Introduction
Angiolymphoid hyperplasia with eosinophilia (ALHE), aka epithelioid hemangioma, is an uncommon benign vasoproliferative disorder, first described in 1969 by Wells and Whimster. The pathogenesis of ALHE is not clearly understood. This condition usually presents with solitary or multiple papules or nodules involving mostly the head and neck region. More rarely, the lesions can appear in other body sites or affect extracutaneous tissues. The objective of the treatment is to attend to cosmetic issues and to allow symptomatic relief. Multiple treatment modalities have been tried, but none proved a reproducible success.[1][2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of ALHE is not fully understood, and it is unclear whether it consists of a vascular neoplasm or a lymphoproliferative process. The first hypothesis is supported by the association with arteriovenous shunts in 43% of the cases and the frequency of preceding trauma (friction, surgery, frostbite, or laceration), similar to pyogenic granuloma.[2][3] Hyperestrogen state seems to contribute to the pathogenesis of this condition. This phenomenon is also observed with various vascular tumors during pregnancy.[4][5] Furthermore, results from a recent study described intracytoplasmic endothelial staining of Wilms tumor-1 in 19 of 20 ALHE specimens. Immunoreactivity for Wilms tumor-1 was also found previously in multiple vascular neoplasms, such as infantile hemangiomas and pyogenic granulomas.[6] A mutation of the endothelial cell tyrosine kinase receptor Tie-2 was present in ALHE and other noninherited vascular anomalies.[7] There have been suggestions that renin, induced by associated arteriovenous shunts, might be a pathogenic factor for ALHE by stimulating new vessel formation and eosinophils activation and migration through angiotensin II production.[8]
The second hypothesis of ALHE as a lymphoproliferative disorder has support from the condition's progressive course and frequent recurrences. Interestingly, T-cell receptor gene rearrangement and monoclonality have been previously observed in ALHE cases, raising the possibility to consider this condition a low-grade T-cell lymphoma.[9] Additionally, there were 2 previous reports of ALHE associated with peripheral T-cell lymphoma. In 1 case, the same monoclonal TCR gene rearrangement was present in both ALHE and T-cell lymphoma.[10][11] A few cases of ALHE showed evidence of follicular mucinosis, but no reports of association with mycosis fungoides exist so far.[12] Of note, some cases of ALHE correlated to human herpesvirus 8 and human T-cell lymphotropic virus.[9][13] Finally, whether ALHE is a true vascular neoplasia with a rich inflammatory component or a lymphoproliferative process with a reactive angiogenic response is yet to be clarified.
Epidemiology
Angiolymphoid hyperplasia with eosinophilia is uncommon but not rare. The exact prevalence is unknown, but it is mostly seen in individuals who are Asian, followed by White individuals, and less frequently in individuals who are Black. ALHE has a wide prevalence range varying from 0.7 months to 91 years. The mean age at presentation is 37 years, occurring in men and women without significant differences.[14]
