Introduction
Over 150,000 people undergo amputations of the lower extremity in the United States each year.[1] This incidence is directly proportional to rates of peripheral arterial occlusive disease, neuropathy, and soft tissue sepsis.[2] This correlation is due to the increased incidence of diabetes mellitus, which is present in 82% of all vascular-related lower extremity amputations in the United States. Patients with diabetes mellitus have an astounding 30 times greater lifetime risk of undergoing an amputation when compared to patients without diabetes mellitus, which translates to an economic strain in healthcare systems of over $4.3 billion in annual costs in the USA alone.[3] Trauma to the lower extremity can lead to amputation in over 20% of patients when associated with severe wound contamination and significant soft tissue loss.[4] Battle-related explosive events can lead to amputation in 93% of cases and approximately 2% of combat casualties least to limb amputation.[5]
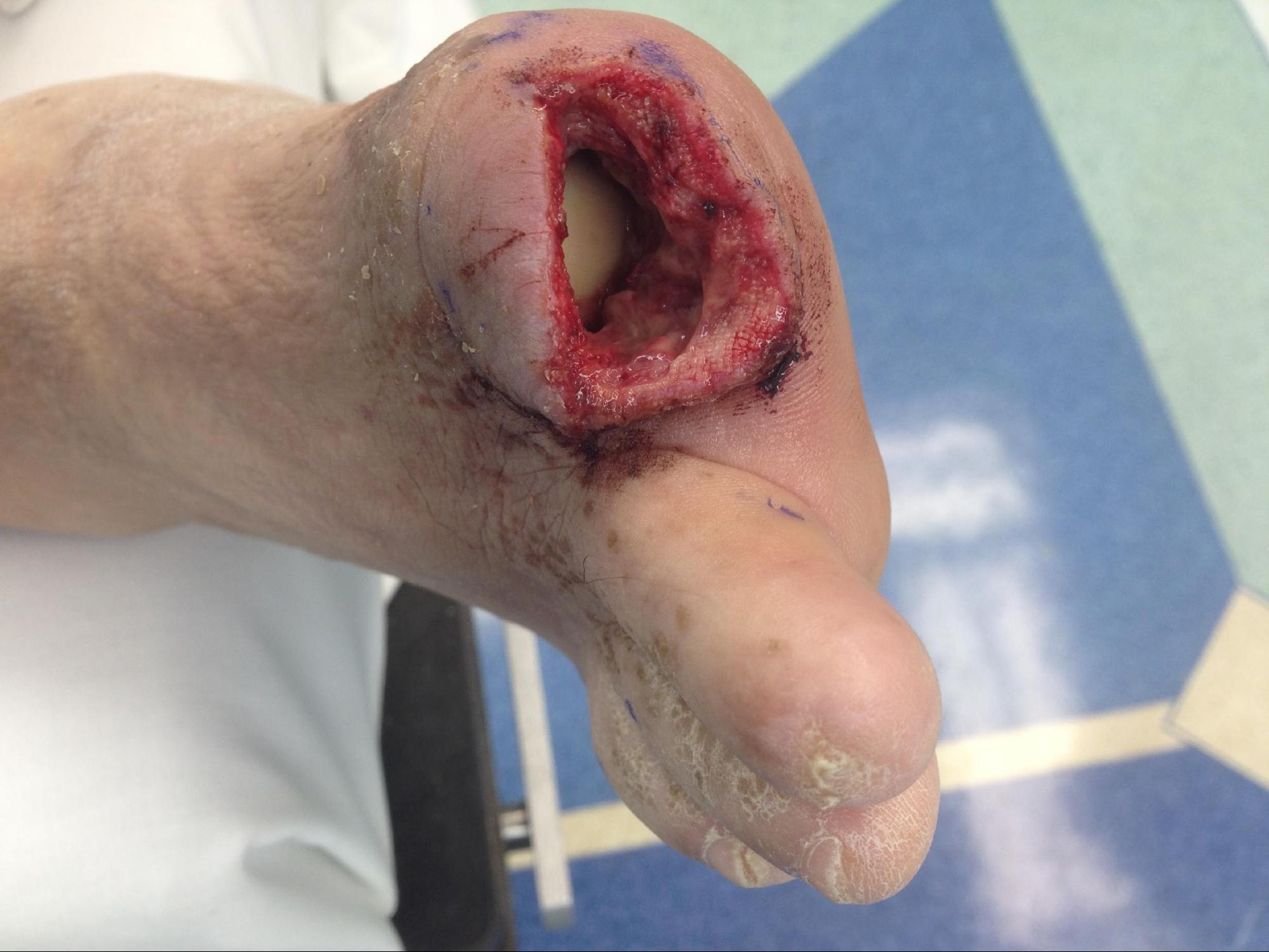
This activity focuses on amputations at the level of the femur and distally. It covers above-knee, through-knee, and below-knee amputations (see Image. Digital Amputation). In addition, it describes the technique for certain foot amputations (Syme, Chopart, Boyd), but the reader is encouraged to seek further in-depth text to review these techniques. Amputations are procedures performed surgically, although on rare occasions and in limited settings, they can be performed employing cryoamputation.[6]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The lower extremity is subdivided into the thigh (between the hip and knee joints), lower leg (between knee and ankle), and the foot (calcaneus and distally).
