Introduction
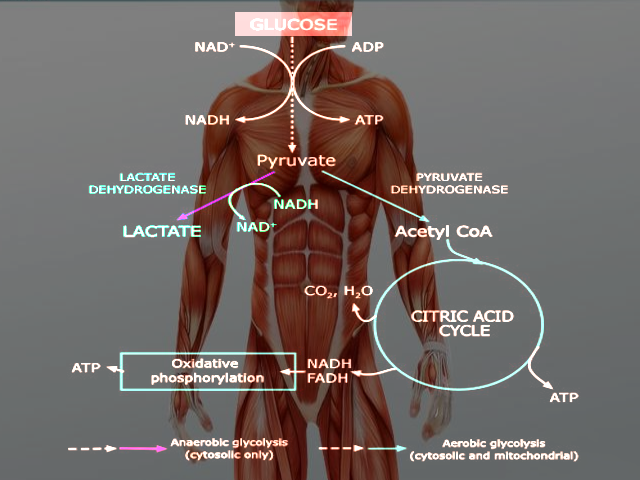
Through the process of glycolysis, one molecule of glucose breaks down to form two molecules of pyruvate. Depending on the microcellular environment (specifically, oxygen availability, energy demand, and the presence or absence of mitochondria), pyruvate has several separate fates:
In mitochondria-containing cells, pyruvate can enter the citric acid cycle within the mitochondrial matrix and undergo oxidative phosphorylation. Aptly named due to its dependence on oxygen as the final electron acceptor, oxidative phosphorylation cannot take place in the absence of oxygen. Moreover, as the enzymes of both the citric acid cycle and electron transport chain are within the mitochondria, cells lacking mitochondria (e.g., erythrocytes) cannot rely on oxidative phosphorylation for energy production.
In erythrocytes and oxygen-deprived tissue, pyruvate remains within the cytoplasm and converts to lactate, a process referred to as anaerobic glycolysis. This final reaction allows for the regeneration of NAD+, a cofactor that must be available in high enough intracellular concentrations for the earlier reactions of glycolysis to remain favorable. Compared to oxidative phosphorylation, however, anaerobic glycolysis is significantly less efficient, providing a net production of only 2 ATP per glucose molecule (versus 32 ATP per glucose molecule produced during oxidative phosphorylation).[1]
Fundamentals
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Fundamentals
Glycolysis is the process by which glucose is broken down within the cytoplasm of a cell to form pyruvate. Under aerobic conditions, pyruvate can diffuse into mitochondria, where it enters the citric acid cycle and generates reducing equivalents in the form of NADH and FADH2. These reducing equivalents then enter the electron transport chain, leading to the production of 32 ATP per molecule of glucose. Because the electron transport chain requires oxygen as the final electron acceptor, inadequate tissue oxygenation inhibits the process of oxidative phosphorylation.
Under anaerobic conditions, pyruvate has a different fate. Instead of entering mitochondria, the cytosolic enzyme lactate dehydrogenase converts pyruvate to lactate. Although lactate itself is not utilized by the cell as a direct energy source, this reaction also allows for the regeneration of NAD+ from NADH. NAD+ is an oxidizing cofactor necessary to maintain the flow of glucose through glycolysis. Glycolysis produces 2 ATP per glucose molecule, and thus provides a direct means of producing energy in the absence of oxygen. This process of breaking down glucose in the absence of oxygen is aptly named anaerobic glycolysis.[1]
Additionally, cells that do not contain mitochondria (e.g., erythrocytes) cannot perform oxidative phosphorylation.[2] The enzymes of the citric acid cycle are in the mitochondrial matrix, and the enzymes of the electron transport chain are embedded within the inner mitochondrial membrane. Consequently, these cells rely on anaerobic glycolysis for ATP production regardless of oxygen concentrations.
Issues of Concern
Relative to oxidative phosphorylation, which maximizes the energy potential of a single glucose molecule (approximately 32 molecules of ATP per 1 molecule of glucose), glycolysis is an inefficient means of energy production. Glycolysis produces only two net molecules of ATP per 1 molecule of glucose. However, in cells lacking mitochondria and/or adequate oxygen supply, glycolysis is the sole process by which such cells can produce ATP from glucose. Additionally, in maximally contracted skeletal muscle, glycolysis is a quick and relatively efficient means of meeting short-term energy goals.
