Introduction
Mitral regurgitation (MR) is one of the most common valvular abnormalities, second only to aortic valve stenosis.[1][2] Treatment depends on the duration and severity of this condition. Acute severe MR, often caused by papillary muscle rupture or leaflet perforation from infective endocarditis, leads to significant hemodynamic instability, acute volume overload, and congestion—necessitating immediate surgical intervention.[3]
Chronic MR can be categorized into 2 types: primary and secondary. Primary MR is caused by a primary abnormality of 1 or more components of the valve apparatus (leaflets, chordae tendineae, papillary muscles, annulus). In contrast, secondary MR is caused by alterations in left ventricular or left atrial function and shape. If mild and asymptomatic, chronic MR can be medically managed and monitored over time. However, patients with symptomatic chronic MR should undergo evaluation for potential surgical intervention.[3][4] In cases of patients who are asymptomatic with chronic MR, surgical consideration may be warranted if they exhibit signs of depressed left ventricular function and dilatation, atrial fibrillation, or pulmonary hypertension.[5][6]
Transthoracic echocardiography (TTE) is the initial imaging modality for screening and evaluating mitral valve morphology and pathology and determining the mechanism of MR. TTE also helps quantify the severity of MR and assess left ventricular function and size, and left atrial size.[3] Various parameters are used for qualitative and quantitative MR assessment, including a 2-dimensional analysis of mitral valve leaflet characteristics, motion, coaptation, MR jet to left atrial area ratio, vena contracta, effective regurgitant orifice area, regurgitant volume, regurgitant area, left ventricular ejection fraction, and left ventricular end-diastolic area.
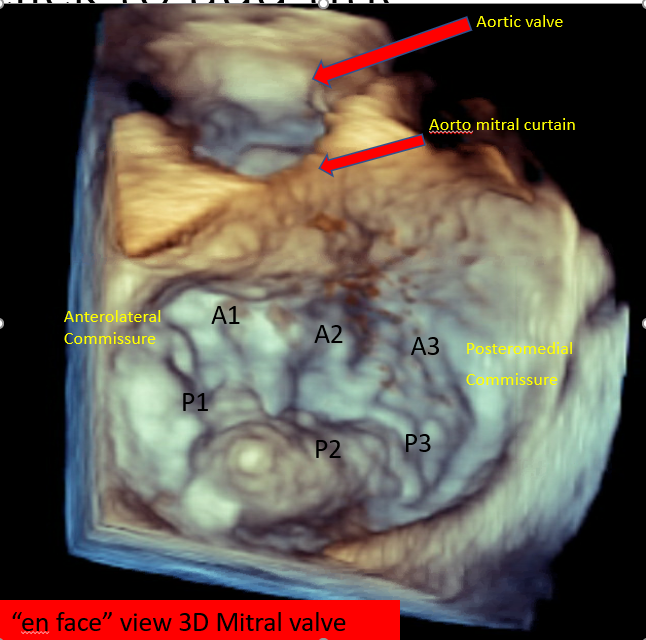
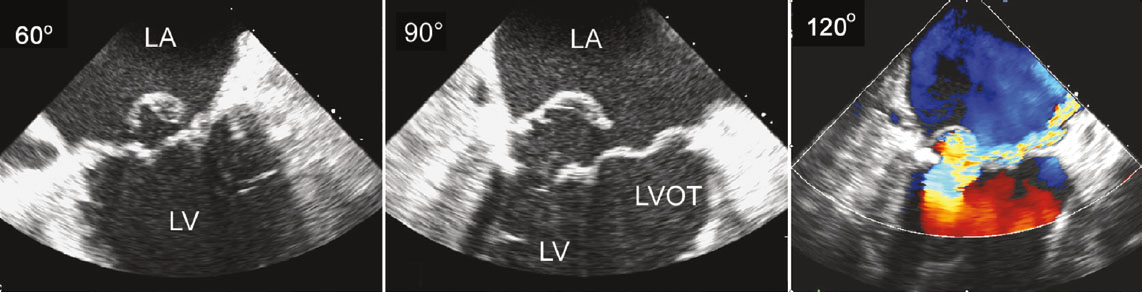
In cases where TTE images do not provide adequate information, transesophageal echocardiography (TEE) can offer a more detailed assessment.[7][8] Three-dimensional TEE can provide an "enface" view of the MV, resembling a surgical inspection, which can greatly aid discussions and preprocedure planning (see Image. Mitral Valve, En Face View). In situations where TEE is contraindicated, cardiac magnetic resonance imaging is an alternative option, providing highly accurate data for MR assessment and evaluation of left ventricle dimensions.
Results from recent studies have shown percutaneous mitral valve repair as a viable alternative for high-surgical-risk patients suffering from severe symptomatic MR. This procedure has demonstrated low morbidity and mortality rates among many patients.[9] The Endovascular Valve Edge-to-Edge Repair Study Trial (EVEREST) 1 laid the groundwork, demonstrating the safety and feasibility of the edge-to-edge repair technique. The subsequent EVEREST 2 randomized control trial compared percutaneous edge-to-edge repair with surgical mitral valve repair/replacement; this suggested the surgical approach's superiority in reducing MR but also supported the long-term safety of the edge-to-edge repair device and its durability in reducing MR.[10][11]
The edge-to-edge leaflet repair device is a minimally invasive, catheter-based therapy based on the principle of the "Alfieri stitch," a surgical technique pioneered by Dr. Ottavio Alfieri, an Italian cardiothoracic surgeon. This technique involves bringing together the 2 flailing leaflets of the MV, resulting in reduced or eliminated regurgitation. Typically, this repair creates a double orifice based on the surgical edge-to-edge Alfieri repair.[12][13]
Many percutaneous options exist for patients with MR and multiple comorbidities, placing them at higher risk for surgical interventions.[14] These percutaneous techniques can be classified based on the specific site of the mitral apparatus they target, such as the leaflets (edge-to-edge repair), annulus (indirect or direct annuloplasty), chordae (neo-chords, percutaneous chord implantation), or left ventricle (percutaneous left ventricle remodeling).[14][15][16]
This article discusses primary and secondary MR and noninvasive catheter management options, including their indications, contraindications, procedural techniques, and complications. The primary focus of the discussion will be on the United States Food and Drug Administration's approved edge-to-edge repair devices.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
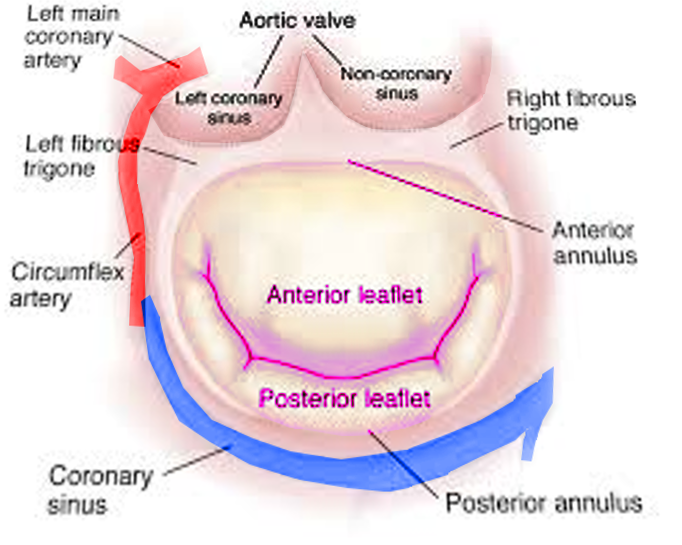
Andreas Vesalius first suggested the name "mitral" for the left-sided atrioventricular valve, given its resemblance with a bishop's miter.[17][18] The mitral valve apparatus is a complex anatomic structure that includes the anterior and posterior mitral leaflets, mitral annulus, subvalvular structures (chordae tendineae, papillary muscles) and left ventricle (see Images. Mitral Valve Anatomy and Mitral Valve, Transverse View). The mitral annulus is a saddle-shaped structure; the competence of the mitral valve depends on the correct interaction of different components of the mitral valve apparatus.[2][18][19] The anterior and posterior leaflets of the mitral valve should "coapt" to prevent MR during systole. An alteration in the functioning of any mitral valve component can lead to the development of MR.[20] The 2 types of MR are primary and secondary. Primary MR is a degenerative valve disease, while secondary MR is characteristically a functional myocardial disease, ie, ventricular remodeling (see Table 1. Etiology of Mitral Regurgitation).
