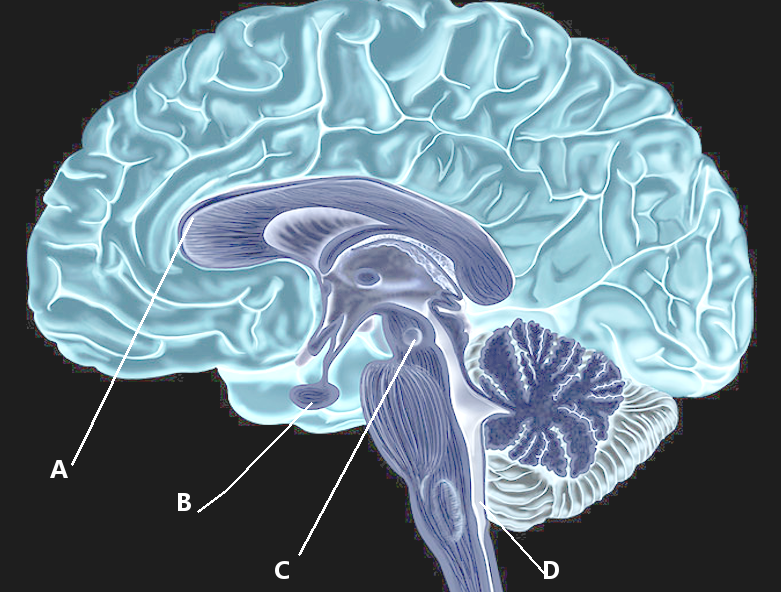
Introduction
The area postrema is a highly vascular paired structure in the medulla oblongata in the brainstem.[1] It lies in the caudal fourth ventricular floor, overlying the inferior portion of vagal trigone while facing the foramen of Magendie and rostral to the obex, the inferior point of the floor of the fourth ventricle. It is between the funiculus separans and the gracilis tubercle, lying adjacent to the nucleus of tractus solitarius. The description of the structure has been as ‘arched wings’ that are open on the sides of the fourth ventricle, as they separate upward from the central canal.[2]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
A semitransparent ridge called the funiculus separans separates the area postrema from the vagal triangle, which lies over the dorsal vagal nucleus, on the caudal end of the fourth ventricle floor. The area postrema is composed of vascular and cellular components. The vascular part is mainly composed of sinusoidal fenestrated capillaries, forming a portal system; with the cellular part composed of flattened ependymal cells, glial cells, and small neurons.[1] A special type of cells called tanycytes made up of thick ependyma, cover the paired structure, and contribute to its essential functions. Many hair-like microvilli cover the cells of the ependyma and frequently intricate microvilli tufts that stretch into the cerebrospinal fluid (CSF) compartment.[3]
A part of the dorsal vagal complex, the area postrema is well studied as the vomiting center. It also is one of the circumventricular organs, which can detect circulating chemical messengers in the blood and incorporate neural inputs in the brainstem.[1] They accomplish this by sensing blood-borne baroreceptor information from the carotid sinus and aorta, along with information from the liver via osmoreceptors. The absence of tight junctions between endothelial cells and its composition of sinusoidal fenestrated capillaries aids in physiologic signaling with its neurons, playing a role in the cardiovascular system and those that regulate feeding and metabolism. It mediates the effect of angiotensin II, which increases the arterial blood pressure without significantly impacting the heart rate. Additionally, neurons in the area postrema can use stretch receptors in the stomach to integrate mechanical information; this aids in somatic growth and in stimulating appetite.[1] These functions are immature are birth and develop over time. A less studied role includes the impact on the respiratory drive: when stimulated, the neurons increase respiratory drive, and when damaged, they contribute to the decrease in respiratory rate.[4]
Embryology
The development of the area postrema in humans begins during weeks 10 to 15 of gestation in the form of two sulci in the wall of the ventricle. There is noticeable vascularity of the parenchyma of area postrema in the late first trimester, which will increase until birth.[5] By weeks 16 to 29, as the vasculature develops, the area projects into the fourth ventricle. It continues to develop, and by weeks 30 to 40 of gestation, the neurons are recognizable and now resemble the adult morphology.[4] In this manner, the thin ependymal structure of the adult develops from a thicker continuous structure present in the second trimester.
