Introduction
Exercise-induced bronchoconstriction (EIB) occurs during physical exertion and involves a narrowing of the airway.[1][2] EIB occurs in 40% to 90% of people with asthma and up to 20% of those without asthma.[1][3][4] The benefits of regular exercise for all people are well established, and activity is an integral part of a healthy lifestyle. People suffering from EIB may avoid exertion due to symptoms of breathlessness, cough, chest tightness, and wheezing. Exercise avoidance has been shown to increase social isolation in adolescents, and it can lead to obesity and poor health.[3] Exercise has paradoxically been shown to improve EIB severity, pulmonary function, and reduce airway inflammation in people with asthma and EIB.[3][2] Early detection, diagnosis confirmed by the change in lung function during exercise, and treatment can improve quality of life and, when managed appropriately, allows patients to participate freely in exercise without limiting competition at the elite level.[3][5] Non-pharmacologic treatments addressing the root cause of EIB, an acute steep increase in ventilation and demand on the respiratory system, including warm-up exercises, and protecting the airway from cold, dry air, pollutants, and allergens is recommended. Pharmacologic treatments aimed at the pathophysiologic processes involved in the symptomatic bronchoconstriction, including short-acting beta-agonists (SABA), inhaled corticosteroids (ICS), leukotriene receptor antagonists (LTRA), and mast cell stabilizing agents (MCSA) are effective and without significant side effects.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
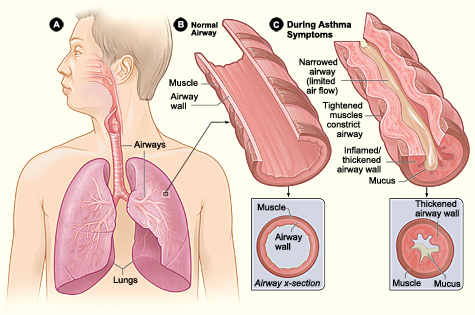
Exercise-induced bronchoconstriction (EIB) describes a transient airway narrowing occurring during physical exertion. The name EIB is favored over exercise-induced asthma (EIA) as recommended by the American Thoracic Society (ATS), and American Academy/College of Allergy, Asthma, and Immunology. EIB can also be denoted as EIB with asthma (EIBa), and EIB without asthma, (EIBwa).[1][2] It is caused by an acute large increase in the amount of air entering the airways that require heating and humidifying. In susceptible individuals, this results in inflammatory, neuronal, and vascular changes ultimately resulting in contraction of the bronchial smooth muscle and symptoms of dyspnea, cough, chest tightness, mucus production, and wheezing.[6]
Epidemiology
Exercise-induced bronchoconstriction occurs in 40% to 90% of people with asthma and up to 20% of the general population without asthma.[1][3][2] Elite athletes have an increased prevalence of 30% to 70%, especially in winter sports athletes and women.[1][3] Athletes frequently seek medical attention for respiratory symptoms.[7] Asthma is a significant health concern, and EIB can indicate poor asthma control.[3] Approximately 400 million people are projected to have asthma in 2024, with a large percentage expected to have EIB.[3][8] Annually, 250,000 people die from asthma complications. There is no cure for asthma, and quality of life is significantly impacted, including sleep, work, school, and exercise. Treatment for asthma is well established and similar to treatment for EIB. The treatment effectively decreases mortality in asthma patients.[8] Therefore, EIB detection, appropriate diagnosis, and treatment can have a significant impact.[3]
EIB occurrence is dependent on what sport is played. High-risk sports include long episodes of exercise greater than 5 to 8 minutes in certain environments such as cold, dry air or chlorinated pools such as long-distance running, cycling, cross country or downhill skiing, ice hockey, ice skating, high altitude sports, swimming, water polo, and triathlons. Medium risk sports include soccer, rugby, football, basketball, volleyball, baseball, cricket, and field hockey, where athletes rarely perform more than 5 to 8 minutes of continuous exercise. Low-risk sports include non-long distance track events inducing sprints, tennis, fencing, gymnastics, boxing, golf, weightlifting, bodybuilding, and martial arts.[2]