Thigh Compartments
Anterior compartment
- Sartorius
- Quadriceps: composed of rectus femoris, vastus lateralis, vastus medius, and vastus intermedius.
- Superficial femoral artery and vein
Medial compartment
- Adductor magnus muscle
- Gracilis muscle
- Deep femoral artery and vein
- Saphenous nerve: located in the subcutaneous tissue of the medial thigh and runs parallel to the intermuscular septum of the anterior and medial compartments
Posterior compartment
- Biceps femoris muscle
- Semitendinosus muscle
- Semimembranosus muscle
- Sciatic nerve
Lower Leg Compartments
Anterior compartment
- Tibialis anterior muscle
- Extensor hallucis longus muscle
- Extensor digitorum longus muscle
- Peroneus tertius muscle
- Anterior Tibial artery
- Anterior tibial vein
- Deep peroneal nerve
Lateral compartment
- Peroneus brevis muscle
- Peroneus longus muscle
Deep posterior compartment
- Tibialis posterior muscle
- Flexor digitorum longus muscle
- Flexor hallucis longus muscle
- Posterior tibial artery
- Posterior tibial vein
- Peroneal artery
- Peroneal vein
- Tibial nerve
Superficial posterior compartment
- Soleus muscle
- Gastrocnemius muscle
- Plantaris muscle
- Sural cutaneous nerve: lesser saphenous vein located in the subcutaneous tissue of the posterior lower leg and runs parallel to the sural nerve
Foot Compartments
The foot comprises 7 tarsal bones, 5 metatarsals, and 14 phalanges. It is subdivided into hindfoot (talus and calcaneus bones), midfoot (cuboid, navicular, 3 cuneiform bones), and forefoot (metatarsals and phalanges). The muscles of the foot can be extrinsic, originating from the anterior or posterior aspect of the lower leg, and intrinsic muscles, originating from the foot.
Indications
Indications for amputation are related to the degree of tissue necrosis or viability, and it is performable in either a single operation or a staged manner (amputation followed by reconstruction). The decision to take either approach depends largely on the clinical status of the patient and the quality of the soft tissues at the desired level of amputation, with the primary goal being to excise the non-viable and infected tissue. In general, soft tissue quality and the ability to obtain bone coverage guide the adequacy of the level of amputation. It is important to note that skin grafts are an acceptable option for patients where adequate muscle coverage is obtainable, where skin coverage is not possible.
Patients with diabetes mellitus can present along a spectrum of diseases, from a non-healing foot wound with underlying osteomyelitis to a grossly infected wound leading to septic shock. In peripheral vascular disease, this decision to amputate is made with the appearance of non-healing wounds when there are no options for the restoration of flow. These patients can generally present in 1 of 2 ways: in the acute setting with infected necrosis (wet gangrene) leading to sepsis or with ischemic necrosis (dry gangrene) where the tissue is necrotic without signs of systemic compromise.
Before deciding to amputate, it is essential to optimize the patient from a medical standpoint. In patients with diabetes mellitus, all efforts should focus on achieving adequate glycemic control and early antibiotic treatment to minimize the risk of surgical site infection and maximize the length of non-infected tissue, respectively. It is reasonable to consider these patients candidates for a single operation should the quality of the soft tissue allow it. In the patient presenting with septic shock, the decision to perform an open (guillotine) amputation with staged reconstruction versus a single operation depends on the clinical status of the patient, and the primary goal should be to obtain adequate source control, leaving reconstruction for a later date. Patients presenting with signs of a systemic inflammatory response and extensive cellulitis may receive initial treatment with intravenous antibiotics. A decrease in cellulitis may allow for a more distal level of amputation than anticipated as well as allowing the operation to take place in a single stage.
High-energy traumatic injuries can lead to amputation at the moment of injury. Alternatively, patients can present to the hospital with a mangled extremity not amenable to reconstruction. Several scoring systems can be utilized to determine whether complex reconstruction options should be pursued. However, the primary focus should have its basis in employing the Advanced Trauma Life Support protocol since it is likely that patients present with concomitant life-threatening injuries. This includes assessment of bleeding from the wound, obtaining hemostasis, and performing adequate resuscitation. The level of amputation depends on the viability of the soft tissues used to obtain bone coverage.[7] It is important to note that victims of severe traumatic lower extremity injury who initially were candidates for limb salvage may become candidates for an amputation due to infection, inability to obtain bone or hardware coverage, persistently high pain levels, or lack of the desire to submit to lengthy reconstructive protocols for poor functional results.
Contraindications
Patients with advanced peripheral vascular disease often have diabetes, are elderly, and have multiple comorbidities with low physiologic reserve. It is, therefore, ideal to medically optimize these patients before a definitive operation. However, an emergency lower extremity amputation may be required to allow for clinical improvement, and the risks of surgery anesthesia must be discussed with the patient and/or designated advocates.
Certain patients are in the intensive care setting receiving vasoactive infusions and heavy sedation with low cardiopulmonary reserve. Amputation may be indicated, but their critically ill state does not allow for such. It is acceptable to wait for clinical optimization before performing an amputation. An alternative to this is cryoamputation, the concept of refrigeration of unsalvageable ischemic limb in critically ill patients. There are many described techniques which include the application of ice bags, ice water immersion, mechanical refrigeration, and utilizing dry ice. Although cumbersome, it can be employed successfully with appropriate training of clinicians and the creation of institutional protocols. A subsequent formal amputation procedure can then follow once the metabolic derangements have resolved and the benefits of the surgery have surpassed the risks.