Blood Supply and Lymphatics
The vessels supplying the area postrema are large and form a closed network. The path of the vasculature contains capillary loops beneath the ependyma of the ventricle. The arteries reach the area postrema externally, whereas the veins leave the structure internally. The arteries and veins rely on the larger vessels located on the dorsal medulla. There is not much consistency in the anastomoses that connects the vasculature of area postrema to that of the choroidal plexus or the underlying nervous tissue.[6]
Although the central nervous system does not have defined lymphatic channels as those present in the body, the CSF, and interstitial fluid drain into the regional lymph nodes.[7]
Nerves
Situated near the nucleus tractus solitarius, the afferent input to the area postrema comes from the glossopharyngeal and vagus nerves and several hypothalamic nuclei. It also receives afferent input from carotid sinus and aortic depressor nerves.[8] Its efferent projections include those to the nucleus tractus solitarius, locus coeruleus, parabrachial nucleus, and the ventral lateral medulla. It also has efferent projections to pericentral dorsal tegmental and dorsolateral tegmental nuclei.[9]
Muscles
Given that the area postrema plays a role in vomiting and impacts the systems of feeding, metabolism, and the heart, it has an indirect effect on the muscles involved. After it relays information to the nucleus tractus solitarius, distinct neural outputs are sent to coordinate effector responses of emesis including swallowing, salivation, cardiovascular, respiration, and gastrointestinal in a structured manner. Some of these results include contraction of the diaphragm via abdominal muscles, external intercostal muscles while internal intercostal muscles relax; esophageal contraction; relaxation of lower esophageal sphincter; gastric dysrhythmia; and duodenal retro-peristaltic activity in duodenum and stomach.[10]
Physiologic Variants
The area postrema, along with the dorsal motor nucleus of the vagus, and the nucleus tractus solitarius form the dorsal vagal complex, where many afferent vagal nerve fibers terminate. Although damage to the area postrema may prevent vomiting in response to most emetic drugs, it does not play a significant role in vomiting induced by motion or through activation of vagal nerve afferents. There is remaining controversy regarding its role in radiation-induced vomiting. Activation of the structure leads to nausea and vomiting as it projects signals to the adjacent nucleus tractus solitarius. Therefore, this nucleus may serve as a common pathway by which various emetic inputs trigger vomiting.[3]
Surgical Considerations
Due to its position and given the lack of surface markers on the floor of the fourth ventricle, the area postrema is challenging to localize during micro-neurosurgical procedures. However, as it may serve as an important landmark during procedures involving the brainstem, there has been some success with fluorescence-enhanced inspection of the fourth ventricle. This success is possible due to the absence of a blood-brain-barrier, and thus fluorescein may flow freely in the interstitial space, revealing the area postrema. The normal anatomical appearance of area postrema appears as two short and thick leaves that merge at the midline.[11]
Clinical Significance
The area postrema may be damaged by lesioning or ablation, which prevents its normal functions, including its variety of physiological reflexes. These reflexes include taste aversion, gastroparesis, and homeostatic functions. Many of them rely on the brain’s ability to monitor the body’s physiologic status through the use of the vagus nerve; thus, lesions to the structure sometimes carry the name of ‘central vagotomy.’[12]
The structure also clinical importance with regards to Parkinson disease. Dopaminergic drugs serving as treatment agents for the disease strongly affect the area postrema, which has a high density of dopamine receptors. As it is a very sensitive chemoreceptor, it senses the foreign poisonous substance, causing it to react defensively and induce nausea and vomiting to prevent further intoxication. Accordingly, a common side effect of antiparkinsonian drugs is nausea.[13]
Other Issues
