Introduction
Prenatal diagnosis enables the diagnosis of a broad spectrum of chromosomal abnormalities, gene disorders, X-linked conditions, neural tube defects, and infections to be made before the birth of the fetus. The various invasive prenatal diagnostic tests are amniocentesis, chorionic villus sampling, and fetal blood sampling or cordocentesis.
Amniocentesis
Amniocentesis is an invasive technique. This technique removes amniotic fluid from the uterine cavity using a needle. This procedure is performed transabdominally and under ultrasound guidance by a trained obstetrician. It was first performed for the diagnosis of genetic diseases (sex determination) of the fetus by Fuchs and Riis in 1956.[1] It is performed for diagnostic and therapeutic purposes, including for diagnostic evaluation in the form of chromosomal, biochemical, histopathological, and microbial assessments. When performed as a therapeutic procedure, it is done to reduce the volume of amniotic fluid in patients with polyhydramnios.[2][3][4]
The amniotic fluid obtained consists of fetal exfoliated cells, transudates, fetal urine, and lung secretions. Amniocentesis can be performed from 15 weeks of gestation to delivery, with an attributable risk of loss in experienced hands of 0.13% in singletons. Earlier the attributable risk is greater than chorion villus sampling (CVS), which provides similar data from 11 to 15 weeks. There is an increased risk of amnionic fluid leakage of 1 to 2%, most of which corresponds to decreased activity in days and fetal demise, which is usually much rarer than the risk of demise attributable to the indication for the procedure, except in low-risk women with no maternal risks indicating the procedure, and talipes equinovarus, presumably from oligohydramnios.[5]
Counseling of the couple is necessary regarding the procedure's indications, risks, benefits, and limitations.[6] This can be complex, as the individual circumstances leading to risk, including maternal age, parental family history, maternal serum screening, sonographic signs of chromosomal and other problems, and population history, can vary significantly. The benefit of possible pregnancy termination also varies depending on the patient's education, religious and ethical preferences, and, at present, state law.
Chorionic Villus Sampling
It is a prenatal invasive procedure and is done under ultrasound guidance. This procedure uses ultrasonography to guide the catheter or needle into the chorion frondosum. It is done abdominally and is followed by tissue aspiration (chorionic villi) for genetic or chromosomal analysis with a syringe containing tissue culture media. It is done in the first trimester for prenatal diagnosis between 10 to 14 weeks. Depending on the position of the uterus and bladder, the patient's gestational age, and placental localization, it can be performed transabdominally or transcervically.
The safer and earlier termination of pregnancy is possible as karyotype results are available within 7 to 10 days, although placental mosaicism is a risk for a false diagnosis or reassurance. It is indicated in chromosomal and genetic disorders. The samples collected are sent for DNA analysis. It is not performed in vaginal bleeding, in cases of cervical abnormalities, and severe infections. The major complications involved in this procedure are limb reduction defects from earlier procedures, chromosomal abnormalities present in the extraembryonic tissue, which are not found in the fetal tissue, intrauterine infections, membrane rupture, and fetal loss. The attributable risk in trained hands is similar to amniocentesis. Both procedures assume direct ultrasound guidance for a safe procedure.
Fetal Blood Sampling or Cordocentesis
It is the technique in which, under ultrasound guidance, fetal blood sampling is performed through the maternal abdomen. It is usually performed after 18 weeks after visualization of cord insertion. As the lumen of the cord at earlier weeks of gestation is narrow, it is considered safer to perform at 20 to 28 weeks of gestation. The blood sample is sent to the laboratory for hematological, immunological, and biochemical analysis. The results are obtained within 24 to 72 hours. There is an increased risk of fetal loss, which is comparatively higher (1 to 3% attributable risk) compared with other invasive procedures. The benefits include conversion to fetal transfusion, which can be life-saving. This is commonly indicated in maternal blood group sensitization from a transfusion or prior or current pregnancy sensitization to fetal cells from delivery or miscarriage of fetomaternal hemorrhage, suspected in the case of aplastic anemia from fetal Parvovirus B19 infection with the maternal acquisition of "Slapped face fever" - more common in teachers, parents, and childcare workers.[7]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Amniotic fluid is a transparent fluid with a light yellowish color. It is present in the amniotic sac. It creates a space for the fetus to grow and survive. It helps permit fetal movements, which are necessary for the effective musculoskeletal development of the fetus. It helps in fetal swallowing and fetal breathing.