Equipment
The procedure occurs in the operating theater in a sterile environment using an appropriately sized tourniquet. The patient is in the supine position and under general anesthesia or regional blockade. Of note, some patients may have no vascular inflow, and therefore, a tourniquet is not necessary. However, careful consideration should focus on the skin by covering it with a cotton roll or stockinette before application of a tourniquet.
A ruler and marking pen are used to demarcate the skin incision and soft tissue flap. A large 15 or 20-blade can incise the skin and soft tissues. Alternatively, electrocautery is an option for the soft tissues and the entire dissection, with fresh blades reserved for nerve transection. A Gigli saw or a power saw is used for transecting the bones. The power saw can also be utilized to soften the edges of the bone once transected. Alternatively, a bone rasper can be used and allow for more control and possibly smoother curvature of the anterior surface of the bone. A drill, a 2.0 mm drill bit, and fiber wire suture are used if performing a myodesis. The tissue is closed in layers. Dressing materials can include petroleum gauze, soft rolls, army battle dressings, and an elastic bandage for compression.
Personnel
Every team performing a lower extremity amputation must include an operating room nurse, a scrub technologist, a surgical assistant, and an anesthesiologist. Post-anesthetic care unit staff are vital in the patient's care in the immediate postoperative period. Face-to-face communication from the surgery team is obligatory during patient hand-off. This is an opportunity to summarize the patient and the reason for the operation performed. It also allows the surgeon to communicate adversities encountered during the case, report on estimated blood loss and discuss resuscitative measures used intra-operatively that may need continuation in the immediate post-operative setting. It is also important to communicate the type of hospital unit the patient goes to thereafter and the need for post-operative laboratory values.
Preparation
The most important part of the preparation after medical optimization is determining the level of amputation. Transcutaneous oxygen tension (TcPO2) is a measure of oxygen tension in the skin derived from the local capillary blood perfusion. This has been utilized as a tool to determine the level of amputation in ischaemic limbs, which demonstrated that patients with primary healing of postoperative wounds had significantly higher values of TcPO2 than patients with failure to heal (37 mmHg; range 15-56 mmHg vs. 18 mmHg; range 8-36 mmHg, p < 0.01).[8] Although useful in the setting of isolated peripheral vascular disease, this tool does not consider the condition of the patient, the condition of the soft tissues, the presence of neuropathy, or the functional status of the patient, all of which are also determinants in selecting amputation level. An accepted approach to amputation level determination in the patient with peripheral vascular disease is the presence of a femoral pulse; this indicates patency of the deep femoral artery, which has general acceptance as appropriate for a transtibial (below-knee) amputation. On the other hand, efforts for revascularization must undergo an assessment before performing an above-knee amputation in the absence of a femoral pulse.[9] Despite many available modalities for assessing healing potential, none has proven more useful than a good physical exam. Pulse, temperature, and hair growth patterns are all useful and guide clinical intuition.
It is imperative to discuss the probability of independence after a major lower extremity amputation with the patient. AMPREDICT is a user-friendly prediction tool for mobility outcomes in individuals undergoing major lower extremity amputation because of complications of diabetes or peripheral vascular disease.[10] Informing the patient of their probability to achieve independence in the 12 months following amputation allows for shared-decision making and, more importantly, allows the patient to understand their mobility prognosis during the strenuous recovery period. Ambulation rates outside the home decrease drastically as the length of amputation decreases. Energy expenditure for ambulation increases significantly as the amputation site moves higher.
More often than not, the level of amputation is determined by the degree of soft tissue compromise/infection despite optimal antibiotic therapy. In patients presenting with gangrene or necrotizing soft tissue infection, there is very little room for discussion, and the primary objective is to preserve life. A secondary objective in this setting is to preserve as much functional limb length as possible, as this has a significant impact on the patient's postoperative functionality.
Technique or Treatment
The use of general anesthesia (GA) versus regional anesthesia (RA) for performing major lower extremity amputation is an area of ongoing debate. There is literature to support the use of RA for major lower extremity amputation with decreased blood loss, need for transfusion, postoperative pain medication, and faster time to oral intake when compared with the general anesthesia group.[11] Another study showed there was no difference in postoperative myocardial infarction or mortality between GA and RA.[12] More recently, the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) was utilized to determine the effect of anesthesia type on major lower extremity amputation outcomes in functionally impaired elderly patients.[13] They reviewed over 3000 patients over 8 years. Fifty-nine percent underwent above-the-knee amputations, and the remainder were below-the-knee. Patients undergoing GA were more apt to have impaired sensorium, be on anticoagulation, or have a bleeding disorder and had a prior operation within 30 days. GA correlated with shorter anesthesia time to surgery but equivalent operative times to RA. There was no difference with regard to postoperative myocardial infarction/cardiac arrest, pulmonary complications, stroke, urinary tract infections, and wound complications. Therefore, deciding which anesthesia type to use should be performed on a case-by-case basis and collectively between the patient, surgeon, and anesthesiologist.