Table 1. Etiology of Mitral Regurgitation
|
Primary MR |
Secondary MR |
| MV prolapse - myxomatous changes (prolapse, filial, ruptured, elongated chordae) | Ischemia (due to coronary artery disease ) |
| Degenerative MR (calcification, thickening) | Nonischemic cardiomyopathy |
|
Infectious (infective endocarditis, vegetation, perforation) Inflammatory (rheumatic, collagen vascular disease) Drug-induced Radiation-induced |
Annular dilation |
| Congenital (parachute mitral valve, cleft) |
Primary and secondary MR are further classified into stages A through D (see Table 2. Stages of Primary Mitral Regurgitation and Table 3. Stages of Secondary Mitral Regurgitation), indicating whether they are mild, moderate, or severe. Several interventions exist to treat severe MR, including surgical and nonsurgical. Patients with severe MR and who are at high or are a prohibitive risk for surgery are currently the only subset of patients recommended for catheter management. If untreated, severe MR can lead to fatal sequelae, including heart failure.[21]
Table 2. Stages of Primary Mitral Regurgitation
| Stage | Definition | Valve Anatomy | Valve Hemodynamics | Hemodynamic Consequences | Symptoms |
| A | At the risk of MR |
Mild mitral valve prolapse with normal coaptation Mild mitral valve thickening and leaflet restriction |
No MR jet or small central jet area <20% left atrium on Doppler Small vena contracta <0.3 cm |
None | Absent |
| B | Progressive MR |
Moderate to severe mitral valve prolapse with normal coaptation Rheumatic valve changes with leaflet restriction and loss of central coaptation Prior infective endocarditis |
Central jet MR 20% to 40% left atrium or late systolic eccentric jet MR Vena contracta <0.7 cm Regurgitant volume <60 mL Regurgitant fraction <50% Effective regurgitant orifice <0.40 cm2 Angiographic grade 1+ to 2+ |
Mild left atrium enlargement Left ventricle normal Normal pulmonary pressure |
Absent |
| C | Asymptomatic severe MR |
Severe MV prolapse with loss of coaptation or flail leaflet Rheumatic valve changes with leaflet restriction and loss of central coaptation Prior infective endocarditis Thickening of leaflets with radiation heart disease |
Central MR >40% left atrium area, holosystolic eccentric jet MR Vena contracta ≥0.7 cm Regurgitant volume ≥60 mL Regurgitant fraction ≥50% Effective regurgitant orifice ≥0.4 cm2 Angiographic grade 3+ to 4+ |
Moderate or severe left atrium enlargement Left ventricle enlargement Pulmonary hypertension may be present at rest or with exercise C1: Left ventricular ejection fraction >60% and left ventricular end-systolic dimension <40 mm C2: Left ventricular ejection fraction ≤60% and/or Left ventricular end-systolic dimension ≥40 mm |
Absent |
| D | Symptomatic severe MR |
Severe mitral valve prolapse with loss of coaptation or flail leaflet Rheumatic valve changes with leaflet restriction and loss of central coaptation Prior infective endocarditis Thickening of leaflets with radiation heart disease |
Central MR >40% left atrial area, holosystolic eccentric jet Vena contracta ≥0.7 cm Regurgitant volume ≥60 mL Regurgitant fraction ≥50% Effective regurgitant orifice ≥0.4 cm2 Angiographic grade 3+ to 4+ |
Moderate or severe left atrial enlargement Left ventricular enlargement Pulmonary hypertension present |
Dyspnea and reduced exercise tolerance |
Several valve hemodynamic criteria are provided for assessing MR, but not all criteria for each category will be present in each patient. Categorization of MR severity as mild, moderate, or severe depends on data quality and integration of these parameters in conjunction with other clinical evidence.[3]
Table 3. Stages of Secondary Mitral Regurgitation
| Stage | Definition | Valve Anatomy | Valve Hemodynamics | Associated Cardiac Findings | Symptoms |
| A | At risk of MR |
Normal mitral valve leaflets, chords, and annulus in a patient with coronary disease or cardiomyopathy |
No MR jet or small central jet area <20% left atrium on Doppler Small vena contracta <0.30 cm |
Normal or mildly dilated left ventricle size with fixed (infarction) or inducible (ischemia) regional wall motion abnormalities Primary myocardial disease with left ventricle dilation and systolic dysfunction |
Symptoms due to coronary ischemia or heart failure may be present that respond to revascularization and appropriate medical therapy. |
| B | Progressive MR | Regional wall motion abnormalities with mild tethering of mitral leafletAnnular dilation with mild loss of central coaptation of the mitral leaflets |
Effective regurgitant orifice <0.40 cm2 Regurgitant volume <60 mL Regurgitant fraction <50% |
Regional wall motion abnormalities with reduced left ventricle systolic function Left ventricle dilation and systolic dysfunction due to primary myocardial disease |
Symptoms due to coronary ischemia or heart failure may be present that respond to revascularization and appropriate medical therapy. |
| C | Asymptomatic severe MR |
Regional wall motion abnormalities and/or left ventricle dilation with severe tethering of mitral leaflet Annular dilation with severe loss of central coaptation of the mitral leaflets |
Effective regurgitant orifice ≥0.40 cm2 Regurgitant volume ≥60 mL Regurgitant fraction ≥50% |
Regional wall motion abnormalities with reduced left ventricle systolic function Left ventricle dilation and systolic dysfunction due to primary myocardial disease |
Symptoms due to coronary ischemia or heart failure may be present that respond to revascularization and appropriate medical therapy. |
| D | Symptomatic severe MR |
Regional wall motion abnormalities and/or left ventricular dilation with severe tethering of mitral leaflet Annular dilation with severe loss of central coaptation of the mitral leaflets |
Effective regurgitant orifice ≥0.40 cm2 Regurgitant volume ≥60 mL Regurgitant fraction ≥50% |
Regional wall motion abnormalities with reduced left ventricle systolic function Left ventricle dilation and systolic dysfunction due to primary myocardial disease |
Heart failure symptoms due to MR persist even after revascularization and optimization of medical therapy. Decreased exercise tolerance Exertional dyspnea |
Several valve hemodynamic criteria are provided for assessing MR severity, but not all criteria for each category will be present in each patient. Categorization of MR severity as mild, moderate, or severe depends on data quality and integration of these parameters in conjunction with other clinical evidence. The measurement of the proximal iso velocity surface area by 2D TTE in patients with secondary MR underestimates the true ERO because of the crescentic shape of the proximal convergence.[3]
Echocardiography is the primary tool used to assess the structure and function of the MV, systolic competence, and nonrestriction during diastole. Various types of mitral valve leaflet motion can indicate the underlying reason for the dysfunction (see Table 4. Mitral Valve Pathology Based on Echocardiography).[22] Severe MR is described as having a color flow jet that may be central and large (>6 cm or >30% of the left atrium area) or smaller, if eccentric, encircling the left atrium. Pulmonary vein flow may show systolic blunting or systolic flow reversal, vena contracta width ≥0.5 cm measured in the parasternal long-axis view, a regurgitant volume of ≥45 mL/beat, regurgitant fraction ≥40%, and/or regurgitant orifice area ≥0.30 cm2 according to the American College of Cardiology and American Heart Association.[23]
Table 4. Mitral Valve Pathology Based on Echocardiography
| Type | Leaflet Motion | Example |
| I | Normal leaflet motion |
Annular dilation without leaflet tethering Cleft or indentation Perforation |
| II | Excessive leaflet motion |
Billowing Prolapse Flail leaflet |
| III | Restricted leaflet motion |
Systolic restriction
Systolic and diastolic restriction
|
| IV | Systolic anterior motion |
Hypertrophic cardiomyopathy Post MV repair |
| V | Mixed conditions | Prolapse of 1 leaflet with restriction of another leaflet |
Preprocedural Anatomical Considerations
Certain conditions must be met to successfully grasp the mitral valve leaflets during transcatheter mitral valve repair. The leaflets must be pliable and noncalcified at the grasping site, and there must be no significant clefts or perforations. The shorter MitraClip clips NT and NTW also require a minimal posterior leaflet length of 6 mm. In comparison, the longer MitraClip clips XT and XTW require a minimal posterior leaflet length of 9 mm.[24] Furthermore, a transmitral gradient of less than 5 mm Hg and a mitral valve area of at least 4 cm2 are desirable to minimize the risk of mitral stenosis. If the mitral valve area is ≤3 cm2, it is considered a contraindication for mitral transcatheter edge-to-edge repair (TEER), and the decision to proceed in borderline cases can be individualized based on the severity and location of MR and the anticipated number of devices needed. The mitral valve area should be measured using 3-dimensional (3D) multiplanar reformatting to avoid overestimation errors.
Patients with an extensive flail, defined as a flail segment width of 15 mm or greater or a flail gap of 10 mm or greater, were not included in the initial clinical trials for TEER therapy, such as EVEREST 2.[10] Despite this, treating degenerative mitral valve disease with a flail leaflet is an important application of TEER therapy; this condition can be associated with higher mortality risk in older individuals.[25] The presence of a flail leaflet segment can predict a greater acute improvement in mean left atrium pressure after TEER. Improvement in left atrium pressure after TEER has been linked to improved functional status.[26] Treating wider and larger flail gaps is now possible with the availability of longer and wider TEER devices. The development of independent leaflet grasping technology has enabled the treatment of larger and wider flail gaps. This technology is available with the MitraClip G4 and the PASCAL transcatheter mitral valve repair systems, allowing for the initial capture of the flail segment, followed by the steering of the delivery system to the nonflail leaflet. (This ensures both leaflets have a sufficient and stable grasp, thus facilitating the treatment of larger and wider flail gaps.)
In its selection criteria, the EVEREST 2 trial only enrolled patients with a primary regurgitant mitral jet originating from the central region of the mitral valve, specifically the A2-P2 segments.[10] However, this approach led to a significant portion of patients with noncentral MR being excluded from TEER treatment, leaving them untreated. Noncentral MR, which originates from the commissures and extends to involve the leaflet edges, constitutes nearly a third of all cases of significant MR.[27][28] When deploying TEER devices in these cases, unique challenges emerge, particularly when dealing with large prolapsing leaflets and flail segments closer to the medial and lateral commissures. The complexity and increased number of chordae tendineae in the commissures elevate the risk of device entanglement and chordal disruption. To mitigate this risk, some operators opt for smaller TEER devices to avoid entanglement, as longer device arms increase the likelihood of such complications. Fortunately, the shorter posterior leaflet length in the commissures often makes short device arms sufficient for achieving adequate tissue grasp (ie, <9 mm). Extensive use of 3D transthoracic echocardiography and unconventional imaging planes proves valuable in visualizing the full extent of the pathology, informing device choice, and ensuring proper orientation.[29]
Patients with severe leaflet prolapse, known as Barlow disease, were not included in the EVEREST trials due to their challenges for a successful TEER. The hypermobility of the leaflets makes them difficult to grasp, necessitating multiple large TEER devices to achieve significant height reduction of the redundant leaflet tissue and a longer-lasting reduction in MR, making the procedure even more challenging.[30]
When considering TEER in secondary MR, it is important to differentiate patients with preserved left ventricular function and annular dilation (atrial functional MR) from those with left ventricular dysfunction and leaflet tethering. A subgroup analysis of the 2018 Cardiovascular Outcomes Assessment of the Mitra Clip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation trial demonstrated that patients with atrial fibrillation who underwent TEER maintained a clinical benefit. However, they exhibited a worse prognosis compared to patients without atrial fibrillation.[31]
Mitral annular calcification is a degenerative process that primarily affects the mitral valve annulus and is often associated with MR.[32] Grasping the thickened and stiffened leaflets in these cases can be challenging. Moreover, patients with reduced mitral valve area at baseline face an increased risk of high diastolic gradients across the mitral annulus. Recent study results suggest that, in select patients with mitral annulus calcification and severe MR, TEER therapy may be a safe and feasible option with comparable midterm outcomes.[33]
A noteworthy concern in mitral valve surgery is that up to 35% of patients who have previously undergone surgical mitral valve repair at high-volume centers of excellence develop moderate to severe MR a decade after their initial repair.[34][35][36] Redo sternotomy in these patients is often associated with a high risk of morbidity and mortality.[37] While results from some studies have demonstrated the safety and feasibility of TEER in these situations, further research is essential to assess its efficacy fully.[38][39][40]
Recognizing individuals who have previously undergone surgical annuloplasty often have a reduced mitral valve area due to the presence of the annuloplasty ring is crucial. Given that more than 1 TEER device is typically needed to reduce MR effectively, the procedural team must exercise caution to prevent a subsequent increase in diastolic inflow gradients. In postsurgical mitral valve repair cases, the posterior mitral valve leaflet is often resected, resulting in a shorter and smaller posterior leaflet that can prove more challenging to grasp during the TEER procedure. An alternative strategy involves grasping the anterior and posterior sections of the annuloplasty ring when the posterior leaflet tissue length is insufficient. However, expertise with this technique is somewhat limited. Furthermore, the visibility of the posterior leaflet is often restricted due to an annuloplasty ring, making leaflet grasping particularly challenging during TEER. In these scenarios, there is an elevated risk of device entanglement, especially in the presence of artificial chords.[41]
Preprocedural TEE examination also helps predict the repair difficulty (see Table 5. Echocardiography Predictors of Transcatheter Edge-to-Edge Repair Difficulties).[8][42][43]
Table 5. Echocardiography Predictors of Transcatheter Edge-to-Edge Repair Difficulties
| Echocardiographic Criterion | Ideal Anatomic Features | Challenging Anatomic Features | Relative Contraindications |
| Anatomic location of pathology | Regurgitation jet in A2-P2 segment | Involves medial or lateral commissure, segments A1-P1, A3-P3 | Perforation, cleft, or severe calcification of leaflet |
| MV area and gradient |
Area >4 cm2 Gradient <4 mm Hg |
Area >3.5 cm2 Gradient ≥4 mm Hg |
Area <3.5 cm2 gradient ≥4 mm Hg |
| Leaflet grasping length | >10 mm | 7 to 10 mm | <7 mm |
| Secondary MR | Coaptation depth <11 mm | Coaptation depth >11 mm | Rheumatic thickening and restriction |
| Primary MR |
Flail width <15 mm Flail gap <10 mm |
Flail width >15 mm with a large valve area | Barlow disease with multisegment involvement |
Indications
The edge-to-edge leaflet repair device is the only recommended intervention to treat MR. Still, there are many emerging technologies, including but not limited to neo-cords, transcatheter mitral valve repair, and rings. The following are currently the indications for the edge-to-edge repair:
Contraindications
A few contraindications to catheter intervention with the edge-to-edge leaflet repair device include the following:
- Inability to tolerate anticoagulation
- Active endocarditis of the mitral valve
- Rheumatic mitral valve disease
- Intracardiac, inferior vena cava, or femoral venous thrombus
- Severe mitral annular calcification involving leaflets
- Presence of significant cleft or perforation in mitral valve leaflets
- Mitral valve stenosis [46][47][48]
Equipment
The following supplies are needed to perform a transcatheter edge-to-edge leaflet repair procedure:
- The edge-to-edge leaflet repair device
- Transeptal puncture kit including catheters, needles, and a radiofrequency wire
- Fluoroscopy machine
- Code cart with a defibrillator
- Sterile gown
- Sterile drape
- Anesthetic
- Transesophageal echocardiography, preferably with 3D imaging capability
- Transducers and equipment for invasive hemodynamic monitoring
- Availability of a perfusionist and a heart-lung machine in case of device embolization
For treating both primary MR and secondary MR, the MitraClip device is the first transcatheter technology to get FDA and Conformité Européenne approval.[49][50] The fourth-generation MitraClip has 4 implant sizes available in 2 different widths and 2 different arm lengths as of 2020.[51] These include the "traditional" width of 4 mm and the newer 6 mm size. Both widths are available in the NT clip with a 9 mm arm length and the XT clip with a 12 mm arm length.