Development
On the 8th day, the trophoblast changes. Bilaminar germ disc formation occurs after the differentiation of embryoblast. On the dorsal aspect of the bilaminar germ disc, there is the presence of tall columnar cells. They are ectodermal in origin.
On the ventral aspect of the bilaminar germ disc, flattened polyhedral endodermal cells are present. Connecting stalk forms the umbilical cord later; it connects the bilaminar germ disc with the trophoblast.
There is the appearance of 2 cavities on either side of the germ disc:
- Amniotic cavity
- Yolk sac
Physiology of Amniotic Fluid
Maternal and fetal compartments are essential in forming amniotic fluid in the first trimester of pregnancy. Fetal skin is non-keratinized in earlier weeks, allowing free transfer of water and other small molecules and solutes through the amnion and chorionic.[8] Amniotic fluid is similar to maternal and fetal extracellular fluid and functions as a nonsterile aqueous electrolyte solution.
During the second trimester of pregnancy, the diffusion process ceases as keratinization of fetal skin occurs, making the fetal skin impervious to water and other solutes.
On transvaginal ultrasonography, urine is observed at nine weeks in the fetal bladder, and on transabdominal sonography, urine is seen at 11 weeks of gestation.[9][10] During this period, the major component of the amniotic fluid is fetal urine. It is hypotonic (80 to 140 mOsm/liter), and as the fetal kidneys mature, they contain increased concentrations of urea, uric acid, and creatinine. At term, a fetus produces 500 to 700 ml of urine per day.[11][12][13]
The average amniotic fluid volume at 12 weeks of gestation is 60 mo.[10] By 16 weeks, the mean volume is 175 ml.[14] From 20 weeks on, amniotic fluid volume varies. Amniotic fluid volume increases steadily throughout pregnancy to a maximum of 400 to 1200 ml at 34 to 38 weeks.[14][15][16] Near term, the net increase of amniotic fluid is only 5 to 10 ml/day in the third trimester. After 38 weeks, fluid volume declines by approximately 125 ml/week to an average volume of 800 ml at 40 weeks.[14][15][16]
Indications
Amniocentesis is a commonly performed procedure for several reasons. It is performed for:
- Diagnostic indications
- Therapeutic indications
Diagnostic Indications
Chromosomal Analysis
Karyotyping and DNA analysis (to diagnose sex-linked disorders, inborn errors of metabolism, and neural tube defects).
- Advanced maternal age (Age > 35years)
- Abnormal biochemical screening markers (maternal alpha-fetoprotein, human chorionic gonadotropin, unconjugated estriol) in 1st or 2nd trimester
- Ultrasound detection of an abnormality or soft tissue markers (Nuchal translucency, nasal bone hypoplasia, nuchal pad edema, etc.)
- Family or personal history of chromosomal abnormalities in previous pregnancies
- Abnormal parental karyotype
- Parental balanced translocation
Genetic Testing
Analysis for Alpha-fetoprotein Level and Acetylcholinesterase
Assessment of Severity of Rh Isoimmunisation
Assessment of Bilirubin Levels in Amniotic Fluid and Grade of the Severity of Alloimmunization
Recently non-invasive tests, including the middle cerebral artery Doppler, have gained more importance. It is usually done in the third trimester of pregnancy.
Diagnosis of Fetal Infections
It can assist in diagnosing TORCH infections.[17] These include:
- CMV
- Parvovirus
- Toxoplasma Gondii
Fetal Lung Maturation (L/S ratio)
Performed in the third trimester of pregnancy.
Diagnosis of Chorioamnionitis
Diagnosis of Inherited Bleeding Disorders
Such as moderate to severe hemophilia A and B and type 3 von Willebrand disease (VWD) in the third trimester of pregnancy. These disorders have increased fetal risk of intracranial bleeding during delivery.[18]
Therapeutic Indications
- In hydramnios, it has a therapeutic role in relieving maternal discomfort and the instillation of intraamniotic drugs.
- Decompression amniocentesis in twin oligohydramnios-polyhydramnios sequence (TOPS): as it decreases the volume of amniotic fluid in the polyhydramnios sac, amniotic fluid pressures are decreased in both the sacs resulting in increased placental thickness thus improving uteroplacental circulation and improving fetal outcome.[19]
- It can help in amnioinfusion in oligohydramnios to prevent fetal lung hypoplasia and umbilical cord compression during labor.
- It is indicated for fetal blood transfusion in fetuses having severe hemolysis.
Contraindications
There are no absolute contraindications for amniocentesis.