There are several components in the preparation of the patient in the operating room which apply to all amputation levels. The patient should be in the supine position with the appropriate use of a tourniquet, as this has been shown to reduce blood loss during lower extremity amputation in the setting of peripheral artery disease.[14] Skin preparation should be performed circumferentially and proximal to the groin. Skin preparation products contain iodophors or chlorhexidine gluconate, both acceptable options.[15] In diabetic foot wounds or gangrene, we recommend maintaining the wound with a dry dressing and covering the affected foot with a sterile impermeable stockinette. An occlusive adhesive dressing can create a seal and further isolate this from the incision site.
Principles of amputation surgery at any level include removal of diseased tissue, providing a residual limb that allows for prosthetic fit, tapering the ends of the bone to avoid sharp edges, providing a conical-shaped limb to allow better prosthetic fit, controlling postsurgical edema, avoiding hematoma formation, allowing for nerve retraction, preservation of length and optimized postoperative pain control.[16] The critical steps in surgical technique for several amputation levels in order of proximal to distal include:
Above-Knee Amputation
- The anterior and posterior flaps should be marked before incision to resemble an ellipse or fishmouth. It is useful to measure the circumference and mark the apices equally. If length is not an issue, the tip of the anterior flap should reach the edge of the patella with a mirror image posterior flap.
- Tourniquet and Esmark bandage is used. An incision is carried through the fascia, and the anterior musculatures are divided with cautery.
- The muscle gets divided to the femur level, and the periosteum is elevated from the femur circumferentially to the level of the incision apex.
- An oscillating saw or Gigli saw is used to divide the femur. The adductor tendon is separated separately from the medial epicondyle and distal femur; this may be preserved for use in a myodesis. The edges are then rasped smooth.
- The femoral artery and femoral vein are clamped and suture ligated with heavy suture.
- The saphenous nerve should be dissected proximally and divided on tension. It is this author's opinion that avoiding ligation helps avoid foreign body reactions and future neuroma formation. The nerve should retract 5 cm to 10 cm.
- If performing a myodesis (preferred by this author), a 2 mm drill bit is used to make a medial and lateral osteotomy on the distal femur.
- The preserved adductor tendon is then secured to the osteotomies medially and laterally with heavy non-absorbable fiber-wire or braided nylon suture.
- If the medullary canal remains open, the elevated periosteum can be reapproximated with a heavy absorbable suture.
- The posterior tissue then gets divided with an amputation knife or cautery.
- The sciatic/tibial nerve is now divided and allowed to retract as above
- The tourniquet is released, and all bleeding is ligated.
- A subfascial drain is left in place if needed but is generally unnecessary.
- The anterior and posterior flap is reapproximated with a heavy absorbable suture at the fascia.
- Skin and subcutaneous tissues are closed in layers.
Through-Knee Amputation
- An elliptical incision similar to the above knee is made with the apices at the medial and lateral epicondyles near the top of the patella if a line were extended around the femur. The anterior flap distal margin extends to the tibial tuberosity. The posterior flap should attempt to mirror the anterior flap.
- A tourniquet is used as above. The surgeon employs a similar technique through the fascia as above.
- The patellar tendon detaches from the tibia, and the surgeon enters the knee joint.
- The joint capsule is incised circumferentially, and the cruciate ligaments are divided from within the knee joint.
- Before dividing the posterior joint capsule and posterior tissues, it is useful to identify the semitendinosus medially and the biceps femoris laterally as they insert into the tibia posteriorly. They should be held with a Kocher clamp to prevent retraction.
- Popliteal artery and vein individually suture ligated.
- The common peroneal and tibial nerves are divided under tension and transected sharply to allow retraction.
- The posterior tissues get divided with an amputation knife or cautery. It is not necessary to preserve any gastrocnemius muscle.
- The specimen is removed at this point.
- An oscillating saw is used to make 6 osteotomies removing all of the articular surfaces while preserving most of the adductor insertion medially; this is cancellous bone with no medullary canal.
- The patella is then everted and removed from the inner surface of the patellar tendon, taking care not to injure the thin skin from the bare area in the mid-tendon.
- The tourniquet is released, and further hemostasis is achieved with ligatures.
- Myodesis then follows by suturing the semitendinosus and biceps to the cruciate remnant posteriorly and the patellar tendon to the cruciate anteriorly. This process is performed with heavy fiber wire or braided nylon.
- Fascia, subcutaneous tissue, and the skin closed as above.
Open Below-Knee Amputation
- This procedure is performed as distal as the soft tissues allow and can take place expeditiously with a Gigli saw through all structures followed by suture ligation of vascular structures. The residual limb is covered with wet-to-dry dressings once hemostasis is obtained.
- The alternative is to use a scalpel and electrocautery for the skin, subcutaneous tissue, and muscle, followed by isolation and control of vascular bundles. A power saw, or the same Gigli saw, can be used for bone. The residual limb is covered with wet-to-dry dressings upon achieving hemostasis.
Formal Below-Knee Amputation
- The level selected depends largely on soft tissue viability, with the ideal length being approximately 12 to 18 cm from the tibial tubercle.[17]
- It is useful to have a standard technique to determine skin flaps. This author prefers to measure the diameter at the transection site and make the anterior flap one-half the circumference and the posterior long flap's full circumference; this prevents unnecessary tension at the time of closure. Additionally, longer residual limb lengths typically involve a smaller circumference and thereby have less chance for tension.
- The incisions are carried through the skin, subcutaneous tissue, and anterior muscle with suture ligation of vasculature identified.