Histopathology
Histopathology displays specific histologic features. The tumors are mainly located in the dermis and less frequently in subcutaneous tissues. A combination vascular-inflammatory component is present. The lesions show a proliferation of capillary-sized vessels lined by prominent, enlarged endothelial cells giving the typical cobblestone appearance. The cells are mostly cuboidal, with intracytoplasmic vacuoles and uniform ovoid nuclei. A dense vascular and interstitial infiltrate of lymphocytes and eosinophils is seen. Mitoses are rarely observed but lack anaplasia and atypical figures. The vascular component predominates in early or actively growing ALHE, while the lymphoid infiltrate is more prominent in the late stages of the disease. Histological evidence of arteriovenous shunts is present in 42% of the cases.[2][15]
History and Physical
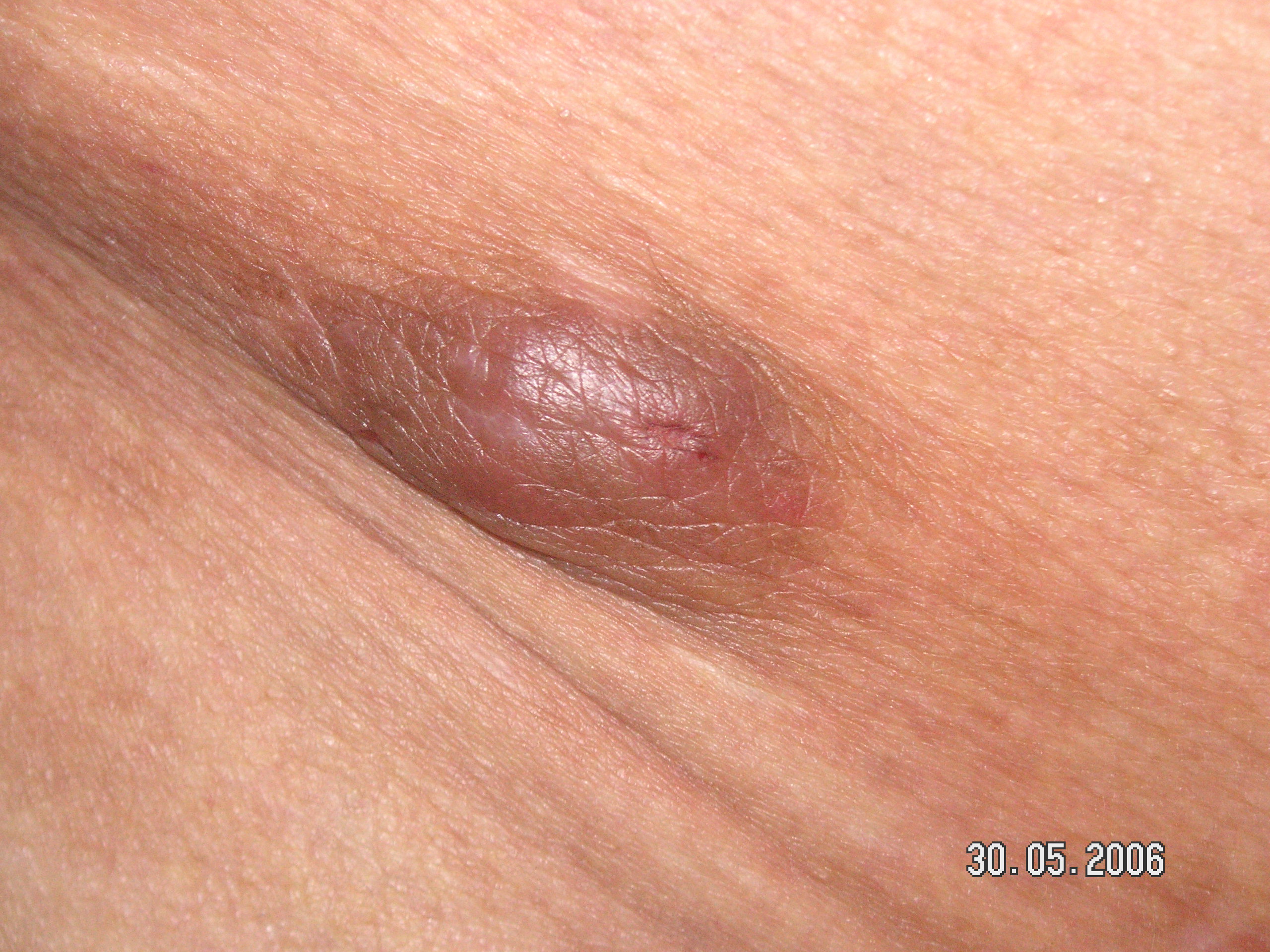
Angiolymphoid hyperplasia with eosinophilia is a benign, locally proliferating disease that tends to arise in the skin of the head and neck, particularly in the periauricular area and the scalp. Nonetheless, lesions can occur on any body site, including extremities, trunk, oral and genital mucosa, and orbit.[14][15] Internal organs may also be involved in a lesser frequency such as heart, colon, kidney, bone, and lung ALHE.[16][17][18][19][20] Several reports exist of a few cases of ALHE arising from arteries. They can present as pulsatile tumors mimicking vascular malformation.[21]
ALHE presents with dermal and/or subcutaneous, single or multiple, pink to red-brown papules and/or nodules of variable size ranging from a few to multiple centimeters. The lesions are most commonly symptomatic, accompanied by pruritus, bleeding, and/or pain; regional lymphadenopathy is rarely associated. The lesions tend to be persistent and recurrent. However, there are also reports of spontaneous remissions. Early age of onset disease characteristically has a longer duration and multiple bilateral lesions, more prone to bleeding, pain, and pruritus.[2][14] The association with nephrotic syndrome has been reported in a few cases, but it is not well-established.[2][22][23] Dermoscopy of ALHE lesions reveals a polymorphous vascular pattern composed of regularly distributed dotted and linear vessels over a concomitant pink-to-red background.[24]
Evaluation
Laboratory analysis includes a complete blood count, which reveals peripheral blood eosinophilia in approximately 20% of the cases.[2] Kidney function tests may be performed to assess eventually associated nephropathy. A biopsy of the lesions is required to establish the diagnosis (see histopathology for findings). An immunohistochemical study reveals a strong reactivity for CD31 and lesser reactivity for CD34 and factor VIII-related antigens.[25] More recently, fosB proto-oncogene, AP-1 transcription factor subunit immunohistochemistry proved sensitive in diagnosing ALHE by allowing differentiation from its histological mimics.[26] Intracytoplasmic endothelial staining of Wilms Tumor 1 can also be useful for diagnosing ALHE.[6] Adequate radiologic workup can be further performed to explore ALHE involving internal organs, arteries, orbit, and bones.