The structure has links to a condition called area postrema syndrome (APS), defined as otherwise unexplained episodes of nausea, hiccups, and vomiting, usually intractable. When coupling this syndrome with the neuroinflammatory involvement of the area postrema, it is characteristic of neuromyelitis optica spectrum disorders (NMOSD). The name APS is only a recent appellation, but there have been many examples in the medical literature from the past. Although non-demyelinating lesions in the medulla or the upper cervical cord might also lead to APS, the syndrome has diagnostic and prognostic significance in relation to NMOSD. The involvement of the medulla with the diagnosis of APS may indicate a more severe disease course of NMOSD.[14]
Media
(Click Image to Enlarge)
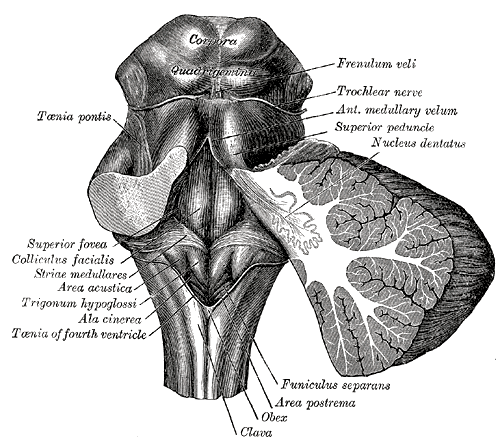
The Hindbrain or Rhombencephalon. Rhomboid fossa, corpora quadrigemina, colliculus facialis, ala cinerea superior peduncle, nucleus dentatus, frenulum veli, trochlear nerve, funiculus separans, area postrema, obex, clava, and taenia pontis.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
References
Sarnat HB, Flores-Sarnat L, Boltshauser E. Area Postrema: Fetal Maturation, Tumors, Vomiting Center, Growth, Role in Neuromyelitis Optica. Pediatric neurology. 2019 May:94():21-31. doi: 10.1016/j.pediatrneurol.2018.12.006. Epub 2018 Dec 25 [PubMed PMID: 30797593]
Beddok A, Faivre JC, Coutte A, Guévelou JL, Welmant J, Clavier JB, Guihard S, Janoray G, Calugaru V, Pointreau Y, Lacout A, Salleron J, Lefranc M, Hasboun D, Duvernoy HM, Thariat J. Practical contouring guidelines with an MR-based atlas of brainstem structures involved in radiation-induced nausea and vomiting. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2019 Jan:130():113-120. doi: 10.1016/j.radonc.2018.08.003. Epub 2018 Aug 29 [PubMed PMID: 30172454]
Miller AD, Leslie RA. The area postrema and vomiting. Frontiers in neuroendocrinology. 1994 Dec:15(4):301-20 [PubMed PMID: 7895890]
Level 3 (low-level) evidenceGokozan HN, Baig F, Corcoran S, Catacutan FP, Gygli PE, Takakura AC, Moreira TS, Czeisler C, Otero JJ. Area postrema undergoes dynamic postnatal changes in mice and humans. The Journal of comparative neurology. 2016 Apr 15:524(6):1259-69. doi: 10.1002/cne.23903. Epub 2015 Dec 17 [PubMed PMID: 26400711]
Level 2 (mid-level) evidencePangestiningsih TW, Hendrickson A, Sigit K, Sajuthi D, Nurhidayat, Bowden DM. Development of the area postrema: an immunohistochemical study in the macaque. Brain research. 2009 Jul 14:1280():23-32. doi: 10.1016/j.brainres.2009.05.028. Epub 2009 May 19 [PubMed PMID: 19460361]
Level 3 (low-level) evidenceDuvernoy H, Koritké JG, Monnier G, Jacquet G. [Vascularization of the area postrema and of the dorsal side of the medulla oblongata in the human]. Zeitschrift fur Anatomie und Entwicklungsgeschichte. 1972:138(1):41-66 [PubMed PMID: 4638677]
Weller RO, Djuanda E, Yow HY, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta neuropathologica. 2009 Jan:117(1):1-14. doi: 10.1007/s00401-008-0457-0. Epub 2008 Nov 11 [PubMed PMID: 19002474]
Level 3 (low-level) evidenceMaolood N, Meister B. Protein components of the blood-brain barrier (BBB) in the brainstem area postrema-nucleus tractus solitarius region. Journal of chemical neuroanatomy. 2009 May:37(3):182-95. doi: 10.1016/j.jchemneu.2008.12.007. Epub 2008 Dec 25 [PubMed PMID: 19146948]
Level 3 (low-level) evidenceVigier D, Rouvière A. Afferent and efferent connections of the area postrema demonstrated by the horseradish peroxidase method. Archives italiennes de biologie. 1979 Nov:117(4):325-39 [PubMed PMID: 550738]
Level 3 (low-level) evidenceBabic T, Browning KN. The role of vagal neurocircuits in the regulation of nausea and vomiting. European journal of pharmacology. 2014 Jan 5:722():38-47. doi: 10.1016/j.ejphar.2013.08.047. Epub 2013 Oct 31 [PubMed PMID: 24184670]
Level 3 (low-level) evidenceLongatti P, Porzionato A, Basaldella L, Fiorindi A, De Caro P, Feletti A. The human area postrema: clear-cut silhouette and variations shown in vivo. Journal of neurosurgery. 2015 May:122(5):989-95. doi: 10.3171/2014.11.JNS14482. Epub 2015 Jan 16 [PubMed PMID: 25594320]
Spencer CM, Eckel LA, Nardos R, Houpt TA. Area postrema lesions attenuate LiCl-induced c-Fos expression correlated with conditioned taste aversion learning. Physiology & behavior. 2012 Jan 18:105(2):151-60. doi: 10.1016/j.physbeh.2011.08.022. Epub 2011 Aug 24 [PubMed PMID: 21889521]
Level 3 (low-level) evidenceTravagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nature reviews. Gastroenterology & hepatology. 2016 Jul:13(7):389-401. doi: 10.1038/nrgastro.2016.76. Epub 2016 May 25 [PubMed PMID: 27251213]
Camara-Lemarroy CR, Burton JM. Area postrema syndrome: A short history of a pearl in demyelinating diseases. Multiple sclerosis (Houndmills, Basingstoke, England). 2019 Mar:25(3):325-329. doi: 10.1177/1352458518813105. Epub 2018 Nov 22 [PubMed PMID: 30463481]