- The tibial bone gets transected with a power saw or Gigli saw, and the tibial edges are blunted with a rasper. The power saw, or rasper can be used to bevel the anterior aspect of the tibia. This process reduces trauma to the posterior flap as it sits along a smooth surface.
- The fibula gets transected in the same manner at approximately 1 cm proximal to the tibial transection, and sharp edges are removed with rasper, giving the residual limb a cornified aspect.
- The posterior tissue is divided with an amputation knife, leaving only a thin portion of the soleus but preserving the gastrocnemius.
- At this point, ligation of the anterior tibial, posterior tibial, and peroneal arteries is confirmed before the tourniquet is released. The tibial, deep and superficial peroneal, and soleus nerves divide on tension.
- The myodesis is performed by bringing the Achilles tendon to the tibia. Three osteotomies are created with a 2 mm drill bit in the anterior portion of the tibia. Fiber-wire or heavy braided nylon is used to secure the Achilles to the tibia utilizing the 3 osteotomies in a mattress fashion.
- Skin and subcutaneous tissues are closed in layers.
ERTL Amputation
- All steps are similar to formal below-knee amputation, except a keyhole incision is used, extending just lateral to the tibial distally and then around the ankle. The tibialis anterior muscle is preserved in the anterior compartment.
- An osteal periosteal graft is raised using a hammer and chisel and left attached to the tibia anteriorly.
- The fibula is transected at the same length as the tibia.
- The soleus muscle and anterolateral compartment muscle are transected. A GIA stapler is useful for this.
- Vascular bundles and nerves receive similar treatment.
- A portion of the fibula is trimmed to create a strut between the tibia and fibula, which gets secured with a tightrope device, plate, or headless screw.
- The periosteal graft is secure around the fibula strut to aid in ossification
- The tibialis anterior is secured over the strut, followed by the Achilles myodesis (pants over vest).
- The closure begins anteriorly, and the skin and subcutaneous tissue are resected as the closure proceeds posteriorly
Syme Amputation
- A weight-bearing amputation through the ankle that involves removal of all of the bones of the foot while preserving the heel pad for weight-bearing
Boyd Amputation
- A weight-bearing amputation through the ankle preserves the calcaneus and heel pad for weight-bearing.
- The calcaneus gets fused to the tibia and is non-mobile
Chopart Amputation
- A weight-bearing amputation at the midtarsal level preserves the additional length of the foot
Complications
Lower extremity amputations involve significant perioperative morbidity and mortality. Thirty-day postoperative mortality rates can range from 4% to 22%.[18] Long-term mortality rates at 1, 3, and 5 years can reach 15, 38, and 68%, respectively.[19] Mortality rates in diabetic lower extremity amputation patients can be as high as 77% at 5 years.[20] Risk factors for death in the perioperative setting include AKA, postoperative cardiac complications, age over 74 years, and acute renal failure.[21] A review of 2879 amputees demonstrated the most common post-surgical complications included pneumonia (22%), acute kidney injury (15%), deep venous thrombosis (15%), acute lung injury/acute respiratory distress syndrome (13%), osteomyelitis (3%) and flap failure (6%).[22]
Wound complications, which include dehiscence, seroma, and hematoma, can occur in 12% to 34% of BKA patients and 6% to 16% of AKA patients.[23] Risk factors for wound complications include sepsis, compartment syndrome, end-stage renal disease, ongoing tobacco use, body mass index over 30 kg/m2, and BKA.[24] A retrospective study showed that the use of incisional negative pressure wound therapy (NPWT) in major limb amputation and revision amputation had demonstrable benefits in decreasing the risk of wound complications.[25]
Phantom limb pain (PLP) is the pain that persists after complete tissue healing and is characterized by dysesthesia at the level of the absent limb. Patients describe this pain as burning, throbbing, stabbing, and sharp, as well as the sensation that the amputated limb is in an abnormal position.[26] This pain can be present in 67% of patients at 6 months and 50% of patients at 5 to 7 years.[27][28] There are several risk factors for developing PLP, which include: the presence of pre-amputation pain, female gender, upper extremity amputations, and bilateral amputations of the upper and/or lower extremities.[26] A multidisciplinary approach, which includes surgical technique, regional analgesia, pharmacological agents, physical therapy, and psychotherapy, are all key components in the peri-operative care of an amputee that can have a strong impact on decreasing the risk of PLP.
Revision amputation procedures can occur in as many as 42% of patients who underwent a below-knee amputation secondary to trauma. Additionally, up to 13% of patients undergo revision to a higher level of amputation. Age, presence of a crush injury, compartment syndrome, and experiencing a major post-surgical complication were significant risk factors of revision amputation.[22]
It is also important to include psychological trauma as a complication of limb loss. A recent review performed by Mckechnie et al. reveals that depression can occur in 20.6 to 63% of patients (3 times higher than the general population) and anxiety in 25% to 57% (approximately the same as the general population) with 83% of patients attending a psychiatric clinic at 1 point after their surgery.[29] Darnall et al. demonstrated an increased risk of depressive symptoms in patients undergoing an amputation secondary to trauma versus vascular disease or cancer.[30] Current research, such as "Amputees Unanimous: A 12-step Program", focuses on a multimodal approach toward the care of an amputee, which aims to provide encouragement, support, and optimism for the future.[31] Further research is needed to determine their impact on this patient population.