Treatment / Management
Although ALHE is a benign disease, its management is particularly challenging considering the frequency of recurrences and the absence of randomized controlled clinical trials. Most of the available treatment evidence exists from case reports and retrospective analyses. There are reports of multiple therapeutic options but none with consistent results.
Surgical excision is considered the treatment of choice in ALHE; this is the most commonly used therapeutic intervention, with the lowest failure rate of 44.2%. Given this high recurrence rate, Mohs micrographic surgery was suggested as a treatment option for lesions with ill-defined margins and when tissue sparing is desirable. Lesions with large cutaneous vessels may respond better if a preoperative embolization is performed.[14][27][28](A1)
Laser therapies are promising alternatives for surgery in patients with multiple lesions, in cosmetic sensitive sites, and in poor surgical candidates. Argon, Nd: YAG, pulsed dye, and carbon-dioxide lasers were previous choices. But the recurrence rate remains high, over 50%. A combination of pulsed dye lasers and carbon dioxide lasers was also considered. The recurrence can be prevented by maintenance treatment.[14][29](A1)
Other interventional treatment options include photodynamic therapy, cryotherapy, electrodesiccation, and radiotherapy.[14][30] Results from one report noted no recurrence up to 8 after brachytherapy.[31] Sclerotherapy with polidocanol or bleomycin was performed in 3 cases of multiple ALHE, achieving complete healing and clinical remission at follow-up.[32][33] Radiofrequency ablation was shown to be efficient in 3 case studies.[34][35][36] A combination of radiofrequency and sclerotherapy was also described in 3 patients and resulted in clinical remission for up to 3 years.[37](A1)
Systemic treatments with systemic corticosteroids, oral isotretinoin, oral dapsone, and oral pentoxifylline were previously used but with high failure rates (80% to 100%).[14] Two cases with refractory orbital and multiple ALHE were successfully managed with methotrexate and thalidomide, respectively.[38][39] Mepolizumab, or anti-interleukin-5 antibody, was administered to patients with ALHE associated with blood eosinophilia. Local pruritus and hypereosinophilia disappeared, while the nodules regressed only slightly.[40][38] Recent data results suggested oral propranolol alone or before surgery to reduce the size or number of lesions.[41][42][43] (A1)
Topical timolol also showed some success in the management of ALHE.[44] Topical and intralesional corticosteroids were the primary therapeutic agents, but high recurrence rates remained.[14] Other local treatment options are topical imiquimod 5%, topical tacrolimus 0.1%, and intralesional interferon alpha-2b. Variable outcomes have been the observed result.[45][46][47][48](A1)
Differential Diagnosis
The major differential diagnosis of ALHE is Kimura disease. These 2 conditions were previously considered as manifestations of the same entity. Epidemiological, clinical, and histopathological particularities subsequently distinguished them from each other. Kimura disease occurs in young Asian men. This condition presents with solitary or multiple large subcutaneous masses with deeper localization. The lesions typically involve the salivary glands in the periauricular or submandibular region and are commonly accompanied by regional lymphadenopathy. Laboratory workup often finds peripheral blood eosinophilia and elevated immunoglobulin E. Nephrotic syndrome is more associated with Kimura disease than with ALHE. On histopathology, hyperplastic lymphoid follicles with prominent germinal centers and eosinophilic infiltrate are conspicuous features of Kimura disease. Vascular proliferation is present but is less notable than in ALHE and lacks the characteristic plump epithelioid endothelial cells.[49][50]
ALHE should also be distinguished from pyogenic granuloma, which presents as an eruptive papule, sometimes eroded or ulcerated, with an epidermal collarette. Histologically, it consists of capillary-sized vessels separated by fibromyxoid stroma, contrasting with the immature blood vessels with hobnailing seen in ALHE.[51] Clinically, ALHE can look very similar to nodular Kaposi sarcoma. However, the histopathological distinction is usually easy. Kaposi sarcoma is composed of spindle cell proliferation with stiltlike vascular spaces that stain positively with human herpesvirus 8.[51]
Another differential diagnosis is angiosarcoma, especially in its epithelioid variant. The latter has more rapid growth, an aggressive course, and histologically shows vigorous mitotic activity, polymorphism, and nuclear hyperchromasia.[15] ALHE can also be confused with epithelioid hemangioendothelioma. The presence of myxohyaline stroma separating strands, cords, and nests of vacuolated endothelial cells, the lack of lymphoid or eosinophilic infiltrate, and the presence of mitoses and nuclear atypia can address the diagnosis.[52] Granuloma faciale usually presents as a red-brown plaque on the face and is refractory to treatments. Eosinophil infiltrate may be present on histology, but chronic leukocytoclastic vasculitis, fibrin deposition, and fibrosis permit the differentiation of the diagnosis.[51]
Prognosis
Angiolymphoid hyperplasia with eosinophilia tends to persist for years and to recur after treatment in more than 40% of the cases. Early age of onset, long disease duration, multiple lesions, and symptomatic disease seem to have a higher recurrence rate. Spontaneous regression can occur occasionally.[14] ALHE is an entirely benign disease. Even though there were cases of T-cell receptor gene rearrangement and monoclonality, no reports of absolute evidence of malignant behavior in ALHE exist thus far.