Relative contraindications for amniocentesis are:
- Hepatitis B and HIV infections can be transmitted from maternal circulation to the fetus during the procedure. It should be deferred in HIV-infected patients until antiretroviral treatment is started and the viral load substantially decreases. Highly active antiretroviral therapy for HIV greatly reduces maternal to fetal transmission rates.[20][21][22]
- Decreased amniotic fluid (oligohydramnios)[23]
- Oral anticoagulation therapy must be stopped 48 to 72 hours before the procedure, and patients may be shifted to low molecular weight heparin.
Equipment
The procedure is done under continuous ultrasound guidance. The following equipment is required:
- Ultrasonography machine
- Sterile swabs and drapes
- Syringes: 2 ml, 10 ml
- Needle 20 gauge to 22 gauge
- Containers for collection and sample transport
- 5% povidone-iodine solution
Personnel
- Couples should undergo genetic counseling.
- Informed written consent should be taken.
- Risks, benefits, indications, procedures, and complications related to the mother and fetus must be explained in detail to the couple. The fetal and maternal risks associated with the procedure should also be discussed in detail. The time required to obtain results, failure to culture cells, and the type of cytogenetic test performed on the sample should be discussed.
- Proper procedure documentation is necessary, and the number of needle insertion attempts should be recorded.
- Ultrasonography before the procedure is done to document the number of fetuses, viability of the fetus, placental location, gestational age, site of cord insertion, and any obvious fetal malformation.
- An operator has to have enough experience of at least 20 supervised amniocentesis procedures done under supervision to do this procedure independently. Operators doing amniocentesis in multiple pregnancy situations should be experts in accurate pregnancy mapping, accurate sampling, and selective termination of the pregnancy by correctly identifying the fetus.
Preparation
- Both the operator and assistant must scrub with an antiseptic solution and use sterile gloves. The exposed abdominal surface area must be cleaned with povidone-iodine with sterile gauze and antiseptic solutions.
- A local anesthetic is not required.
- Prophylactic antibiotics are not required.
- In Rh-negative women, anti-D immunoglobulin must be administered.[24]
- The ultrasound probe must be covered with a sterile plastic cover. The gel must be kept at the inner surface, as it helps better transmit ultrasound waves. Ideally, sterile gel should be used to decrease the risk and spread of contamination.
Technique or Treatment
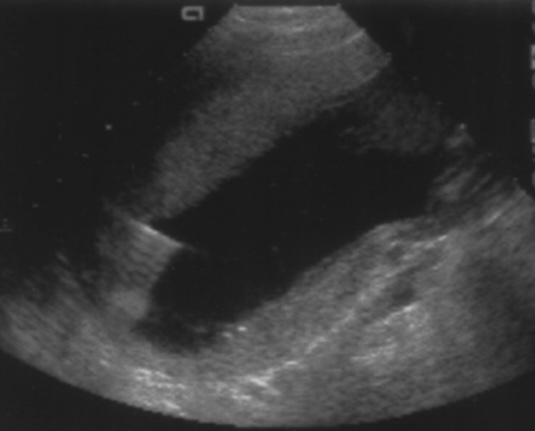
Amniocentesis is done as an outpatient procedure. After proper genetic counseling and informed written consent, a detailed ultrasound is performed to assess the gestational age, placental localization, gross congenital anomalies, maximum vertical pocket (MVP) of amniotic fluid, fetal position, fetal movements, fetal cardiac activity, and amniotic fluid volume. Skin is prepared with povidone-iodine, and sterile ultrasonography gel is applied. After confirming the prerequisites and once the preparation is complete, amniocentesis is performed using the aseptic technique. A 20- to 22-gauge spinal needle is used to enter the amniotic cavity under continuous ultrasound guidance. The entire procedure should be performed in real-time sonographic monitoring with continuous, direct visualization of the spinal needle. The needle is directed to the clear region in the deepest amniotic fluid pocket. Needle insertion in the upper third of the uterus is less painful.[25]
The needle, in ideal conditions, is inserted perpendicular to the skin. The transducer is placed in a way that the ultrasound beam is directed at a 15 to 20-degree angle from the planned track of the needle. Figure 1 shows the ultrasound picture of the inserted needle during the amniocentesis procedure. It must be ensured that fetal parts, umbilical cord, or placenta are not present in the region of needle insertion. Transplacental puncture is usually not the preferred point of entry.[26] Transplacental entry is strictly avoided in cases of alloimmunization or infections to the mother, like human immunodeficiency virus (HIV), hepatitis B virus (HBV), or hepatitis C virus (HCV).[27][28]
A firm entry into the amniotic cavity is recommended to prevent the tenting of the amniotic membrane.[29] Once entry into the cavity is confirmed, amniotic fluid is slowly aspirated. The initial 1 ml to 2 ml of amniotic fluid is discarded because it has the highest chance of maternal cell contamination.[29] Approximately 18 ml to 20 ml of amniotic fluid is required for karyotype testing, and 2 ml to 5 ml is required to test for enzyme deficiency testing. The needle is removed after adequate amniotic fluid has been obtained. Fetal wellbeing is confirmed by assessing fetal cardiac activity regularity and fetal movements. This should be witnessed by the patient on ultrasound post-procedure and documented.