The MitraClip comprises 2 rigid arms of cobalt-chromium alloy featuring flexible nitinol-based "grippers." These grippers are equipped with either 4 (in the case of NT/NTW) or 6 (XT/XTW) small hooks, often referred to as "frictional elements," arranged longitudinally. Notably, the longer clip arms (XT/XTW) extend beyond the strict anatomical and morphological criteria set forth by the EVEREST trials, allowing for the treatment of more extensive coaptation gaps and leaflet flails.[52]
Expanding the use of XT/XTW devices to patients with more complex anatomies has raised concerns about potential risks, such as leaflet injuries and single leaflet device attachment. These concerns arise from increased leaflet tension resulting from the grasping of more tissue with the XT/XTW devices and the presence of an active locking mechanism and device stiffness. Such increased tension forces have been observed to cause leaflet injury in various anatomical scenarios, including cases with calcified leaflets.[52]
A comprehensive examination of the EXPAND registry did not reveal higher rates of adverse leaflet events associated with the long-arm XTR clip system compared to the smaller NTR device.[24] The fourth-generation MitraClip device now offers the advantage of autonomous and controlled gripper actuation. This feature allows for the confirmation and optimization of leaflet gripping while enabling continuous left atrial pressure monitoring through the guiding catheter.
The PASCAL transcatheter mitral valve repair technology was first introduced in 2016 and has since been evaluated in a compassionate-use cohort of 23 patients with challenging anatomical characteristics for transcatheter edge-to-edge repair.[53] The latest iteration of the PASCAL system, its second version, has been released, incorporating three integrated catheters: a 22 Fr steerable guide sheath, a maneuverable catheter, and an implant catheter with the device preattached at the distal end. This innovative design allows for a wide range of motion and enhances maneuverability within the left atrium.
The PASCAL P10 implant, constructed from nitinol, features a central spacer and two spring-loaded curved paddles, offering a gripping length of 26 mm when opened to 180°, along with 2 clasps (each measuring 10 mm). The central spacer is strategically designed to fill specific coaptation gaps within the primary MR jet area, thus reducing stresses on the mitral valve leaflets. The nitinol clasps possess a horizontal line of small hooks, referred to as "retention elements," at the distal end, which can be adjusted independently. This feature allows for either simultaneous or independent leaflet capture.
A smaller version (the PASCAL Ace) maintains a gripping breadth comparable to the PASCAL P10 implant. However, the paddles on the PASCAL Ace are only 6 mm wide, making them suitable for smaller anatomies and enabling various implant techniques. Both PASCAL implants offer the flexibility of separate leaflet gripping, allowing for either "leaflet optimization" or "staged leaflet capture." Notably, in August 2022, the second-generation PASCAL Precision platform was unveiled, featuring enhancements to the catheter system that improve device stability and steerability.[54]
Personnel
The key personnel required to adequately and safely perform catheter-based MR treatment with the TEER device include:
- Interventional cardiologist
- Echocardiographer, either a cardiac anesthesiologist or a cardiologist
- Cardiac anesthesiologist
- First-assist, for the proceduralist
- Nursing and technical staff for the procedure
- Cardiac surgeon and operating room staff, on standby in case of emergent complication
- Perfusionist if there is a need for cardiopulmonary bypass
Preparation
The TEER procedure is typically performed in a catheterization lab or a hybrid operating room with fluoroscopy capabilities. The procedure also necessitates real-time transthoracic echocardiography, TEE, which is pivotal in confirming the pathology, guiding the procedure, and ensuring repair effectiveness. Given the critical role of TEE, TEER is usually performed under general anesthesia to facilitate TEE evaluation and prevent any accidental patient movement, which could have severe consequences. Before commencing the TEER procedure, preoperative TEE should be conducted to thoroughly assess the mitral valve lesion and evaluate the feasibility of repair. In some cases, it may be necessary to obtain other forms of cardiac imaging performed by a trained cardiovascular interventionalist or cardiovascular imaging specialist.
Additionally, a preoperative anesthetic evaluation is essential to optimize patients for general anesthesia, ensuring their safety and comfort throughout the procedure. Collaboration among the structural heart team, which comprises key members, including the interventional cardiologist, cardiac anesthesiologist, operating room personnel, and nursing staff, is crucial for the procedure's success. Adequate preparation, including readily available equipment and devices, is paramount. Conducting a preprocedure time-out is recommended to ensure effective communication within the team and confirm that all necessary components are in place.
Sterility is a critical aspect of preparation for any medical procedure, and this is no exception. Before the procedure, a sterile field is established. The catheter insertion site is thoroughly sterilized in line with standard catheterization protocols. All personnel near the sterile field should follow strict sterile techniques, including scrubbing and wearing full gowns, hats, masks, and gloves. The procedure area should be cleaned and appropriately draped before commencement to maintain a sterile environment and ensure patient safety.[55]
Device Selection
When using 3DE, it is crucial to carefully assess the underlying etiology, baseline mitral valve area, mean transmitral gradient, and anatomical complexity before selecting the appropriate device. The primary criteria to be considered when choosing a device are shown below (see Table 6. Mitral Valve Criteria for Device Selection).
Table 6. Mitral Valve Criteria for Device Selection
| Mitral valve anatomy | NT | NTW | XT | XTW | PASCAL P10 | PASCAL ACE |
| Length of the leaflet grasping zone <9 mm | + | + | + | + | ||
| Length of the leaflet grasping zone >9 mm | + | + | + | + | ||
| Barlow disease | + | + | + | + | ||
| Thin leaflet structure | + | + | + | + | ||
| Broad gap size | + | + | + | |||
| Commissural jet | + | + | + | |||
| MV area <4.0 cm2 | + | + | + |
The accurate quantification of the mitral valve area, MVA, ideally involves multiplanar reconstruction using high-resolution 3D volumes of the mitral valve. When deploying a PASCAL P10 device, the MVA has been observed to be reduced by approximately 47%. However, using rigid implants with extended arms (XTW/XT) is expected to impact the baseline MVA substantially. Specifically, the MVA reduction achieved with the NTR and XTR implants was 52% and 57%, respectively.[56] It's important to note that the extent of MVA reduction depends on the precise location of the device along the line of coaptation, with the A2/P2 position experiencing the most significant reduction and commissural placement resulting in the least reduction.[56]
Two pivotal factors influencing the selection of devices for TMVR are the chosen treatment strategy and the localization of the regurgitant jet. Notably, individuals with discrete jets, in whom the expectation is to implant 2 distant clips to address the issue, necessitate a larger baseline mitral valve area, around 6 cm2, to prevent the development of significant MV stenosis.[56]
In cases where significant flail gaps or wide prolapses are present, particularly when multiple implants are required for stabilization, devices with extended arms (XTW, XT, or PASCAL) have shown increased effectiveness in reducing mitral regurgitation.[57] However, when a multiple-clip strategy is considered, using PASCAL P10 is not recommended due to the concave design of its paddles, which may complicate the precise alignment of 2 implants. For isolated commissural lesions, the preference should be for implants with smaller arms (NTW/NT) and dependable steering capabilities.[57]
Conducting a thorough assessment of the length and thickness of the leaflet tissue is essential. When annular calcifications with leaflet infiltration are identified as predictors of a higher transmitral gradient after TEER, opting for smaller, more flexible devices is advisable.[26][58] In cases of secondary MR involving a short or thin tethered posterior leaflet, it is advisable to avoid devices with extended arms, like the MitraClip XT and XTW, to minimize the risk of single leaflet device attachment or leaflet injury. The PASCAL devices, with their flexible nitinol construction and horizontal positioning of the gripping components, are often preferred, especially when dealing with a short posterior leaflet, as they apply gripping force at the leaflet base, often referred to as the "hinge point" with the mitral annulus.[54]
Technique or Treatment
The Edge-to-Edge Leaflet Repair Device
The structural heart team comprises an interventional cardiologist, cardiac surgeon, cardiac anesthesiologist, and operating room nurse who is required to perform the transcatheter mitral valve repair with the edge-to-edge leaflet repair device. Procedural rooms are specialized with fluoroscopic capability. The procedure involves using fluoroscopic and TEE guidance to image the heart before, during, and after the procedure.[59] The patient will be under general anesthesia for ease and comfort and to avoid patient movement during the procedure.
The following table demonstrates the procedure's major steps; the TEE views to guide those steps, and potential complications with each step (see Table 7. Transcatheter Edge-to-Edge Leaflet Repair Procedure Overview).