Function
Anaerobic glycolysis serves as a means of energy production in cells that cannot produce adequate energy through oxidative phosphorylation. In poorly oxygenated tissue, glycolysis produces 2 ATP by shunting pyruvate away from mitochondria and through the lactate dehydrogenase reaction.[1] In rapidly contracting skeletal muscle cells with energy demand exceeding what can be produced by oxidative phosphorylation alone, anaerobic glycolysis allows for the more rapid production of ATP.[3] (Glycolysis is approximately 100 times faster than oxidative phosphorylation.) In cells lacking mitochondria altogether, pyruvate cannot undergo oxidative phosphorylation regardless of oxygen levels.
Mature erythrocytes do not contain mitochondria and thus rely exclusively on anaerobic glycolysis for ATP production.[2] Other tissues, such as the cornea and lens of the eye and inner medulla of the kidney, are poorly vascularized and rely heavily on anaerobic glycolysis despite the presence of mitochondria.[4][5]
Mechanism
The steps of glycolysis are as follows:
- Glucose gets phosphorylated by hexokinase, forming glucose-6-phosphate. This step requires one molecule of ATP.
- Glucose-6-phosphate is isomerized by phosphoglucose isomerase to form fructose-6-phosphate.
- Fructose-6-phosphate is phosphorylated by phosphofructokinase to form fructose-1,6-bisphosphate. This step requires one molecule of ATP.
- Fructose-1,6-bisphosphate is split into two separate sugar molecules, dihydroxyacetone phosphate and glyceraldehyde-3-phosphate, by aldolase.
- The molecule of dihydroxyacetone phosphate is isomerized by triosephosphate isomerase to form a second glyceraldehyde-3-phosphate.
- Glyceraldehyde-3-phosphate is phosphorylated by glyceraldehyde-3-phosphate dehydrogenase to form 1,3-bisphosphoglycerate. This step requires NAD+ as a cofactor.
- 1,3-bisphosphoglycerate is converted to 3-phosphoglycerate by phosphoglycerate kinase. This step involves the transfer of a phosphate molecule to ADP to form 1 molecule of ATP.
- 3-phosphoglycerate rearranges to form 2-phosphoglycerate by the enzyme phosphoglycerate mutase.
- 2-phosphoglycerate is dehydrated to produce phosphoenolpyruvate by the enzyme enolase.
- Phosphoenolpyruvate is converted to pyruvate by pyruvate kinase. This step involves the transfer of a phosphate molecule to ADP to form 1 molecule of ATP.
The microenvironment of the cell determines the fate of pyruvate following the initial ten steps of glycolysis. If a cell lacks mitochondria, is poorly oxygenated, or energy demand has rapidly increased to exceed the rate at which oxidative phosphorylation can provide sufficient ATP, pyruvate can be converted to lactate by the enzyme lactate dehydrogenase.[1] This step involves the oxidation of NADH to NAD+, allowing glycolysis to continue through the glyceraldehyde-3-phosphate dehydrogenase reaction (step #6, see above).