Amniocentesis in twin pregnancies requires the determination of chorionicity and amnionicity. In dichorionic twins, there are two methods: single-needle technique and two-needle insertion technique. In the single needle technique, after collecting amniotic fluid from one sac, the syringe with the amniotic fluid of the first fetus is removed, and the needle pierces through the septum separating the two amniotic sacs to collect the amniotic fluid from the amniotic sac of the second fetus. This technique has a risk of contamination from the first sac. In the two-needle insertion technique, after collecting amniotic fluid from one sac, a small amount of blue-colored indigo carmine dye is injected into the sac of the first twin. Then the needle has pierced the skin and is placed into the sac of the second twin, and fluid is withdrawn. Repeat sampling of blue amniotic fluid allows the operator to know if the amniotic fluid of the first fetus is sampled twice.[30][31]
The patient should be observed after amniocentesis for a short time. She should be instructed to report if there is any vaginal fluid leaking, vaginal bleeding, severe uterine pain, or fever. Routine activities can be resumed after the procedure, but the patient should avoid strenuous exercise.
Amniocentesis done without ultrasound guidance has 5 to 10 percent failure rates, where no amniotic fluid is aspirated.[32] Sometimes when amniocentesis is attempted using a suprapubic needle insertion, there is doubt of the fluid aspirated, be it maternal urine or amniotic fluid. The presence of urea and potassium in maternal urine and crystalline arborization pattern of dried amniotic fluid in slide observed under magnification confirms the fluid origin.[33][34]
Complications
Both maternal and fetal complications can occur with amniocentesis.
Maternal Complications
- There is an estimated 2.6% risk of fetomaternal hemorrhage.
- Maternal isoimmunization in Rh-negative cases.
- There is minimal chance of introducing skin bacteria into the amniotic cavity. The risk of chorioamnionitis and uterine infections is less than 0.1%.
- The procedure increases the risk of preterm labor in the third-trimester amniocentesis. Preterm premature rupture of membrane and oligohydramnios are common complications encountered following amniocentesis.
- There is a 2% to 3% risk of vaginal bleeding.
- Post-procedure pain and maternal discomfort: Mean pain intensity described is 1.6+/-1.3 when noted on a scale of 0 to 7.[35]
- Amniotic fluid embolism, very rare
- There can be hematoma of maternal skin or intestinal or internal organ injuries.
Fetal Complications
- On average, the fetal loss rate associated with amniocentesis is 0.11%. The loss is 0.56% within 28 days and 0.09% within 42 days.[29]
- The risk of fetal loss is higher in women who are otherwise at a higher risk of miscarriage, such as women carrying fetuses with structural malformations, fibroids, obese women, and women with vaginal infections at the time of the procedure. Amniocentesis in up to 86.0% of the patients is safe and free from complications.[36] The risk of fetal loss in case of multiple pregnancies is higher.
- Amniotic fluid leak: 1% to 2%, and usually associated with spontaneous sealing of membranes.[29] It may also result in:
- Fetal lung hypoplasia
- Respiratory distress
- Fetal injuries like bleeding from the cord, ocular injuries, and postural deformities like talipes equinovarus (clubfoot) might occur.
- The risk of complications is high when more than or equal to 3 pricks are used to obtain amniotic fluid. In ideal conditions, if an adequate fluid sample is not obtained in two pricks, the procedure should be abandoned for 24 hours, after which it can be re-attempted. The risk is less in experienced hands, i.e., people who perform more than 300 procedures/year.
Clinical Significance
Amniocentesis procedure is relatively safe, with fewer complications among experienced hands. The location of the placenta is an important factor in amniocentesis. While performing the procedure, one should try to avoid penetration of the placenta. The anterior and fundal placenta is associated with more complications, including multiple pricks and blood-stained liquor; however, it is not associated with increased fetal loss rates.[37] Passing the needle through the placenta is slightly associated with increased preterm birth rates.[38]
After the amniocentesis procedure, the sample of amniotic fluid is taken to a laboratory for testing. Results usually take ten days to three weeks depending upon the laboratory. In the laboratory, genetic and chemical tests are done. For genetic tests, specific chromosomes and genes undergo analysis.
