Introduction
Anemia is generally defined as a hemoglobin level of less than 13 g/dL in men and less than 12 g/dL in women.[1] Anemia of chronic renal disease, also known as anemia of chronic kidney disease (CKD), is a type of normocytic and normochromic anemia and hypoproliferative anemia, which is common in patients with renal disease. Among other complications of CKD, it is frequently associated with poor outcomes, decreased quality of life, and increased mortality.[2]
In 1836, anemia was first linked to renal disease by Richard Bright, also known as the "Father of Nephrology."[3][4] As kidney disease progresses, the prevalence of anemia increases, affecting almost all patients with stage 5 CKD. The primary mechanisms behind anemia of CKD, including end-stage renal disease (ESRD), involve decreased erythropoietin production, decreased gastrointestinal iron absorption due to chronic inflammation, and a decreased lifespan of red blood cells (RBCs).[2]
The treatment of anemia of CKD has advanced considerably in the last 2 decades. Before the therapeutic options currently available, the mainstay of treatment was blood transfusion, which came with numerous complications, including infections, hemosiderosis, fluid overload, and transfusion reactions. In addition, frequent blood transfusions increase the risk of allosensitization, which can worsen renal transplant outcomes if a transplant is an option.
In the 1970s, androgens were used to avoid transfusion in patients with CKD; however, this practice is now strongly discouraged.[5][6] In the late 1980s, the development of recombinant erythropoietin, followed by erythropoiesis-stimulating agents (ESAs), revolutionized the management of anemia of CKD.[7] Initially introduced to avoid transfusions, these treatments were soon found to have various positive effects, including improved survival and quality of life, improved cardiac function, reduced hospitalizations, and lower overall costs.[8][9][10]
The mean hemoglobin level of dialysis patients increased from 9.6 g/dL in 1991 to 12.5 g/dL in 2005, and transfusion requirements decreased considerably.[11] However, in 1998, the Normal Hematocrocrit Trial raised concerns about adverse events associated with higher hemoglobin or hematocrit goals.[12] Subsequently, multiple trials have assessed the benefits of targeting higher versus lower hemoglobin ranges. The discovery of adverse effects of ESAs raised questions about their overall benefits and led to increased interest in finding alternative management strategies for anemia of CKD.
Anemia of CKD is highly associated with adverse outcomes such as cardiovascular events and increased mortality. Additionally, the severity of anemia correlates with decreased quality of life and increased hospitalizations. Understanding the diverse mechanisms involved, recommended treatment guidelines, and new therapeutic developments is crucial for managing this condition effectively.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Anemia of chronic renal disease is of multifactorial origin, with a primary etiology being decreased renal production of erythropoietin, the hormone responsible for stimulating RBC production, coupled with abnormal iron metabolism due to chronic inflammation. Decreased erythropoietin has recently been linked to the downregulation of hypoxia-inducible factor (HIF)—a transcription factor that regulates gene expression of erythropoietin.[13][14] Other mechanisms include uremia (leading to RBC deformity and hemolysis), folate and vitamin B12 deficiency, bleeding due to dysfunctional platelets, and blood loss from hemodialysis.[15]
Erythropoietin deficiency is a hallmark of kidney disease. Erythropoietin is produced by peritubular type 1 interstitial cells in the renal cortex and outer medulla and aids in the differentiation of erythroid cells. Its absence leads to programmed apoptosis of erythroid precursors. Additionally, proinflammatory cytokines inhibit erythropoietin production and decrease the proliferation of erythroid progenitor cells.[1]
Iron deficiency also plays a significant role in the anemia of CKD, attributable to both absolute iron deficiency and relative iron deficiency caused by chronic inflammation inhibiting iron release from cellular stores. Iron is first absorbed from the gastrointestinal tract and bound by transferrin. Bound iron is then transported to the liver and spleen, where it is stored in ferritin or transported to the bone marrow for erythropoiesis. Iron is also recycled by macrophages phagocytosing senescent RBCs, another erythropoietin-dependent process.[16][17] Although anemia of CKD is usually described as normochromic, significant iron deficiency can also cause hypochromia and microcytosis.
Hepcidin is a crucial hormone in iron metabolism. Synthesized by the liver, hepcidin regulates iron absorption from the gastrointestinal system and releases stored iron. Macrophages and adipocytes also release small amounts of hepcidin. Hepcidin decreases the expression of ferroportin (the cell-surface iron exporter), and its production is upregulated by chronic inflammation, infection, and renal failure. In addition, it decreases iron absorption, facilitates iron storage, and impedes erythroid progenitor cell proliferation. Since hepcidin is also renally cleared, increased levels are seen as the glomerular filtration rate (GFR) falls.[2][18] Please see Figure. Iron Metabolism and Erythropoiesis for more information.
HIF is a transcription factor and a key regulator of cellular responses to hypoxia. Composed of an oxygen-binding α-unit and a stable β-unit, HIF regulates erythropoietin (EPO) and other iron-metabolism genes. When oxygen levels are normal, prolyl-4-hydroxylase domain-containing proteins 1-3 (PHD 1–3) hydroxylate HIF-α, which allows the von Hippel Lindau protein complex to ubiquitinate HIF-α, leading to its degradation. In hypoxemic conditions, HIF-α is stabilized, resulting in increased erythropoietin transcription. HIF also indirectly decreases hepcidin levels through increased erythroferrone secretion by erythroblasts.[16][17][19]
Epidemiology
Anemia of CKD typically develops when the GFR falls below 60 mL/min/1.73 m2, with up to 20% of patients at stage 3 CKD demonstrating anemia. At least 90% of patients who become dialysis-dependent will eventually develop anemia.[1][4]
Anemia becomes more prevalent and severe with a declining GFR. The National Health and Nutrition Examination Survey (NHANES) from 2007 to 2008 and 2009 to 2010 observed that anemia was twice as prevalent in CKD patients as in the general population.[14][20] Similar data were observed in the CKD Prognosis Consortium.[21]
Pathophysiology
Both absolute and functional iron deficiencies are present in anemia of CKD. Absolute iron deficiency can result from poor nutrition, decreased iron absorption, losses from frequent phlebotomy, and intra-dialytic blood losses (with an estimated annual intra-dialytic iron loss of 161 mg).[22][23][24] Functional iron deficiency arises from an inability to utilize iron stores effectively. Anemia of CKD, also known as reticuloendothelial cell iron blockade, can be caused by any inflammatory state, including CKD. In addition, exogenous erythropoietin administration can deplete readily available iron faster than it can be released from storage cells, leading to a supply/demand mismatch.[16][17]
As discussed above, anemia of CKD is primarily due to erythropoietin deficiency and impaired iron metabolism. However, other mechanisms, as mentioned below, may also contribute to the development of anemia in patients with chronic renal disease.
- Hypocellular bone marrow has been observed in over 50% of CKD patients, though specific inhibitors have not been identified.[1][25]
- The shortened lifespan of RBCs also contributes to anemia, as observed in radioisotope labeling studies. Contributing mechanisms include uremia and other unknown factors.[26][27]
- Nutritional deficiencies, such as vitamin B12 and folate, due to dialysate losses or anorexia, can contribute to anemia. Routine supplementation of water-soluble vitamins is standard in hemodialysis patients, but micronutrients may still be lost in the process.