Clinical Significance
An amputation not only reduces mobility but can cause significant impairment in quality of life. There is increased energy expenditure with higher levels of amputation.[32] The mean oxygen consumption in unilateral below-knee amputees compared to unimpaired subjects has been shown to increase by 9%. This increase is even more notable in above-knee amputees at approximately 49% and 280% in those with bilateral above-knee amputees.[33] The rate of metabolic expenditure is higher in dysvascular amputees compared to traumatic amputees.[34] The extent of the disease dictates the level of the amputation. Through-knee amputation is a reasonable alternative to above-knee amputation should perfusion and soft tissue conditions allow. TKA has demonstrated similar morbidity and mortality compared to AKA and can provide a better end-weight-bearing residual limb, improved stability with adductor preservation, and improved prosthetic comfort.[35] An above-knee prosthetic can sit on the ischium and can require removal for activities of daily life, such as sitting on the toilet. The through-knee amputation is generally preferable in younger patients or any patients with ambulatory potential, with the above-knee amputations preserved for those non-ambulatory bed-bound patients or those with advanced vascular disease.
Technological advancements in materials and ways to optimize the coupling between the residual limb and socket drive prosthetic development. Gel liners protect the skin while allowing for the suction suspension of the prosthetic. An active suction device can also be implemented as a mechanical pump that activates during ambulation. Improper fit leading to discomfort is the most common reason for rejection of prosthetic devices. However, patients may also be very self-conscious of the appearance of their residual limb, which may disincentivize its use. Therefore, a silicone cover or sleeve that mirrors the contralateral limb (including tattoos and hair) can be applied.[36] A prosthetist must be involved early in the post-operative care to assist with stump sock fitting and evaluation for an eventual prosthetic limb that adjusts to the patient's needs.
Enhancing Healthcare Team Outcomes
An interprofessional approach is necessary for the care of an amputee. Performing an amputation is a stressful period for most patients. If the procedure is elective, a mental health clinician should consult with the patient. Also, a prosthetic professional should consult with the patient to explain the different prosthetics and when the patient gets fitted with the appliance. After surgery, a wound care clinician must follow the patient to ensure complete healing before fitting any prosthetic device. Pharmacists support glycemic control in patients with diabetes, pain control, and overall medication reconciliation, reporting any issues to the rest of the interprofessional team.
Providers caring for this patient population should include an interprofessional team working closely to provide the best outcome. Every clinician is responsible for ensuring that the patient still can function in society. The social worker must ensure that the home is suitable and that the patient has adequate resources and support services.
The care of an amputee should be individualized based on the patient's overall health status. Clear communication with the patient and their families regarding their treatment course and expectations must be established in the pre-operative setting to facilitate post-operative care and improve the patient-physician relationship. Only through a thorough interprofessional team effort, extending across many months, can patients receiving a lower extremity amputation achieve optimal results and maintain an adequate quality of life.
Nursing, Allied Health, and Interprofessional Team Interventions
The care of an amputee begins and ends with the healthcare team's actions and interventions. Diabetic or peripheral vascular disease patients with foot or leg wounds can often undergo wound care with the assistance of a home healthcare team. Should there be a lack of progression in wound healing or early signs of infection, they are the first to refer the patient to a medical provider and, in some instances, the emergency department. After that, an ER or hospital clinician is generally the first to greet a patient and obtain vital signs, which can indicate the severity of the disease and, therefore, dictate the hospital setting where this patient receives their care. With the assistance of a clinician, the wound is exposed, and the patient is assessed from head-to-toe looking for pressure ulcers, ecchymosis, or hematomas in patients who are wheelchair or bed-bound. Other actions include obtaining adequate intravenous access, medication dispensing and administration, serial symptom assessment including pain, monitoring vital signs, providing wound care and patient hygiene, and liaising between patients, their families, and medical providers.
Nursing, Allied Health, and Interprofessional Team Monitoring
The care of an amputee involves an interprofessional approach. Monitoring in the post-operative setting includes obtaining and recording vital signs, pain scores, and laboratory values. Serial wound assessments allow for early detection of postoperative bleeding and allow the clinician to perform temporizing maneuvers (digital pressure, dressing reinforcement). Pain scores are diligently obtained to titrate the patients' pain medication accordingly and provide the best condition for early mobilization. Close communication between the interprofessional team is necessary to provide patients individualized care based on their needs.