Complications
Angiolymphoid hyperplasia with eosinophilia is a benign disease. Reports exist of concomitant nephropathy in a few cases, but the association is not all that common. According to the location of the lesions, complications may present. Rectal bleeding was reported in colonic ALHE. Orbital ALHE can present with proptosis, diplopia, and blurred vision.
Arterial ALHE can result in compressing proximal nerves: there are prior reports of entrapment of the ulnar nerve resulting in paresthesia and tactile dysesthesia of the involved area. Arterial ALHE can also cause complete occlusion of the vessel lumen leading to chronic ischemia. A case of cardiac ALHE presented with supraventricular arrhythmia.[18][53][54][55][56]
Deterrence and Patient Education
Patients with ALHE should be aware of the chronic course of the disease and the possibility of recurrence after treatment. But they should also be reassured about the benign nature of the condition. Inform patients of the different treatment options and involve them in decision-making about their care.
Enhancing Healthcare Team Outcomes
The management of ALHE requires an interprofessional team. Patients often present with unique or multiple cutaneous papules and/or nodules, therefore, primary care clinicians generally refer patients to a dermatologist. In many other cases, ALHE can present in any site of the body and present with several symptoms. Thus, different specialists such as ophthalmologists, cardiologists, gastroenterologists, neurologists, and otorhinolaryngologists can be involved in making the diagnosis and organizing a treatment plan. The role of the radiologist can also be vital in some cases where the lesions are not accessible, especially in ALHE with arterial involvement.
A biopsy is recommended to confirm the diagnosis and requires a trained pathologist. Many patients with disfiguring lesions develop depression and anxiety, so psychological counseling is useful for these individuals. Given the high rate of recurrence, the treatment of ALHE can be tricky and challenging. Hence, meticulous discussion and planning between surgeons, radiotherapists, and pharmacists is required.
Media
(Click Image to Enlarge)
References
Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. The British journal of dermatology. 1969 Jan:81(1):1-14 [PubMed PMID: 5763634]
Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia. A clinicopathologic study of 116 patients. Journal of the American Academy of Dermatology. 1985 May:12(5 Pt 1):781-96 [PubMed PMID: 4008683]
Wollina U, Langner D, França K, Gianfaldoni S, Lotti T, Tchernev G. Pyogenic Granuloma - A Common Benign Vascular Tumor with Variable Clinical Presentation: New Findings and Treatment Options. Open access Macedonian journal of medical sciences. 2017 Jul 25:5(4):423-426. doi: 10.3889/oamjms.2017.111. Epub 2017 Jul 13 [PubMed PMID: 28785323]
Moy RL, Luftman DB, Nguyen QH, Amenta JS. Estrogen receptors and the response to sex hormones in angiolymphoid hyperplasia with eosinophilia. Archives of dermatology. 1992 Jun:128(6):825-8 [PubMed PMID: 1599273]
Level 3 (low-level) evidenceWalker JL, Wang AR, Kroumpouzos G, Weinstock MA. Cutaneous tumors in pregnancy. Clinics in dermatology. 2016 May-Jun:34(3):359-67. doi: 10.1016/j.clindermatol.2016.02.008. Epub 2016 Feb 9 [PubMed PMID: 27265074]
Tokat F, Lehman JS, Sezer E, Cetin ED, Ince U, Durmaz EO. Immunoreactivity of Wilms tumor 1 (WT1) as an additional evidence supporting hemangiomatous rather than inflammatory origin in the etiopathogenesis of angiolymphoid hyperplasia with eosinophilia. Dermatology practical & conceptual. 2018 Jan:8(1):28-32. doi: 10.5826/dpc.0801a06. Epub 2018 Jan 31 [PubMed PMID: 29445571]