- Hemodialysis is believed to remove copper, which is a crucial component of ferroxidase enzymes (ie, ceruloplasmin and hephaestin) involved in iron processing.[17]
- Fibroblast growth factor 23 (FGF23) is a hormone produced by osteocytes and osteoblasts and is markedly elevated in CKD due to metabolic bone disease. Studies have shown that it suppresses erythropoiesis and erythropoietin production, and FGF23 antagonists improve renal anemia in animal studies.[17][24]
- Medications commonly given to CKD patients may also contribute to anemia. This is particularly true for patients with kidney transplants on anti-rejection medications, which can cause bone marrow hypocellularity.[1]
In summary, anemia of chronic renal disease is a multifactorial condition attributable to relative erythropoietin deficiency, uremia-induced erythropoiesis inhibitors, shortened erythrocyte lifespan, disordered iron homeostasis, and other contributing factors.
History and Physical
The clinical presentation of anemia of chronic renal disease is similar to anemia from other causes. Common symptoms include:
- Generalized weakness
- Fatigue
- Dyspnea
- Decreased concentration
- Dizziness
- Chest pain (mostly with severe anemia)
- Headaches
- Dyspnea
- Reduced exercise tolerance
Commonly observable signs include:
- Skin and conjunctival pallor
- Respiratory distress
- Tachycardia
- Heart failure (usually with chronic and severe anemia)
Evaluation
Common tests required to diagnose anemia of chronic renal disease include:
- Complete blood count (CBC) with differential
- Peripheral smear
- Vitamin B12, folate, haptoglobin, and thyroid studies (to rule out other causes of anemia)
- Iron indices (iron, ferritin, total-iron binding capacity [TIBC], and transferrin saturation [TSAT]) include:[16]
- Iron (serum iron level): Normal 60 to 170 mcg/dL for adults
- Ferritin (serum ferritin level): Normal 11 to 300 ng/mL
- TIBC (calculated as transferrin × 1.389): Normal 240 to 450 mcg/dL
- TSAT (calculated as serum iron/TIBC × 100): Normal 20% to 40%
Pure iron deficiency anemia usually results in a decreased serum iron level, decreased ferritin, elevated TIBC, and decreased TSAT.[16]
With anemia of CKD, serum ferritin levels are usually elevated due to chronic inflammation, and the serum iron indices often do not align with normal ranges. Common findings include decreased or normal iron and TIBC, elevated ferritin, and decreased TSAT.[28][29] The Dialysis Patients' Response to IV Iron With Elevated Ferritin (DRIVE) study demonstrated that intravenous (IV) iron is beneficial in dialysis patients even with ferritin levels as high as 1200 ng/mL if the TSAT is less than 30%.[30] Low ferritin levels are highly suggestive of iron deficiency, but high ferritin levels do not rule out iron deficiency when CKD or chronic inflammation is present.
Various reticulocyte indices can also be used to measure functional iron deficiency and the body's response to iron repletion. Reticulocytes typically mature in the bone marrow for 1 to 3 days and then circulate in peripheral blood for 1 to 2 days before becoming mature erythrocytes. The reticulocyte hemoglobin content indirectly measures the amount of iron available for RBC production over the last 3 to 4 days. This may correlate more accurately with actual iron stores than serum iron, ferritin, or MCV values.[31][32] However, this test must be conducted within a certain timeframe and may be inaccurate in patients with thalassemias.[16]
Measuring serum erythropoietin levels in CKD is generally discouraged, as they do not influence treatment decisions. This is due to a phenomenon known as 'relative erythropoietin deficiency,' where there is insufficient increase in erythropoietin levels relative to the severity of anemia.[33][34] Hepcidin levels are not typically measured, as they do not affect treatment options.
In CKD-associated anemia, a peripheral blood smear typically shows normocytic and normochromic anemia and peripheral reticulocytopenia. Hypochromia may also be observed in cases of iron deficiency. Measuring the percentage of hypochromic RBCs can help diagnose iron deficiency, with values over 4.3% often used as an indicator of iron deficiency.[1]
Bone marrow biopsy is not commonly performed but is considered the gold standard for diagnosing iron deficiency anemia. It may reveal erythroid hypoplasia or absent iron stores, which correlates with the reported resistance of bone marrow to erythropoietin.[35]
Treatment / Management
Erythropoiesis-Stimulating Agents
The erythropoietin analogs epoetin alfa and darbepoetin alfa are the 2 ESAs generally used in managing anemia of CKD. Produced by recombinant DNA technology through cell cultures, they have similar efficacy and adverse effect profiles, except for the longer half-life of darbepoetin alfa, which allows for less frequent dosing.[36][37][38](B3)
As per Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, ESAs are typically considered in patients with CKD when hemoglobin levels drop below 10 g/dL. However, ESA treatment is individualized based on factors such as anemia symptoms, transfusion requirements, the rate of hemoglobin decline, and response to iron therapy. Erythropoietin (50-100 units/kg IV or subcutaneously [SC]) is usually administered every 1 to 2 weeks, while darbepoetin alfa is dosed every 2 to 4 weeks. For dialysis patients, erythropoietin is given with each dialysis session (3 times a week), whereas darbepoetin alfa is administered once weekly.