Table 7. Transcatheter Edge-to-Edge Leaflet Repair Procedure Overview
| Major Steps in the Procedure | Things to Consider for a Successful Procedure | TEE View | Images | Potential Complications |
|
Preprocedure: Cardiac assessment and evaluation of MV by TEE [60]
|
The valvular pathology must be confirmed Intracardiac thrombus must be ruled out before starting the procedure Favorable echocardiographic features for a successful procedure are as follows:
|
4-chamber midesophageal, 2-chamber, modified bicaval, midesophageal long axis, and LA appendage-focused views to rule out intracardiac thrombus Color and pulse wave Doppler of the LA appendage Evaluate interatrial septum anatomy Determine the baseline MR severity, MR mechanism, Doppler gradients across the MV, MVA measurement, presence of any pericardial effusion, and pulmonary vein Doppler profile [61] Determine if any pericardial effusions are present before starting the procedure and quantify the effusion. |
Mitral Valve, En Face View Mitral Valve, Echocardiograph Posterior Mitral Leaflet With Flail Central Scallop (P2) Flail Mitral Valve Prolapse, Echocardiograph |
None |
|
Femoral venous access and cannulation |
Use ultrasound guidance for femoral venous access Follow the wire in under fluoroscopy to ensure no kinking or malposition Assess the size of the vessel to be able to accommodate the catheter and delivery system Check vein compressibility and the color Doppler to rule out femoral vein thrombus Consider a micropuncture kit followed by the sheath and percutaneous suture devices for cannulation |
Transgastric IVC views in short and long axes to visualize the wire as it advances into IVC to the RA
|
Pelvic Veins |
Bleeding at the vein puncture site Retroperitoneal hemorrhage Femoral arterial injury Injury to surrounding structures |
|
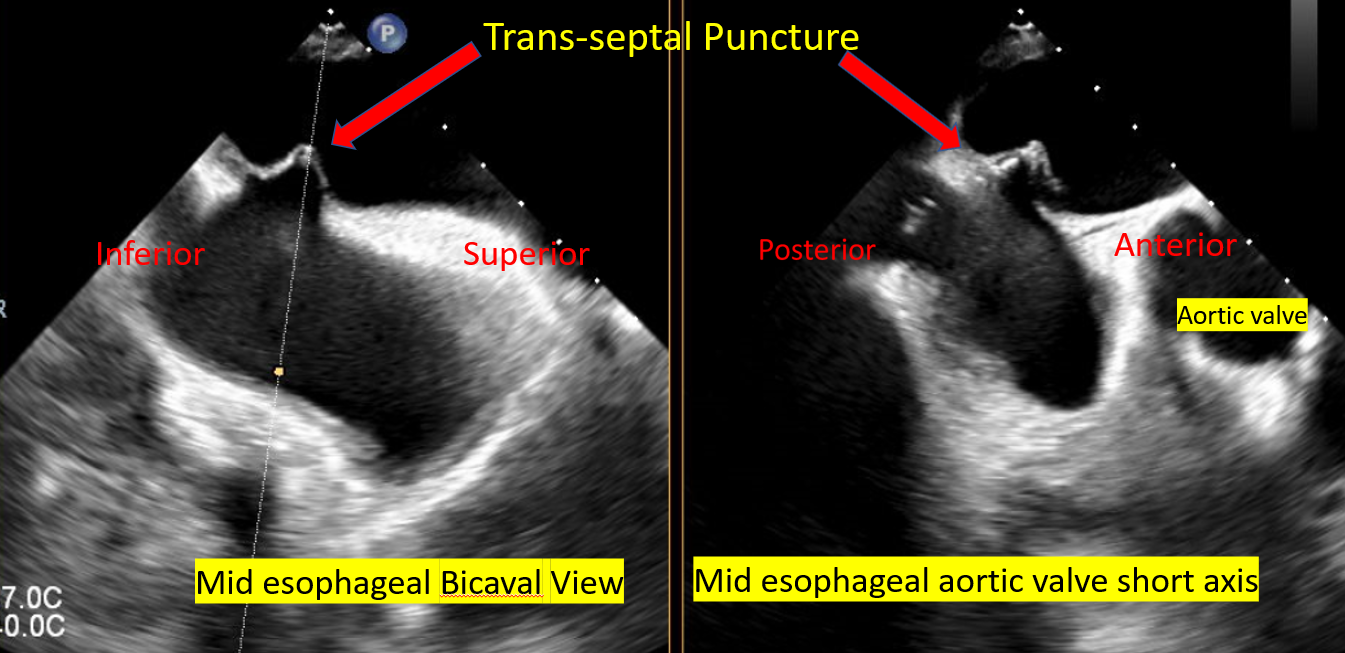
Transseptal puncture
|
Septal puncture is performed in the posterior superior portion of the IAS The tip of the transseptal puncture needle on the IAS appears like an indentation (tenting) The tip should point toward the LA and confirm the anterior-posterior and superior-inferior orientation, as well as the height of the puncture above the mitral annular plane before crossing the septum The height of the transeptal puncture should be 4 to 5 cm from the mitral annular plane:
A transeptal puncture at a higher height is used for medial jets, while a lower height is used for lateral jets [62] De-airing of device delivery system to avoid air embolism Consider a radiofrequency transseptal needle if the septum is very "floppy," fibrotic, or lipomatous Use real-time TEE monitoring during transseptal puncture to ensure the puncture needle is not directed toward the aorta or posterior LA
Achieve an ACT >250 to decrease the risk of clot formation. Heparinize and monitor ACT every 15 to 30 min. |
Midesophageal bicaval view to guide transseptal puncture needle into the RA Midesophageal bicaval 120° to obtain superior-inferior orientation with tenting of the fossa, ie, superior towards the SVC, inferior towards the IVC The midesophageal aortic valve short axis shows the anterior-posterior portion of the IAS, ie, the anterior part is towards the aorta/aortic valve 4-chamber midesophageal view shows the distance from the point of septal puncture to the mitral annular plane |
Transseptal Puncture for Mitra Clip Procedure |
If a transseptal puncture is too anterior, it can cause injury to the aortic valve, ascending aorta, and aortic root. Cardiac tamponade Air embolism |
|
Insertion of the SGC |
An extra stiff Amplatz wire is placed in the left upper pulmonary vein under TEE, and fluoroscopic guidance, and an SGC and dilator are advanced over it. The dilator is identified by its cone-shaped tip The bright radiopaque double-ring echo identifies the SGC Once the SGC is placed in the LA, the extra stiff Amplatz wire is retrieved, followed by the dilator |
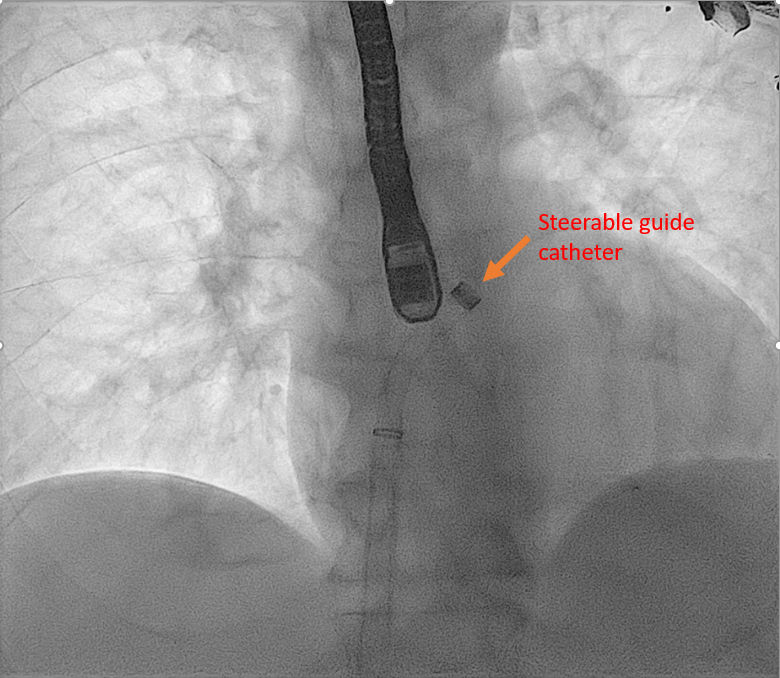
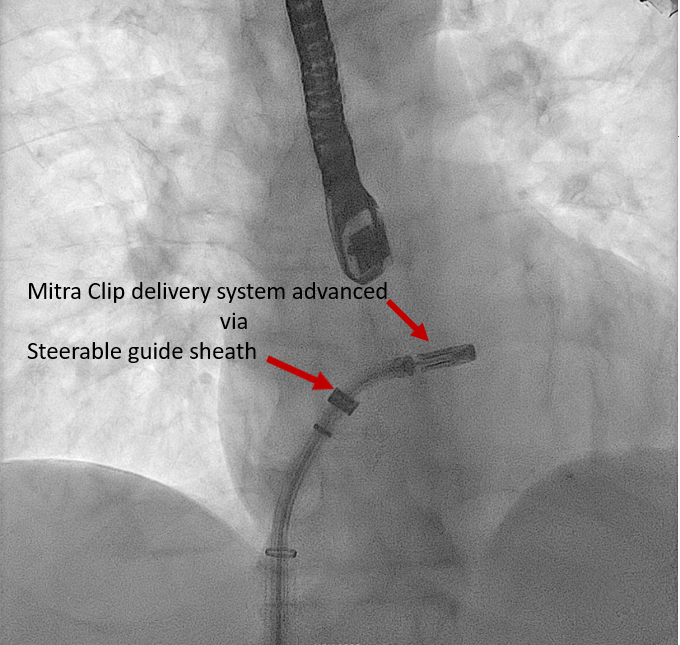
Real-time 3D imaging from the aortic valve short axis or mitral commissural views is used to get a 3D assessment of the SGC and delivery system in the LA. | Mitra Clip Steerable Guide Catheter | |
|
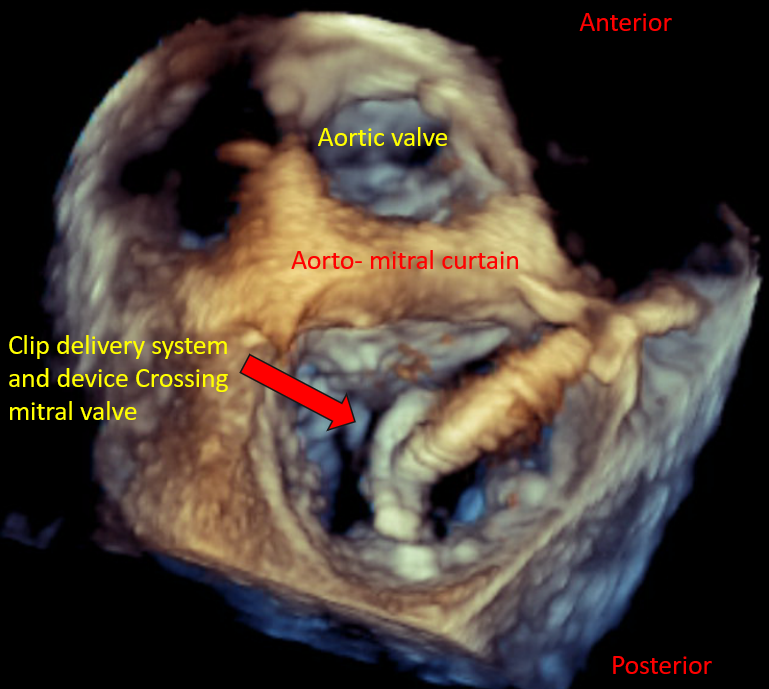
Introduction of the edge-to-edge repair device clip delivery system into the LA |
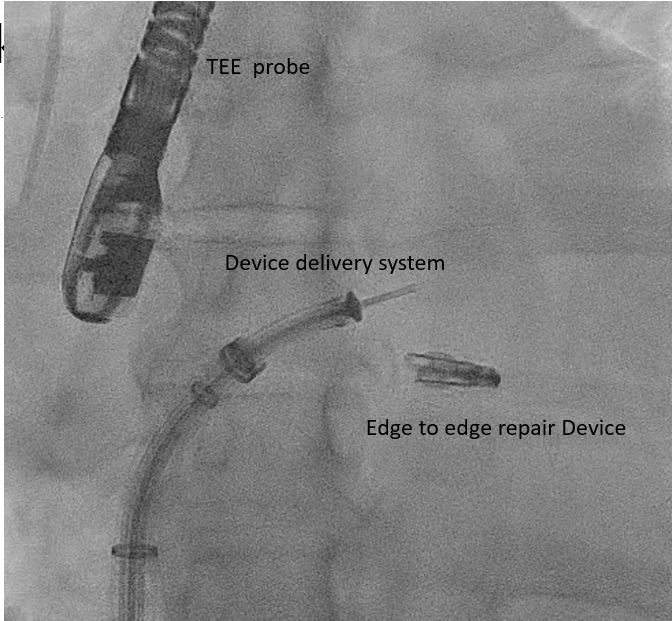
The edge-to-edge repair device delivery system is advanced through the steerable guide catheter under TEE and fluoroscopic guidance [63] To position the edge-to-edge repair device delivery system above the MV, posterior torque on SGC and medial deflection of the edge-to-edge repair device delivery system with retraction of the whole system is required. Edge-to-edge repair device alignment and adjustments are monitored medial-lateral and anterior-posterior under TEE and fluoroscopic guidance. Direct the tip of the edge-to-edge repair device towards the largest regurgitant area. |
Real-time 3D imaging from the aortic valve short axis or mitral commissural views is used to get a 3D assessment of the SGC and delivery system in the LA. |
Transcatheter Edge-to-Edge Valve Repair Device MitraClip Delivery System |
|
| Axial alignment of the edge-to-edge repair device clip delivery system | The guide catheter is advanced into the LA oriented perpendicular to the coaptation line of the MV |
The midesophageal mitral commissural view shows medial and lateral orientation on the MV. The midesophageal long-axis view shows anteroposterior orientation on the MV. 3D en face view of the MV helps guide the edge-to-edge repair device delivery system and orient it to the MR jet. A real-time 3D en face view can help guide the trajectory and motion of the edge-to-edge repair device delivery system from the LA through the MV into the LV. |