A. For chromosomal and genetic testing, the amniotic fluid is sent for a conventional cell culture report, which is obtained in 14 days. Rapid chromosomal preparations are available that give results in 1 to 2 days, including fluorescent in-situ hybridization (FISH) and quantitative fluorescence polymerase chain reaction (QF-PCR).
Commonly Performed Tests
1. Rapid test: A rapid test looks for abnormalities on specific chromosomes. This test is almost 100% accurate. A rapid test can identify some chromosomal conditions that cause physical and mental abnormalities. These are:
- Down's syndrome: caused by extra chromosome 21
- Edward's syndrome: caused by extra chromosome 18
- Patau's syndrome: caused by an extra chromosome 13
2. Full karyotype:
The cells in the sample of amniotic fluid are grown for up to 10 days in a laboratory before being examined under a microscope to check for:
- Number of chromosomes
- Appearance of chromosomes
Results from a full karyotype will usually be available in two or three weeks. Amniocentesis in the third trimester has higher cell culture failure rates.
Uncommon Tests
- Chromosomal microarray: Chromosomal microarray analysis detects a pathogenic copy number variant in approximately 1.7% of patients with a normal karyotype and normal ultrasound examination findings. It can detect chromosomal abnormalities in approximately 6% of the fetuses with normal karyotype and structural abnormalities on ultrasound. Hence, all women opting for invasive prenatal diagnostic testing should be offered chromosomal microarray testing.[39]
- Molecular amniotic fluid testing helps diagnose single-gene conditions, including X-linked, recessive, and dominant conditions. Parental mutation should be known preferably before offering prenatal molecular testing for a fetal diagnosis. Ultrasound diagnosis usually indicates molecular testing of a particular condition.[40][41]
- Various laboratory tests with special testing requirements, such as peroxisomal, biochemical, or methylation conditions, are useful for diagnosing specific genetic conditions. Methylation study in amniotic fluid helps diagnose fragile X syndrome.[42]
B. Amniotic fluid is analyzed for the presence of proteins, minerals, and other compounds. Amniocentesis results will either be positive or negative. Acetylcholinesterase (AChE), in conjunction with elevated alpha-fetoprotein (AFP) in amniotic fluid, is considered diagnostic for open neural tube defects.[43]
C. Turbid amniotic fluid should be sent for microbiological culture to detect the presence of any organism. Antibiotic coverage should be given to the patient. Occasionally, brown or green amniotic fluid is aspirated during amniocentesis due to intra- amniotic hemorrhage before the amniocentesis procedure due to the breakdown of blood products.[44] Various studies have shown higher fetal loss rates with the aspiration of such discolored fluid.[45][5]
For most chromosomal conditions, there is no cure, so the couple needs to be appropriately counseled regarding the continuation of pregnancy.
Enhancing Healthcare Team Outcomes
The decision to perform amniocentesis and to convey the results to the couple requires communication between geneticists and fetal medicine experts operating as an interdisciplinary and interprofessional team. Geneticists must counsel the patient to know the possibility of the fetus being affected by a genetic disease. The genetic counselors should educate themselves and verify the availability of the required laboratory testing facilities prior to suggesting a prenatal diagnostic test to the patient. The operator should be aware of the type of samples required to be collected, the type of procedure to be performed, knowledge of the sample collection instrument, sample storage and handling instructions, and prescribed ways to transport the samples.
Following the counseling session, the patient is referred for the procedure. While performing the procedure, coordination amongst the team is required. Once the needle is inside the amniotic cavity, the assistant should carefully and promptly withdraw the amniotic fluid so that the procedure is performed in the minimal time possible and with minimal risk of needle displacement. Following the test, the implications of the report should be conveyed to the patient and her family by the geneticists. Consultation with the pediatrician and disability center counselors can be arranged for the patient if required. Meetings with the diagnosed disability social support group and families dealing with a similar disability in a family member help the patient make a proper decision.
