161/CIRCULATIONAHA.108.190811. Epub 2008 Nov 7 [PubMed PMID: 18997168]
Level 1 (high-level) evidenceYang MC, Wu JR. Recent review of transcatheter closure of atrial septal defect. The Kaohsiung journal of medical sciences. 2018 Jul:34(7):363-369. doi: 10.1016/j.kjms.2018.05.001. Epub 2018 May 30 [PubMed PMID: 30063008]
Hopkins RA, Bert AA, Buchholz B, Guarino K, Meyers M. Surgical patch closure of atrial septal defects. The Annals of thoracic surgery. 2004 Jun:77(6):2144-9; author reply 2149-50 [PubMed PMID: 15172284]
Fasting H, Axelsen F, Søndergaard T. Atrial septal defect, primum type. Results of surgical closure in 46 patients. Scandinavian journal of thoracic and cardiovascular surgery. 1980:14(2):165-8 [PubMed PMID: 7433935]
Sadeghi AM, Laks H, Pearl JM. Primum atrial septal defect. Seminars in thoracic and cardiovascular surgery. 1997 Jan:9(1):2-7 [PubMed PMID: 9109219]
Ryan WH, Cheirif J, Dewey TM, Prince SL, Mack MJ. Safety and efficacy of minimally invasive atrial septal defect closure. The Annals of thoracic surgery. 2003 May:75(5):1532-4 [PubMed PMID: 12735575]
Liang T, XiangJun Z, XiaoJing M, Yun L, Leng CY. New minimally invasive technique to occlude secundum atrial septal defect in 53 patients. The Annals of thoracic surgery. 2006 Apr:81(4):1417-9 [PubMed PMID: 16564284]
Vida VL, Zanotto L, Zanotto L, Tessari C, Padalino MA, Zanella F, Pittarello D, Stellin G. Minimally invasive surgery for atrial septal defects: a 20-year experience at a single centre. Interactive cardiovascular and thoracic surgery. 2019 Jun 1:28(6):961-967. doi: 10.1093/icvts/ivz017. Epub [PubMed PMID: 30726938]
Vistarini N, Aiello M, Mattiucci G, Alloni A, Cattadori B, Tinelli C, Pellegrini C, D'Armini AM, Viganò M. Port-access minimally invasive surgery for atrial septal defects: a 10-year single-center experience in 166 patients. The Journal of thoracic and cardiovascular surgery. 2010 Jan:139(1):139-45. doi: 10.1016/j.jtcvs.2009.07.022. Epub 2009 Aug 25 [PubMed PMID: 19709683]
Galal MO, Wobst A, Halees Z, Hatle L, Schmaltz AA, Khougeer F, De Vol E, Fawzy ME, Abbag F, Fadley F. Peri-operative complications following surgical closure of atrial septal defect type II in 232 patients--a baseline study. European heart journal. 1994 Oct:15(10):1381-4 [PubMed PMID: 7821316]
Gatzoulis MA, Freeman MA, Siu SC, Webb GD, Harris L. Atrial arrhythmia after surgical closure of atrial septal defects in adults. The New England journal of medicine. 1999 Mar 18:340(11):839-46 [PubMed PMID: 10080846]