Introduction
Anasarca is severe generalized fluid accumulation in the interstitial space. This generalized edema can result either when capillary filtration exceeds the fluid removed via lymphatic drainage, when intravascular hydrostatic pressure increases, when the permeability of the endothelial barrier increases, when oncotic pressure within the capillary decreases, or when oncotic pressure changes from low protein states. In contrast to peripheral edema, which is localized to specific areas, anasarca is characterized by massive and generalized swelling. Various clinical conditions can cause anasarca, including heart failure, renal failure, liver failure, or impairment of the lymphatic system. The presentation of anasarca in patients may vary, but it typically becomes clinically apparent when the interstitial volume exceeds 2.5 to 3L.[1][2][3] The characteristic feature is a significant accumulation of fluid in the interstitial spaces, resulting in noticeable swelling in multiple body areas, including the legs, arms, face, abdomen, and elsewhere.
Anasarca is not a standalone disease but rather a symptom of an underlying medical condition, primarily diagnosed through clinical evaluation. While diagnostic tests can aid in assessing the extent and areas of swelling, their primary purpose is to identify the root cause. Treatment typically involves the use of diuretics and addressing the underlying cause. Therefore, it is crucial to diagnose and treat the underlying condition promptly.[4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Anasarca is a condition characterized by severe generalized edema of the body. Due to various underlying pathologies, the etiology can be varied. It usually occurs due to the shift in the Starling forces that regulate fluid movement between blood vessels and the interstitium. A multitude of conditions can potentially lead to anasarca, including the following:
- Heart failure: One of the most common causes of anasarca is congestive heart failure. Due to impaired ejection fraction, increased afterload, and decreased preload, this causes vascular fluid leakage into the interstitium.[5]
- Kidney failure: Renal diseases (eg, glomerulonephritis) may lead to essential protein loss, resulting in decreased capillary oncotic pressure.[6] Consequent fluid leakage can cause widespread edema. In some cases of kidney diseases, edema is attributable to salt and water retention in the body.
- Liver diseases: Albumin helps maintain the oncotic pressure of the capillaries; therefore, hypoalbuminemia resulting from liver diseases (eg, cirrhosis) can cause fluid movement from blood vessels to the interstitial tissues.
- Malnutrition: Severe protein or caloric deficiency can cause hypoalbuminemia and fluid retention, potentially leading to anasarca.
- Protein-losing enteropathy: Some gastrointestinal diseases (eg, celiac disease) cause intestinal protein loss, resulting in vascular fluid leakage.[7]
- Endocrinopathies: Fluid retention from some endocrinological conditions (eg, hypothyroidism) can cause generalized nonpitting edema.[8]
- Collagenopathies: Certain inflammatory conditions, including systemic lupus erythematosus, dermatomyositis, or rheumatoid arthritis, can cause anasarca due to increased vascular wall permeability.[9]
- Medications: Severe fluid retention may be caused by some medications (eg, corticosteroids, nonsteroidal anti-inflammatory drugs, or calcium channel blockers).[4]
Epidemiology
Research on the precise occurrence of anasarca is quite scarce. Peripheral edema, a more commonly observed clinical condition, is estimated to affect roughly 20% of adults aged >50 years.[4] In contrast, one study reported that the incidence of anasarca in postoperative patients undergoing abdominal surgery was approximately 29.87%.[10]
Pathophysiology
The primary pathophysiological mechanisms underlying anasarca involve several factors: an elevation in capillary hydrostatic pressure, increased capillary permeability, a lower plasma oncotic pressure, lymphatic obstruction, or a combination of these. These mechanisms collectively lead to severe fluid retention in the interstitial space. Conditions that can cause increased capillary hydrostatic pressure include heart failure, kidney disease, early cirrhosis, pregnancy, medications (eg, amlodipine), or conditions characterized by venous obstruction or insufficiencies, such as deep venous thrombosis or hepatic venous congestion. Increased capillary permeability may result from burns, trauma, sepsis, allergic reactions, or malignant ascites. Hypoalbuminemia, which causes decreased plasma oncotic pressure, is associated with conditions like nephrotic syndrome, liver disease, and malnutrition, while malignancy and lymphatic dissection can lead to lymphatic obstruction.[11][12]
The physiologic abnormalities underlying anasarca stem from an imbalance in the forces that regulate fluid movement between vasculature and surrounding tissues, resulting in widespread fluid accumulation in the interstitial spaces throughout the body. Plasma oncotic pressure is primarily determined by the osmotic pressure exerted by blood proteins, especially albumin, in the blood vessels.[13] These proteins help retain fluid within the vessels, preventing excessive leakage into the tissues. Conditions that lead to low levels of plasma proteins can lead to decreased oncotic pressure and fluid retention in tissues. Another mechanism contributing to severe edema is an increase in vascular hydrostatic pressure. Reduced capillary hydrostatic or interstitial oncotic pressure favors fluid movement into the capillaries, known as absorption. However, when capillary hydrostatic or interstitial oncotic pressure rises, fluid shifts out of capillaries, a process called filtration. Additionally, an increase in the permeability of capillary walls can also lead to an increased outflow of fluid and proteins.[14]
As a result of these pathophysiological mechanisms, fluid moves from the vascular space to the interstitium, reducing plasma volume. This decrease in tissue perfusion triggers renal retention of sodium and water, setting off a cascade of effects that worsen interstitial fluid retention. Some excess fluid gained will be retained in the intravascular compartment, but the alteration in capillary hemodynamics causes most of the retained fluid to enter the interstitium, eventually becoming apparent as edema or anasarca.