Ye C, Pan L, Huang Y, Ye R, Han A, Li S, Li X, Wang S. Somatic mutations in exon 17 of the TEK gene in vascular tumors and vascular malformations. Journal of vascular surgery. 2011 Dec:54(6):1760-8. doi: 10.1016/j.jvs.2011.06.098. Epub 2011 Oct 1 [PubMed PMID: 21962923]
Level 2 (mid-level) evidenceOnishi Y, Ohara K. Angiolymphoid hyperplasia with eosinophilia associated with arteriovenous malformation: a clinicopathological correlation with angiography and serial estimation of serum levels of renin, eosinophil cationic protein and interleukin 5. The British journal of dermatology. 1999 Jun:140(6):1153-6 [PubMed PMID: 10354088]
Level 3 (low-level) evidenceKempf W, Haeffner AC, Zepter K, Sander CA, Flaig MJ, Mueller B, Panizzon RG, Hardmeier T, Adams V, Burg G. Angiolymphoid hyperplasia with eosinophilia: evidence for a T-cell lymphoproliferative origin. Human pathology. 2002 Oct:33(10):1023-9 [PubMed PMID: 12395376]
Gonzalez-Cuyar LF, Tavora F, Zhao XF, Wang G, Auerbach A, Aguilera N, Burke AP. Angiolymphoid hyperplasia with eosinophilia developing in a patient with history of peripheral T-cell lymphoma: evidence for multicentric T-cell lymphoproliferative process. Diagnostic pathology. 2008 May 29:3():22. doi: 10.1186/1746-1596-3-22. Epub 2008 May 29 [PubMed PMID: 18510751]
Andreae J, Galle C, Magdorf K, Staab D, Meyer L, Goldman M, Querfeld U. Severe atherosclerosis of the aorta and development of peripheral T-cell lymphoma in an adolescent with angiolymphoid hyperplasia with eosinophilia. The British journal of dermatology. 2005 May:152(5):1033-8 [PubMed PMID: 15888166]
Level 3 (low-level) evidenceGutte R, Doshi B, Khopkar U. Angiolymphoid hyperplasia with eosinophilia with follicular mucinosis. Indian journal of dermatology. 2013 Mar:58(2):159. doi: 10.4103/0019-5154.108081. Epub [PubMed PMID: 23716828]
Nagy K, Marschalkó M, Kemény B, Horváth A. Localization of human T-cell lymphotropic virus-1 gag proviral sequences in dermato-immunological disorders with eosinophilia. Acta microbiologica et immunologica Hungarica. 2005:52(3-4):385-96 [PubMed PMID: 16400878]
Adler BL, Krausz AE, Minuti A, Silverberg JI, Lev-Tov H. Epidemiology and treatment of angiolymphoid hyperplasia with eosinophilia (ALHE): A systematic review. Journal of the American Academy of Dermatology. 2016 Mar:74(3):506-12.e11. doi: 10.1016/j.jaad.2015.10.011. Epub 2015 Dec 11 [PubMed PMID: 26685720]
Level 1 (high-level) evidenceGuo R, Gavino AC. Angiolymphoid hyperplasia with eosinophilia. Archives of pathology & laboratory medicine. 2015 May:139(5):683-6. doi: 10.5858/arpa.2013-0334-RS. Epub [PubMed PMID: 25927152]
Wang SX, Zou WZ, Lü JC, Su T, Zhou FD, Wang HY. [Angiolymphoid hyperplasia with eosinophilia involving the kidney: a report of three cases with literature review]. Zhonghua nei ke za zhi. 2007 Oct:46(10):827-30 [PubMed PMID: 18218234]
Level 3 (low-level) evidenceBui MM, Draper NL, Dessureault S, Nasir NA, Cooper H, Nasir A, Coppola D. Colonic angiolymphoid hyperplasia with eosinophilia masquerading as malignancy: a case report and review of the literature. Clinical colorectal cancer. 2010 Jul:9(3):179-82. doi: 10.3816/CCC.2010.n.025. Epub [PubMed PMID: 20643624]
Level 3 (low-level) evidenceMachado I, Chong A, Serrano A, Naranjo Ugalde AM, Pineda D, Savón L, Olivera E, Llombart-Bosch A. Epithelioid Hemangioma (Angiolymphoid Hyperplasia With Eosinophilia) of the Heart With Peripheral Eosinophilia and Nephrotic Syndrome. International journal of surgical pathology. 2016 Feb:24(1):59-65. doi: 10.1177/1066896915604254. Epub 2015 Sep 2 [PubMed PMID: 26338719]