Mitral Valve, En Face View Device Orientation in Percutaneous Mitral Valve Repair |
Arrhythmias Atrial wall injury Cardiac tamponade Injury to MV apparatus and surrounding structures Air embolism |
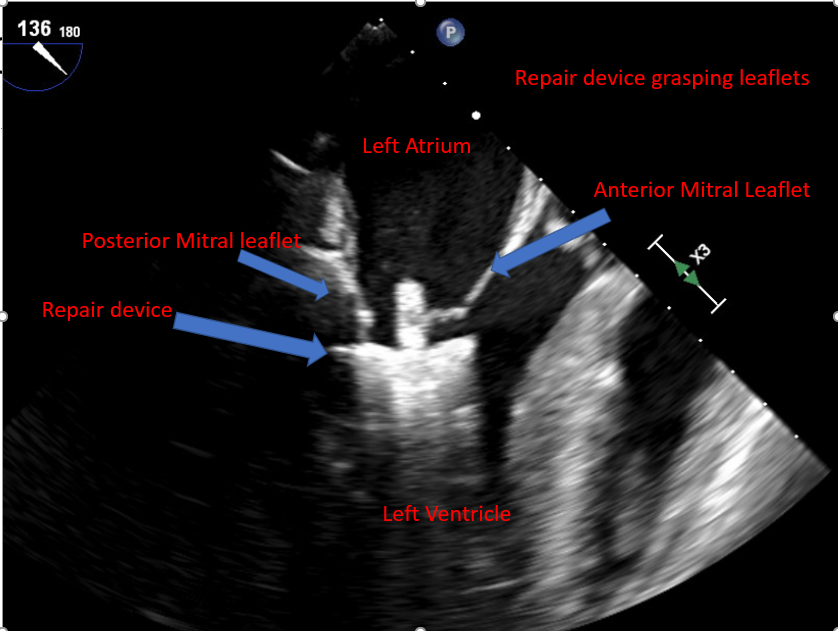
| Advancement of the device into the LV and leaflet grasping |
To avoid injury, the device should be continuously visualized, especially the delivery system's tip The delivery device should be perpendicular to the MV's regurgitant orifice and coaptation line Confirm the position in relation to the regurgitant jet and MV leaflets as the delivery system and device enter the LV Ensure no entanglement exists in the subvalvular MV apparatus and chordae tendineae Rapid pacing or ventilation holding may be needed to make the procedure more precise |
3D en face view of the MV, looking from the LA and LV perspectives Biplane imaging of midesophageal mitral commissural view and midesophageal long axis view, with and without color Doppler, can be used to evaluate the orientation of MV's anterior and posterior leaflets to the edge-to-edge repair device arms. Fluoroscopy can be used to aid orientation along with TEE. |
Mitral Valve, En Face View MitraClip Delivery System Mitral Valve Leaflet Grasping |
Arrhythmias Injury to the MV Entanglement of the device delivery system and the device in subvalvular apparatus |
| Assessment of leaflet capture and edge-to-edge repair device deployment |
Integrate echo and fluoroscopic images Orient the device before entering the LV and avoid too much manipulation, as it can lead to edge-to-edge repair device entanglement and injury to the subvalvular apparatus Consider rapid ventricular pacing or holding mechanical ventilation to decrease motion and achieve a better leaflet grasp [43] If the coaptation defect width is significant, consider using the "zip and clip" technique, where the first edge-to-edge repair device can be deployed immediately medial or lateral to the largest coaptation defect and thus facilitate leaflet grip in the area of bigger coaptation defect. Monitor for spontaneous echo contrast and monitor ACT to ensure no thrombus formation in the LA. |
Obtaining a 2D midesophageal mitral commissural view and a long axis view simultaneously allows for visualization of the length of leaflets within the edge-to-edge repair device arms and restriction of leaflet motion with edge-to-edge repair device grasping the leaflets. Fluoroscopy is used to confirm the position and stability of the device. |
None |
Edge-to-edge repair device detachment Injury to MV apparatus MV leaflet injury causing severe MR not amenable to percutaneous repair Mitral stenosis |
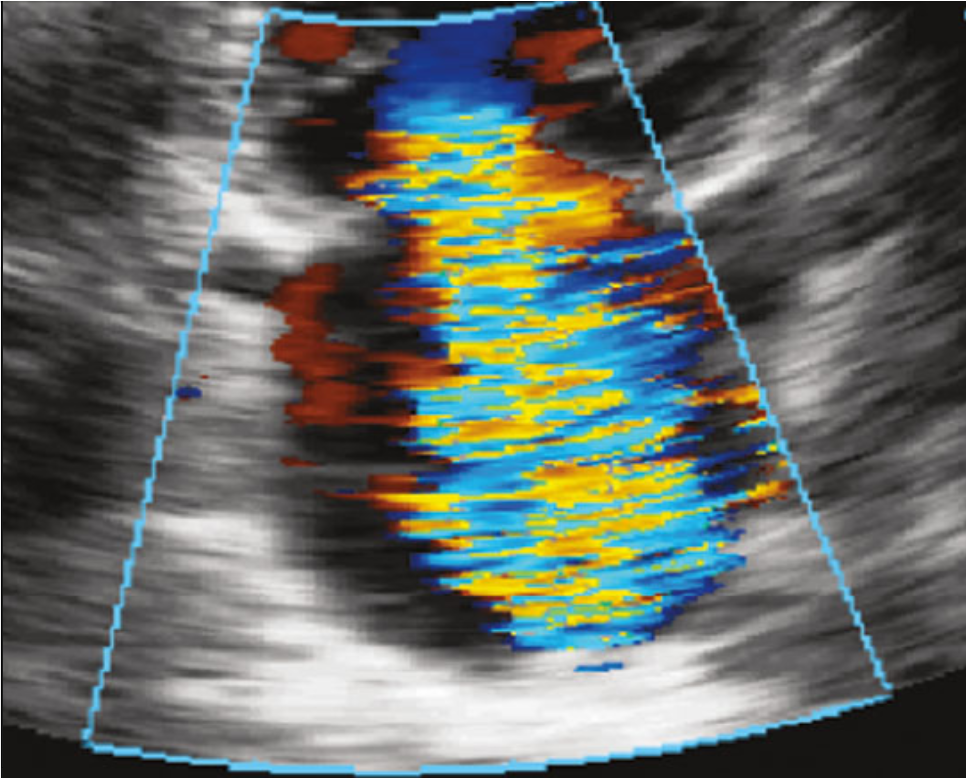
| Assessment after edge-to-edge repair device deployment |
Assess for any complications Assess for the stability of leaflet capture and the device Assess for residual MR, mitral stenosis, and edge-to-edge repair device dislodgement The transmitral gradient should be <5 mm Hg |
Simultaneous assessment of the 2D midesophageal commissural view and the long-axis view 3D en face view from the perspective of both the LA and LV Doppler assessment of the gradient at the MV after deployment of edge-to-edge repair device |
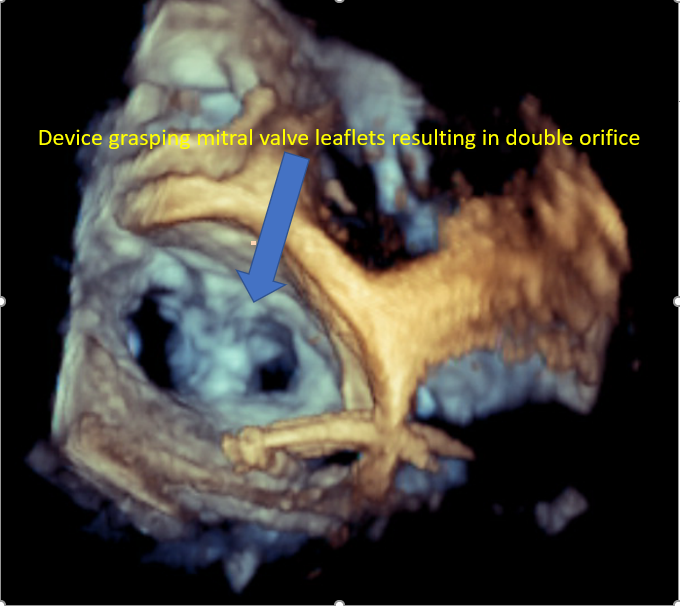
Mitral Valve Repair, Edge-to-Edge Percutaneous Device |
Leaflet injury Leaflet perforation Creation of mitral stenosis Injury to subvalvular mitral apparatus Residual MR necessitates the placement of an additional edge-to-edge repair device. |
| Withdrawal of the edge-to-edge repair device delivery system and SGC from the patient |
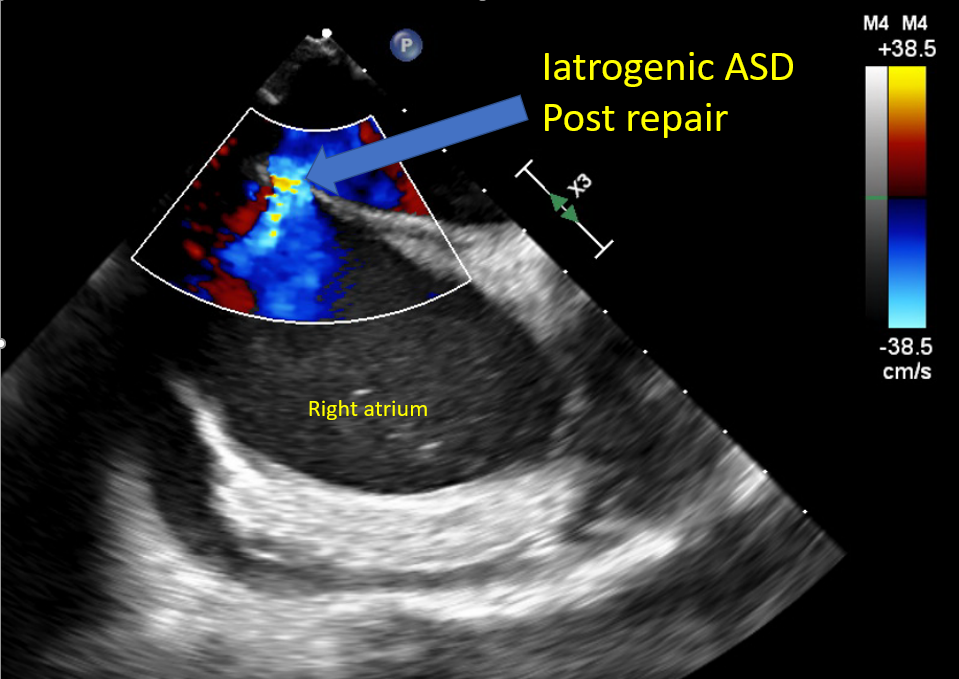
Evaluate for iatrogenic ASD Reverse heparin with protamine to avoid bleeding and help with hemostasis Monitor for protamine reaction Monitor femoral venous sites for bleeding A complete postprocedure echocardiographic evaluation should be done to assess the repair and rule out complications |
4-chamber midesophageal view, midesophageal commissural view, midesophageal long axis view, all with and without color Doppler Midesophageal view assessing pulmonary venous flow to assess the reduction in MR Continuous wave Doppler assessment of MV to assess for mitral stenosis The 3D en face view of the repaired MV from the LA and LV view ensures leaflet grip and device stability 3D reconstruction and multiplanar reconstruction to assess the area of the repaired double orifice MV [64][65] Midesophageal bicaval view and 3D imaging of IAS to assess for iatrogenic ASD [66] |
Mitral Valve Repair, Edge-to-Edge Percutaneous Device Iatrogenic Atrial Septal Defect |
Cardiac tamponade Iatrogenic ASD Injury to IVC or femoral veins Protamine reaction |
| Postprocedural monitoring | Monitoring for postprocedural complications should be done in the PACU or ICU. | None | None |
Respiratory issues Postoperative nausea and vomiting Hemodynamic instability Cardiac tamponade Bleeding from the femoral site |
IVC, inferior vena cava; LA, left atrium; LV, left ventricle; RA, right atrium; IAS, interatrial septum; SVC, superior vena cava; ACT, activated clotting time; SGC, steerable guide catheter; ASD, atrial septal defect; PACU, post-anesthesia care unit; ICU, intensive care unit; MV, mitral valve; IAS, iatrogenic atrial septa; TEER, transcatheter edge-to-edge repair; MR, mitral regurgitation; TEE, transesophageal echocardiography
Complications
Despite the significant comorbidities among the patients being treated, TEER is a safe operation with a low likelihood of major consequences. The table below summarizes the most common complications and their relative occurrence rates (see Table 8. TEER Complications).