History and Physical
Clinical Presentation
Anasarca is extensive and generalized fluid accumulation in various body tissues, which may affect multiple areas, including subcutaneous tissues, lungs, abdomen, and extremities. The clinical presentation of anasarca can include the following:
- Significant swelling throughout the body, involving the face, limbs, abdomen, dependent areas, and the genital area [15]
- Restricted movement in swollen extremities
- Increased body weight due to fluid retention
- Pulmonary edema, resulting in shortness of breath that is worse when lying down, cough, and chest pain [4]
- Ascites and abdominal distension [16]
- Oliguria or anuria
- Fatigue
- Dermatological changes over swollen areas (eg, erythema, stretching, shiny, weeping, and taut) [17]
- Hemosiderin deposits and venous ulcers [8]
- Hypothyroid-associated myxedema [18]
The patient's history should be comprehensive, including medical, surgical, and medication history. Some medications (eg, amlodipine), postsurgical complications, and comorbidities can result in edema.[19] Clinicians should also inquire about the history of anasarca, including symptom onset and duration, affected areas, associated pain, and positional effects (eg, improvement of edema with elevation). Other symptoms (eg, dyspnea, chest pain) should be included in a detailed history, along with the onset or exacerbation of systemic conditions (eg, congestive heart failure, renal disease, or hepatic disease) that can cause chronic fluid accumulation. An accurate symptom history can help guide clinicians to the correct underlying etiology. For example, venous insufficiency causing dependent edema is often observed to improve with elevation. Conversely, edema caused by decreased plasma oncotic pressure associated with malabsorption or nephrotic syndrome does not improve with positional changes. Therefore, obtaining a careful history can help exclude differential diagnoses.[4]
Physical Examination
Anasarca is primarily diagnosed through characteristic findings on physical examination. Vital signs may reflect conditions secondary to fluid overload (eg, tachycardia, tachypnea, decreased oxygen saturation).[20] Physical examination should focus on identifying the edema pattern, such as peripheral versus generalized and pitting versus nonpitting edema. Clinical findings can also aid in establishing the underlying etiology, which will help guide management. For instance, pitting reflects the movement of excess interstitial water in response to pressure, usually seen in dependent areas, typically the lower extremities in ambulatory patients, and over the sacrum in bed-bound patients. Nonpitting edema more commonly suggests lymphatic obstruction or myxedema associated with hypothyroidism. Ascites suggest a primary hepatic disease, while signs of volume overload or heart failure may indicate cardiovascular-associated anasarca.[4] The following are common exam findings with anasarca:
- Face: nonpitting periorbital edema [8]
- Pulmonary: inspiratory crackles, rhonchi [8]
- Cardiovascular: S3 gallop, jugular venous distension [8]
- Extremities: pitting or nonpitting edema, erythema, venous ulceration, myxedema over the tibia or dorsum of the foot [18]
- Abdominal: ascites, distended abdominal wall veins, and splenomegaly [8]
- Skin: verrucous and hyperkeratotic changes, hyperpigmented, cold or warm to touch over edematous areas [8]
- Genital: scrotal or vulvar edema [21]
Evaluation
The primary focus of anasarca evaluation is identifying the underlying cause and excluding differential diagnoses. Clinicians from various specialties (eg, cardiology, nephrology, gastroenterology, and oncology) frequently must collaborate to determine the primary etiology because multiple organ systems (eg, urinary, cardiovascular, and respiratory) are affected simultaneously.[22] Assessment may include the following studies to diagnose potential etiologies leading to anasarca.