Screening for EIB is not supported by quality evidence, and further research and development of a symptom measurement tool is needed.[1][6]
Pathophysiology
Exercise-induced bronchoconstriction results from the alteration of normal lung physiology occurring with evaporative water loss, thermal changes, and irritant exposure induced by a large increase in minute ventilation and demand on the respiratory system to heat and humidify air with exercise-related hyperpnea.[9][10][1] Ventilation increases by 200 L/min, and airway cooling with mucosal dehydration occur. As cells are dehydrated, there is an increase in osmolality, and the cells shrink in size, leading to an increase in cough, mucus, and loss of the physical barrier function of the epithelium.[9] Decreased osmolality and increased electrolyte concentrations are thought to cause a regulatory increase in cell volume pulling fluid from the submucosal layer resulting in edema and release of inflammatory mediators, including histamine, leukotrienes, cysteine, tryptase, prostaglandins and mast cell degranulation. Bronchial blood flow is increased.[1][10][9][6] Reactive oxygen species are generated with increased lipid peroxidation.[8] Sensory and autonomic nerves are activated.[1] The results are direct and neuronal activation of bronchoconstriction.[1][10]
Also, nasal breathing becomes insufficient, leading to mouth breathing and increased exposure of lung surfaces to pollutants, irritants, and allergens, which result in the generation of reactive oxygen species and a neutrophilic inflammatory response.[3][2][11] Some research shows people genetically unable to counteract oxidative stress with glutathione may have an increased risk of EIB.[11]
Changes in mucus composition and decrease function of mucociliary clearance can lead to mucus plugging and bacterial growth.[9] Intense physical training can independently lead to transient immune suppression with a shift to T2 lymphocyte response and has been linked to increased atopy and viral upper respiratory infection (URI).[2]
Long term exposure can lead to epithelial damage and shedding, with remodeling and inflammation resembling asthma.[3] The process is partially reversible as studies have shown the lung damage and hyperresponsiveness improves over weeks to years when exposure and exercise are stopped.[3][2]
The osmotic theory is supported by evidence that EIB severity is directly related to the rate of water loss in the airways, EIB can be prevented by inhaling fully humidified air at body temperature, and bronchoconstriction can also be induced by inhalation of hyperosmolar saline.[9] Studies have shown an increased number of epithelial cells in sputum samples and elevated exhaled nitric oxide indicative of increased airway inflammation, damage, and shedding in those with EIB.[1] Exposure to foreign substances as a contributor to EIB is supported by evidence that pollutants such as fossil fuels and ozone contribute to EIB in environments such as ice rinks or practice fields close to major roadways. Competitive swimmers have increased EIB likely due to the inhalation of trichloramine.[1][2] Exercising in areas with high pollen counts can also increase EIB.[1]
Interestingly, EIB has not been proven to affect exercise or athletic performance negatively.[3][12]
History and Physical
Symptoms of exercise-induced bronchoconstriction can include mild to moderate symptoms of chest tightness, wheezing, coughing, and dyspnea that occurs within 15 minutes after 5 to 8 minutes of high-intensity aerobic training.[1][3][2] Reports of severe symptoms with respiratory failure and death occur rarely. Symptoms may occur more often in specific environments with cold, dry air or high concentration of respiratory irritants. Symptoms usually resolve spontaneously within 30 to 90 minutes and induce a refractory period of 1 to 3 hours, where continued exercise does not produce bronchoconstriction.[1] Patients may also be asymptomatic, and therefore EIB may be underdiagnosed.