Table 8 TEER Complications
| Complication | Rate of Complication | References |
| Single leaflet device attachment | 1.5%-5.1% | [11][67] |
| Injury to the MV leaflet | 0%-2% | [51][52] |
| Device embolization | 0.05%-0.7% | [11][68] |
| Transmitral gradient >5 mmHg | Up to 15% | [52] |
| Residual MR >2+ | 3.4%-17.0% | [51] [52] |
| Pericardial effusion or tamponade | 0%-0.5% | [69] |
| Major vascular complications | 1.4%-4.0% | [69] |
| Severe bleeding requiring blood transfusion | 0%-17% | [69] |
| Stroke | 0%-1% | [69] |
| Myocardial infarction | 0%-3% | [69] |
The heightened leaflet perforation, tear, or single leaflet device attachment risk in patients with long-standing secondary MR and calcified leaflets raises significant concerns. The percutaneous retrieval of embolized devices can pose challenges, particularly when larger clips are involved.[70] While afterload mismatch may occur in individuals with reduced left ventricle function, it is an infrequent and transient event typically managed with inotropic medications, often not necessitating mechanical support. Despite its rarity, afterload mismatch may adversely impact long-term outcomes, possibly indicating an advanced stage of heart failure. In some cases of secondary MR with severely impaired left ventricle function, thrombus development in the left atrium or ventricle may occur. Early and intensive anticoagulant therapy may be deemed necessary for these patients.[71]
The multidisciplinary team must reassess the indication for mitral valve surgery or reintervention when confronted with residual or recurrent MR. A repeat transesophageal echocardiography is typically warranted to comprehend the underlying disease, identify residual leaflet anatomy for potential device implantation, and assess the risk of significant mitral stenosis. In case series with limited safety data, alternative interventional strategies for managing substantial para-clip or inter-clip residual MR have been explored. Examples include using an Amplatzer vascular plug (Abbott), originally designed for peripheral vasculature embolization, or an enlarged polytetrafluoroethylene double-disk occluder, initially intended for closing atrial septal defects.[38][72]
According to a large multicenter registry, implant failure due to leaflet perforation, tear, or loss affects 3.5% of patients and is associated with increased in-hospital and long-term mortality.[73] Redo TEER is a viable option and may be preferable to surgery in anatomically suitable patients with primary or secondary MR, especially when surgical outcomes are suboptimal.[74]
Clinical Significance
Catheter management of MR represents a significant recent innovation, marking a considerable advancement in cardiology; it provides a corrective option for patients with severe MR and high surgical risks. Recent studies indicate that catheter management may, in certain circumstances, outperform surgical intervention.[20]
Following mitral valve repair with the edge-to-edge device, left ventricle contractility and cardiac output remain stable, although total ejection fraction and global strain may decrease. This decline is likely attributable to a reduced regurgitant volume postrepair, leading to decreased left ventricular end-diastolic volume. This reduction in myocardial oxygen demand can contribute to an improved New York Heart Association functional class after 3 months.[75]
Enhancing Healthcare Team Outcomes
Catheter management of mitral valve regurgitation demands a comprehensive approach from an interprofessional team to ensure patient-centered care, optimize outcomes, enhance patient safety, and improve team performance. Physicians, advanced practitioners, nurses, pharmacists, and other health professionals play pivotal roles in various aspects of this procedure. Physicians, particularly highly trained cardiovascular interventionalists, lead the procedural aspects. They collaborate with advanced practitioners to ensure thorough patient assessments and communicate risks and benefits effectively.
Nurses specializing in cardiology are essential for preoperative, operative, and postoperative monitoring, patient education, and coordination of follow-up care. Interprofessional communication is facilitated by cardiovascular imaging specialists, structuralists, and anesthesiologists who contribute their expertise in optimizing lung and heart function, providing specialized imaging, and ensuring the patient's readiness for anesthesia.
Pharmacists are crucial for pharmaceutical consultation, addressing postoperative pain management, antiemetics, and blood thinners. This multidisciplinary approach, driven by effective communication and care coordination, is vital for a successful catheter management procedure. Emphasizing this interprofessional collaboration enhances patient safety, improves outcomes, and contributes to overall team performance in the complex landscape of mitral valve regurgitation interventions.
Media
(Click Image to Enlarge)

Pelvic Veins. Pelvic veins include the inferior vena cava, internal iliac vein, common iliac vein, external iliac vein, femoral vein, deep circumflex iliac vein, middle sacral vein, and lateral sacral vein.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Video to Play)
Mitral Valve Prolapse. The echocardiograph reveals a mitral valve prolapse.
Contributed by I Ahmed
References
Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. European heart journal. 2003 Jul:24(13):1231-43 [PubMed PMID: 12831818]
Level 3 (low-level) evidenceQuader N, Rigolin VH. Two and three dimensional echocardiography for pre-operative assessment of mitral valve regurgitation. Cardiovascular ultrasound. 2014 Oct 25:12():42. doi: 10.1186/1476-7120-12-42. Epub 2014 Oct 25 [PubMed PMID: 25344779]
Writing Committee Members, Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C, ACC/AHA Joint Committee Members, O'Gara PT, Beckman JA, Levine GN, Al-Khatib SM, Armbruster A, Birtcher KK, Ciggaroa J, Deswal A, Dixon DL, Fleisher LA, de Las Fuentes L, Gentile F, Goldberger ZD, Gorenek B, Haynes N, Hernandez AF, Hlatky MA, Joglar JA, Jones WS, Marine JE, Mark D, Palaniappan L, Piano MR, Spatz ES, Tamis-Holland J, Wijeysundera DN, Woo YJ. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. The Journal of thoracic and cardiovascular surgery. 2021 Aug:162(2):e183-e353. doi: 10.1016/j.jtcvs.2021.04.002. Epub 2021 May 8 [PubMed PMID: 33972115]
Level 1 (high-level) evidenceGuarracino F, Baldassarri R, Ferro B, Giannini C, Bertini P, Petronio AS, Di Bello V, Landoni G, Alfieri O. Transesophageal echocardiography during MitraClip® procedure. Anesthesia and analgesia. 2014 Jun:118(6):1188-96. doi: 10.1213/ANE.0000000000000215. Epub [PubMed PMID: 24842173]
Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W, ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Revista espanola de cardiologia (English ed.). 2022 Jun:75(6):524. doi: 10.1016/j.rec.2022.05.006. Epub [PubMed PMID: 35636831]
Kang DH, Kim JH, Rim JH, Kim MJ, Yun SC, Song JM, Song H, Choi KJ, Song JK, Lee JW. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation. 2009 Feb 17:119(6):797-804. doi: 10.1161/CIRCULATIONAHA.108.802314. Epub 2009 Feb 2 [PubMed PMID: 19188506]
Sidebotham DA, Allen SJ, Gerber IL, Fayers T. Intraoperative transesophageal echocardiography for surgical repair of mitral regurgitation. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2014 Apr:27(4):345-66. doi: 10.1016/j.echo.2014.01.005. Epub 2014 Feb 15 [PubMed PMID: 24534653]
Bonow RO, O'Gara PT, Adams DH, Badhwar V, Bavaria JE, Elmariah S, Hung JW, Lindenfeld J, Morris AA, Satpathy R, Whisenant B, Woo YJ. 2020 Focused Update of the 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation: A Report of the American College of Cardiology Solution Set Oversight Committee. Journal of the American College of Cardiology. 2020 May 5:75(17):2236-2270. doi: 10.1016/j.jacc.2020.02.005. Epub 2020 Feb 14 [PubMed PMID: 32068084]
Level 3 (low-level) evidenceFeldman T, Kar S, Rinaldi M, Fail P, Hermiller J, Smalling R, Whitlow PL, Gray W, Low R, Herrmann HC, Lim S, Foster E, Glower D, EVEREST Investigators. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. Journal of the American College of Cardiology. 2009 Aug 18:54(8):686-94. doi: 10.1016/j.jacc.2009.03.077. Epub [PubMed PMID: 19679246]
Feldman T, Kar S, Elmariah S, Smart SC, Trento A, Siegel RJ, Apruzzese P, Fail P, Rinaldi MJ, Smalling RW, Hermiller JB, Heimansohn D, Gray WA, Grayburn PA, Mack MJ, Lim DS, Ailawadi G, Herrmann HC, Acker MA, Silvestry FE, Foster E, Wang A, Glower DD, Mauri L, EVEREST II Investigators. Randomized Comparison of Percutaneous Repair and Surgery for Mitral Regurgitation: 5-Year Results of EVEREST II. Journal of the American College of Cardiology. 2015 Dec 29:66(25):2844-2854. doi: 10.1016/j.jacc.2015.10.018. Epub [PubMed PMID: 26718672]
Level 1 (high-level) evidenceFeldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, Loghin C, Trento A, Skipper ER, Fudge T, Letsou GV, Massaro JM, Mauri L, EVEREST II Investigators. Percutaneous repair or surgery for mitral regurgitation. The New England journal of medicine. 2011 Apr 14:364(15):1395-406. doi: 10.1056/NEJMoa1009355. Epub 2011 Apr 4 [PubMed PMID: 21463154]
Level 1 (high-level) evidenceAlfieri O, Maisano F, De Bonis M, Stefano PL, Torracca L, Oppizzi M, La Canna G. The double-orifice technique in mitral valve repair: a simple solution for complex problems. The Journal of thoracic and cardiovascular surgery. 2001 Oct:122(4):674-81 [PubMed PMID: 11581597]
Mack MJ, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant BK, Grayburn PA, Rinaldi MJ, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Rogers JH, Marx SO, Cohen DJ, Weissman NJ, Stone GW, COAPT Investigators. 3-Year Outcomes of Transcatheter Mitral Valve Repair in Patients With Heart Failure. Journal of the American College of Cardiology. 2021 Mar 2:77(8):1029-1040. doi: 10.1016/j.jacc.2020.12.047. Epub [PubMed PMID: 33632476]
Khatib D, Neuburger PJ, Pan S, Rong LQ. Transcatheter Mitral Valve Interventions for Mitral Regurgitation: A Review of Mitral Annuloplasty, Valve Replacement, and Chordal Repair Devices. Journal of cardiothoracic and vascular anesthesia. 2022 Oct:36(10):3887-3903. doi: 10.1053/j.jvca.2022.05.005. Epub 2022 May 8 [PubMed PMID: 35871885]