Media
(Click Image to Enlarge)
References
Eddleman KA, Malone FD, Sullivan L, Dukes K, Berkowitz RL, Kharbutli Y, Porter TF, Luthy DA, Comstock CH, Saade GR, Klugman S, Dugoff L, Craigo SD, Timor-Tritsch IE, Carr SR, Wolfe HM, D'Alton ME. Pregnancy loss rates after midtrimester amniocentesis. Obstetrics and gynecology. 2006 Nov:108(5):1067-72 [PubMed PMID: 17077226]
Piantelli G, Bedocchi L, Cavicchioni O, Verrotti C, Cavallotti D, Fieni S, Gramellini D. Amnioreduction for treatment of severe polyhydramnios. Acta bio-medica : Atenei Parmensis. 2004:75 Suppl 1():56-8 [PubMed PMID: 15301292]
Elliott JP, Urig MA, Clewell WH. Aggressive therapeutic amniocentesis for treatment of twin-twin transfusion syndrome. Obstetrics and gynecology. 1991 Apr:77(4):537-40 [PubMed PMID: 2002975]
Nizard J. Amniocentesis: technique and education. Current opinion in obstetrics & gynecology. 2010 Apr:22(2):152-4. doi: 10.1097/GCO.0b013e32833723a0. Epub [PubMed PMID: 20098324]
Level 3 (low-level) evidenceOdibo AO, Gray DL, Dicke JM, Stamilio DM, Macones GA, Crane JP. Revisiting the fetal loss rate after second-trimester genetic amniocentesis: a single center's 16-year experience. Obstetrics and gynecology. 2008 Mar:111(3):589-95. doi: 10.1097/AOG.0b013e318162eb53. Epub [PubMed PMID: 18310360]
Level 2 (mid-level) evidenceQuinlan MP. Amniocentesis: indications and risks. The virtual mentor : VM. 2008 May 1:10(5):304-6. doi: 10.1001/virtualmentor.2008.10.5.cprl1-0805. Epub 2008 May 1 [PubMed PMID: 23211983]
Kirchesch H. [Infection with parvovirus B19: typical course of erythema infectiosum and its complications]. Zeitschrift fur Hautkrankheiten. 1990 Nov:65(11):1007-10 [PubMed PMID: 1964320]
Lind T, Kendall A, Hytten FE. The role of the fetus in the formation of amniotic fluid. The Journal of obstetrics and gynaecology of the British Commonwealth. 1972 Apr:79(4):289-98 [PubMed PMID: 5025135]
Bronshtein M, Yoffe N, Brandes JM, Blumenfeld Z. First and early second-trimester diagnosis of fetal urinary tract anomalies using transvaginal sonography. Prenatal diagnosis. 1990 Oct:10(10):653-66 [PubMed PMID: 2274490]
Abramovich DR. Fetal factors influencing the volume and composition of liquor amnii. The Journal of obstetrics and gynaecology of the British Commonwealth. 1970 Oct:77(10):865-77 [PubMed PMID: 4919671]
Abramovich DR, Garden A, Jandial L, Page KR. Fetal swallowing and voiding in relation to hydramnios. Obstetrics and gynecology. 1979 Jul:54(1):15-20 [PubMed PMID: 377164]
van Otterlo LC, Wladimiroff JW, Wallenburg HC. Relationship between fetal urine production and amniotic fluid volume in normal pregnancy and pregnancy complicated by diabetes. British journal of obstetrics and gynaecology. 1977 Mar:84(3):205-9 [PubMed PMID: 843496]
Wladimiroff JW, Campbell S. Fetal urine-production rates in normal and complicated pregnancy. Lancet (London, England). 1974 Feb 2:1(7849):151-4 [PubMed PMID: 4129720]
Brace RA, Wolf EJ. Normal amniotic fluid volume changes throughout pregnancy. American journal of obstetrics and gynecology. 1989 Aug:161(2):382-8 [PubMed PMID: 2764058]
ELLIOTT PM, INMAN WH. Volume of liquor amnii in normal and abnormal pregnancy. Lancet (London, England). 1961 Oct 14:2(7207):835-40 [PubMed PMID: 13889956]
Queenan JT, Thompson W, Whitfield CR, Shah SI. Amniotic fluid volumes in normal pregnancies. American journal of obstetrics and gynecology. 1972 Sep 1:114(1):34-8 [PubMed PMID: 4637037]
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 88, December 2007. Invasive prenatal testing for aneuploidy. Obstetrics and gynecology. 2007 Dec:110(6):1459-67 [PubMed PMID: 18055749]