Khanna G, Sharma S, Rajni. Angiolymphoid hyperplasia with eosinophilia involving bone. Indian journal of pathology & microbiology. 2007 Oct:50(4):844-6 [PubMed PMID: 18306582]
Level 3 (low-level) evidenceRibeiro L, Souto M, Loureiro A. Angiolymphoid Hyperplasia With Eosinophilia of the Lung. Archivos de bronconeumologia. 2018 Jun:54(6):340-342. doi: 10.1016/j.arbres.2017.12.013. Epub 2018 Feb 3 [PubMed PMID: 29402550]
Vandy F, Izquierdo L, Liu J, Criado E. Angiolymphoid hyperplasia involving large arteries. Journal of vascular surgery. 2008 May:47(5):1086-9. doi: 10.1016/j.jvs.2007.12.004. Epub [PubMed PMID: 18455650]
Level 3 (low-level) evidenceAzizzadeh M, Namazi MR, Dastghaib L, Sari-Aslani F. Angiolymphoid hyperplasia with eosinophilia and nephrotic syndrome. International journal of dermatology. 2005 Mar:44(3):242-4 [PubMed PMID: 15807737]
Level 3 (low-level) evidenceIto S, Oda T, Matsuo A, Takechi H, Uchida T, Watanabe A, Kono T, Shimazaki H, Tamai S, Oshima N, Kumagai H. Observation of Angiolymphoid Hyperplasia with Eosinophilia (ALHE) at Three Arterial Sites and Its Association with Membranous Nephropathy. Internal medicine (Tokyo, Japan). 2015:54(15):1933-9. doi: 10.2169/internalmedicine.54.4031. Epub 2015 Aug 1 [PubMed PMID: 26234240]
Rodríguez-Lomba E, Avilés-Izquierdo JA, Molina-López I, Parra-Blanco V, Lázaro-Ochaita P, Suárez-Fernández R. Dermoscopic features in 2 cases of angiolymphoid hyperplasia with eosinophilia. Journal of the American Academy of Dermatology. 2016 Jul:75(1):e19-21. doi: 10.1016/j.jaad.2016.02.1145. Epub [PubMed PMID: 27317536]
Level 3 (low-level) evidenceFetsch JF, Sesterhenn IA, Miettinen M, Davis CJ Jr. Epithelioid hemangioma of the penis: a clinicopathologic and immunohistochemical analysis of 19 cases, with special reference to exuberant examples often confused with epithelioid hemangioendothelioma and epithelioid angiosarcoma. The American journal of surgical pathology. 2004 Apr:28(4):523-33 [PubMed PMID: 15087672]
Level 3 (low-level) evidenceOrtins-Pina A, Llamas-Velasco M, Turpin S, Soares-de-Almeida L, Filipe P, Kutzner H. FOSB immunoreactivity in endothelia of epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia). Journal of cutaneous pathology. 2018 Jun:45(6):395-402. doi: 10.1111/cup.13141. Epub 2018 Apr 6 [PubMed PMID: 29527734]
Miller CJ, Ioffreda MD, Ammirati CT. Mohs micrographic surgery for angiolymphoid hyperplasia with eosinophilia. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2004 Aug:30(8):1169-73 [PubMed PMID: 15274714]
Level 3 (low-level) evidenceBazakas A, Solander S, Ladizinski B, Halvorson EG. Transarterial embolization followed by surgical excision of skin lesions as treatment for angiolymphoid hyperplasia with eosinophilia. Journal of the American Academy of Dermatology. 2013 Feb:68(2):e48-9. doi: 10.1016/j.jaad.2012.07.023. Epub [PubMed PMID: 23317987]
Level 3 (low-level) evidenceSagi L, Halachmi S, Levi A, Amitai DB, Enk CD, Lapidoth M. Combined pulsed dye and CO2 lasers in the treatment of angiolymphoid hyperplasia with eosinophilia. Lasers in medical science. 2016 Aug:31(6):1093-6. doi: 10.1007/s10103-016-1954-3. Epub 2016 May 16 [PubMed PMID: 27184154]
Sotiriou E, Apalla Z, Patsatsi A, Panagiotidou DD, Ioannides D. Angiolymphoid hyperplasia with eosinophilia: good response to photodynamic therapy. Clinical and experimental dermatology. 2009 Dec:34(8):e629-31. doi: 10.1111/j.1365-2230.2009.03348.x. Epub 2009 Jun 22 [PubMed PMID: 19549233]
Level 3 (low-level) evidenceZhang J, Li Y, Wen G, Deng Y, Yao H. Novel Application of (32)P Brachytherapy: Treatment of Angiolymphoid Hyperplasia with Eosinophilia in the Right Auricle with 8-Year Follow-Up. Cancer biotherapy & radiopharmaceuticals. 2018 Sep:33(7):282-284. doi: 10.1089/cbr.2018.2468. Epub 2018 Jun 29 [PubMed PMID: 29957026]