Media
(Click Image to Enlarge)
References
Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Archives of physical medicine and rehabilitation. 2005 Mar:86(3):480-6 [PubMed PMID: 15759232]
Level 2 (mid-level) evidenceBeckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002 May 15:287(19):2570-81 [PubMed PMID: 12020339]
Moxey PW, Gogalniceanu P, Hinchliffe RJ, Loftus IM, Jones KJ, Thompson MM, Holt PJ. Lower extremity amputations--a review of global variability in incidence. Diabetic medicine : a journal of the British Diabetic Association. 2011 Oct:28(10):1144-53. doi: 10.1111/j.1464-5491.2011.03279.x. Epub [PubMed PMID: 21388445]
Bosse MJ, MacKenzie EJ, Kellam JF, Burgess AR, Webb LX, Swiontkowski MF, Sanders RW, Jones AL, McAndrew MP, Patterson BM, McCarthy ML, Travison TG, Castillo RC. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. The New England journal of medicine. 2002 Dec 12:347(24):1924-31 [PubMed PMID: 12477942]
Isaacson BM, Weeks SR, Pasquina PF, Webster JB, Beck JP, Bloebaum RD. The road to recovery and rehabilitation for injured service members with limb loss: a focus on Iraq and Afghanistan. U.S. Army Medical Department journal. 2010 Jul-Sep:():31-6 [PubMed PMID: 21181652]
Chen SL, Kuo IJ, Kabutey NK, Fujitani RM. Physiologic Cryoamputation in Managing Critically Ill Patients with Septic, Advanced Acute Limb Ischemia. Annals of vascular surgery. 2017 Jul:42():50-55. doi: 10.1016/j.avsg.2016.11.006. Epub 2017 Mar 7 [PubMed PMID: 28279723]
MacKenzie EJ, Bosse MJ, Kellam JF, Burgess AR, Webb LX, Swiontkowski MF, Sanders R, Jones AL, McAndrew MP, Patterson B, McCarthy ML, Rohde CA, LEAP Study Group. Factors influencing the decision to amputate or reconstruct after high-energy lower extremity trauma. The Journal of trauma. 2002 Apr:52(4):641-9 [PubMed PMID: 11956376]
Level 2 (mid-level) evidencePoredos P, Rakovec S, Guzic-Salobir B. Determination of amputation level in ischaemic limbs using tcPO2 measurement. VASA. Zeitschrift fur Gefasskrankheiten. 2005 May:34(2):108-12 [PubMed PMID: 15968892]
Level 1 (high-level) evidenceBunt TJ. Gangrene of the immediate postoperative above-knee amputation stump: role of emergency revascularization in preventing death. Journal of vascular surgery. 1985 Nov:2(6):874-7 [PubMed PMID: 4057446]
Czerniecki JM, Turner AP, Williams RM, Thompson ML, Landry G, Hakimi K, Speckman R, Norvell DC. The development and validation of the AMPREDICT model for predicting mobility outcome after dysvascular lower extremity amputation. Journal of vascular surgery. 2017 Jan:65(1):162-171.e3. doi: 10.1016/j.jvs.2016.08.078. Epub 2016 Oct 14 [PubMed PMID: 27751738]
Level 1 (high-level) evidenceMann RA, Bisset WI. Anaesthesia for lower limb amputation. A comparison of spinal analgesia and general anaesthesia in the elderly. Anaesthesia. 1983 Dec:38(12):1185-91 [PubMed PMID: 6419632]
Level 1 (high-level) evidenceChery J, Semaan E, Darji S, Briggs WT, Yarmush J, D'Ayala M. Impact of regional versus general anesthesia on the clinical outcomes of patients undergoing major lower extremity amputation. Annals of vascular surgery. 2014 Jul:28(5):1149-56. doi: 10.1016/j.avsg.2013.07.033. Epub 2013 Dec 14 [PubMed PMID: 24342828]
Level 2 (mid-level) evidenceMoreira CC, Farber A, Kalish JA, Eslami MH, Didato S, Rybin D, Doros G, Siracuse JJ. The effect of anesthesia type on major lower extremity amputation in functionally impaired elderly patients. Journal of vascular surgery. 2016 Mar:63(3):696-701. doi: 10.1016/j.jvs.2015.09.050. Epub 2015 Nov 6 [PubMed PMID: 26553953]
Choksy SA, Lee Chong P, Smith C, Ireland M, Beard J. A randomised controlled trial of the use of a tourniquet to reduce blood loss during transtibial amputation for peripheral arterial disease. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2006 Jun:31(6):646-50 [PubMed PMID: 16750790]
Level 1 (high-level) evidenceLiu Z, Dumville JC, Norman G, Westby MJ, Blazeby J, McFarlane E, Welton NJ, O'Connor L, Cawthorne J, George RP, Crosbie EJ, Rithalia AD, Cheng HY. Intraoperative interventions for preventing surgical site infection: an overview of Cochrane Reviews. The Cochrane database of systematic reviews. 2018 Feb 6:2(2):CD012653. doi: 10.1002/14651858.CD012653.pub2. Epub 2018 Feb 6 [PubMed PMID: 29406579]
Level 3 (low-level) evidenceSchnur D, Meier RH 3rd. Amputation surgery. Physical medicine and rehabilitation clinics of North America. 2014 Feb:25(1):35-43. doi: 10.1016/j.pmr.2013.09.013. Epub [PubMed PMID: 24287238]
Pinzur MS, Gottschalk FA, Pinto MA, Smith DG, American Academy of Orthopaedic Surgeons. Controversies in lower-extremity amputation. The Journal of bone and joint surgery. American volume. 2007 May:89(5):1118-27 [PubMed PMID: 17523257]