Risk factors include a personal or family history of asthma, a personal history of atopy or allergic rhinitis, exposure to cigarette smoke, participating in high-risk sports (see epidemiology), living and practicing in areas with high levels of pollution, and female gender. Some small studies suggest sugar-sweetened beverages may increase risk by increasing inflammation and adiposity.[13][11]
Evaluation
Clinical diagnosis by symptoms has low sensitivity and specificity, and some patients are asymptomatic. Standardized testing for diagnosis includes direct and indirect methods and usually involves spirometry measurement of FEV1 changes from baseline expressed as a percent decrease.[14][1][3]
Direct stimulation of smooth muscle receptors by methacholine to induce bronchoconstriction is well established. Sensitivity at predicting EIB has been reported to be 58.6% to 91.1%.[14]
Indirect testing, which is more specific for EIB, can involve aerobic exercise in a controlled environment with cold, dry air as these conditions are known to precipitate EIB in susceptible individuals. Alternatives to exercise testing include eucapnic voluntary hyperpnea or hyperventilation of dry air, and airway provocation testing, including hyperosmolar 4.5% saline or dry powder mannitol, which act to dehydrate the respiratory epithelium to induce EIB. The sensitivity and specificity of the alternatives are not well established and may vary by the lab.[14][1][6]
Exercise Challenge Testing
The ATS recommends exercise challenge testing in a controlled dry environment. Exercise testing parameters outlined by the ATS include recommendations on ventilation level, heart rate, time at maximal capacity, and medications to hold before testing, including caffeine. The patient must avoid entering the refractory period before exercise testing. Serial measurements of spirometry, specifically FEV1, are recorded during exercise at 5,10,15, and 30 minutes. FEV1 has good repeatability and is recommended by ATS to diagnose EIB.[1] A fall in FEV1 of greater than or equal to 10% is diagnostic for EIB, with mild at 10% to less than 25%, moderate at 25% to less than 50%, and severe at 50% or greater.[1][3] Some labs use 15% as the minimum reduction for diagnosis as it is more specific. Some patients may require multiple exercise sessions to confirm the diagnosis as the reproducibility is 76%.[1]
Pulmonary Function Testing
CHEST guidelines provide 2B recommendation for pulmonary function tests (PFT), exercise or bronchoprovocation studies as described above, and allergy testing for common airborne allergens to distinguish between the most common causes of exercise-induced cough.[4]
Fractional Excretion of Nitric Oxide (FENO) Testing
A few smaller studies suggest fractional excretion of nitric oxide (FENO) may replace FEV1 to diagnose and measure the severity of EIB instead of spirometry with FEV1 percent change. The FENO can be used with direct or indirect testing and can be easily performed by younger children. FENO measures the T helper cell type 2 (Th2) inflammatory response, versus airway hyperresponsiveness, and may be better suited to distinguish EIB from another etiology of symptoms. Cut off values of 27 to 46 ppb FENO have been suggested as diagnostic, with greater than 46 ppb being 100% specific.[15][16][17]
Treatment / Management
Short-Acting Beta Agonists (SABA)
American Thoracic Society (ATS) guidelines from 2013 provide a strong recommendation with high-quality evidence for short-acting beta 2 agonists (SABA) use 5 to 20 minutes (optimally 15) minutes before exercise.[1] The bronchodilation is of rapid onset and can last 2 to 4 hours. Tolerance can develop with frequent SABA use and is likely due to the downregulation of the beta 2 receptors. SABA's are the preferred first-line treatment and have limited side effects.[1][18] Mechanism of action is through relaxation of the airway smooth muscles and inhibition of mast cell degranulation.[19](A1)
Inhaled Corticosteroids (ICS)
If symptoms are not well controlled with the SABA or patient is using SABA daily, additional medications can be added after medication adherence, and proper use has been confirmed. It is estimated that 15% to 20% of the patients will not respond to SABA treatment. ATS provides a strong recommendation with moderate-quality evidence for daily inhaled corticosteroids (ICS).[1][18] ICS may take 2 to 4 weeks for the maximal benefit. ICS appears to be more effective in patients with underlying asthma and are dose-dependent. ICS is not effective when used intermittently before exercise.[1] ICS has multiple well-studied benefits in patients with asthma, including a reduction in mortality.[18](A1)
Leukotriene Receptor Antagonists (LTRA)