De Backer O, Piazza N, Banai S, Lutter G, Maisano F, Herrmann HC, Franzen OW, Søndergaard L. Percutaneous transcatheter mitral valve replacement: an overview of devices in preclinical and early clinical evaluation. Circulation. Cardiovascular interventions. 2014 Jun:7(3):400-9. doi: 10.1161/CIRCINTERVENTIONS.114.001607. Epub [PubMed PMID: 24944303]
Level 3 (low-level) evidenceOoms JF, Van Mieghem NM. Transcatheter Repair and Replacement Technologies for Mitral Regurgitation: a European Perspective. Current cardiology reports. 2021 Jul 16:23(9):125. doi: 10.1007/s11886-021-01556-6. Epub 2021 Jul 16 [PubMed PMID: 34269914]
Level 3 (low-level) evidenceCondado JA, Vélez-Gimón M. Catheter-based approach to mitral regurgitation. Journal of interventional cardiology. 2003 Dec:16(6):523-34 [PubMed PMID: 14632950]
Perloff JK, Roberts WC. The mitral apparatus. Functional anatomy of mitral regurgitation. Circulation. 1972 Aug:46(2):227-39 [PubMed PMID: 5046018]
Blanke P, Naoum C, Webb J, Dvir D, Hahn RT, Grayburn P, Moss RR, Reisman M, Piazza N, Leipsic J. Multimodality Imaging in the Context of Transcatheter Mitral Valve Replacement: Establishing Consensus Among Modalities and Disciplines. JACC. Cardiovascular imaging. 2015 Oct:8(10):1191-1208. doi: 10.1016/j.jcmg.2015.08.004. Epub [PubMed PMID: 26481845]
Level 3 (low-level) evidenceEl Sabbagh A, Reddy YNV, Nishimura RA. Mitral Valve Regurgitation in the Contemporary Era: Insights Into Diagnosis, Management, and Future Directions. JACC. Cardiovascular imaging. 2018 Apr:11(4):628-643. doi: 10.1016/j.jcmg.2018.01.009. Epub [PubMed PMID: 29622181]
Level 3 (low-level) evidenceDziadzko V, Clavel MA, Dziadzko M, Medina-Inojosa JR, Michelena H, Maalouf J, Nkomo V, Thapa P, Enriquez-Sarano M. Outcome and undertreatment of mitral regurgitation: a community cohort study. Lancet (London, England). 2018 Mar 10:391(10124):960-969. doi: 10.1016/S0140-6736(18)30473-2. Epub [PubMed PMID: 29536860]
Shah PM, Raney AA. Echocardiography in mitral regurgitation with relevance to valve surgery. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2011 Oct:24(10):1086-91. doi: 10.1016/j.echo.2011.08.017. Epub [PubMed PMID: 21933744]
Shiota T. Role of echocardiography for catheter-based management of valvular heart disease. Journal of cardiology. 2017 Jan:69(1):66-73. doi: 10.1016/j.jjcc.2016.09.015. Epub 2016 Nov 15 [PubMed PMID: 27863908]
Asch FM, Little SH, Mackensen GB, Grayburn PA, Sorajja P, Rinaldi MJ, Maisano F, Kar S. Incidence and standardised definitions of mitral valve leaflet adverse events after transcatheter mitral valve repair: the EXPAND study. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2021 Dec 3:17(11):e932-e941. doi: 10.4244/EIJ-D-21-00012. Epub 2021 Dec 3 [PubMed PMID: 34031024]
Avierinos JF, Tribouilloy C, Grigioni F, Suri R, Barbieri A, Michelena HI, Ionico T, Rusinaru D, Ansaldi S, Habib G, Szymanski C, Giorgi R, Mahoney DW, Enriquez-Sarano M, Mitral regurgitation International DAtabase (MIDA) Investigators. Impact of ageing on presentation and outcome of mitral regurgitation due to flail leaflet: a multicentre international study. European heart journal. 2013 Sep:34(33):2600-9. doi: 10.1093/eurheartj/eht250. Epub 2013 Jul 12 [PubMed PMID: 23853072]
Thaden JJ, Malouf JF, Nkomo VT, Pislaru SV, Holmes DR Jr, Reeder GS, Rihal CS, Eleid MF. Mitral Valve Anatomic Predictors of Hemodynamic Success With Transcatheter Mitral Valve Repair. Journal of the American Heart Association. 2018 Jan 13:7(2):. doi: 10.1161/JAHA.117.007315. Epub 2018 Jan 13 [PubMed PMID: 29331957]
Estévez-Loureiro R, Franzen O, Winter R, Sondergaard L, Jacobsen P, Cheung G, Moat N, Ihlemann N, Ghione M, Price S, Duncan A, Streit Rosenberg T, Barker S, Di Mario C, Settergren M. Echocardiographic and clinical outcomes of central versus noncentral percutaneous edge-to-edge repair of degenerative mitral regurgitation. Journal of the American College of Cardiology. 2013 Dec 24:62(25):2370-2377. doi: 10.1016/j.jacc.2013.05.093. Epub 2013 Sep 4 [PubMed PMID: 24013059]
Level 2 (mid-level) evidenceLapenna E, De Bonis M, Sorrentino F, La Canna G, Grimaldi A, Torracca L, Maisano F, Alfieri O. Commissural closure for the treatment of commissural mitral valve prolapse or flail. The Journal of heart valve disease. 2008 May:17(3):261-6 [PubMed PMID: 18592922]
Level 2 (mid-level) evidenceKatz WE, Conrad Smith AJ, Crock FW, Cavalcante JL. Echocardiographic evaluation and guidance for MitraClip procedure. Cardiovascular diagnosis and therapy. 2017 Dec:7(6):616-632. doi: 10.21037/cdt.2017.07.04. Epub [PubMed PMID: 29302467]
Khan F, Winkel M, Ong G, Brugger N, Pilgrim T, Windecker S, Praz F, Fam N. Percutaneous Mitral Edge-to-Edge Repair: State of the Art and a Glimpse to the Future. Frontiers in cardiovascular medicine. 2019:6():122. doi: 10.3389/fcvm.2019.00122. Epub 2019 Sep 18 [PubMed PMID: 31620446]
Gertz ZM, Herrmann HC, Lim DS, Kar S, Kapadia SR, Reed GW, Puri R, Krishnaswamy A, Gersh BJ, Weissman NJ, Asch FM, Grayburn PA, Kosmidou I, Redfors B, Zhang Z, Abraham WT, Lindenfeld J, Stone GW, Mack MJ. Implications of Atrial Fibrillation on the Mechanisms of Mitral Regurgitation and Response to MitraClip in the COAPT Trial. Circulation. Cardiovascular interventions. 2021 Apr:14(4):e010300. doi: 10.1161/CIRCINTERVENTIONS.120.010300. Epub 2021 Mar 15 [PubMed PMID: 33719505]
Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Mitral Annulus Calcification. Journal of the American College of Cardiology. 2015 Oct 27:66(17):1934-41. doi: 10.1016/j.jacc.2015.08.872. Epub [PubMed PMID: 26493666]
Fernández-Peregrina E, Pascual I, Freixa X, Tirado-Conte G, Estévez-Loureiro R, Carrasco-Chinchilla F, Benito-González T, Asmarats L, Sanchís L, Jiménez-Quevedo P, Avanzas P, Caneiro-Queija B, Molina-Ramos AI, Fernández-Vázquez F, Li CH, Flores-Umanzor E, Sans-Roselló J, Nombela-Franco L, Arzamendi D. Transcatheter edge-to-edge mitral valve repair in patients with mitral annulus calcification. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2022 Mar 18:17(16):1300-1309. doi: 10.4244/EIJ-D-21-00205. Epub [PubMed PMID: 34483091]
Flint N, Raschpichler M, Rader F, Shmueli H, Siegel RJ. Asymptomatic Degenerative Mitral Regurgitation: A Review. JAMA cardiology. 2020 Mar 1:5(3):346-355. doi: 10.1001/jamacardio.2019.5466. Epub [PubMed PMID: 31995124]
Flameng W, Herijgers P, Bogaerts K. Recurrence of mitral valve regurgitation after mitral valve repair in degenerative valve disease. Circulation. 2003 Apr 1:107(12):1609-13 [PubMed PMID: 12668494]
Kim JH, Lee SH, Joo HC, Youn YN, Yoo KJ, Chang BC, Lee S. Effect of Recurrent Mitral Regurgitation After Mitral Valve Repair in Patients With Degenerative Mitral Regurgitation. Circulation journal : official journal of the Japanese Circulation Society. 2017 Dec 25:82(1):93-101. doi: 10.1253/circj.CJ-17-0380. Epub 2017 Jul 20 [PubMed PMID: 28724839]
Mehaffey HJ, Hawkins RB, Schubert S, Fonner C, Yarboro LT, Quader M, Speir A, Rich J, Kron IL, Ailawadi G. Contemporary outcomes in reoperative mitral valve surgery. Heart (British Cardiac Society). 2018 Apr:104(8):652-656. doi: 10.1136/heartjnl-2017-312047. Epub 2017 Oct 5 [PubMed PMID: 28982718]
Niikura H, Bae R, Gössl M, Lin D, Jay D, Sorajja P. Transcatheter therapy for residual mitral regurgitation after MitraClip therapy. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2019 Aug 9:15(6):e491-e499. doi: 10.4244/EIJ-D-18-01148. Epub 2019 Aug 9 [PubMed PMID: 31217145]
Braun D, Frerker C, Körber MI, Gaemperli O, Patzelt J, Schaefer U, Hammerstingl C, Boekstegers P, Ott I, Ince H, Thiele H, Hausleiter J. Percutaneous Edge-to-Edge Repair of Recurrent Severe Mitral Regurgitation After Surgical Mitral Valve Repair. Journal of the American College of Cardiology. 2017 Jul 25:70(4):504-505. doi: 10.1016/j.jacc.2017.05.045. Epub [PubMed PMID: 28728696]
Grasso C, Ohno Y, Attizzani GF, Cannata S, Immè S, Barbanti M, Pistritto AM, Ministeri M, Caggegi A, Chiarandà M, Dipasqua F, Ronsivalle G, Mangiafico S, Scandura S, Capranzano P, Capodanno D, Tamburino C. Percutaneous mitral valve repair with the MitraClip system for severe mitral regurgitation in patients with surgical mitral valve repair failure. Journal of the American College of Cardiology. 2014 Mar 4:63(8):836-8. doi: 10.1016/j.jacc.2013.09.045. Epub 2013 Oct 23 [PubMed PMID: 24161329]
Level 3 (low-level) evidenceKanda BS, Jay D, Farivar RS, Sorajja P. Leaflet-to-Annuloplasty Ring Clipping for Severe Mitral Regurgitation. JACC. Cardiovascular interventions. 2016 Apr 11:9(7):e63-4. doi: 10.1016/j.jcin.2015.12.272. Epub 2016 Mar 4 [PubMed PMID: 26952908]
Wu IY, Barajas MB, Hahn RT. The MitraClip Procedure-A Comprehensive Review for the Cardiac Anesthesiologist. Journal of cardiothoracic and vascular anesthesia. 2018 Dec:32(6):2746-2759. doi: 10.1053/j.jvca.2018.05.020. Epub 2018 Sep 27 [PubMed PMID: 30268642]
Hahn RT. Transcathether Valve Replacement and Valve Repair: Review of Procedures and Intraprocedural Echocardiographic Imaging. Circulation research. 2016 Jul 8:119(2):341-56. doi: 10.1161/CIRCRESAHA.116.307972. Epub [PubMed PMID: 27390336]
Hahn RT, Chan V, Adams DH. Current Indications for Transcatheter Edge-to-Edge Repair in a Patient With Primary Mitral Regurgitation. Circulation. 2022 Oct 25:146(17):1263-1265. doi: 10.1161/CIRCULATIONAHA.122.061495. Epub 2022 Oct 24 [PubMed PMID: 36279415]
Lee G, Chikwe J, Milojevic M, Wijeysundera HC, Biondi-Zoccai G, Flather M, Gaudino MFL, Fremes SE, Tam DY. ESC/EACTS vs. ACC/AHA guidelines for the management of severe aortic stenosis. European heart journal. 2023 Mar 7:44(10):796-812. doi: 10.1093/eurheartj/ehac803. Epub [PubMed PMID: 36632841]
Russell EA, Walsh WF, Costello B, McLellan AJA, Brown A, Reid CM, Tran L, Maguire GP. Medical Management of Rheumatic Heart Disease: A Systematic Review of the Evidence. Cardiology in review. 2018 Jul/Aug:26(4):187-195. doi: 10.1097/CRD.0000000000000185. Epub [PubMed PMID: 29608495]
Level 1 (high-level) evidenceVan Praet KM, Stamm C, Sündermann SH, Meyer A, Unbehaun A, Montagner M, Nazari Shafti TZ, Jacobs S, Falk V, Kempfert J. Minimally Invasive Surgical Mitral Valve Repair: State of the Art Review. Interventional cardiology (London, England). 2018 Jan:13(1):14-19. doi: 10.15420/icr.2017:30:1. Epub [PubMed PMID: 29593831]