Cutler J, Chappell LC, Kyle P, Madan B. Third trimester amniocentesis for diagnosis of inherited bleeding disorders prior to delivery. Haemophilia : the official journal of the World Federation of Hemophilia. 2013 Nov:19(6):904-7. doi: 10.1111/hae.12247. Epub 2013 Aug 6 [PubMed PMID: 23919291]
Bruner JP, Crean DM. Equalization of amniotic fluid volumes after decompression amniocentesis for treatment of the twin oligohydramnios-polyhydramnios sequence. Fetal diagnosis and therapy. 1999 Mar-Apr:14(2):80-5 [PubMed PMID: 10085504]
Floridia M, Masuelli G, Meloni A, Cetin I, Tamburrini E, Cavaliere AF, Dalzero S, Sansone M, Alberico S, Guerra B, Spinillo A, Chiadò Fiorio Tin M, Ravizza M, Italian Group on Surveillance on Antiretroviral Treatment in Pregnancy. Amniocentesis and chorionic villus sampling in HIV-infected pregnant women: a multicentre case series. BJOG : an international journal of obstetrics and gynaecology. 2017 Jul:124(8):1218-1223. doi: 10.1111/1471-0528.14183. Epub 2016 Jun 20 [PubMed PMID: 27319948]
Level 2 (mid-level) evidenceEkoukou D, Khuong-Josses MA, Ghibaudo N, Mechali D, Rotten D. Amniocentesis in pregnant HIV-infected patients. Absence of mother-to-child viral transmission in a series of selected patients. European journal of obstetrics, gynecology, and reproductive biology. 2008 Oct:140(2):212-7. doi: 10.1016/j.ejogrb.2008.04.004. Epub 2008 Jun 27 [PubMed PMID: 18584937]
Level 2 (mid-level) evidenceSimões M, Marques C, Gonçalves A, Pereira AP, Correia J, Castela J, Guerreiro C. Amniocentesis in HIV pregnant women: 16 years of experience. Infectious diseases in obstetrics and gynecology. 2013:2013():914272. doi: 10.1155/2013/914272. Epub 2013 Jul 21 [PubMed PMID: 23970821]
Level 2 (mid-level) evidenceDubil EA, Magann EF. Amniotic fluid as a vital sign for fetal wellbeing. Australasian journal of ultrasound in medicine. 2013 May:16(2):62-70. doi: 10.1002/j.2205-0140.2013.tb00167.x. Epub 2015 Dec 31 [PubMed PMID: 28191176]
Wysowski DK, Flynt JW Jr, Goldberg MF, Connell FA. Rh hemolytic disease. Epidemiologic surveillance in the United States, 1968 to 1975. JAMA. 1979 Sep 28:242(13):1376-9 [PubMed PMID: 113567]
Lekskul N, Tannirandorn Y. The location of needle insertion effect on maternal pain in amniocentesis. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2006 Oct:89 Suppl 4():S137-41 [PubMed PMID: 17725150]
Level 3 (low-level) evidenceTabor A, Philip J, Bang J, Madsen M, Obel EB, Nørgaard-Pedersen B. Needle size and risk of miscarriage after amniocentesis. Lancet (London, England). 1988 Jan 23:1(8578):183-4 [PubMed PMID: 2893021]
Level 3 (low-level) evidenceLópez M, Coll O. Chronic viral infections and invasive procedures: risk of vertical transmission and current recommendations. Fetal diagnosis and therapy. 2010:28(1):1-8. doi: 10.1159/000309155. Epub 2010 Jun 19 [PubMed PMID: 20558971]
Davies G, Wilson RD, Désilets V, Reid GJ, Shaw D, Summers A, Wyatt P, Young D, Society of Obstetricians and Gynaecologists of Canada. RETIRED: Amniocentesis and women with hepatitis B, hepatitis C, or human immunodeficiency virus. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstetrique et gynecologie du Canada : JOGC. 2003 Feb:25(2):145-48, 149-52 [PubMed PMID: 12577132]
Level 1 (high-level) evidenceGhi T, Sotiriadis A, Calda P, Da Silva Costa F, Raine-Fenning N, Alfirevic Z, McGillivray G, International Society of Ultrasound in Obstetrics and Gynecology (ISUOG). ISUOG Practice Guidelines: invasive procedures for prenatal diagnosis. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2016 Aug:48(2):256-68. doi: 10.1002/uog.15945. Epub [PubMed PMID: 27485589]
Level 1 (high-level) evidenceVink J, Wapner R, D'Alton ME. Prenatal diagnosis in twin gestations. Seminars in perinatology. 2012 Jun:36(3):169-74. doi: 10.1053/j.semperi.2012.02.008. Epub [PubMed PMID: 22713497]