van Netten JJ, Fortington LV, Hinchliffe RJ, Hijmans JM. Early Post-operative Mortality After Major Lower Limb Amputation: A Systematic Review of Population and Regional Based Studies. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2016 Feb:51(2):248-57. doi: 10.1016/j.ejvs.2015.10.001. Epub 2015 Nov 14 [PubMed PMID: 26588994]
Level 1 (high-level) evidenceLarsson J, Agardh CD, Apelqvist J, Stenström A. Long-term prognosis after healed amputation in patients with diabetes. Clinical orthopaedics and related research. 1998 May:(350):149-58 [PubMed PMID: 9602814]
Fortington LV, Geertzen JH, van Netten JJ, Postema K, Rommers GM, Dijkstra PU. Short and long term mortality rates after a lower limb amputation. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2013 Jul:46(1):124-31. doi: 10.1016/j.ejvs.2013.03.024. Epub 2013 Apr 28 [PubMed PMID: 23628328]
Level 2 (mid-level) evidenceLópez-Valverde ME, Aragón-Sánchez J, López-de-Andrés A, Guerrero-Cedeño V, Tejedor-Méndez R, Víquez-Molina G, Jiménez-García R. Perioperative and long-term all-cause mortality in patients with diabetes who underwent a lower extremity amputation. Diabetes research and clinical practice. 2018 Jul:141():175-180. doi: 10.1016/j.diabres.2018.05.004. Epub 2018 May 17 [PubMed PMID: 29777746]
Low EE, Inkellis E, Morshed S. Complications and revision amputation following trauma-related lower limb loss. Injury. 2017 Feb:48(2):364-370. doi: 10.1016/j.injury.2016.11.019. Epub 2016 Nov 18 [PubMed PMID: 27890336]
Morisaki K, Yamaoka T, Iwasa K. Risk factors for wound complications and 30-day mortality after major lower limb amputations in patients with peripheral arterial disease. Vascular. 2018 Feb:26(1):12-17. doi: 10.1177/1708538117714197. Epub 2017 Jun 6 [PubMed PMID: 28587576]
O'Brien PJ, Cox MW, Shortell CK, Scarborough JE. Risk factors for early failure of surgical amputations: an analysis of 8,878 isolated lower extremity amputation procedures. Journal of the American College of Surgeons. 2013 Apr:216(4):836-42; discussion 842-4. doi: 10.1016/j.jamcollsurg.2012.12.041. Epub [PubMed PMID: 23521969]
Zayan NE, West JM, Schulz SA, Jordan SW, Valerio IL. Incisional Negative Pressure Wound Therapy: An Effective Tool for Major Limb Amputation and Amputation Revision Site Closure. Advances in wound care. 2019 Aug 1:8(8):368-373. doi: 10.1089/wound.2018.0935. Epub 2019 Jul 25 [PubMed PMID: 31346491]
Level 3 (low-level) evidenceAhuja V, Thapa D, Ghai B. Strategies for prevention of lower limb post-amputation pain: A clinical narrative review. Journal of anaesthesiology, clinical pharmacology. 2018 Oct-Dec:34(4):439-449. doi: 10.4103/joacp.JOACP_126_17. Epub [PubMed PMID: 30774224]
Level 3 (low-level) evidenceJensen TS, Krebs B, Nielsen J, Rasmussen P. Phantom limb, phantom pain and stump pain in amputees during the first 6 months following limb amputation. Pain. 1983 Nov:17(3):243-256. doi: 10.1016/0304-3959(83)90097-0. Epub [PubMed PMID: 6657285]
Bloomquist T. Amputation and phantom limb pain: a pain-prevention model. AANA journal. 2001 Jun:69(3):211-7 [PubMed PMID: 11759564]
Mckechnie PS, John A. Anxiety and depression following traumatic limb amputation: a systematic review. Injury. 2014 Dec:45(12):1859-66. doi: 10.1016/j.injury.2014.09.015. Epub 2014 Sep 28 [PubMed PMID: 25294119]
Level 1 (high-level) evidenceDarnall BD, Ephraim P, Wegener ST, Dillingham T, Pezzin L, Rossbach P, MacKenzie EJ. Depressive symptoms and mental health service utilization among persons with limb loss: results of a national survey. Archives of physical medicine and rehabilitation. 2005 Apr:86(4):650-8 [PubMed PMID: 15827913]
Level 2 (mid-level) evidenceAmorelli C, Yancosek K, Morris R. Amputees Unanimous: A 12-step program. Prosthetics and orthotics international. 2019 Jun:43(3):293-300. doi: 10.1177/0309364619836027. Epub 2019 Mar 19 [PubMed PMID: 30887887]
Waters RL, Perry J, Antonelli D, Hislop H. Energy cost of walking of amputees: the influence of level of amputation. The Journal of bone and joint surgery. American volume. 1976 Jan:58(1):42-6 [PubMed PMID: 1249111]
Huang CT, Jackson JR, Moore NB, Fine PR, Kuhlemeier KV, Traugh GH, Saunders PT. Amputation: energy cost of ambulation. Archives of physical medicine and rehabilitation. 1979 Jan:60(1):18-24 [PubMed PMID: 420566]
Waters RL, Mulroy S. The energy expenditure of normal and pathologic gait. Gait & posture. 1999 Jul:9(3):207-31 [PubMed PMID: 10575082]
Lim S, Javorski MJ, Halandras PM, Aulivola B, Crisostomo PR. Through-knee amputation is a feasible alternative to above-knee amputation. Journal of vascular surgery. 2018 Jul:68(1):197-203. doi: 10.1016/j.jvs.2017.11.094. Epub 2018 Mar 19 [PubMed PMID: 29567029]
Fergason J, Keeling JJ, Bluman EM. Recent advances in lower extremity amputations and prosthetics for the combat injured patient. Foot and ankle clinics. 2010 Mar:15(1):151-74. doi: 10.1016/j.fcl.2009.10.001. Epub [PubMed PMID: 20189122]
Level 3 (low-level) evidence