Taramasso M, Gaemperli O, Maisano F. Treatment of degenerative mitral regurgitation in elderly patients. Nature reviews. Cardiology. 2015 Mar:12(3):177-83. doi: 10.1038/nrcardio.2014.210. Epub 2014 Dec 23 [PubMed PMID: 25533801]
Asch FM, Grayburn PA, Siegel RJ, Kar S, Lim DS, Zaroff JG, Mishell JM, Whisenant B, Mack MJ, Lindenfeld J, Abraham WT, Stone GW, Weissman NJ, COAPT Investigators. Echocardiographic Outcomes After Transcatheter Leaflet Approximation in Patients With Secondary Mitral Regurgitation: The COAPT Trial. Journal of the American College of Cardiology. 2019 Dec 17:74(24):2969-2979. doi: 10.1016/j.jacc.2019.09.017. Epub 2019 Sep 28 [PubMed PMID: 31574303]
Nishimura RA, Vahanian A, Eleid MF, Mack MJ. Mitral valve disease--current management and future challenges. Lancet (London, England). 2016 Mar 26:387(10025):1324-34. doi: 10.1016/S0140-6736(16)00558-4. Epub [PubMed PMID: 27025438]
Chakravarty T, Makar M, Patel D, Oakley L, Yoon SH, Stegic J, Singh S, Skaf S, Nakamura M, Makkar RR. Transcatheter Edge-to-Edge Mitral Valve Repair With the MitraClip G4 System. JACC. Cardiovascular interventions. 2020 Oct 26:13(20):2402-2414. doi: 10.1016/j.jcin.2020.06.053. Epub 2020 Sep 30 [PubMed PMID: 33011141]
Praz F, Braun D, Unterhuber M, Spirito A, Orban M, Brugger N, Brinkmann I, Spring K, Moschovitis A, Nabauer M, Blazek S, Pilgrim T, Thiele H, Lurz P, Hausleiter J, Windecker S. Edge-to-Edge Mitral Valve Repair With Extended Clip Arms: Early Experience From a Multicenter Observational Study. JACC. Cardiovascular interventions. 2019 Jul 22:12(14):1356-1365. doi: 10.1016/j.jcin.2019.03.023. Epub 2019 May 22 [PubMed PMID: 31129091]
Level 2 (mid-level) evidencePraz F, Spargias K, Chrissoheris M, Büllesfeld L, Nickenig G, Deuschl F, Schueler R, Fam NP, Moss R, Makar M, Boone R, Edwards J, Moschovitis A, Kar S, Webb J, Schäfer U, Feldman T, Windecker S. Compassionate use of the PASCAL transcatheter mitral valve repair system for patients with severe mitral regurgitation: a multicentre, prospective, observational, first-in-man study. Lancet (London, England). 2017 Aug 19:390(10096):773-780. doi: 10.1016/S0140-6736(17)31600-8. Epub [PubMed PMID: 28831993]
Hausleiter J, Stocker TJ, Adamo M, Karam N, Swaans MJ, Praz F. Mitral valve transcatheter edge-to-edge repair. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2023 Jan 23:18(12):957-976. doi: 10.4244/EIJ-D-22-00725. Epub [PubMed PMID: 36688459]
Huang EY, Chen C, Abdullah F, Aspelund G, Barnhart DC, Calkins CM, Cowles RA, Downard CD, Goldin AB, Lee SL, St Peter SD, Arca MJ, 2011 American Pediatric Surgical Association Outcomes and Clinical Trials Committee. Strategies for the prevention of central venous catheter infections: an American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. Journal of pediatric surgery. 2011 Oct:46(10):2000-11. doi: 10.1016/j.jpedsurg.2011.06.017. Epub [PubMed PMID: 22008341]
Level 2 (mid-level) evidenceKassar M, Praz F, Hunziker L, Pilgrim T, Windecker S, Seiler C, Brugger N. Anatomical and Technical Predictors of Three-Dimensional Mitral Valve Area Reduction After Transcatheter Edge-To-Edge Repair. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2022 Jan:35(1):96-104. doi: 10.1016/j.echo.2021.08.021. Epub 2021 Oct 11 [PubMed PMID: 34506920]
Singh GD, Smith TW, Rogers JH. Multi-MitraClip therapy for severe degenerative mitral regurgitation: "anchor" technique for extremely flail segments. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2015 Aug:86(2):339-46. doi: 10.1002/ccd.25811. Epub 2015 Feb 12 [PubMed PMID: 25559345]
Level 3 (low-level) evidenceOguz D, Padang R, Rashedi N, Pislaru SV, Nkomo VT, Mankad SV, Malouf JF, Guerrero M, Reeder GS, Eleid MF, Rihal CS, Thaden JJ. Risk for Increased Mean Diastolic Gradient after Transcatheter Edge-to-Edge Mitral Valve Repair: A Quantitative Three-Dimensional Transesophageal Echocardiographic Analysis. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2021 Jun:34(6):595-603.e2. doi: 10.1016/j.echo.2021.01.018. Epub 2021 Jan 29 [PubMed PMID: 33524491]
Naqvi TZ. Echocardiography in percutaneous valve therapy. JACC. Cardiovascular imaging. 2009 Oct:2(10):1226-37. doi: 10.1016/j.jcmg.2009.08.004. Epub [PubMed PMID: 19833314]
Hahn RT, Saric M, Faletra FF, Garg R, Gillam LD, Horton K, Khalique OK, Little SH, Mackensen GB, Oh J, Quader N, Safi L, Scalia GM, Lang RM. Recommended Standards for the Performance of Transesophageal Echocardiographic Screening for Structural Heart Intervention: From the American Society of Echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2022 Jan:35(1):1-76. doi: 10.1016/j.echo.2021.07.006. Epub 2021 Jul 17 [PubMed PMID: 34280494]
Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL, Scientific Document Committee of the European Association of Cardiovascular Imaging. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. European heart journal. Cardiovascular Imaging. 2013 Jul:14(7):611-44. doi: 10.1093/ehjci/jet105. Epub 2013 Jun 3 [PubMed PMID: 23733442]
Alkhouli M, Rihal CS, Holmes DR Jr. Transseptal Techniques for Emerging Structural Heart Interventions. JACC. Cardiovascular interventions. 2016 Dec 26:9(24):2465-2480. doi: 10.1016/j.jcin.2016.10.035. Epub [PubMed PMID: 28007198]
Sherif MA, Paranskaya L, Yuecel S, Kische S, Thiele O, D'Ancona G, Neuhausen-Abramkina A, Ortak J, Ince H, Öner A. MitraClip step by step; how to simplify the procedure. Netherlands heart journal : monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation. 2017 Feb:25(2):125-130. doi: 10.1007/s12471-016-0930-7. Epub [PubMed PMID: 27933588]
Swaans MJ, Van den Branden BJ, Van der Heyden JA, Post MC, Rensing BJ, Eefting FD, Plokker HW, Jaarsma W. Three-dimensional transoesophageal echocardiography in a patient undergoing percutaneous mitral valve repair using the edge-to-edge clip technique. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2009 Dec:10(8):982-3. doi: 10.1093/ejechocard/jep101. Epub 2009 Aug 4 [PubMed PMID: 19654135]
Level 3 (low-level) evidenceMaslow A, Mahmood F, Poppas A, Singh A. Three-dimensional echocardiographic assessment of the repaired mitral valve. Journal of cardiothoracic and vascular anesthesia. 2014 Feb:28(1):11-17. doi: 10.1053/j.jvca.2013.05.007. Epub 2013 Sep 25 [PubMed PMID: 24075641]
Saitoh T, Izumo M, Furugen A, Tanaka J, Miyata-Fukuoka Y, Gurudevan SV, Tolstrup K, Siegel RJ, Kar S, Shiota T. Echocardiographic evaluation of iatrogenic atrial septal defect after catheter-based mitral valve clip insertion. The American journal of cardiology. 2012 Jun 15:109(12):1787-91. doi: 10.1016/j.amjcard.2012.02.023. Epub 2012 Apr 3 [PubMed PMID: 22475361]
Writing Committee Members, Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2021 Feb 2:77(4):450-500. doi: 10.1016/j.jacc.2020.11.035. Epub 2020 Dec 17 [PubMed PMID: 33342587]
Nickenig G, Estevez-Loureiro R, Franzen O, Tamburino C, Vanderheyden M, Lüscher TF, Moat N, Price S, Dall'Ara G, Winter R, Corti R, Grasso C, Snow TM, Jeger R, Blankenberg S, Settergren M, Tiroch K, Balzer J, Petronio AS, Büttner HJ, Ettori F, Sievert H, Fiorino MG, Claeys M, Ussia GP, Baumgartner H, Scandura S, Alamgir F, Keshavarzi F, Colombo A, Maisano F, Ebelt H, Aruta P, Lubos E, Plicht B, Schueler R, Pighi M, Di Mario C, Transcatheter Valve Treatment Sentinel Registry Investigators of the EURObservational Research Programme of the European Society of Cardiology. Percutaneous mitral valve edge-to-edge repair: in-hospital results and 1-year follow-up of 628 patients of the 2011-2012 Pilot European Sentinel Registry. Journal of the American College of Cardiology. 2014 Sep 2:64(9):875-84. doi: 10.1016/j.jacc.2014.06.1166. Epub [PubMed PMID: 25169171]
Level 3 (low-level) evidenceSchnitzler K, Hell M, Geyer M, Kreidel F, Münzel T, von Bardeleben RS. Complications Following MitraClip Implantation. Current cardiology reports. 2021 Aug 13:23(9):131. doi: 10.1007/s11886-021-01553-9. Epub 2021 Aug 13 [PubMed PMID: 34387748]
Sticchi A, Bartkowiak J, Brugger N, Weiss S, Windecker S, Praz F. Retrograde Retrieval of a Novel Large Mitral Clip After Embolization Into the Left Ventricle. JACC. Case reports. 2021 Oct 20:3(14):1561-1568. doi: 10.1016/j.jaccas.2021.08.024. Epub 2021 Oct 20 [PubMed PMID: 34729501]
Level 3 (low-level) evidenceHamm K, Barth S, Diegeler A, Kerber S. Stroke and thrombus formation appending to the MitraClip: what is the appropriate anticoagulation regimen? The Journal of heart valve disease. 2013 Sep:22(5):713-5 [PubMed PMID: 24383386]
Level 3 (low-level) evidenceNakajima Y, Kar S. First experience of the usage of a GORE CARDIOFORM Septal Occluder device for treatment of a significant residual commissural mitral regurgitation jet following a MitraClip procedure. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2018 Sep 1:92(3):607-610. doi: 10.1002/ccd.27438. Epub 2017 Dec 8 [PubMed PMID: 29219253]
Mangieri A, Melillo F, Montalto C, Denti P, Praz F, Sala A, Winkel MG, Taramasso M, Tagliari AP, Fam NP, Rubbio AP, De Marco F, Bedogni F, Toggweiler S, Schofer J, Brinkmann C, Sievert H, Van Mieghem NM, Ooms JF, Paradis JM, Rodés-Cabau J, Brochet E, Himbert D, Perl L, Kornowski R, Ielasi A, Regazzoli D, Baldetti L, Masiero G, Tarantini G, Latib A, Laricchia A, Gattas A, Tchetchè D, Dumonteil N, Francesco G, Agricola E, Montorfano M, Lurz P, Crimi G, Maisano F, Colombo A. Management and Outcome of Failed Percutaneous Edge-to-Edge Mitral Valve Plasty: Insight From an International Registry. JACC. Cardiovascular interventions. 2022 Feb 28:15(4):411-422. doi: 10.1016/j.jcin.2021.11.040. Epub [PubMed PMID: 35210047]
Alessandrini H, Dreher A, Harr C, Wohlmuth P, Meincke F, Hakmi S, Ubben T, Kuck KH, Hassan K, Willems S, Schmoeckel M, Geidel S. Clinical impact of intervention strategies after failed transcatheter mitral valve repair. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2021 Apr 20:16(17):1447-1454. doi: 10.4244/EIJ-D-20-01008. Epub [PubMed PMID: 33074154]
Lavall D, Reil JC, Segura Schmitz L, Mehrer M, Schirmer SH, Böhm M, Laufs U. Early Hemodynamic Improvement after Percutaneous Mitral Valve Repair Evaluated by Noninvasive Pressure-Volume Analysis. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2016 Sep:29(9):888-98. doi: 10.1016/j.echo.2016.05.012. Epub 2016 Jun 29 [PubMed PMID: 27372560]