Weisz B, Rodeck CH. Invasive diagnostic procedures in twin pregnancies. Prenatal diagnosis. 2005 Sep:25(9):751-8 [PubMed PMID: 16170858]
Simpson NE, Dallaire L, Miller JR, Siminovich L, Hamerton JL, Miller J, McKeen C. Prenatal diagnosis of genetic disease in Canada: report of a collaborative study. Canadian Medical Association journal. 1976 Oct 23:115(8):739-48 [PubMed PMID: 61796]
Guibaud S, Bonnet M, Dury A, Thoulon JM, Dumont M. Letter: Amniotic fluid or maternal urine? Lancet (London, England). 1976 Apr 3:1(7962):746 [PubMed PMID: 56559]
Level 3 (low-level) evidenceElias S, Martin AO, Patel VA, Gerbie A, Simpson JL. Analysis for amniotic fluid crystallization in second-trimester amniocentesis. American journal of obstetrics and gynecology. 1979 Feb 15:133(4):401-4 [PubMed PMID: 434004]
Level 1 (high-level) evidenceHarris A, Monga M, Wicklund CA, Robbins-Furman PJ, Strecker MN, Doyle NM, Mastrobattista J. Clinical correlates of pain with amniocentesis. American journal of obstetrics and gynecology. 2004 Aug:191(2):542-5 [PubMed PMID: 15343234]
Jummaat F, Ahmad S, Mohamed Ismail NA. 5-Year review on amniocentesis and its maternal fetal complications. Hormone molecular biology and clinical investigation. 2019 Sep 20:40(2):. pii: /j/hmbci.2019.40.issue-2/hmbci-2019-0006/hmbci-2019-0006.xml. doi: 10.1515/hmbci-2019-0006. Epub 2019 Sep 20 [PubMed PMID: 31539354]
Kalogiannidis I, Prapa S, Dagklis T, Karkanaki A, Petousis S, Prapas Y, Prapas N. Amniocentesis-related adverse outcomes according to placental location and risk factors for fetal loss after midtrimester amniocentesis. Clinical and experimental obstetrics & gynecology. 2011:38(3):239-42 [PubMed PMID: 21995155]
Chaksuwat P, Wanapirak C, Piyamongkol W, Sirichotiyakul S, Tongprasert F, Srisupundit K, Luewan S, Traisrisilp K, Jatavan P, Tongsong T. A comparison of pregnancy outcomes after second-trimester amniocentesis between cases with penetration of the placenta and nonpenetration. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2021 Dec:34(23):3883-3888. doi: 10.1080/14767058.2019.1702017. Epub 2020 Apr 16 [PubMed PMID: 32299277]
Level 3 (low-level) evidence. Practice Bulletin No. 162 Summary: Prenatal Diagnostic Testing for Genetic Disorders. Obstetrics and gynecology. 2016 May:127(5):976-978. doi: 10.1097/AOG.0000000000001438. Epub [PubMed PMID: 27101119]
Eisenberg B, Wapner RJ. Clinical proceduress in prenatal diagnosis. Best practice & research. Clinical obstetrics & gynaecology. 2002 Oct:16(5):611-27 [PubMed PMID: 12475543]
South ST, Chen Z, Brothman AR. Genomic medicine in prenatal diagnosis. Clinical obstetrics and gynecology. 2008 Mar:51(1):62-73. doi: 10.1097/GRF.0b013e3181616509. Epub [PubMed PMID: 18303500]
McConkie-Rosell A, Finucane B, Cronister A, Abrams L, Bennett RL, Pettersen BJ. Genetic counseling for fragile x syndrome: updated recommendations of the national society of genetic counselors. Journal of genetic counseling. 2005 Aug:14(4):249-70 [PubMed PMID: 16047089]
Cheschier N, ACOG Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin. Neural tube defects. Number 44, July 2003. (Replaces committee opinion number 252, March 2001). International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2003 Oct:83(1):123-33 [PubMed PMID: 14626221]
Level 1 (high-level) evidenceHankins GD, Rowe J, Quirk JG Jr, Trubey R, Strickland DM. Significance of brown and/or green amniotic fluid at the time of second trimester genetic amniocentesis. Obstetrics and gynecology. 1984 Sep:64(3):353-8 [PubMed PMID: 6205335]
Cruikshank DP, Varner MW, Cruikshank JE, Grant SS, Donnelly E. Midtrimester amniocentesis. An analysis of 923 cases with neonatal follow-up. American journal of obstetrics and gynecology. 1983 May 15:146(2):204-11 [PubMed PMID: 6189400]
Level 3 (low-level) evidence