Akdeniz N, Kösem M, Calka O, Bilgili SG, Metin A, Gelincik I. Intralesional bleomycin for angiolymphoid hyperplasia. Archives of dermatology. 2007 Jul:143(7):841-4 [PubMed PMID: 17638726]
Level 3 (low-level) evidenceWang Y, Tu Y, Tao J, Li Y. Angiolymphoid hyperplasia with eosinophilia responsive to sclerotherapy. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2014 Sep:40(9):1042-3. doi: 10.1097/01.DSS.0000452634.06834.bc. Epub [PubMed PMID: 25099288]
Level 3 (low-level) evidenceTambe SA, Nayak CS. Successful Management of Angiolymphoid Hyperplasia with Eosinophilia by Radiofrequency. Journal of cutaneous and aesthetic surgery. 2017 Apr-Jun:10(2):116-118. doi: 10.4103/JCAS.JCAS_91_16. Epub [PubMed PMID: 28852302]
Yadav D, Singh S, Bhari N, Gupta S. Angiolymphoid hyperplasia of external ear treated with intralesional radiofrequency ablation. BMJ case reports. 2018 Apr 20:2018():. pii: bcr-2017-223447. doi: 10.1136/bcr-2017-223447. Epub 2018 Apr 20 [PubMed PMID: 29678818]
Level 3 (low-level) evidenceSingh S, Dayal M, Walia R, Arava S, Sharma R, Gupta S. Intralesional radiofrequency ablation for nodular angiolymphoid hyperplasia on forehead: a minimally invasive approach. Indian journal of dermatology, venereology and leprology. 2014 Sep-Oct:80(5):419-21. doi: 10.4103/0378-6323.140300. Epub [PubMed PMID: 25201842]
Level 3 (low-level) evidenceKhunger N, Pahwa M, Jain RK. Angiolymphoid hyperplasia with eosinophilia treated with a novel combination technique of radiofrequency ablation and sclerotherapy. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2010 Mar:36(3):422-5. doi: 10.1111/j.1524-4725.2009.01460.x. Epub 2010 Jan 19 [PubMed PMID: 20100250]
Level 3 (low-level) evidenceBaker MS, Avery RB, Johnson CR, Allen RC. Methotrexate as an alternative treatment for orbital angiolymphoid hyperplasia with eosinophilia. Orbit (Amsterdam, Netherlands). 2012 Oct:31(5):324-6. doi: 10.3109/01676830.2011.584932. Epub [PubMed PMID: 23030406]
Level 3 (low-level) evidenceRongioletti F, Cecchi F, Pastorino C, Scaparro M. Successful management of refractory angiolymphoid hyperplasia with eosinophilia with thalidomide. Journal of the European Academy of Dermatology and Venereology : JEADV. 2016 Mar:30(3):527-9. doi: 10.1111/jdv.12925. Epub 2015 Jan 15 [PubMed PMID: 25588972]
Level 3 (low-level) evidenceBraun-Falco M, Fischer S, Plötz SG, Ring J. Angiolymphoid hyperplasia with eosinophilia treated with anti-interleukin-5 antibody (mepolizumab). The British journal of dermatology. 2004 Nov:151(5):1103-4 [PubMed PMID: 15541097]
Level 3 (low-level) evidenceMcClintic EA, Ting A, Yeatts RP. Oral Propranolol as an Alternative Therapy for Orbital Angiolymphoid Hyperplasia With Eosinophilia. Ophthalmic plastic and reconstructive surgery. 2016 Nov/Dec:32(6):e139-e140 [PubMed PMID: 26954109]
Zarbafian M, Ward CE, Zhang J, Macdonald J. Angiolymphoid Hyperplasia With Eosinophilia-Combination Treatment With Propranolol and Surgical Resection. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2018 Aug:44(8):1147-1149. doi: 10.1097/DSS.0000000000001442. Epub [PubMed PMID: 30045145]
Horst C, Kapur N. Propranolol: a novel treatment for angiolymphoid hyperplasia with eosinophilia. Clinical and experimental dermatology. 2014 Oct:39(7):810-2. doi: 10.1111/ced.12412. Epub 2014 Jul 28 [PubMed PMID: 25065811]
Level 3 (low-level) evidenceArora P, Meena N, Sharma PK, Bhardwaj M. Angiolymphoid Hyperplasia with Eosinophilia and its Response to the Combination of Radiofrequency Ablation and Topical Timolol. Indian dermatology online journal. 2017 Jul-Aug:8(4):267-270. doi: 10.4103/idoj.IDOJ_293_16. Epub [PubMed PMID: 28761845]