Laboratory Studies
- CBC: Abnormalities may indicate systemic conditions that need evaluation. Also, the necessity for further hematology studies (eg, peripheral blood smear) is guided by initial abnormal findings (eg, thrombocytopenia, leukocytosis).[10][20]
- Comprehensive metabolic panel: Depending on the results, these findings can help assess renal function, albumin level, and liver function. For instance, decreased glomerular filtration rate in combination with hypoalbuminemia could indicate an underlying renal pathology as opposed to raised liver enzymes indicative of liver disease as the cause of anasarca.[4]
- Urinanalysis: Dipstick testing principally detects albumin. A urine protein-to-creatinine ratio or 24-hour urine markedly positive for protein in combination with hypoalbuminemia and clinical edema is virtually diagnostic of nephrotic syndrome.[23]
- Brain natriuretic peptide: An elevated level may indicate a diagnosis of CHF.[24]
- Thyroid studies: Depending on the thyroid impairment, results will help diagnose hyperthyroid or hypothyroid conditions.[4]
Imaging Studies
- Chest X-ray: May help assess patients with findings of cardiac enlargement, pulmonary edema, and pleural effusions.[25]
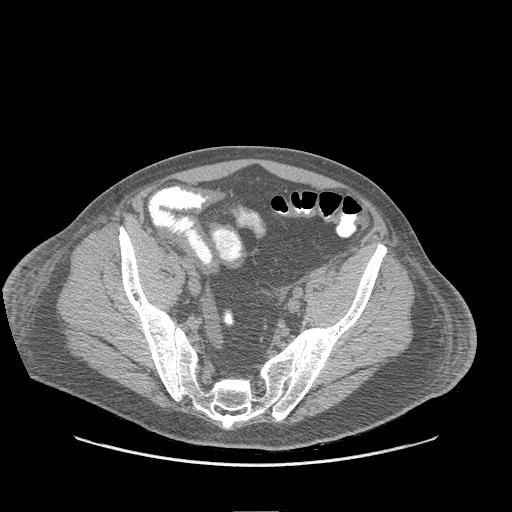
- CT: Anasarca appears as fluid within affected interstitial tissues, including the axilla, chest wall, pelvis, and pleural and cardiac effusions (see Image. Submucosal Edema in the Abdomen on CT).[22]
- Echocardiogram: If clinically indicated, an echocardiogram can evaluate ventricular function, assess for pericardial effusion, and diagnose cardiac disease. Echocardiography is also recommended to evaluate pulmonary hypertension in patients known or suspected to have obstructive sleep apnea.[8]
- Venous ultrasound: The preferred imaging method in evaluating suspected deep vein thrombosis. A duplex ultrasound may be used for diagnostic confirmation of chronic venous insufficiency.[8]
- Renal ultrasound: The most frequently used imaging modality in patients with renal dysfunction or proteinuria. Ultrasonography clinicians use this to characterize kidney size and assess for cystic renal disease and hydronephrosis.[26]
- Lymphoscintigraphy: The preferred modality to assess lymphedema if clinical evaluation is insufficient.[8]
- MRI: This may be used to evaluate underlying musculoskeletal causes of anasarca. T1-weighted magnetic resonance lymphangiography may also be used to assess lymphedema.[8]
Treatment / Management
Anasarca is a symptomatic manifestation of an underlying condition; therefore, managing the cause is the primary treatment. Additionally, a majority of patients with anasarca also require the administration of diuretics to help resolve systemic fluid overload. Particularly in volume-overloaded patients with pulmonary edema, recognition of a fluid imbalance by clinicians prompting urgent treatment is critical, as pulmonary edema not treated immediately may lead to poor outcomes, including increased mortality.[4][27]
Anasarca involves various body tissues and may involve the lungs. For anasarca without respiratory distress, secondary to pulmonary edema, is not acutely life-threatening, and removal of the excess fluid can proceed more slowly. However, pulmonary edema can develop quickly, becoming life-threatening, and requires immediate therapy. In patients with generalized edema due to heart failure, nephrotic syndrome, or primary sodium retention, the excess fluid can be mobilized rapidly with the help of loop or thiazide diuretics. According to American Heart Association (AHA) guidelines, fluid-overloaded patients with heart failure presenting with shortness of breath should receive immediate treatment with diuretics, as earlier and more aggressive intervention is associated with improved outcomes.[27][28](A1)
Diuretic therapy can lead to volume depletion in patients with localized edema due to venous or lymphatic obstruction or malignant ascites and, therefore, may not be helpful. However, in severely generalized edematous states, such as congestive heart failure, initial therapy is recommended to begin with a loop diuretic, such as furosemide or bumetanide.[29] Treatment for anasarca due to cirrhosis can begin with spironolactone alone or a combination of spironolactone and a loop diuretic like furosemide. The recommended initial dosage of spironolactone is 100 mg/day, titrating up to 400 mg/day according to patient response; furosemide can be initiated at a dosage between 20 and 40 mg/day. The European Association for the Study of the Liver (EASL) recommends an aldosterone antagonist alone as first-line therapy for the first episode of grade 2 cirrhotic ascites. In patients with a poor response (ie, a body weight reduction of <2 kg/wk) or those who develop hyperkalemia, furosemide should be started at a dosage of 40 mg/day and increased by 40 mg/day as needed, up to a maximum daily dose of 160 mg. Patients with recurrent or long-standing ascites should be started on an antimineralocorticoid and furosemide combination.[30](A1)