ATS provides a strong recommendation with moderate-quality evidence for daily leukotriene receptor antagonist (LTRA) to address the inflammatory mediator release involved in EIB.[1][18] LTRAs may take 2-4 weeks for the maximal benefit.[1] LTRAs, including montelukast, zafirlukast, and zileuton, provide longer-lasting bronchodilation and are not associated with tolerance. The effect on FEV1 reduction is less than with ICS or SABA. The choice between adding ICS of LTRA is patient-specific.[1](A1)
Mast Cell Stabilizing Agents (MCSA)
ATS also makes strong recommendations with high-quality evidence for adding mast cell stabilizer (MCSA) before exercise. Mast cell degranulation plays a key role in EIB pathology. There is no additional benefit when MCSA is combined with SABA, and MCSAs are less effective than SABA. MCSA is not widely available in the U.S.[1](A1)
Short-acting Muscarinic Antagonists (SAMA)
Inhaled anticholinergic agents are weakly recommended with low-quality evidence. Short-acting muscarinic antagonists (SAMA) are less effective than SABA; however, they can be used in combination when SABA tolerance develops.[1][18](A1)
Antihistamine
An antihistamine may be beneficial in patients with underlying allergies.[1](A1)
Long-acting Beta Agonists (LABA)
ATS strongly recommends against, with high-quality evidence, the daily use of long-acting beta 2 agonists (LABA) because the potential side effects do not outweigh the benefits.[1](A1)
Non-pharmacologic Interventions
Nonpharmacologic treatment approaches are strongly recommended with moderate-quality evidence.[1] The first is to induce a refractory period by performing 10 to 15 minutes of vigorous activity, decreasing EIB for the next 2 hours. However, high or low-intensity exercise does not induce the refractory period.[1][20] The refractory period is theorized to occur due to an increase in bronchodilating PGE2 and/or desensitization to bronchoconstriction mediators.[9][20](A1)
Secondly, masks to promote warming and humidification of the air with exercise are weakly recommended with low-quality evidence and may be as effective as using a SABA.[1][20] The severity of bronchoconstriction is related to the humidity of the air inhaled, and new heat and moisture exchanger masks may reduce the severity and SABA use.[20](A1)
Thirdly, wearing a mechanical barrier mask and/or avoiding exercise in environments high in pollen, allergens, ozone, exhaust, and high levels of chlorine can reduce EIB. Alternatives for pool disinfection are available.[3][20] The patient can also choose a sport with a lower risk of EIB, where 5 to 8 minutes of sustained intense activity is less likely to occur.[2]
Increasing general exercise tolerance and endurance and decreasing bodyweight if the patient is obese are helpful, and a Cochrane review found exercise to improve EIB severity, pulmonary function, and reduce airway inflammation in people with asthma and EIB.[3][2][1][21] Exercise has been shown to independently reduce markers of airway inflammation and reduce the severity of EIB in some studies.[3](A1)
Caffeine may offer protection against bronchoconstriction and decreased ventilatory dead space and decreases exercise-induced hypoxemia and respiratory muscle fatigue when used before exercise.[20]
Low salt diet and supplementation with fish oil and vitamin C may be beneficial in some patients.[1] A Cochrane review of vitamins C and E to protect from oxidative damage found insufficient evidence to make a recommendation.[8] ATS recommends against lycopene supplementation.[1](A1)
Non-invasive positive pressure ventilation (NIPPV)
Treatment with non-invasive positive pressure ventilation (NIPPV) support has been shown to decrease airway reactivity in children with asthma and was evaluated in combination with respiratory physical therapy in one study for EIB in asthmatics with positive results in a Brazilian study. The benefit of NIPPV was postulated to involve the stretch mechanism in airways and with the resulting induction of an inhibitory pathway that stopped the cycle of inflammation and promoted bronchodilation. Participants completed 10 1-hr sessions twice a week. The first 20 minutes were spent completing respiratory exercises while sitting and supine, followed by either continuous positive airway pressure (CPAP) at 8 cmH2O, inspiratory muscle training, or bilevel positive airway pressure (BiPAP) at 8/12 cm H2O. Results included exercise FEV1 measurements as outlined by ATS, FENO to measure airway inflammation, and a questionnaire for symptom measurement. PPV both bilevel and CPAP reduced EIB. Respiratory muscle training (RMT) was shown in the same study to reduce medication use and increase respiratory muscle strength.[10] This recent study offers additional nonpharmacologic options for the treatment of EIB.(B3)
Treatment to reduce the perception of symptoms