Isohisa T, Masuda K, Nakai N, Takenaka H, Katoh N. Angiolymphoid hyperplasia with eosinophilia treated successfully with imiquimod. International journal of dermatology. 2014 Jan:53(1):e43-4. doi: 10.1111/j.1365-4632.2011.05284.x. Epub 2012 May 16 [PubMed PMID: 22591376]
Level 3 (low-level) evidenceNouchi A, Hickman G, Battistella M, Estève E, Bagot M, Vignon-Pennamen D, Petit A. [Treatment of angiolymphoid hyperplasia with eosinophilia (ALHE) using topical tacrolimus: Two cases]. Annales de dermatologie et de venereologie. 2015 May:142(5):360-6. doi: 10.1016/j.annder.2015.02.006. Epub 2015 Mar 13 [PubMed PMID: 25778634]
Level 3 (low-level) evidenceFink CM, Maggio KL. Angiolymphoid hyperplasia with eosinophilia revisited: lack of durable response to intralesional interferon alfa-2a. Archives of dermatology. 2011 Apr:147(4):507-8. doi: 10.1001/archdermatol.2011.69. Epub [PubMed PMID: 21482908]
Level 3 (low-level) evidenceOguz O, Antonov M, Demirkesen C. Angiolymphoid hyperplasia with eosinophilia responding to interferon-alpha2B. Journal of the European Academy of Dermatology and Venereology : JEADV. 2007 Oct:21(9):1277-8 [PubMed PMID: 17894734]
Level 3 (low-level) evidenceBastos JT, Rocha CRMD, Silva PMCE, Freitas BMP, Cassia FF, Avelleira JCR. Angiolymphoid hyperplasia with eosinophilia versus Kimura's disease: a case report and a clinical and histopathological comparison. Anais brasileiros de dermatologia. 2017 May-Jun:92(3):392-394. doi: 10.1590/abd1806-4841.20175318. Epub [PubMed PMID: 29186256]
Level 3 (low-level) evidenceFite-Trepat L, Martos-Fernandez M, Alberola-Ferranti M, Pablo-Garcia-Cuenca A, Bescosatin C. Angiolymphoid Hyperplasia with Eosinophilia Involving the Occipital Artery: Case Report and Review of Literature. Journal of clinical and diagnostic research : JCDR. 2017 Mar:11(3):ZD21-ZD23. doi: 10.7860/JCDR/2017/23323.9569. Epub 2017 Mar 1 [PubMed PMID: 28511526]
Level 3 (low-level) evidenceCole CM. Angiolymphoid hyperplasia with eosinophilia. Cutis. 2013 Sep:92(3):110;117-8 [PubMed PMID: 24153147]
Flucke U, Vogels RJ, de Saint Aubain Somerhausen N, Creytens DH, Riedl RG, van Gorp JM, Milne AN, Huysentruyt CJ, Verdijk MA, van Asseldonk MM, Suurmeijer AJ, Bras J, Palmedo G, Groenen PJ, Mentzel T. Epithelioid Hemangioendothelioma: clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases. Diagnostic pathology. 2014 Jul 1:9():131. doi: 10.1186/1746-1596-9-131. Epub 2014 Jul 1 [PubMed PMID: 24986479]
Level 3 (low-level) evidenceMukherjee B, Kadaskar J, Priyadarshini O, Krishnakumar S, Biswas J. Angiolymphoid Hyperplasia with Eosinophilia of the Orbit and Adnexa. Ocular oncology and pathology. 2015 Sep:2(1):40-7. doi: 10.1159/000433545. Epub 2015 Jul 16 [PubMed PMID: 27171790]
Berney DM, Griffiths MP, Brown CL. Angiolymphoid hyperplasia with eosinophilia in the colon: a novel cause of rectal bleeding. Journal of clinical pathology. 1997 Jul:50(7):611-3 [PubMed PMID: 9306946]
Level 3 (low-level) evidenceDi Vitantonio H, De Paulis D, Ricci A, Raysi SD, Marzi S, Del Maestro M, Galzio RJ. Angiolymphoid hyperplasia with eosinophilia and entrapment of the ulnar nerve. Surgical neurology international. 2016:7(Suppl 5):S160-3. doi: 10.4103/2152-7806.177896. Epub 2016 Mar 2 [PubMed PMID: 27069750]
Krapohl BD, Machens HG, Reichert B, Mailänder P. A rare vasoproliferative lesion: angiolymphoid hyperplasia with eosinophilia of the hand. British journal of plastic surgery. 2003 Mar:56(2):168-70 [PubMed PMID: 12791366]
Level 3 (low-level) evidence