However, the American Association for the Study of Liver Diseases (AASLD) recommends combination therapy initially with a starting dosage of spironolactone 100 mg/day and furosemide 40 mg/day. Starting both drugs together is preferred as it achieves rapid natriuresis while maintaining normokalemia. When excess fluid is adequately reduced, the dosage should be tapered to maintain minimal or no ascites. The percentage of patients with cirrhotic ascites that experience adverse effects from diuretics is 20% to 40%. Angeli et al concluded that combined diuretic treatment is the better management approach for moderate ascites without renal failure.[31] The AASLD and EASL management recommendations have minimal differences; both approaches have merits in different situations.(A1)
Furthermore, in patients with conditions causing a significant accumulation of fluid (eg, cirrhosis or heart failure), 2 nonpharmacologic mainstays of treatment include sodium and fluid restriction. The AHA recommends a fluid restriction of 2 L/day in most patients with fluid overload; increased restrictions may be required for patients with hyponatremia or diuretic resistance.[27][28] The International Ascites Club has recommended a sodium intake of 2000 mg daily, while AHA guidelines state that some level of sodium restriction should be considered dependent on the patient's stage of heart failure. In some cases, addressing nutritional deficiencies and achieving a balanced diet helps the management of anasarca. In some patients, intravenous albumin infusions may help draw excess fluid back into the bloodstream and reduce edema. If anasarca is due to an underlying infection, appropriate antimicrobial therapy may be necessary. In patients where kidney failure is the underlying cause of anasarca, hemodialysis may be required to remove excess fluid.[14][27] Nevertheless, these additions to diuretic therapy are critical to preventing the recurrence of anasarca.[32][33][28](A1)
Following initial management, clinicians must adequately monitor patients. Diuretic dosages can be monitored and titrated using a random "spot" urine sodium concentration, increasing dosages until the urine sodium concentration exceeds that of potassium.[34] Higher dosages of <1 mg/kg/day of furosemide and 2 mg/kg/day of spironolactone may be required to treat nephrotic syndrome.[35][36] Some cases of idiopathic edema are medication-induced; in these individuals, initial management consists of medication cessation and nonpharmacologic therapies.[8](A1)
Some ancillary therapies for anasarca are targeted to areas affected by edema. Mechanical therapies (eg, leg elevation and compression stockings) are useful treatments for lower-extremity edema. Leg elevation reduces swelling by assisting fluid return to the heart. A supportive pressure between 30 to 40 mm Hg is often recommended for patients using compression stockings, though not for individuals with peripheral arterial disease.[8] Treatment of areas with lymphedema typically involves lymphatic massage combined with compressive bandages to increase lymphatic drainage. Compression stockings or pneumatic compression devices can then be used for therapeutic maintenance. However, diuretics are not effective in the treatment of lymphedema.[8][37]
Differential Diagnosis
The differential diagnoses of anasarca include any condition that can lead to widespread severe edema, including the following:[38][39][4]
- Heart failure
- Kidney diseases, including glomerular pathologies (eg, IgA nephropathy, glomerulonephritis, and nephrotic syndrome)
- Autoimmune disease (eg, juvenile dermatomyositis)
- Hematological disorders (eg, acute myeloid leukemia)
- Thrombocytopenia, anasarca, fever, reticulin fibrosis, renal insufficiency, and organomegaly (TAFRO) syndrome [40]
- Cirrhosis
- Hypoproteinemia (eg, malnutrition or protein-losing enteropathies)
- Hypothyroidism
- Cellulitis or sepsis
- Deep vein thrombosis
- Medications (eg, calcium channel blockers or nonsteroidal anti-inflammatory drugs) [19]
- Severe allergic reactions
- Lymphedema
- Pregnancy
- Some malignancies, particularly advanced stages
- Amyloidosis [41]
Prognosis