Another treatment approach involves reducing the perception of symptoms. Breathing control, including yoga, or supervised breathing training, has been shown in some small studies to decrease symptoms, reduce medication use, decrease anxiety and depression associated with EIB, and increase the quality of life. Further research and adaptation for use during EIB events are needed.[20] Respiratory muscle training involves first ensuring proper breathing technique and then using a device to increase inspiratory and expiratory muscle strength. A Cochrane review of a small number of studies reported no evidence for or against respiratory muscle training. It has shown promise with COPD, exercise-induced laryngeal obstruction (EILO), and stridor and offers an additional inexpensive treatment for EIB.[20][21]
Differential Diagnosis
Symptoms of chest tightness, wheezing, coughing, and dyspnea occurring with exercise can indicate pathology along the entire airway.[7] Exercise-induced bronchoconstriction is not easily diagnosed by clinical symptoms, and objective data of a decrease in lung function with exercise is required.[1]
Nasal Airway
The differential includes diseases of the nasal airway, including exercise-induced rhinosinusitis, allergic rhinitis, upper airway cough syndrome, upper respiratory infection (URI), and anatomic abnormalities. The nasal airway participates in filtering, humidifying, and regulating airway resistance and, therefore, nasal airway conditions can be comorbid with EIB.[7][22] Treatment for upper airway diseases includes avoidance of triggers and irritants, MCSA, and LTRA’s, used for EIB in addition to intranasal corticosteroids, decongestants, and immunotherapy.[22][1][4][2][7]
Pharynx and Larynx
The differential also includes diseases of the pharynx and larynx, including EILO, previously called exercise-induced vocal cord dysfunction. Athletes with EILO present similarly to EIB; however, treatment is not, and differentiation is required with appropriate testing, including visualization of the larynx with exercise.[1][4][2][7]
Lower Airways
Lastly, the differential included diseases of the airways, including asthma, respiratory tract infection (RTI), and gastroesophageal reflux disease (GERD).[1][4][2][7]
Cardiac
Cardiac causes of exertional dyspnea should be investigated, especially in children.[6]
Gastrointestinal
GERD
Others
CHEST guidelines recommend the evaluation of cough in adolescent athletes include asthma, EIB, respiratory tract infection, upper airway cough syndrome, and environmental exposures.[4]
Less common causes include exercise-induced pulmonary edema, mainly seen in water immersion sports or high-altitude winter sports, PE, saltwater aspiration syndrome, and smoking. No studies were identified by the authors of CHEST guidelines for exercise-induced cough caused by cardiac abnormalities.[4]
Prognosis
With appropriate treatment, athletes can perform at the same level as peers and compete and win a medal in the Olympics and other international competitions.[2]
Complications
Complications involve sequela of poorly managed asthma, reduction in physical activity, and a sedentary lifestyle.
Pearls and Other Issues
It is important to consider the anti-doping regulations present in many athletic programs. Some medications utilized for Exercise-induced bronchoconstriction may require a therapeutic use exemption (TUE). ICS, LTRA's, MCSA's, inhaled anticholinergics, SABA (including albuterol and formoterol), antibiotics, 1st generation antihistamines with or without oral decongestant, nasal ipratropium, dextromethorphan, nasal corticosteroids, topical decongestants, and proton pump inhibitors (PPIs) in appropriate doses do not enhance performance and therefore do not require a TUE.[1] Oral steroids and terbutaline are banned and require TUE.[1][4]
Enhancing Healthcare Team Outcomes
Care coordination between primary care providers, pulmonologists, ENT, sports medicine practitioners, and coaches is required to ensure proper diagnosis and treatment of Exercise-induced bronchoconstriction. Sports coaches play an important role in identifying athletes who are experiencing symptoms during the practice or who express a desire to quit the sport due to poor fitness, as it can be a sign of EIB. The education of coaches is important to ensure adherence to face protection from cold, dry air, exposure to pollutants, particulate matter, and allergens during practice. Coaches can also work with school administration to ensure practice locations and pool chemicals are safe for practice. Primary care sports medicine practitioners may be the first contact for athletes with symptoms. Proper testing for diagnosis is required as clinical symptoms are not sensitive or specific, and some patients are asymptomatic. Differential diagnosis includes the entire airway, and each part may contribute to symptoms requiring referral to an otolaryngologist. Coordination with pulmonology for testing may be required. Pulmonology may already be involved in asthma management in hard to control patients. Communication and coordination will lead to an optimal diagnosis, treatment, treatment adherence, and control of bronchoconstriction, allowing patients to participate in an activity as they desire.