An alternative to the above ESAs is epoetin alfa-epbx—a genetically engineered recombinant human erythropoietin—approved by the US Food and Drug Administration (FDA) for treating anemia in patients with CKD in 2018. Studies have found similar rates of effectiveness and adverse events when compared to epoetin alfa. This biosimilar product has been available in Europe since 2007 and may lead to cost savings if widely utilized.[39][40][41](A1)
Continuous erythropoiesis receptor activator (CERA) is a newer, longer-acting ESA that may be preferred over other ESAs due to its lower administration frequency. This compound has a methoxy polyethylene glycol chain bound to epoetin beta. CERA has a lower affinity for the soluble erythropoietin receptor and possibly reduced cellular proliferation activity. CERA is available in the US since 2007, has a significantly increased half-life of about 130 hours, and can be administered SC every 2 to 4 weeks. So far, no specific evidence supports or detracts from its use compared to other ESAs.[42][43] Generally, the peak rise in RBCs in response to ESAs occurs at 8 to 12 weeks. However, in about 10% to 20% of cases, anemia can be resistant to ESAs. Relative iron deficiency should always be considered in this situation. Please see StatPearls' companion resource, "Epoetin Alfa," for more information.(A1)
In all patients with CKD, regardless of the need for dialysis, the goal hemoglobin using ESAs is less than 11.5 g/dL. Multiple trials, including CHOIR, NHCT, and TREAT, have assessed the superiority of targeting hemoglobin to 'normal' versus lower ranges. These trials demonstrated higher mortality, thrombosis, and adverse cerebrovascular and cardiovascular events with higher levels of ESAs. The FDA has also issued a warning regarding the increased risk of death, severe adverse cardiovascular events, and stroke when ESAs are administered to target Hb levels above 11 g/dL.[44][45][46][47][48] This appears to be the higher ESA dose rather than the resulting higher hemoglobin levels, causing the adverse effects, possibly related to ESAs' effects on vascular remodeling and vasoconstriction.[49] (A1)
Another concern with ESA use is the potential effect on malignancy. Some neoplastic cells express erythropoietin receptors, making them susceptible to increased growth with ESA administration. A meta-analysis suggested increased mortality with ESA administration.[50] KDIGO guidelines suggest using ESAs cautiously in CKD patients with active malignancy (grade 1B), a history of stroke (grade 1B), or a history of malignancy (grade 2C).[38](A1)
A rare but severe adverse effect of ESA use is an allergic reaction or the development of anti-erythropoietin antibodies. Early cases reported from 1998 to 2006 are thought to be related to a prior epoetin formulation. The allergic component could be the recombinant erythropoietin or another agent in the drug. Erythropoietin-neutralizing antibodies can attack both the recombinant and endogenous erythropoietin, causing pure red cell aplasia that may worsen with ESA administration. This condition is more likely with SC rather than IV injection and is associated with anti-erythropoietin antibodies. The anti-erythropoietin antibodies include neutralizing anti-erythropoietin antibodies, and their titers correlate with the degree of anemia. In pure red cell aplasia, a bone marrow biopsy may show absent erythroid precursors or arrested development of the precursors. This condition is usually treated with immunosuppressive agents, but discontinuing the ESA may be sufficient.[51][52][53][54](B3)
Treatment with Iron
Patients with renal disease face an increased risk of iron deficiency due to factors such as impaired dietary iron absorption, chronic bleeding from platelet dysfunction caused by uremia, frequent phlebotomy, and blood trapped in the dialysis apparatus. In addition to the depletion of circulating iron from erythropoiesis stimulated by ESAs, this deficiency makes iron supplementation essential in treating anemia of CKD. Due to elevated hepcidin levels, oral iron supplementation is largely ineffective, making IV iron the preferred choice for hemodialysis patients and those with advanced CKD.[55][56](A1)
KDIGO recommends a target TSAT between 20% and 30% and ferritin levels between 100 to 500 ng/mL in patients with CKD and anemia. The European Renal Best Practice Guidelines (2013) propose a ceiling for TSAT at 30% and ferritin at 500 ng/mL. Additionally, dialysis centers often have their own specific goals and protocols.[16] Guidelines from the National Institute for Healthcare and Excellence (2015) and the Renal Association (2017) suggest using a ferritin ceiling of 800 ng/mL.[57](A1)
Data from recent trials, including the Randomized Trial Comparing Proactive, High-Dose versus Reactive, Low-Dose IV Iron Supplementation in Hemodialysis (PIVOTAL) trial, suggest that using even more liberal guidelines for iron administration may be warranted. The PIVOTAL trial used a cutoff of 40% for TSAT and 700 ng/mL for ferritin to hold the administration of IV iron sucrose. Findings were a lower incidence of death, hospitalization, and nonfatal cardiovascular events in the high-cutoff treatment arm, as well as considerably lower ESAs and transfusion requirements. Notably, infection rates did not differ between both study arms. The results of DRIVE I and DRIVE II showed similar improvements with high-cutoff levels. Notably, the average mean ferritin level for dialysis patients in the United States in 2013 was 800 ng/mL, with 18% of patients exceeding 1200 ng/mL. Therefore, understanding the implications of very high ferritin levels is an area needing further research.[2][11]
Concerns about administering IV iron with high ferritin or TSAT levels include potential risks of iron overload, which may increase the risk of infection, damage from oxidative stress, and iron deposition in tissues. While studies and meta-analyses on the impact of IV iron on mortality and morbidity in ESRD patients have yielded mixed results, the theoretical risk of infection or neutrophil impairment has not been substantiated by observational studies.[16][47] Anaphylaxis remains a concern, particularly with iron dextran (less commonly used now), but can also occur with iron gluconate, iron sucrose, or ferumoxytol. The risk is estimated at 24 to 68 per 100,000 for all IV iron formulations combined. Most dialysis centers mitigate this risk by administering a test dose or carefully initiating IV treatments as a precaution.[16] Another significant concern is that certain IV iron formulations may increase FGF23 levels due to interactions with the carbohydrate shell surrounding the iron.[2][58](A1)
New-generation IV iron compounds, such as ferumoxytol, ferric derisomaltose, and ferric carboxymaltose, have become widely used in clinical practice. Their key advantage is the highly stable carbohydrate shell, which prevents the uncontrolled release of toxic-free iron and allows for complete replacement doses in just 1 or 2 infusions. Another essential feature is that the polynuclear iron core in these agents is stable with a low redox potential, thus minimizing the risk of harmful oxidative stress reactions.[17]
Novel Iron Therapies
Ferric citrate, FDA-approved for treating iron-deficiency anemia in patients with CKD or ESRD, also functions as a phosphate binder. This compound forms insoluble complexes with phosphates in the acidic environment of the stomach and releases ferric ions in the alkaline duodenum. The oral formulation allows a more physiological repletion of iron, and its dual role as a phosphate binder may reduce the total pill burden for patients.[16][59] Studies have shown that ferric citrate is as effective as both calcium-based and non-calcium–based phosphate binders. Additionally, ferric citrate lowers FGF23 levels in both dialysis-dependent and non-dialysis–dependent patients, independent of its phosphorus-lowering effects. Given that high levels of FGF23 are independently associated with anemia and cardiovascular mortality, this could have significant implications.[17](B3)
Ferric maltol is a novel oral iron therapy that consists of a stable complex of ferric iron and maltol–a naturally occurring sugar derivative. This formulation allows bioavailable iron to be released in the neutral pH of the intestinal tract and possesses both hydrophilic and lipophilic properties. Upon oral administration, ferric iron is delivered to the intestinal mucosa complexed with maltol, potentially enhancing the uptake of ferric iron into enterocytes compared to ferrous iron salts.[59] As it bypasses stomach metabolism, it has fewer gastrointestinal adverse effects and has been studied for use in irritable bowel syndrome. Ferric maltol is approved for treating iron-deficient anemia in the United States and the European Union.(B3)
Sucrosomial iron is an oral iron preparation featuring ferric pyrophosphate encased in a phospholipid bilayer membrane, forming a "sucrosome." This structure allows the iron to bypass the stomach and be absorbed by intestinal enterocytes. Sucrosome is also absorbed independently of hepcidin regulation, enhancing bioavailability. An open-label study found sucrosomial iron to be as effective as IV ferrous gluconate in the short term, with fewer adverse effects.[17][59](B3)
Ferric pyrophosphate is a novel water-soluble, carbohydrate-free, complex iron salt administered via the dialysate during hemodialysis and was FDA-approved in 2015. This compound is designed to be added to the bicarbonate concentrate of every dialysis treatment, delivering about 7 mg of iron per treatment. Donating iron directly to transferrin may help avoid iron sequestration in reticuloendothelial macrophages. The CRUISE 1 and 2 trials showed that ferric pyrophosphate significantly increases iron indices compared to placebo without significant adverse events.[60][61]
Hypoxia-Inducible Factor–Prolyl Hydroxylase Inhibitors
HIF–prolyl hydroxylase inhibitors (HIF–PHIs) are a novel class of therapeutic agents that raise erythropoietin levels by stabilizing HIF levels, thereby increasing endogenous erythropoietin production. HIF–PHIs also decrease hepcidin levels. In 2023, daprodustat was approved by the FDA for use in patients on dialysis for longer than 4 months. This compound is currently not approved for use in non-dialysis patients.[62][63][62](B3)
The FDA has rejected several other drugs in this class, which are still currently used in other countries. This class of medication can be taken orally rather than IV or SC. Preliminary evidence comparing ESAs to HIF–PHIs suggests the possibility of increased cardiovascular events in non-dialysis-dependent CKD patients (but not dialysis-dependent patients).[48] These medications are thought to have similar adverse effects to ESAs, including the risk of perpetuating malignant cells.[59][64] In addition, HIF is thought to contribute to angiogenesis, which could worsen conditions such as retinopathy. The HIF activation pathways also contribute to cyst formation in polycystic kidney disease, and effects on cyst growth are also unknown. So far, data has not shown an increased risk above ESAs, but given the relative newness of this class of medications, the full effects may not yet be known.[2][64](B3)
Ziltivekimab
Ziltivekimab is a human immunoglobulin G (IgG) monoclonal antibody targeting interleukin (IL)-6–an inflammatory cytokine. In patients with CKD stages 3 to 5, Ziltivekimab has demonstrated the ability to reduce inflammation, increase albumin and hemoglobin levels, and improve iron indices compared to placebo. IL-6 is associated with increased hepcidin expression, which may explain the therapeutic benefits of Ziltivekimab.[1][65][66](A1)
Differential Diagnosis
When diagnosing anemia of chronic renal disease, the following conditions should be considered:
- Alcohol use disorder
- Aplastic anemia
- Dialysis-related blood loss
- Hypothyroidism
- Gastrointestinal losses
- Medication-induced anemia
- Methemoglobinemia
- Myelophthisic anemia
- Sickle cell anemia
- Systemic lupus erythematosus
- Panhypopituitarism
- Primary and secondary hyperparathyroidism
Prognosis
Many patients with renal failure may not respond to erythropoietin, which is a significant predictor of adverse cardiac events. Iron deficiency and inflammation are the 2 key contributing factors to unresponsiveness. Elevated levels of CRP are associated with resistance to erythropoietin in dialysis patients.