The prognosis for anasarca depends on the underlying cause and how promptly it can be managed. Anasarca can be a reversible symptom if the etiologic factors can be treated effectively. For instance, anasarca caused by medications or infection has a good prognosis as treatment such as drug cessation or antibiotic therapy will reverse resulting pathophysiological changes.[19][22] However, anasarca due to chronic conditions (eg, advanced heart failure, severe kidney disease, or liver failure) may have a grave prognosis because the underlying disease's severity and advanced progression guide prognostic assessment.[42]
Complications
Anasarca may result in complications involving various organ systems, including the following:[43][22][14][22]
- Organ dysfunction
- Skin ulcers
- Skin infections
- Reduced mobility
- Nutritional deficiencies
- Weight gain
- Impaired wound healing
- Deep vein thrombosis
- Respiratory distress
- Pericardial effusion
- Pericardial tamponade
- Complications associated with underlying etiologies or diuretic treatment
Deterrence and Patient Education
Early intervention is crucial to effectively prevent anasarca's development. Furthermore, patient education plays a pivotal role in averting anasarca and its associated complications, given that it often manifests as a late-onset symptom stemming from poorly managed underlying conditions. Patients should possess awareness regarding chronic medical conditions that may lead to anasarca if not diligently managed, such as heart failure, kidney disease, liver disease, and specific inflammatory disorders. Familiarity with risk factors and early warning signs can motivate individuals to seek timely medical attention. Additionally, lifestyle modifications frequently prove indispensable in preventing and managing conditions contributing to anasarca, such as hypertension, diabetes, and obesity.
Compliance with prescribed medications is paramount, especially for patients with preexisting medical conditions that elevate the risk of anasarca, like heart failure. Furthermore, patients should receive education about the significance of limiting sodium and fluid intake. Clinicians should educate patients about the initial indicators of anasarca, which include pronounced swelling in the legs, ankles, and face, accompanied by shortness of breath. Additionally, appropriate patients should be informed about the benefits of using compression stockings to enhance circulation and reduce swelling. Clinicians should also offer guidance on self-care techniques for managing edema, such as elevating the legs when sitting or lying down and engaging in gentle exercises that promote circulation. For patients with a history of anasarca, identifying triggers that exacerbate their condition, such as avoiding excessive salt intake or specific allergens, can be highly beneficial.
Enhancing Healthcare Team Outcomes
Anasarca is a severe condition characterized by fluid accumulation throughout the body. Due to the diverse underlying causes of anasarca, its management necessitates a comprehensive and collaborative approach involving a team of healthcare professionals. Primary care providers play a crucial role in the early detection of the underlying disease because they are often the first to encounter patients and can identify anasarca at an earlier stage of illness. Early referral to the appropriate specialist is vital for achieving better outcomes, particularly when a patient's initial symptoms are nonspecific, and they first present to a primary care clinician or internist. Clinicians from various specialties, such as cardiologists, hepatologists, nephrologists, and internists, frequently collaborate and play a crucial role in diagnosing and managing the root cause of anasarca.
Once a patient is admitted to the hospital or discharged back into the community, healthcare professionals, including inpatient and outpatient nurses and home health nurses, become crucial in monitoring the patient's condition, administering medications, regulating fluid intake and output, and providing patient education. Dietitians also play a central role, particularly in cases involving malnutrition or high-sodium diets, as they can develop appropriate nutritional plans. Pharmacists ensure the safe prescription of medications, while physical and occupational therapists work with patients whose mobility and lifestyle have been affected by anasarca. Respiratory therapists assist in managing the patient's breathing and oxygen needs if respiratory distress is present. Therefore, the collaborative approach of an interprofessional healthcare team aims to optimize treatment, enhance the patient's quality of life, and address any complications associated with anasarca.