Media
(Click Image to Enlarge)
References
Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell KW, Hull JH, Storms WW, Weiler JM, Cheek FM, Wilson KC, Anderson SD, American Thoracic Society Subcommittee on Exercise-induced Bronchoconstriction. An official American Thoracic Society clinical practice guideline: exercise-induced bronchoconstriction. American journal of respiratory and critical care medicine. 2013 May 1:187(9):1016-27. doi: 10.1164/rccm.201303-0437ST. Epub [PubMed PMID: 23634861]
Level 1 (high-level) evidenceBonini M, Silvers W. Exercise-Induced Bronchoconstriction: Background, Prevalence, and Sport Considerations. Immunology and allergy clinics of North America. 2018 May:38(2):205-214. doi: 10.1016/j.iac.2018.01.007. Epub 2018 Mar 2 [PubMed PMID: 29631730]
Jayasinghe H, Kopsaftis Z, Carson K. Asthma Bronchiale and Exercise-Induced Bronchoconstriction. Respiration; international review of thoracic diseases. 2015:89(6):505-12. doi: 10.1159/000433559. Epub 2015 Jun 11 [PubMed PMID: 26068579]
Boulet LP, Turmel J, Irwin RS, CHEST Expert Cough Panel. Cough in the Athlete: CHEST Guideline and Expert Panel Report. Chest. 2017 Feb:151(2):441-454. doi: 10.1016/j.chest.2016.10.054. Epub 2016 Nov 16 [PubMed PMID: 27865877]
Vlietstra RE, Holmes DR Jr. PTCA in acute ischemic syndromes. Current problems in cardiology. 1987 Dec:12(12):703-62 [PubMed PMID: 2963730]
Aggarwal B, Mulgirigama A, Berend N. Exercise-induced bronchoconstriction: prevalence, pathophysiology, patient impact, diagnosis and management. NPJ primary care respiratory medicine. 2018 Aug 14:28(1):31. doi: 10.1038/s41533-018-0098-2. Epub 2018 Aug 14 [PubMed PMID: 30108224]
Olin JT, Hull JH. Exercise and the Total Airway: A Call to Action. Immunology and allergy clinics of North America. 2018 May:38(2):xv-xix. doi: 10.1016/j.iac.2018.02.001. Epub 2018 Feb 19 [PubMed PMID: 29631744]
Wilkinson M, Hart A, Milan SJ, Sugumar K. Vitamins C and E for asthma and exercise-induced bronchoconstriction. The Cochrane database of systematic reviews. 2014 Jun 17:2014(6):CD010749. doi: 10.1002/14651858.CD010749.pub2. Epub 2014 Jun 17 [PubMed PMID: 24936673]
Level 1 (high-level) evidenceKippelen P, Anderson SD, Hallstrand TS. Mechanisms and Biomarkers of Exercise-Induced Bronchoconstriction. Immunology and allergy clinics of North America. 2018 May:38(2):165-182. doi: 10.1016/j.iac.2018.01.008. Epub [PubMed PMID: 29631728]
David MMC, Gomes ELFD, Mello MC, Costa D. Noninvasive ventilation and respiratory physical therapy reduce exercise-induced bronchospasm and pulmonary inflammation in children with asthma: randomized clinical trial. Therapeutic advances in respiratory disease. 2018 Jan-Dec:12():1753466618777723. doi: 10.1177/1753466618777723. Epub [PubMed PMID: 29865929]
Level 3 (low-level) evidenceRundell KW, Smoliga JM, Bougault V. Exercise-Induced Bronchoconstriction and the Air We Breathe. Immunology and allergy clinics of North America. 2018 May:38(2):183-204. doi: 10.1016/j.iac.2018.01.009. Epub [PubMed PMID: 29631729]