Anemia of chronic renal disease is associated with cardiorenal anemia syndrome. A study observed that for every 1 g decrease in hemoglobin concentration, a 42% increase in left ventricular dilatation is seen in patients with stage 5 CKD.[67] Cardiovascular disease remains the most common cause of mortality in these patients, significantly exceeding the rate seen in the general population.[68]
The Dialysis Outcomes Practice Pattern Study (DOPPS), conducted across various countries, reported that a decrease in hemoglobin to below 11 g/dL is associated with increased hospitalization and mortality in CKD patients.[69]
Complications
Anemia of chronic renal disease is an independent risk factor for death, and it has been shown to promote faster progression of left ventricular hypertrophy, increase peripheral oxygen demand, and worsen cardiac outcomes. In addition, anemia in renal failure can lead to depression, fatigue, stroke, reduced exercise tolerance, and an increased rate of hospital re-admission.[70]
Deterrence and Patient Education
Healthcare providers should educate patients about the causes and treatments of anemia associated with chronic renal disease. Dietary changes can help prevent or manage anemia, and consultation with a dietitian can be highly beneficial. All patients with CKD should be encouraged to inform their healthcare providers if they notice any bleeding or experience symptoms of anemia.
Patients should follow the manufacturer's storage instructions when administering an ESA at home, as some products require refrigeration.
Pearls and Other Issues
Key facts to keep in mind regarding anemia of chronic renal disease include:
- This condition is prevalent and primarily results from decreased erythropoietin production and abnormal iron metabolism.
- Commonly found iron indices in anemia of CKD include normal or low iron and TIBC, low TSAT, and increased ferritin levels.
- Reticulocyte hemoglobin content and the percentage of hypochromic RBCs may provide more accurate measurements of recent iron stores compared to traditional iron indices.
- Current KDIGO guidelines suggest implementing IV iron therapy when ferritin is less than 500 ng/mL and TSAT is less than 30%. However, newer studies indicate that IV iron may also be beneficial with ferritin levels as high as 1200 ng/mL.
- ESAs are typically started when hemoglobin is below 10 g/dL, but this threshold can be adjusted based on patient-specific factors such as symptoms, the rate of hemoglobin decrease, or patient preference after discussing risks and benefits.
- Newer and potentially less expensive ESA alternatives include epoetin alfa-epbx, a genetically engineered recombinant human erythropoietin, and CERA.
- HIF–PHIs are a novel class of therapeutic agents that raise erythropoietin levels by stabilizing HIF, which in turn boosts endogenous erythropoietin production. Currently, daprodustat is the only FDA-approved medication in this class.
- Several other new agents are available, including ferric pyrophosphate (delivered through dialysate), ferric citrate (which also acts as an oral phosphate binder), ziltivekimab (an anti-inflammatory antibody), and ferric maltol/sucrosomial iron (which bypasses stomach absorption and minimizes gastrointestinal effects).
- Blood transfusions should be avoided as much as possible, especially for patients who may be renal transplant candidates, to prevent allosensitization.
Enhancing Healthcare Team Outcomes
The management of anemia of CKD in patients is complex, and a thorough workup is essential to determine the cause. Clinicians must be aware of current guidelines, as deviations can result in unfavorable outcomes. Policies should be reviewed frequently for the latest evidence-based suggestions. Treating patients with renal disease and anemia requires an integrated approach by an interprofessional healthcare team, including nephrologists, primary care providers, hematologists, nurse practitioners, physician assistants, nurses, and pharmacists, to achieve the best possible outcomes. Dialysis nurses should monitor vital signs and obtain total blood counts to assess anemia, while dialysis technicians are essential for administering medications appropriately. Nutritionists and dieticians are crucial in optimizing patients' nutritional states and avoiding confounding factors that can worsen anemia.
A strategic approach is equally crucial, involving evidence-based strategies to optimize treatment plans and minimize adverse effects. Ethical considerations must guide decision-making, ensuring informed consent and respecting patient autonomy in treatment choices. Each healthcare professional must know their responsibilities and contribute their unique expertise to the patient's care plan, fostering a multidisciplinary approach. Effective interprofessional communication is paramount, allowing seamless information exchange and collaborative decision-making among the team members. Care coordination is pivotal in ensuring that the patient's journey from diagnosis to treatment and follow-up is well-managed, thereby minimizing errors and enhancing patient safety. By embracing these principles of skill, strategy, ethics, responsibilities, interprofessional communication, and care coordination, healthcare professionals can deliver patient-centered care, ultimately improving patient outcomes and enhancing team performance in the management of anemia of CKD.