Media
References
Wang CS, Greenbaum LA. Nephrotic Syndrome. Pediatric clinics of North America. 2019 Feb:66(1):73-85. doi: 10.1016/j.pcl.2018.08.006. Epub [PubMed PMID: 30454752]
Bonney KM, Luthringer DJ, Kim SA, Garg NJ, Engman DM. Pathology and Pathogenesis of Chagas Heart Disease. Annual review of pathology. 2019 Jan 24:14():421-447. doi: 10.1146/annurev-pathol-020117-043711. Epub 2018 Oct 24 [PubMed PMID: 30355152]
Wang G, Cao WG, Zhao TL. Fluid management in extensive liposuction: A retrospective review of 83 consecutive patients. Medicine. 2018 Oct:97(41):e12655. doi: 10.1097/MD.0000000000012655. Epub [PubMed PMID: 30313055]
Level 2 (mid-level) evidencePatel H, Skok C, DeMarco A. Peripheral Edema: Evaluation and Management in Primary Care. American family physician. 2022 Nov:106(5):557-564 [PubMed PMID: 36379502]
Abassi Z, Khoury EE, Karram T, Aronson D. Edema formation in congestive heart failure and the underlying mechanisms. Frontiers in cardiovascular medicine. 2022:9():933215. doi: 10.3389/fcvm.2022.933215. Epub 2022 Sep 27 [PubMed PMID: 36237903]
Kallash M, Mahan JD. Mechanisms and management of edema in pediatric nephrotic syndrome. Pediatric nephrology (Berlin, Germany). 2021 Jul:36(7):1719-1730. doi: 10.1007/s00467-020-04779-x. Epub 2020 Nov 20 [PubMed PMID: 33216218]
Craven MD, Washabau RJ. Comparative pathophysiology and management of protein-losing enteropathy. Journal of veterinary internal medicine. 2019 Mar:33(2):383-402. doi: 10.1111/jvim.15406. Epub 2019 Feb 14 [PubMed PMID: 30762910]
Level 2 (mid-level) evidenceTrayes KP, Studdiford JS, Pickle S, Tully AS. Edema: diagnosis and management. American family physician. 2013 Jul 15:88(2):102-10 [PubMed PMID: 23939641]
Schildt EE, De Ranieri D. Anasarca as the presenting symptom of juvenile dermatomyositis: a case series. Pediatric rheumatology online journal. 2021 Aug 13:19(1):120. doi: 10.1186/s12969-021-00604-3. Epub 2021 Aug 13 [PubMed PMID: 34389019]
Level 2 (mid-level) evidenceMeena SP, Sairam MV, Puranik AK, Badkur M, Sharma N, Lodha M, Rohda MS, Kothari N. Risk Factors and Patient Outcomes Associated With Immediate Post-operative Anasarca Following Major Abdominal Surgeries: A Prospective Observational Study From 2019 to 2021. Cureus. 2021 Dec:13(12):e20631. doi: 10.7759/cureus.20631. Epub 2021 Dec 23 [PubMed PMID: 34963874]
Level 2 (mid-level) evidenceKlanderman RB, Bosboom JJ, Migdady Y, Veelo DP, Geerts BF, Murphy MF, Vlaar APJ. Transfusion-associated circulatory overload-a systematic review of diagnostic biomarkers. Transfusion. 2019 Feb:59(2):795-805. doi: 10.1111/trf.15068. Epub 2018 Nov 29 [PubMed PMID: 30488959]
Level 1 (high-level) evidenceGradalski T. Edema of Advanced Cancer: Prevalence, Etiology, and Conservative Management-A Single Hospice Cross-Sectional Study. Journal of pain and symptom management. 2019 Feb:57(2):311-318. doi: 10.1016/j.jpainsymman.2018.11.005. Epub 2018 Nov 17 [PubMed PMID: 30453053]
Krogh A, Landis EM, Turner AH. THE MOVEMENT OF FLUID THROUGH THE HUMAN CAPILLARY WALL IN RELATION TO VENOUS PRESSURE AND TO THE COLLOID OSMOTIC PRESSURE OF THE BLOOD. The Journal of clinical investigation. 1932 Jan:11(1):63-95 [PubMed PMID: 16694035]
Ellis D. Pathophysiology, Evaluation, and Management of Edema in Childhood Nephrotic Syndrome. Frontiers in pediatrics. 2015:3():111. doi: 10.3389/fped.2015.00111. Epub 2016 Jan 11 [PubMed PMID: 26793696]
Sabar R, Safadi W. Relieving the burden: palliative centesis of an oedematous scrotal wall due to anasarca in end-stage heart failure. BMJ case reports. 2013 Sep 6:2013():. doi: 10.1136/bcr-2013-009388. Epub 2013 Sep 6 [PubMed PMID: 24014556]