Price OJ, Hull JH, Backer V, Hostrup M, Ansley L. The impact of exercise-induced bronchoconstriction on athletic performance: a systematic review. Sports medicine (Auckland, N.Z.). 2014 Dec:44(12):1749-61. doi: 10.1007/s40279-014-0238-y. Epub [PubMed PMID: 25129699]
Level 1 (high-level) evidenceEmerson SR, Rosenkranz SK, Rosenkranz RR, Kurti SP, Harms CA. The potential link between sugar-sweetened beverage consumption and post-exercise airway narrowing across puberty: a longitudinal cohort study. Public health nutrition. 2016 Sep:19(13):2435-40. doi: 10.1017/S1368980015003109. Epub 2015 Oct 30 [PubMed PMID: 26514591]
Dreßler M, Friedrich T, Lasowski N, Herrmann E, Zielen S, Schulze J. Predictors and reproducibility of exercise-induced bronchoconstriction in cold air. BMC pulmonary medicine. 2019 May 16:19(1):94. doi: 10.1186/s12890-019-0845-3. Epub 2019 May 16 [PubMed PMID: 31097027]
Dreßler M, Salzmann-Manrique E, Zielen S, Schulze J. Exhaled NO as a predictor of exercise-induced asthma in cold air. Nitric oxide : biology and chemistry. 2018 Jun 1:76():45-52. doi: 10.1016/j.niox.2018.03.004. Epub 2018 Mar 8 [PubMed PMID: 29526567]
Kim K, Cho HJ, Yoon JW, Choi SH, Sheen YH, Han MY, Baek H. Exhaled nitric oxide and mannitol test to predict exercise-induced bronchoconstriction. Pediatrics international : official journal of the Japan Pediatric Society. 2018 Aug:60(8):691-696. doi: 10.1111/ped.13599. Epub [PubMed PMID: 29786927]
Alving K. FeNO and the Prediction of Exercise-Induced Bronchoconstriction. The journal of allergy and clinical immunology. In practice. 2018 May-Jun:6(3):863-864. doi: 10.1016/j.jaip.2017.12.038. Epub [PubMed PMID: 29747989]
Backer V, Mastronarde J. Pharmacologic Strategies for Exercise-Induced Bronchospasm with a Focus on Athletes. Immunology and allergy clinics of North America. 2018 May:38(2):231-243. doi: 10.1016/j.iac.2018.01.011. Epub [PubMed PMID: 29631732]
Bonini M, Di Mambro C, Calderon MA, Compalati E, Schünemann H, Durham S, Canonica GW. Beta₂-agonists for exercise-induced asthma. The Cochrane database of systematic reviews. 2013 Oct 2:(10):CD003564. doi: 10.1002/14651858.CD003564.pub3. Epub 2013 Oct 2 [PubMed PMID: 24089311]
Level 1 (high-level) evidenceDickinson J, Amirav I, Hostrup M. Nonpharmacologic Strategies to Manage Exercise-Induced Bronchoconstriction. Immunology and allergy clinics of North America. 2018 May:38(2):245-258. doi: 10.1016/j.iac.2018.01.012. Epub [PubMed PMID: 29631733]
Weatherald J, Lougheed MD, Taillé C, Garcia G. Mechanisms, measurement and management of exertional dyspnoea in asthma: Number 5 in the Series "Exertional dyspnoea" Edited by Pierantonio Laveneziana and Piergiuseppe Agostoni. European respiratory review : an official journal of the European Respiratory Society. 2017 Jun 30:26(144):. doi: 10.1183/16000617.0015-2017. Epub 2017 Jun 14 [PubMed PMID: 28615308]
Steelant B, Hox V, Hellings PW, Bullens DM, Seys SF. Exercise and Sinonasal Disease. Immunology and allergy clinics of North America. 2018 May:38(2):259-269. doi: 10.1016/j.iac.2018.01.014. Epub 2018 Mar 2 [PubMed PMID: 29631734]