Media
(Click Image to Enlarge)
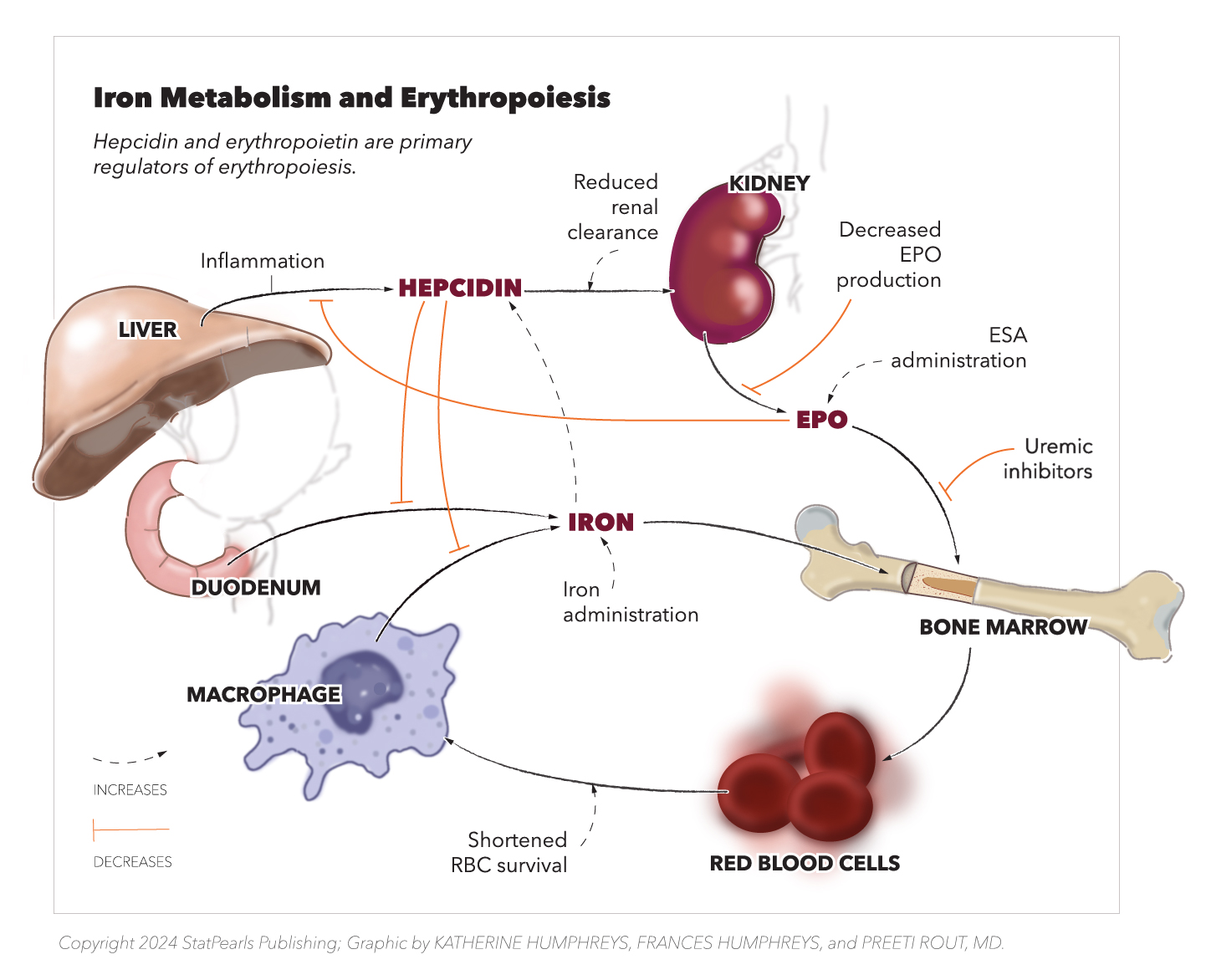
Iron Metabolisms and Erythropoiesis. This diagram depicts the key interactions between erythropoietin, hepcidin and iron and their effects on end organs and red blood cell availability. Hepcidin decreases the availability of iron to be released from macrophages and the liver. It also decreases iron absorption from the duodenum. Decreased erythropoietin from the kidney further decreases total red blood cell formation from the bone marrow.
Copyright StatPearls 2024. Created by K Humphreys, F Humphreys, and P Rout, MD
References
Badura K, Janc J, Wąsik J, Gnitecki S, Skwira S, Młynarska E, Rysz J, Franczyk B. Anemia of Chronic Kidney Disease-A Narrative Review of Its Pathophysiology, Diagnosis, and Management. Biomedicines. 2024 May 27:12(6):. doi: 10.3390/biomedicines12061191. Epub 2024 May 27 [PubMed PMID: 38927397]
Level 3 (low-level) evidenceBabitt JL, Eisenga MF, Haase VH, Kshirsagar AV, Levin A, Locatelli F, Małyszko J, Swinkels DW, Tarng DC, Cheung M, Jadoul M, Winkelmayer WC, Drüeke TB, Conference Participants. Controversies in optimal anemia management: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney international. 2021 Jun:99(6):1280-1295. doi: 10.1016/j.kint.2021.03.020. Epub 2021 Apr 8 [PubMed PMID: 33839163]
. Cases and Observations Illustrative of Renal Disease, Accompanied with the Secretion of Albuminous Urine. The Medico-chirurgical review. 1836 Jul 1:25(49):23-35 [PubMed PMID: 29918407]
Level 3 (low-level) evidenceFishbane S, Coyne DW. How I treat renal anemia. Blood. 2020 Aug 13:136(7):783-789. doi: 10.1182/blood.2019004330. Epub [PubMed PMID: 32556307]
DeGowin RL, Lavender AR, Forland M, Charleston D, Gottschalk A. Erythropoiesis and erythropoietin in patients with chronic renal failure treated with hemodialysis and testosterone. Annals of internal medicine. 1970 Jun:72(6):913-8 [PubMed PMID: 5448750]
Richardson JR Jr, Weinstein MB. Erythropoietic response of dialyzed patients to testosterone administration. Annals of internal medicine. 1970 Sep:73(3):403-7 [PubMed PMID: 5455991]
Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. The New England journal of medicine. 1987 Jan 8:316(2):73-8 [PubMed PMID: 3537801]
Level 1 (high-level) evidenceMacdougall IC, Lewis NP, Saunders MJ, Cochlin DL, Davies ME, Hutton RD, Fox KA, Coles GA, Williams JD. Long-term cardiorespiratory effects of amelioration of renal anaemia by erythropoietin. Lancet (London, England). 1990 Mar 3:335(8688):489-93 [PubMed PMID: 1968526]
Perkins R, Olson S, Hansen J, Lee J, Stiles K, Lebrun C. Impact of an anemia clinic on emergency room visits and hospitalizations in patients with anemia of CKD pre-dialysis. Nephrology nursing journal : journal of the American Nephrology Nurses' Association. 2007 Mar-Apr:34(2):167-73, 182 [PubMed PMID: 17486947]
Level 2 (mid-level) evidenceMaddux FW, Shetty S, del Aguila MA, Nelson MA, Murray BM. Effect of erythropoiesis-stimulating agents on healthcare utilization, costs, and outcomes in chronic kidney disease. The Annals of pharmacotherapy. 2007 Nov:41(11):1761-9 [PubMed PMID: 17895328]
Level 2 (mid-level) evidenceCharytan DM, Pai AB, Chan CT, Coyne DW, Hung AM, Kovesdy CP, Fishbane S, Dialysis Advisory Group of the American Society of Nephrology. Considerations and challenges in defining optimal iron utilization in hemodialysis. Journal of the American Society of Nephrology : JASN. 2015 Jun:26(6):1238-47. doi: 10.1681/ASN.2014090922. Epub 2014 Dec 26 [PubMed PMID: 25542967]
Coyne DW. The health-related quality of life was not improved by targeting higher hemoglobin in the Normal Hematocrit Trial. Kidney international. 2012 Jul:82(2):235-41. doi: 10.1038/ki.2012.76. Epub 2012 Mar 21 [PubMed PMID: 22437411]
Level 2 (mid-level) evidenceBernhardt WM, Wiesener MS, Scigalla P, Chou J, Schmieder RE, Günzler V, Eckardt KU. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. Journal of the American Society of Nephrology : JASN. 2010 Dec:21(12):2151-6. doi: 10.1681/ASN.2010010116. Epub 2010 Nov 29 [PubMed PMID: 21115615]
Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proceedings of the National Academy of Sciences of the United States of America. 1995 Jun 6:92(12):5510-4 [PubMed PMID: 7539918]
Agarwal AK. Practical approach to the diagnosis and treatment of anemia associated with CKD in elderly. Journal of the American Medical Directors Association. 2006 Nov:7(9 Suppl):S7-S12; quiz S17-21 [PubMed PMID: 17098634]
Batchelor EK, Kapitsinou P, Pergola PE, Kovesdy CP, Jalal DI. Iron Deficiency in Chronic Kidney Disease: Updates on Pathophysiology, Diagnosis, and Treatment. Journal of the American Society of Nephrology : JASN. 2020 Mar:31(3):456-468. doi: 10.1681/ASN.2019020213. Epub 2020 Feb 10 [PubMed PMID: 32041774]
Lee KH, Ho Y, Tarng DC. Iron Therapy in Chronic Kidney Disease: Days of Future Past. International journal of molecular sciences. 2021 Jan 20:22(3):. doi: 10.3390/ijms22031008. Epub 2021 Jan 20 [PubMed PMID: 33498292]
Babitt JL, Huang FW, Xia Y, Sidis Y, Andrews NC, Lin HY. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. The Journal of clinical investigation. 2007 Jul:117(7):1933-9 [PubMed PMID: 17607365]