Level 3 (low-level) evidenceNobbe AM, McCurdy HM. Management of the Adult Patient with Cirrhosis Complicated by Ascites. Critical care nursing clinics of North America. 2022 Sep:34(3):311-320. doi: 10.1016/j.cnc.2022.04.005. Epub 2022 Jul 20 [PubMed PMID: 36049850]
Thakrar DB, Sultan MJ. Cellulitis: diagnosis and differentiation. Journal of wound care. 2021 Dec 2:30(12):958-965. doi: 10.12968/jowc.2021.30.12.958. Epub [PubMed PMID: 34881996]
Sabanova EA, Fadeyev VV, Potekaev NN, Lvov AN. [Pretibial myxedema: pathogenetic features and clinical aspects]. Problemy endokrinologii. 2019 Jun 30:65(2):134-138. doi: 10.14341/probl9848. Epub 2019 Jun 30 [PubMed PMID: 31271716]
Sener D, Halil M, Yavuz BB, Cankurtaran M, Arioğul S. Anasarca edema with amlodipine treatment. The Annals of pharmacotherapy. 2005 Apr:39(4):761-3 [PubMed PMID: 15728328]
Level 3 (low-level) evidenceMontazeripouragha A, Campbell CM, Russell J, Medvedev N, Owen DR, Harris A, Donnellan F, McCormick I, Fajgenbaum DC, Chen LYC. Thrombocytopenia, anasarca, and severe inflammation. American journal of hematology. 2022 Oct:97(10):1374-1380. doi: 10.1002/ajh.26651. Epub 2022 Jul 19 [PubMed PMID: 35794839]
Nakaya H, Okamoto R, Nagashima K, Sugino Y, Ogihara Y, Sakuma H, Dohi K. Elderly Man With "Overalls" Edema. Circulation journal : official journal of the Japanese Circulation Society. 2022 Jan 25:86(2):333. doi: 10.1253/circj.CJ-21-0700. Epub 2021 Sep 15 [PubMed PMID: 34526441]
Gbadamosi WA, Melvin J, Lopez M. Atypical Case of Minoxidil-Induced Generalized Anasarca and Pleuropericardial Effusion. Cureus. 2021 Jun:13(6):e15424. doi: 10.7759/cureus.15424. Epub 2021 Jun 3 [PubMed PMID: 34249570]
Level 3 (low-level) evidenceJinadasa AGRG, Srimantha LASM, Siriwardhana ID, Gunawardana KB, Attanayake AP. Optimization of 25% Sulfosalicylic Acid Protein-to-Creatinine Ratio for Screening of Low-Grade Proteinuria. International journal of analytical chemistry. 2021:2021():6688941. doi: 10.1155/2021/6688941. Epub 2021 Jan 28 [PubMed PMID: 33574847]
Kuwahara K. The natriuretic peptide system in heart failure: Diagnostic and therapeutic implications. Pharmacology & therapeutics. 2021 Nov:227():107863. doi: 10.1016/j.pharmthera.2021.107863. Epub 2021 Apr 21 [PubMed PMID: 33894277]
Zhou L, Yin X, Zhang T, Feng Y, Zhao Y, Jin M, Peng M, Xing C, Li F, Wang Z, Wei G, Jia X, Liu Y, Wu X, Lu L. Detection and Semiquantitative Analysis of Cardiomegaly, Pneumothorax, and Pleural Effusion on Chest Radiographs. Radiology. Artificial intelligence. 2021 Jul:3(4):e200172. doi: 10.1148/ryai.2021200172. Epub 2021 May 19 [PubMed PMID: 34350406]
Dopp H, Maagh P, Meissner A. [Heart Failure Despite Low BNP-Level: Paradoxon or Pathfinder? - - A Case of Pericarditis constrictiva]. Deutsche medizinische Wochenschrift (1946). 2018 May:143(10):731-734. doi: 10.1055/a-0600-1645. Epub 2018 May 4 [PubMed PMID: 29727888]
Level 3 (low-level) evidenceClaure-Del Granado R, Mehta RL. Fluid overload in the ICU: evaluation and management. BMC nephrology. 2016 Aug 2:17(1):109. doi: 10.1186/s12882-016-0323-6. Epub 2016 Aug 2 [PubMed PMID: 27484681]
WRITING COMMITTEE MEMBERS, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013 Oct 15:128(16):e240-327. doi: 10.1161/CIR.0b013e31829e8776. Epub 2013 Jun 5 [PubMed PMID: 23741058]
Level 1 (high-level) evidenceBellomo R, Prowle JR, Echeverri JE. Diuretic therapy in fluid-overloaded and heart failure patients. Contributions to nephrology. 2010:164():153-163. doi: 10.1159/000313728. Epub 2010 Apr 20 [PubMed PMID: 20428001]