Testing
Lactic acid, the end product of anaerobic glycolysis, is commonly measured in the inpatient setting. Because anaerobic glycolysis predominates when tissue is poorly oxygenated or perfused, lactic acid levels are useful in directing the management of severe sepsis, shock, blood loss, anemia, or heart failure. Hyperlactatemia and lactic acidosis are indicative of inefficient cardiac output and are associated with increased morbidity and mortality.[6][7][8]
Clinical Significance
- Serum Lactic Acid: Lactic acid levels increase when oxygen demand exceeds oxygen supply/delivery, such as in anemia, heart failure, severe infection (sepsis), and shock. Lactic acid measurements are useful for diagnosing and directing the management of such conditions.[6][7][8]
- Anaerobic Exercise: During periods of high-intensity exercise in which oxygen demand exceeds oxygen supply, muscles rely on anaerobic glycolysis for ATP production. Although oxidative phosphorylation produces approximately 15 times more ATP than glycolysis, glycolysis occurs at a rate approximately 100 times faster.[3]
- The Warburg Effect: One hallmark of cancer is the shift from aerobic to anaerobic metabolism seen within tumor cells, referred to as the Warburg Effect. As tumors grow, they expand beyond the capabilities of local blood supply. To combat the inadequate tissue perfusion and oxygenation, cancerous cells shift away from oxidative metabolism and instead rely heavily on anaerobic glycolysis.[9]
- Fibromyalgia: Fibromyalgia is a chronic pain condition characterized by diffuse tender points on the body in the absence of abnormal diagnostic testing. Some studies have revealed an increase in pyruvate and lactate production in individuals with fibromyalgia compared to healthy controls, as well as a decrease in ATP production. Subjects with fibromyalgia also expressed lactate dehydrogenase in lower concentrations.[10][11]
Media
References
Granchi C, Bertini S, Macchia M, Minutolo F. Inhibitors of lactate dehydrogenase isoforms and their therapeutic potentials. Current medicinal chemistry. 2010:17(7):672-97 [PubMed PMID: 20088761]
Minakami S, Yoshikawa H. Studies on erythrocyte glycolysis. II. Free energy changes and rate limitings steps in erythrocyte glycolysis. Journal of biochemistry. 1966 Feb:59(2):139-44 [PubMed PMID: 4223318]
Peek CB, Levine DC, Cedernaes J, Taguchi A, Kobayashi Y, Tsai SJ, Bonar NA, McNulty MR, Ramsey KM, Bass J. Circadian Clock Interaction with HIF1α Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell metabolism. 2017 Jan 10:25(1):86-92. doi: 10.1016/j.cmet.2016.09.010. Epub 2016 Oct 20 [PubMed PMID: 27773696]
Chhabra M, Prausnitz JM, Radke CJ. Modeling corneal metabolism and oxygen transport during contact lens wear. Optometry and vision science : official publication of the American Academy of Optometry. 2009 May:86(5):454-66. doi: 10.1097/OPX.0b013e31819f9e70. Epub [PubMed PMID: 19357551]
Level 3 (low-level) evidenceChen Y, Fry BC, Layton AT. Modeling glucose metabolism and lactate production in the kidney. Mathematical biosciences. 2017 Jul:289():116-129. doi: 10.1016/j.mbs.2017.04.008. Epub 2017 May 8 [PubMed PMID: 28495544]
Suetrong B, Walley KR. Lactic Acidosis in Sepsis: It's Not All Anaerobic: Implications for Diagnosis and Management. Chest. 2016 Jan:149(1):252-61. doi: 10.1378/chest.15-1703. Epub 2016 Jan 6 [PubMed PMID: 26378980]
Di Mauro FM, Schoeffler GL. Point of Care Measurement of Lactate. Topics in companion animal medicine. 2016 Mar:31(1):35-43. doi: 10.1053/j.tcam.2016.05.004. Epub 2016 May 21 [PubMed PMID: 27451047]
Level 3 (low-level) evidencePéronnet F, Aguilaniu B. [Physiological significance and interpretation of plasma lactate concentration and pH in clinical exercise testing]. Revue des maladies respiratoires. 2014 Jun:31(6):525-51. doi: 10.1016/j.rmr.2014.04.002. Epub 2014 May 17 [PubMed PMID: 25012038]
Schwartz L, Supuran CT, Alfarouk KO. The Warburg Effect and the Hallmarks of Cancer. Anti-cancer agents in medicinal chemistry. 2017:17(2):164-170 [PubMed PMID: 27804847]
. Is fibromyalgia caused by a glycolysis impairment? Nutrition reviews. 1994 Jul:52(7):248-50 [PubMed PMID: 8090378]
Eisinger J, Plantamura A, Ayavou T. Glycolysis abnormalities in fibromyalgia. Journal of the American College of Nutrition. 1994 Apr:13(2):144-8 [PubMed PMID: 8006296]