Nakai T, Iwamura Y, Kato K, Hirano I, Matsumoto Y, Tomioka Y, Yamamoto M, Suzuki N. Drugs activating hypoxia-inducible factors correct erythropoiesis and hepcidin levels via renal EPO induction in mice. Blood advances. 2023 Aug 8:7(15):3793-3805. doi: 10.1182/bloodadvances.2023009798. Epub [PubMed PMID: 37146271]
Level 3 (low-level) evidenceSt Peter WL, Guo H, Kabadi S, Gilbertson DT, Peng Y, Pendergraft T, Li S. Prevalence, treatment patterns, and healthcare resource utilization in Medicare and commercially insured non-dialysis-dependent chronic kidney disease patients with and without anemia in the United States. BMC nephrology. 2018 Mar 15:19(1):67. doi: 10.1186/s12882-018-0861-1. Epub 2018 Mar 15 [PubMed PMID: 29544446]
Inker LA, Grams ME, Levey AS, Coresh J, Cirillo M, Collins JF, Gansevoort RT, Gutierrez OM, Hamano T, Heine GH, Ishikawa S, Jee SH, Kronenberg F, Landray MJ, Miura K, Nadkarni GN, Peralta CA, Rothenbacher D, Schaeffner E, Sedaghat S, Shlipak MG, Zhang L, van Zuilen AD, Hallan SI, Kovesdy CP, Woodward M, Levin A, CKD Prognosis Consortium. Relationship of Estimated GFR and Albuminuria to Concurrent Laboratory Abnormalities: An Individual Participant Data Meta-analysis in a Global Consortium. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2019 Feb:73(2):206-217. doi: 10.1053/j.ajkd.2018.08.013. Epub 2018 Oct 19 [PubMed PMID: 30348535]
Level 1 (high-level) evidenceKDOQI, National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2006 May:47(5 Suppl 3):S11-145 [PubMed PMID: 16678659]
Level 2 (mid-level) evidenceTsukamoto T, Matsubara T, Akashi Y, Kondo M, Yanagita M. Annual Iron Loss Associated with Hemodialysis. American journal of nephrology. 2016:43(1):32-8. doi: 10.1159/000444335. Epub 2016 Feb 18 [PubMed PMID: 26885949]
Park MY, Le Henaff C, Sitara D. Administration of α-Klotho Does Not Rescue Renal Anemia in Mice. Frontiers in pediatrics. 2022:10():924915. doi: 10.3389/fped.2022.924915. Epub 2022 Jun 23 [PubMed PMID: 35813388]
Eschbach JW. The anemia of chronic renal failure: pathophysiology and the effects of recombinant erythropoietin. Kidney international. 1989 Jan:35(1):134-48 [PubMed PMID: 2651751]
Level 3 (low-level) evidenceVos FE, Schollum JB, Coulter CV, Doyle TC, Duffull SB, Walker RJ. Red blood cell survival in long-term dialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011 Oct:58(4):591-8. doi: 10.1053/j.ajkd.2011.03.031. Epub 2011 Jun 29 [PubMed PMID: 21715072]
Level 2 (mid-level) evidenceEschbach JW Jr, Funk D, Adamson J, Kuhn I, Scribner BH, Finch CA. Erythropoiesis in patients with renal failure undergoing chronic dialysis. The New England journal of medicine. 1967 Mar 23:276(12):653-8 [PubMed PMID: 6018456]
Tessitore N, Solero GP, Lippi G, Bassi A, Faccini GB, Bedogna V, Gammaro L, Brocco G, Restivo G, Bernich P, Lupo A, Maschio G. The role of iron status markers in predicting response to intravenous iron in haemodialysis patients on maintenance erythropoietin. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2001 Jul:16(7):1416-23 [PubMed PMID: 11427634]
Level 2 (mid-level) evidenceMittman N, Sreedhara R, Mushnick R, Chattopadhyay J, Zelmanovic D, Vaseghi M, Avram MM. Reticulocyte hemoglobin content predicts functional iron deficiency in hemodialysis patients receiving rHuEPO. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1997 Dec:30(6):912-22 [PubMed PMID: 9398141]
Coyne DW, Kapoian T, Suki W, Singh AK, Moran JE, Dahl NV, Rizkala AR, DRIVE Study Group. Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: results of the Dialysis Patients' Response to IV Iron with Elevated Ferritin (DRIVE) Study. Journal of the American Society of Nephrology : JASN. 2007 Mar:18(3):975-84 [PubMed PMID: 17267740]
Level 1 (high-level) evidenceMast AE, Blinder MA, Dietzen DJ. Reticulocyte hemoglobin content. American journal of hematology. 2008 Apr:83(4):307-10 [PubMed PMID: 18027835]
Karagülle M, Gündüz E, Sahin Mutlu F, Olga Akay M. Clinical significance of reticulocyte hemoglobin content in the diagnosis of iron deficiency anemia. Turkish journal of haematology : official journal of Turkish Society of Haematology. 2013 Jun:30(2):153-6. doi: 10.4274/Tjh.2012.0107. Epub 2013 Jun 5 [PubMed PMID: 24385778]
Radtke HW, Claussner A, Erbes PM, Scheuermann EH, Schoeppe W, Koch KM. Serum erythropoietin concentration in chronic renal failure: relationship to degree of anemia and excretory renal function. Blood. 1979 Oct:54(4):877-84 [PubMed PMID: 476305]
Level 3 (low-level) evidenceKorte W, Cogliatti SB, Jung K, Riesen W. Mild renal dysfunction is sufficient to induce erythropoietin deficiency in patients with unexplained anaemia. Clinica chimica acta; international journal of clinical chemistry. 2000 Feb 25:292(1-2):149-54 [PubMed PMID: 10686284]
Level 3 (low-level) evidencePhiri KS, Calis JC, Kachala D, Borgstein E, Waluza J, Bates I, Brabin B, van Hensbroek MB. Improved method for assessing iron stores in the bone marrow. Journal of clinical pathology. 2009 Aug:62(8):685-9. doi: 10.1136/jcp.2009.064451. Epub [PubMed PMID: 19638538]
Carrera F, Burnier M. Use of darbepoetin alfa in the treatment of anaemia of chronic kidney disease: clinical and pharmacoeconomic considerations. NDT plus. 2009 Jan:2(Suppl_1):i9-i17 [PubMed PMID: 19461859]
Egrie JC, Browne JK. Development and characterization of novel erythropoiesis stimulating protein (NESP). Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2001:16 Suppl 3():3-13 [PubMed PMID: 11402085]