Level 3 (low-level) evidenceEuropean Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. Journal of hepatology. 2018 Aug:69(2):406-460. doi: 10.1016/j.jhep.2018.03.024. Epub 2018 Apr 10 [PubMed PMID: 29653741]
Level 1 (high-level) evidenceAngeli P, Fasolato S, Mazza E, Okolicsanyi L, Maresio G, Velo E, Galioto A, Salinas F, D'Aquino M, Sticca A, Gatta A. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010 Jan:59(1):98-104. doi: 10.1136/gut.2008.176495. Epub [PubMed PMID: 19570764]
Level 1 (high-level) evidenceMoore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G, Jimenez W, Planas R, Arroyo V. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology (Baltimore, Md.). 2003 Jul:38(1):258-66 [PubMed PMID: 12830009]
Level 3 (low-level) evidencePerri GA. Ascites in patients with cirrhosis. Canadian family physician Medecin de famille canadien. 2013 Dec:59(12):1297-9; e538-40 [PubMed PMID: 24336542]
Level 3 (low-level) evidenceRunyon BA, AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology (Baltimore, Md.). 2013 Apr:57(4):1651-3. doi: 10.1002/hep.26359. Epub [PubMed PMID: 23463403]
Level 1 (high-level) evidenceTapia C, Bashir K. Nephrotic Syndrome. StatPearls. 2024 Jan:(): [PubMed PMID: 29262216]
Kodner C. Diagnosis and Management of Nephrotic Syndrome in Adults. American family physician. 2016 Mar 15:93(6):479-85 [PubMed PMID: 26977832]
Pereira de Godoy JM, Pereira de Godoy HJ, Lopes Pinto R, Facio FN Jr, Guerreiro Godoy MF. Maintenance of the Results of Stage II Lower Limb Lymphedema Treatment after Normalization of Leg Size. International journal of vascular medicine. 2017:2017():8515767. doi: 10.1155/2017/8515767. Epub 2017 Aug 1 [PubMed PMID: 28835857]
Zedan M, El-Ayouty M, Abdel-Hady H, Shouman B, El-Assmy M, Fouda A. Anasarca: not a nephrotic syndrome but dermatomyositis. European journal of pediatrics. 2008 Jul:167(7):831-4. doi: 10.1007/s00431-008-0716-z. Epub 2008 Apr 15 [PubMed PMID: 18414893]
Level 3 (low-level) evidenceNara M, Komatsuda A, Itoh F, Kaga H, Saitoh M, Togashi M, Kameoka Y, Wakui H, Takahashi N. Two Cases of Thrombocytopenia, Anasarca, Fever, Reticulin Fibrosis/Renal Failure, and Organomegaly (TAFRO) Syndrome with High Serum Procalcitonin Levels, Including the First Case Complicated with Adrenal Hemorrhaging. Internal medicine (Tokyo, Japan). 2017:56(10):1247-1252. doi: 10.2169/internalmedicine.56.7991. Epub 2017 May 15 [PubMed PMID: 28502946]
Level 3 (low-level) evidenceNishimura Y, Fajgenbaum DC, Pierson SK, Iwaki N, Nishikori A, Kawano M, Nakamura N, Izutsu K, Takeuchi K, Nishimura MF, Maeda Y, Otsuka F, Yoshizaki K, Oksenhendler E, van Rhee F, Sato Y. Validated international definition of the thrombocytopenia, anasarca, fever, reticulin fibrosis, renal insufficiency, and organomegaly clinical subtype (TAFRO) of idiopathic multicentric Castleman disease. American journal of hematology. 2021 Oct 1:96(10):1241-1252. doi: 10.1002/ajh.26292. Epub 2021 Jul 28 [PubMed PMID: 34265103]
Oka S, Ono K. Successful treatment of systemic AL amyloidosis with autologous hematopoietic stem cell transplantation combined with cell-free and concentrated ascites reinfusion therapy. Clinical case reports. 2023 May:11(5):e7233. doi: 10.1002/ccr3.7233. Epub 2023 May 9 [PubMed PMID: 37180320]
Level 3 (low-level) evidencePeng Y, Qi X, Guo X. Child-Pugh Versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies. Medicine. 2016 Feb:95(8):e2877. doi: 10.1097/MD.0000000000002877. Epub [PubMed PMID: 26937922]
Level 1 (high-level) evidenceKumar P, Khan IA, Das A, Shah H. Chronic venous disease. Part 1: pathophysiology and clinical features. Clinical and experimental dermatology. 2022 Jul:47(7):1228-1239. doi: 10.1111/ced.15143. Epub 2022 Apr 5 [PubMed PMID: 35167156]