Level 3 (low-level) evidenceFishbane S, Spinowitz B. Update on Anemia in ESRD and Earlier Stages of CKD: Core Curriculum 2018. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2018 Mar:71(3):423-435. doi: 10.1053/j.ajkd.2017.09.026. Epub 2018 Jan 11 [PubMed PMID: 29336855]
Fishbane S, Singh B, Kumbhat S, Wisemandle WA, Martin NE. Intravenous Epoetin Alfa-epbx versus Epoetin Alfa for Treatment of Anemia in End-Stage Kidney Disease. Clinical journal of the American Society of Nephrology : CJASN. 2018 Aug 7:13(8):1204-1214. doi: 10.2215/CJN.11631017. Epub 2018 Jun 19 [PubMed PMID: 29921734]
Wish JB, Rocha MG, Martin NE, Reyes CRD, Fishbane S, Smith MT, Nassar G. Long-term Safety of Epoetin Alfa-epbx for the Treatment of Anemia in ESKD: Pooled Analyses of Randomized and Open-label Studies. Kidney medicine. 2019 Sep-Oct:1(5):271-280. doi: 10.1016/j.xkme.2019.06.009. Epub 2019 Aug 28 [PubMed PMID: 32734207]
Level 1 (high-level) evidenceFishbane S, Spinowitz BS, Wisemandle WA, Martin NE. Randomized Controlled Trial of Subcutaneous Epoetin Alfa-epbx Versus Epoetin Alfa in End-Stage Kidney Disease. Kidney international reports. 2019 Sep:4(9):1235-1247. doi: 10.1016/j.ekir.2019.05.010. Epub 2019 May 22 [PubMed PMID: 31517143]
Level 1 (high-level) evidenceMacdougall IC, Walker R, Provenzano R, de Alvaro F, Locay HR, Nader PC, Locatelli F, Dougherty FC, Beyer U, ARCTOS Study Investigators. C.E.R.A. corrects anemia in patients with chronic kidney disease not on dialysis: results of a randomized clinical trial. Clinical journal of the American Society of Nephrology : CJASN. 2008 Mar:3(2):337-47. doi: 10.2215/CJN.00480107. Epub 2008 Feb 20 [PubMed PMID: 18287255]
Level 1 (high-level) evidencePanchapakesan U, Sumual S, Pollock C. Nanomedicines in the treatment of anemia in renal disease: focus on CERA (Continuous Erythropoietin Receptor Activator). International journal of nanomedicine. 2007:2(1):33-8 [PubMed PMID: 17722510]
Szczech LA, Barnhart HX, Inrig JK, Reddan DN, Sapp S, Califf RM, Patel UD, Singh AK. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney international. 2008 Sep:74(6):791-8. doi: 10.1038/ki.2008.295. Epub 2008 Jul 2 [PubMed PMID: 18596733]
Level 1 (high-level) evidenceBesarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. The New England journal of medicine. 1998 Aug 27:339(9):584-90 [PubMed PMID: 9718377]
Level 1 (high-level) evidenceBello NA, Lewis EF, Desai AS, Anand IS, Krum H, McMurray JJ, Olson K, Solomon SD, Swedberg K, van Veldhuisen DJ, Young JB, Pfeffer MA. Increased risk of stroke with darbepoetin alfa in anaemic heart failure patients with diabetes and chronic kidney disease. European journal of heart failure. 2015 Nov:17(11):1201-7. doi: 10.1002/ejhf.412. Epub 2015 Oct 1 [PubMed PMID: 26423928]
Ye Y, Liu H, Chen Y, Zhang Y, Li S, Hu W, Yang R, Zhang Z, Lv L, Liu X. Hemoglobin targets for the anemia in patients with dialysis-dependent chronic kidney disease: a meta-analysis of randomized, controlled trials. Renal failure. 2018 Nov:40(1):671-679. doi: 10.1080/0886022X.2018.1532909. Epub [PubMed PMID: 30741617]
Level 1 (high-level) evidenceKu E, Del Vecchio L, Eckardt KU, Haase VH, Johansen KL, Nangaku M, Tangri N, Waikar SS, Więcek A, Cheung M, Jadoul M, Winkelmayer WC, Wheeler DC, for Conference Participants. Novel anemia therapies in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney international. 2023 Oct:104(4):655-680. doi: 10.1016/j.kint.2023.05.009. Epub 2023 May 24 [PubMed PMID: 37236424]
Fishbane S, Berns JS. Hemoglobin cycling in hemodialysis patients treated with recombinant human erythropoietin. Kidney international. 2005 Sep:68(3):1337-43 [PubMed PMID: 16105069]
Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke MJ, Weingart O, Kluge S, Piper M, Napoli M, Rades D, Steensma D, Djulbegovic B, Fey MF, Ray-Coquard I, Moebus V, Thomas G, Untch M, Schumacher M, Egger M, Engert A. Erythropoietin or Darbepoetin for patients with cancer--meta-analysis based on individual patient data. The Cochrane database of systematic reviews. 2009 Jul 8:2009(3):CD007303. doi: 10.1002/14651858.CD007303.pub2. Epub 2009 Jul 8 [PubMed PMID: 19588423]
Level 1 (high-level) evidenceBadiu I, Diena D, Guida G, Ferrando C, Rapezzi D, Besso L. Cutaneous allergic reaction correlates with anti-erythropoietin antibodies in dialysis patient developing pure red cell aplasia. Clinical case reports. 2022 Apr:10(4):e05554. doi: 10.1002/ccr3.5554. Epub 2022 Apr 4 [PubMed PMID: 35414924]
Level 3 (low-level) evidenceCasadevall N. Antibodies against rHuEPO: native and recombinant. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2002:17 Suppl 5():42-7 [PubMed PMID: 12091607]
McKoy JM, Stonecash RE, Cournoyer D, Rossert J, Nissenson AR, Raisch DW, Casadevall N, Bennett CL. Epoetin-associated pure red cell aplasia: past, present, and future considerations. Transfusion. 2008 Aug:48(8):1754-62. doi: 10.1111/j.1537-2995.2008.01749.x. Epub 2008 May 14 [PubMed PMID: 18482185]
Macdougall IC, Casadevall N, Locatelli F, Combe C, London GM, Di Paolo S, Kribben A, Fliser D, Messner H, McNeil J, Stevens P, Santoro A, De Francisco AL, Percheson P, Potamianou A, Foucher A, Fife D, Mérit V, Vercammen E, PRIMS study group. Incidence of erythropoietin antibody-mediated pure red cell aplasia: the Prospective Immunogenicity Surveillance Registry (PRIMS). Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2015 Mar:30(3):451-60. doi: 10.1093/ndt/gfu297. Epub 2014 Sep 19 [PubMed PMID: 25239637]
Fudin R, Jaichenko J, Shostak A, Bennett M, Gotloib L. Correction of uremic iron deficiency anemia in hemodialyzed patients: a prospective study. Nephron. 1998:79(3):299-305 [PubMed PMID: 9678430]
Level 1 (high-level) evidenceMarkowitz GS, Kahn GA, Feingold RE, Coco M, Lynn RI. An evaluation of the effectiveness of oral iron therapy in hemodialysis patients receiving recombinant human erythropoietin. Clinical nephrology. 1997 Jul:48(1):34-40 [PubMed PMID: 9247776]
Level 1 (high-level) evidenceMikhail A, Brown C, Williams JA, Mathrani V, Shrivastava R, Evans J, Isaac H, Bhandari S. Renal association clinical practice guideline on Anaemia of Chronic Kidney Disease. BMC nephrology. 2017 Nov 30:18(1):345. doi: 10.1186/s12882-017-0688-1. Epub 2017 Nov 30 [PubMed PMID: 29191165]
Level 1 (high-level) evidenceWolf M, Rubin J, Achebe M, Econs MJ, Peacock M, Imel EA, Thomsen LL, Carpenter TO, Weber T, Brandenburg V, Zoller H. Effects of Iron Isomaltoside vs Ferric Carboxymaltose on Hypophosphatemia in Iron-Deficiency Anemia: Two Randomized Clinical Trials. JAMA. 2020 Feb 4:323(5):432-443. doi: 10.1001/jama.2019.22450. Epub [PubMed PMID: 32016310]
Level 1 (high-level) evidencePergola PE, Fishbane S, Ganz T. Novel Oral Iron Therapies for Iron Deficiency Anemia in Chronic Kidney Disease. Advances in chronic kidney disease. 2019 Jul:26(4):272-291. doi: 10.1053/j.ackd.2019.05.002. Epub [PubMed PMID: 31477258]
Level 3 (low-level) evidenceFishbane SN, Singh AK, Cournoyer SH, Jindal KK, Fanti P, Guss CD, Lin VH, Pratt RD, Gupta A. Ferric pyrophosphate citrate (Triferic™) administration via the dialysate maintains hemoglobin and iron balance in chronic hemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2015 Dec:30(12):2019-26. doi: 10.1093/ndt/gfv277. Epub 2015 Jul 13 [PubMed PMID: 26175145]
Fell LH, Fliser D, Heine GH. Ferric pyrophosphate: good things come to those who wait? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2015 Dec:30(12):1942-4. doi: 10.1093/ndt/gfv287. Epub 2015 Jul 21 [PubMed PMID: 26203048]
Singh AK, Carroll K, McMurray JJV, Solomon S, Jha V, Johansen KL, Lopes RD, Macdougall IC, Obrador GT, Waikar SS, Wanner C, Wheeler DC, Więcek A, Blackorby A, Cizman B, Cobitz AR, Davies R, DiMino TL, Kler L, Meadowcroft AM, Taft L, Perkovic V, ASCEND-ND Study Group. Daprodustat for the Treatment of Anemia in Patients Not Undergoing Dialysis. The New England journal of medicine. 2021 Dec 16:385(25):2313-2324. doi: 10.1056/NEJMoa2113380. Epub 2021 Nov 5 [PubMed PMID: 34739196]
Haider MU, Furqan M, Mehmood Q. Daprodustat: A potential game-changer in renal anemia therapy-A perspective. Frontiers in pharmacology. 2023:14():1249492. doi: 10.3389/fphar.2023.1249492. Epub 2023 Aug 10 [PubMed PMID: 37637409]
Level 3 (low-level) evidenceXu Q, Huang J, Liu Q, Wang X, Liu H, Song Y, Dou F, Lv S, Liu G. Short-term effect of low-dose roxadustat combined with erythropoiesis-stimulating agent treatment for erythropoietin-resistant anemia in patients undergoing maintenance hemodialysis. Frontiers in endocrinology. 2024:15():1372150. doi: 10.3389/fendo.2024.1372150. Epub 2024 Jul 1 [PubMed PMID: 39010898]
Pergola PE, Devalaraja M, Fishbane S, Chonchol M, Mathur VS, Smith MT, Lo L, Herzog K, Kakkar R, Davidson MH. Ziltivekimab for Treatment of Anemia of Inflammation in Patients on Hemodialysis: Results from a Phase 1/2 Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Journal of the American Society of Nephrology : JASN. 2021 Jan:32(1):211-222. doi: 10.1681/ASN.2020050595. Epub 2020 Dec 3 [PubMed PMID: 33272965]
Level 1 (high-level) evidencePergola PE, Davidson M, Jensen C, Mohseni Zonoozi AA, Raj DS, Andreas Schytz P, Tuttle KR, Perkovic V. Effect of Ziltivekimab on Determinants of Hemoglobin in Patients with CKD Stage 3-5: An Analysis of a Randomized Trial (RESCUE). Journal of the American Society of Nephrology : JASN. 2024 Jan 1:35(1):74-84. doi: 10.1681/ASN.0000000000000245. Epub 2023 Dec 13 [PubMed PMID: 38088558]
Level 1 (high-level) evidenceFishbane S, Block GA, Loram L, Neylan J, Pergola PE, Uhlig K, Chertow GM. Effects of Ferric Citrate in Patients with Nondialysis-Dependent CKD and Iron Deficiency Anemia. Journal of the American Society of Nephrology : JASN. 2017 Jun:28(6):1851-1858. doi: 10.1681/ASN.2016101053. Epub 2017 Jan 12 [PubMed PMID: 28082519]
Sarnak MJ, Levey AS. Epidemiology, diagnosis, and management of cardiac disease in chronic renal disease. Journal of thrombosis and thrombolysis. 2000 Oct:10(2):169-80 [PubMed PMID: 11005939]
Pisoni RL, Bragg-Gresham JL, Young EW, Akizawa T, Asano Y, Locatelli F, Bommer J, Cruz JM, Kerr PG, Mendelssohn DC, Held PJ, Port FK. Anemia management and outcomes from 12 countries in the Dialysis Outcomes and Practice Patterns Study (DOPPS). American journal of kidney diseases : the official journal of the National Kidney Foundation. 2004 Jul:44(1):94-111 [PubMed PMID: 15211443]
Del Fabbro P, Luthi JC, Carrera E, Michel P, Burnier M, Burnand B. Anemia and chronic kidney disease are potential risk factors for mortality in stroke patients: a historic cohort study. BMC nephrology. 2010 Oct 16:11():27. doi: 10.1186/1471-2369-11-27. Epub 2010 Oct 16 [PubMed PMID: 20950484]
Level 2 (mid-level) evidence